Abstract
Plant development is characterized by a profound ability to regenerate and form tissues with new axes of polarity. An unsolved question concerns how the position within a tissue and cues from neighboring cells are integrated to specify the polarity of individual cells. The canalization hypothesis proposes a feedback effect of the phytohormone auxin on the directionality of intercellular auxin flow as a means to polarize tissues. Here we identify a cellular and molecular mechanism for canalization. Local auxin application, wounding, or auxin accumulation during de novo organ formation lead to rearrangements in the subcellular polar localization of PIN auxin transport components. This auxin effect on PIN polarity is cell-specific, does not depend on PIN transcription, and involves the Aux/IAA-ARF (indole-3-acetic acid-auxin response factor) signaling pathway. Our data suggest that auxin acts as polarizing cue, which links individual cell polarity with tissue and organ polarity through control of PIN polar targeting. This feedback regulation provides a conceptual framework for polarization during multiple regenerative and patterning processes in plants.
Keywords: Auxin signaling, auxin transport, cell and tissue polarity, lateral root development, phyllotaxis, vasculature development
The exceptional flexibility of plant development can be seen as an adaptation to the sessile lifestyle and as alternative to behavioral responses of animals. It is mainly characterized by post-embryonic de novo organ formation and a high capacity for regeneration. Examples of these events include the initiation of new leaves, flowers or roots, regeneration of organs from undifferentiated callus, and regeneration of tissues following wounding (Steeves and Sussex 1989).
New organs or regenerated tissues are formed at distinct positions relative to pre-existing structures and frequently display new axes of polarity. Tissue repatterning can occur also in mature tissues and often includes the complete respecification of former tissue polarity. The process of tissue polarization inevitably encompasses de novo specification of individual cell polarity in cells within a polarizing tissue. An intriguing problem of these phenomena is how the activities of surrounding cells and the orientation of the whole tissue or organ are integrated to specify the orientation of a single cell (Sachs 1991). Common models of tissue patterning do not suffice here, as gene activities that underlie cell specification and differentiation are, as such, not directional. Therefore, additional intercellular polarizing signals are required. A long history of elegant physiological experiments established the plant signaling molecule auxin as a key player in organogenesis and vascular tissue formation (for review, see Reinhardt 2005). This, in conjunction with the unique property of auxin being polarly transported from cell to cell through whole tissues, led to the formulation of the “canalization hypothesis.” This theory proposes a polarizing effect of auxin on tissues based on a feedback effect that auxin exerts on the polarity of its transport at the single cell level (Sachs 1981, 1991). This gradual reinforcement of cellular polarity by polar auxin flow itself would thus provide the connection between cellular and tissue polarity. It has been proposed that such a mechanism could account for polarizing events in multiple developmental processes including regeneration following wounding, establishment of vascular venation pattern, embryo axis formation, and organogenesis (Berleth and Sachs 2001). A recent study by Scarpella et al. (2006) provides evidence for an auxin transport-driven mechanism in leaf venation patterning. Also several recent mathematical models for the phyllotactic pattern of leaf initiation all incorporate an influence of auxin on the directionality of its own transport as a necessary parameter (de Reuille et al. 2006; Jönsson et al. 2006; Smith et al. 2006). Nonetheless, the critical requirement for these models—the auxin effect on the polarity of its own transport or the underlying cellular mechanism—has not been demonstrated experimentally.
Here we identify the cellular and molecular mechanisms underlying the proposed models. Auxin influences the polarity of its own flow at the single cell level via Aux/IAA-ARF (indole-3-acetic acid-auxin response factor)-dependent regulation of polar targeting of PIN auxin transport components. We demonstrate that this feedback regulation underlies regeneration and de novo formation of vascular strands as well as post-embryonic organ formation.
Results
Auxin is sufficient to change subcellular PIN polarity in roots
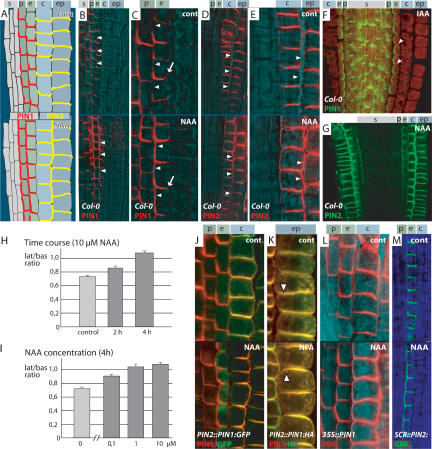
Physiological experiments suggested that auxin is needed to maintain the capacity for polar auxin transport, although the underlying mechanisms remain elusive (Morris 2000). We became interested whether auxin not only was required to maintain polar auxin transport capacity, but also could act on the directionality of its transport by modifying the polarity of PIN localization. PIN proteins are critical components of cellular auxin efflux (Petrášek et al. 2006) and their subcellular polarity has been shown to determine directionality of auxin flow (Wiśniewska et al. 2006). We used Arabidopsis thaliana primary roots as a model to examine potential effects of auxin on PIN polarity. PIN1 is localized at the basal (lower) side of stele cells; in pericycle and endodermis cells it is basal with some spreading to the inner lateral side (Fig. 1A–C, upper panels; Friml et al. 2002). PIN2 is localized at the apical (upper) side of epidermis cells; in young cortex cells it is mainly at the basal side with weak lateral spreading (Fig. 1A,D,E; Müller et al. 1998). A recent study on Arabidopsis root meristem regeneration using laser-based ablation of cells did not reveal any perturbations of PIN polarity following ablation-induced changes in auxin distribution in wild-type roots (Xu et al. 2006). However, direct incubation of roots in solutions with biologically active auxins such as IAA or NAA (naphthalene-1-acetic acid) led to a clear change of PIN1 and PIN2 polarity in particular cell types, which have not been primarily investigated in the ablation study (Fig. 1A–G; Supplementary Fig. 1A). PIN1 was not affected in the stele, but in pericycle and endodermis cells it relocated to the inner lateral side (Fig. 1A–C, cf. arrowheads in lower and upper panels) almost completely after prolonged treatment (6 h) (Fig. 1F, arrowheads indicate loss of PIN1 signal at basal cell sides). On the other hand, PIN2 was not affected in epidermis cells, but relocated to the outer lateral side in cortex cells (Fig. 1A,D,E, cf. arrowheads in lower and upper panels). After prolonged treatments (12 h), PIN2 was strongly localized to the outer side of cortex cells, and also ectopically up-regulated in endodermis and stele cells (Fig. 1G). In addition to biologically active auxins, treatment with auxin efflux inhibitors such as 1-N-naphthylphthalamic acid (NPA) also led to similar changes in PIN polarity (Supplementary Fig. 1B), probably as a consequence of increased cellular auxin levels. To evaluate the auxin effect on PIN polarity quantitatively, we measured the ratio between PIN1 fluorescence intensity at the basal and the inner lateral membrane in endodermis cells (see Materials and Methods for detailed description). This allowed us to assess the concentration-and time-dependency of the auxin effect on PIN polarity. The relocation effect was significant after 2 h (P < 0.005) and more pronounced after 4 h (P < 0.001) of auxin treatment (Fig. 1H; see Supplementary Table 1 for actual values). Already 0.1 μM NAA led to a significant lateralization (P < 0.001), which further increased at 1 μM (P < 0.005). There was no further increase in the effect (P = 0.42) at 10 μM, suggesting saturation (Fig. 1I; see Supplementary Table 1 for actual values). In all following experiments, we used 10 μM of NAA for 4 h as standard auxin treatment. Taken together, these results demonstrate that auxin, besides influencing PIN transcription (Vieten et al. 2005), also induces changes of PIN1 and PIN2 polarity in certain cell types in a time-and concentration-dependent manner already at low concentrations.
Figure 1.
Auxin-dependent changes of PIN polarity in Arabidopsis roots. PIN1 or PIN2 subcellular localization; upper panels show mock, lower panels show auxin treatments (10 μM NAA for 4 h, unless otherwise stated). (A) Scheme of PIN1 localization (red) and PIN2 (yellow) in root meristem. Indexes indicate stele (s), peri-cycle (p), endodermis (e), cortex (c), and epidermis (ep) cell files. Black rectangle indicates approximate position of micro-graphs. (B) Overview picture of PIN1 relocation after auxin treatment to the entire inner endodermis cell side (lower panel, arrowheads). (C) Like B, close up. PIN1 relocation after auxin treatment to the entire inner endodermis cell side (lower panel, arrowheads), loss of signal at the outer side (arrow). (D) Overview picture of PIN2 relocation to the outer cortex cell side after auxin treatment (lower panel, arrowheads). (E) Like D, close up. PIN2 relocation to the outer cortex cell side after auxin treatment (lower panel, arrowheads). (F) Almost complete PIN1 relocation in endodermis cells following 6 h of 10 μM IAA treatment. (G) PIN2 relocation after 6 h of 10 μM NAA treatment. (H,I) Quantitative evaluation of auxin-depen-dent PIN1 lateralization in endodermis cells. Graph shows mean ratio of lateral to basal signal intensity; bars indicate standard error (see Materials and Methods section for detailed description). Time (H) and concentration (I) dependence of auxin effect using 10 μM NAA for different time points or 4-h treatments with different NAA concentrations, respectively. (J,K) Auxin-dependent relocation of ectopically expressed PIN1 in cortex of PIN2::PIN1:GFP-3 after 24 h 5 μM NAA treatment (J), in epidermis of PIN2::PIN1:HA after 12 h 20 μM NPA treatment; arrowheads indicate switch from basal to apical localization (K) and in 35S::PIN1 (L). (M) Relocation of ectopically expressed PIN2 in endodermis of SCR::PIN2:GFP. Immunolocalization of PIN1 or PIN2 (B–G,J–L) with signal in red (B–E,L) or green (F,G); colocalization of PIN1 (red) and GFP (green) (J); colocalization of PIN1 (green) and HA (red) (K); PIN2:GFP signal green (M); transmitted light images in cyan or blue. Genotype and antibodies (color coded) are given in the lower left corner and treatment in the upper right corner.
Changes in PIN polarity are independent of transcriptional control and depend on cell type-specific determinants
Auxin treatment leads to relocation of PIN1 to the inner and PIN2 to the outer cell sides. This clear difference in polarity raises the question whether it results from inherent properties of the two PIN proteins or if the direction of lateralization is dependent on the cell type. A laser ablation-based study had previously linked auxin-dependent changes of PIN polarity with cell type-deter-mining factors (Xu et al. 2006). To clarify this further, we examined PIN1 relocation in transgenic lines, which ec-topically express PIN1 (35S::PIN1) and PIN1:GFP (PIN2::PIN1:GFP-3) in cortex cells. After treatment with auxin, PIN1:GFP (Fig. 1J) and PIN1 (Fig. 1L) relocated to the outer cortex sides similarly to PIN2. In a complementary experiment, we tested the auxin effect on PIN2:GFP expressed in endodermis cells (SCR::PIN2:GFP, Xu et al. 2006). Here, the originally predominantly basally localized PIN2:GFP relocated to the inner cell side following auxin treatment (Fig. 1M), exhibiting the same behavior as PIN1 in this cell type. Thus, the decision about lateral polarity induced by auxin appears to be cell type-specific and not strictly dependent on inherent signals in the PIN proteins. In addition, ectopically expressed PIN1 (PIN2::PIN1:HA), which is mislocalized in epidermis cells to the basal (lower) side (Wiśniewska et al. 2006), largely recovers its “PIN2-like” localization to the apical (upper) side following auxin or NPA treatment (Fig. 1K). This result reveals that epidermis cells are also competent for auxin-dependent PIN relocation, but in this specific case to the apical cell side. This explains compensatory expression and correct localization of PIN1 in epidermis cells of the pin2 mutant, which is the base for their functional redundancy (Vieten et al. 2005).
Importantly, the experiments with 35S::PIN1 and SCR::PIN2:GFP show that the change in PIN polarity in response to auxin is not a consequence of auxin-depen-dent up-regulation of PIN transcription (Schrader et al. 2003; Vieten et al. 2005), as neither of these promoters are regulated by auxin.
Known components of polarity and cycling are not required for auxin-dependent changes in PIN polarity
Next we addressed the cellular and molecular mechanism underlying the auxin-dependent changes in PIN polarity. Inhibition of protein synthesis by cycloheximide (CHX) led to a change in PIN1 localization. The preferential localization to the inner lateral sides in endodermis and pericycle cells was abolished and PIN1 localized to both inner and outer lateral sides in these cells, resulting in a U-shaped signal (Supplementary Fig. 1C, cf. A and B). Coincubation with auxin changed neither PIN localization nor signal strength (Supplementary Fig. 1C). This finding suggests that the preferential localization of PIN1 protein to one side of the cell requires the constant synthesis of an unknown regulator, and this or another labile factor is also needed for auxin-dependent changes of PIN polarity.
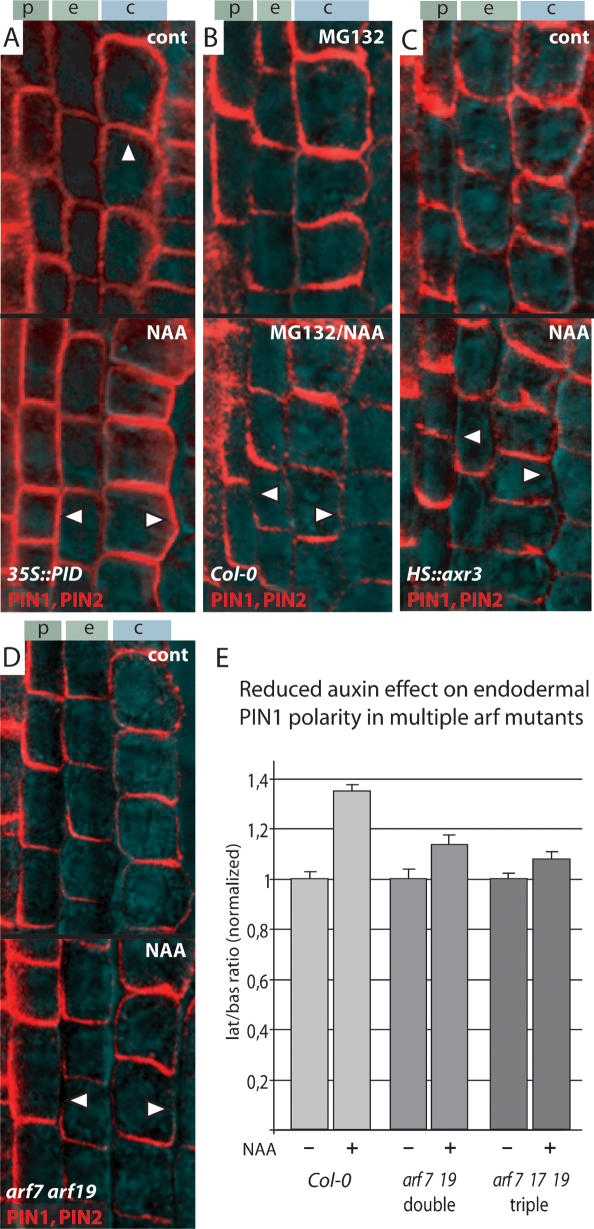
PIN proteins are known to cycle constitutively between the plasma membrane and endosomal compartments (Geldner et al. 2001). These subcellular dynamics require the ARF-GEF protein GNOM as endosomal regulator of vesicle trafficking (Shevell et al. 1994; Geldner et al. 2003). Some instances of rapid PIN relocalization (Benková et al. 2003; Friml et al. 2003b; Reinhardt et al. 2003; Heisler et al. 2005) possibly depend on GNOM-mediated PIN cycling. Furthermore, auxin itself influences PIN cycling by a yet unknown pathway involving the protein BIG (Paciorek et al. 2005). Apart from these, the only identified component of PIN polar targeting is the protein kinase PINOID (PID) (Christensen et al. 2000; Friml et al. 2004). Its expression is regulated by auxin (Benjamins et al. 2001), which provides a potential link between auxin and PIN polarity. We tested the auxin effect on PIN1 and PIN2 polarity in mutant or transgenic lines, where GNOM, BIG, or PID function was genetically altered. A partial gnom loss-of-function allele (van7), which shows strong defects in seedling development (Koizumi et al. 2000), did not visibly interfere with auxin-dependent lateralization of PIN1 and PIN2 (Supplementary Fig. 1D). Also, the big allele umbrella (Kanyuka et al. 2003), which is impaired in auxin-depen-dent inhibition of PIN cycling (Paciorek et al. 2005), showed normal responses of PIN1 and PIN2 polarities to auxin (Supplementary Fig. 1E). Finally, constitutive (35S::PID) or conditional (pINTAM>>PID) overexpression of PID, which causes a complete switch of PIN polarity to the apical cell sides (Friml et al. 2004), did not affect auxin-induced PIN1 and PIN2 lateralization (Fig. 2A). These data imply that auxin does not strictly require GNOM-, BIG-, or PID-dependent pathways for its effect on PIN polarity.
Figure 2.
Molecular requirements for auxin-dependent changes of PIN polarity. Immunolocalization of PIN1 and PIN2; upper panels show mock, lower panels show 10 μM NAA treatment for 4 h. (A) Overexpression of PINOID protein kinase in 35S::PID leads to apicalization of PIN polarity (upper panel, arrowhead) but has no effect on auxin-dependent relocation to the lateral cell sides (lower panel, arrowheads). (B) Pretreatment with 26S proteasome inhibitor MG132 reduces lateral PIN relocation after auxin treatment (lower panel, arrowheads; cf. A). (C) Heat-shock-induced overexpression of axr3 in HS::axr3-1 abolishes the lateral PIN relocation after auxin treatment (lower panel, arrowheads; cf. A). (D) PIN lateralization after auxin treatment is reduced in arf7 arf19 double mutants (lower panel, arrowheads; cf. A). (E) Quantitative evaluation confirms reduction of auxin-dependent PIN1 relocation in endodermis cells of arf7 arf19 double and arf7 arf17 arf19 triple mutants. Graph shows mean ratio of lateral to basal signal intensity of PIN1 in endodermis cells, standardized to the corresponding untreated control; bars indicate standard error (see Materials and Methods for detailed description). (A–D) Immunolocalization signals in red, transmitted light images cyan. Genotype and antibodies (color-coded) are given in the lower left corner and treatment in the upper right corner.
Auxin utilizes an Aux/IAA-ARF-dependent pathway for modulation of PIN polarity
A molecularly well characterized mechanism of cellular auxin signaling is the SCFTIR1 ubiquitin ligase-depen-dent pathway for targeted degradation of transcriptional repressors of the Aux/IAA class (for review, see Woodward and Bartel 2005). Auxin binds directly to the F-box protein TIR1 (Dharmasiri et al. 2005; Kepinski and Leyser 2005) and promotes the interaction with Aux/IAAs, which then are presumably ubiquitinated and degraded by the 26S proteasome. Subsequently, ARFs are released from their inhibition and mediate auxin-dependent gene expression. The auxin effect on PIN polarity requires the function of the 26S proteasome, since pretreatment of the roots with MG-132, a 26S proteasome inhibitor, strongly reduced auxin-dependent lateralization of PIN1 and PIN2 (Fig. 2B). In addition, in gain-of-function alleles of IAA17 (axr3) and an inducible overexpressing line HS::axr3, which both are impaired in auxin signaling (Knox et al. 2003), PIN1 and PIN2 relocation in response to auxin was abolished (Fig. 2C; Supplementary Fig. 1F). As Aux/IAA proteins act as upstream repressors of ARF transcription factors, we investigated whether arf loss-of-function mutants exhibited reduced PIN relocation in response to auxin. We chose nph4/arf7 single, arf7 arf19 double and arf7 arf17 arf19 triple mutants because for ARF7 and ARF19, expression in the primary root—and also some degree of functional redundancy in organogenic processes—has been shown (Wilmoth et al. 2005). While a single nph4/arf 7 mutant showed no detectable reduction (data not shown), we found that in arf7 arf19 double and arf7 arf16 arf19 or arf7 arf17 arf19 triple mutants, auxin-dependent PIN relocation was reduced (Fig. 2D; data not shown). Quantification of the auxin-depen-dent lateralization of PIN1 in endodermis cells (see Materials and Methods section for details) confirmed a significant (P < 0.001) decrease in auxin-induced lateralization in the double mutant, which was even more pronounced (P < 0.001) in the triple mutant (Fig. 2E; see Supplementary Table 1 for actual values). In summary, these data show that the auxin effect on PIN polarity involves the Aux/IAA-ARF-dependent auxin signaling pathway.
Wounding induces PIN expression and polarity changes for regeneration of vasculature
Regeneration of vascular tissue following its disruption by wounding is a prime process, which requires repolarization of adult tissue and has been the basis for formulation of the canalization hypothesis (Sachs 1991). The classical experiments to investigate polarity during regeneration and de novo formation of vascular tissue were performed in pea (Pisum sativum) epicotyls (Sachs 1981). Unfortunately, comparable treatments in Arabidopsis hypocotyls led to a different reaction, mainly to induction of adventitious roots (data not shown). Therefore, to investigate PIN polarity changes in the course of tissue polarization during vascular tissue regeneration, we also made use of pea epicotyls and monitored the subcellular distribution of endogenous pea PINs by immunocyto-chemistry. From all available Arabidopsis anti-PIN antibodies, we found that anti-PIN1 (Paciorek et al. 2005) also detected a PIN1-homologous protein in pea, as evidenced by comparable expression and subcellular localization patterns (Supplementary Fig. 1G,H).
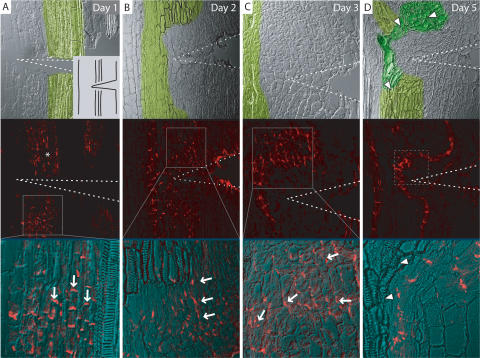
Twenty-four hours after disruption of the existing vasculature by wounding, PsPIN1 (in following PIN1) lost its basal polarity above the wound (Fig. 3A, around the asterisk) but remained basally localized below the wound in cells accompanying the vasculature (Fig. 3A), identical to the control situation (data not shown). After 2 d, PIN1 became ectopically expressed above the cut in a relatively large field of tissue, with a pronounced sub-cellular lateral orientation facing the central part (Fig. 3B). After 3 d, the field of cells ectopically expressing PIN1 became narrower, and stronger expression levels were constricted to inner cells of the field (Fig. 3C). The field of cells expressing PIN1 was oriented around the wound with PIN1 polarity lateral above the wound, and gradually becoming basal at the position of the wound itself. After 5 d, the field of cells expressing PIN1 had narrowed down to only a file of cells and new vasculature (morphologically distinguishable by tracheary elements) had formed next to these cells (Fig. 3D). Thus, the regeneration of new vasculature is preceded temporally and spatially by differential expression of PIN auxin transport components, and its polarity is preceded by the concerted gradual establishment of PIN polarities in each individual cell.
Figure 3.
Rearrangement of PIN1 polarity in wounded pea epicotyls. (A–D) Immunolocalization of putative PsPIN1 in wounded pea epicotyls. (A) Day 1: Loss of basally localized PIN1 signal above the wound (around the asterisk) and retained basally localized PIN1 below the wound (arrows). Inset shows experimental setup. (B) Day 2: Ectopic expression of PIN1 in parenchyma cells above the wound with lateral localization (arrows). (C) Day 3: Field of PIN1-expressing cells narrows, PIN1 polarity orients around the wound (arrows). (D) Day 5: PIN1 expression is restricted to a file of cells trailing around the wound; new vasculature has formed along the PIN1-marked channel, as visualized by xylem formation in adjacent cells (upper panel, arrowheads). Upper panels show transmitted light image, with old vasculature false-colored light green and new vasculature dark green. Wedge-shaped wound indicated by dotted lines. Solid boxes in A–C represent magnifications and dotted box in D approximate position of close-up from a different epicotyl. PIN1 signal is in red.
Local auxin application induces PIN polarity for de novo formation of vasculature
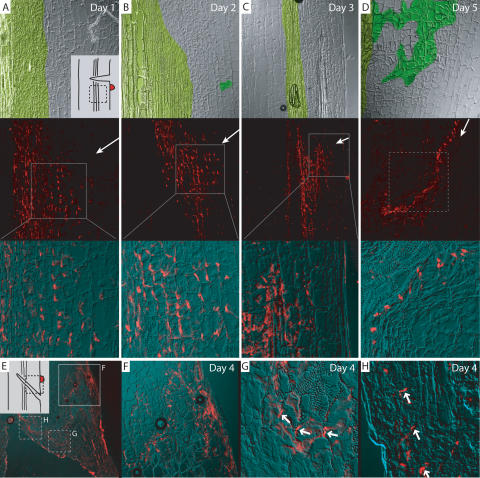
To test whether auxin is a sufficient signal underlying the changes in PIN expression and polarity that precede the induction of vascular strands, we locally applied auxin to the side of a wounded epicotyl below the cut (similar to Sachs 1981) (Fig. 4A, inset). Auxin application to a nonwounded epicotyl had no effects on PIN1 expression or polarity (data not shown); presumably the wounding confers competence for vasculature formation. With wounding and auxin application, we detected ectopic expression of PIN1 after 24 h with a subcellular localization facing away from the site of auxin application (Fig. 4A). Ectopic expression increased and also extended further into the direction of the auxin source after 2 d (Fig. 4B), with unchanged subcellular PIN polarity directing away from the source. After 3 d, the expression of PIN1 extended as far as the epidermis and the field of PIN1 expressing cells narrowed (Fig. 4C). After 5 d, a single file of cells expressing PIN1 had formed, connecting the site of auxin application with the central vasculature. Additionally, the cells neighboring this file of cells had differentiated into tracheary elements (Fig. 4D). These results indicate that auxin is a sufficient signal to induce PIN expression and polarity in competent tissues, which is then followed by differentiation of new vasculature along the PIN1-marked auxin channel.
Figure 4.
Rearrangement of PIN1 polarity in pea epicotyls after local auxin application. (A–D) Immunolocalization of putative PsPIN1 in pea epicotyls after wounding and local auxin application. (A, inset) Experimental setup with source of auxin as red dot and approximate micrograph position marked by dotted box. (A) Day 1: Ectopic PIN1 expression with lateral polarity facing away from source of auxin. (B) Day 2: Increase in PIN1 ectopic expression. (C) Day 3: PIN1 expression extends to outer layers and field of PIN1-expressing cells narrows. (D) Day 5: PIN1 expression has narrowed to a file of cells, which makes contact with the original vasculature. New vasculature has formed along the PIN1-marked channel, as visualized by xylem formation in adjacent cells. Image assembly and color coding as in Figure 3; arrows in middle panels indicate position of applied auxin. (E–H) PIN1 immunolocalization 4 d after a transversal cut and auxin application. (E) Overview picture; inset shows experimental setup. (F) From application site, a channel has formed trailing downward. (G) At the margin of the wound, PIN polarity reverses gradually (arrows). (H) Completely reversed polarity along the margin of the wound (arrows). Solid boxes in E represent magnifications and dotted boxes approximate position of close-ups from different epicotyl sections.
In an additional experiment, a diagonal cut was made at a 45° angle, disrupting the vasculature, and auxin was applied above the cut (Fig. 4E, inset). After 4 d, we observed the expression and localization of PIN1 at different positions in the tissue. Next to the site of auxin application, a file of cells expressing PIN1 trailed downward, until it reached the upper margin of the wound (Fig. 4F). At this point, PIN1 polarity, which had been basal, reversed and became lateral (Fig. 4G) and eventually apical (Fig. 4G,H). Again, the PIN1-expressing cell file was neighbored by differentiated tracheary elements. This illustrates that local auxin application can completely override and reverse original tissue polarity and can lead to repolarization of tissue as evidenced by the resulting directional auxin flow through the tissue.
Aux/IAA pathway is required for PIN polarity changes during organogenesis
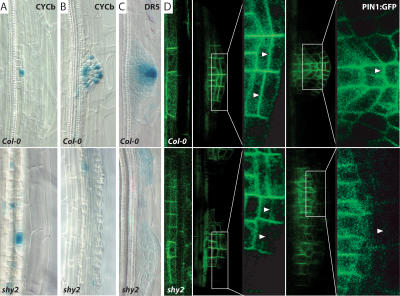
It has been hypothesized that feedback regulation between auxin transport and polarization of auxin flow could represent a general mechanism of plant development, which, besides regenerative processes, governs also the establishment of polar growth axes during organogenesis (Berleth and Sachs 2001). We tested this hypothesis on the model of lateral root development in Arabidopsis. De novo formation of lateral roots requires local auxin accumulation in founder cells, initiation of cell division (Fig. 5A,B), and a gradual change in the direction of auxin flow. Consequently, auxin flows toward the primordium tip, where it accumulates (Fig. 5C). This finally leads to the establishment of a new polar growth axis perpendicular to the main apical–basal axis. These processes are accompanied with lateral relocation of PIN1 (Fig. 5D), which precedes the establishment of the new growth axis (Benková et al. 2003).
Figure 5.
Aux/IAA-dependent PIN1 relocation during lateral root formation. Upper panels show control and lower panels show shy2/iaa3 mutants. (A,B) Cell division marker CYCb shows extensive founder cell establishment (A) but no organized development of primordia (B) in shy2 mutants. (C) Gradient of auxin response (as monitored by DR5::GUS) with maximum at the tip is not established in shy2 roots. (D) Relocation of PIN1:GFP to the distal lateral sides (arrowheads) is compromised in shy2 mutants.
We tested whether Aux/IAA-dependent signaling is required for the establishment of new polar growth axes during lateral root formation. It has been shown that Aux/IAA-dependent signaling is required very early, as dominant negative alleles of iaa14 (solitary root, slr) (Fukaki et al. 2002) or iaa3 (data not shown) block already the initiation step well before changes of PIN polarity are required. Therefore, we screened different aux/ iaa mutants for defects specifically at subsequent stages of lateral root formation. In dominant negative iaa3 (shy2-2) alleles, many founder cells are established and start to divide (as monitored by cell morphology and division marker CycB); however, the polar development of discrete primordia accompanied by an auxin maximum at the primordium tip is severely compromised (Fig. 5A–C). Strikingly similar defects were observed, when auxin transport or relocation of PIN polarity during primordia development was chemically or genetically interfered with (Benková et al. 2003; Geldner et al. 2004). Indeed, also in shy2-2 mutants, the underlying defect was a failure to relocate PIN1 to the outer (distal) lateral cell sides at early stages of primordium development (Fig. 5D, arrowheads). Therefore, auxin accumulation at the distal end of the primordium axis was not established and polar growth did not occur. These results suggest that the Aux/IAA pathway is utilized for auxin-dependent changes of PIN polarity and thus redirection of auxin flow, resulting in establishment of a new primordium axis during lateral root formation. As initiation of other organs is also triggered by auxin and accompanied with rearrangements of PIN polarity (Friml et al. 2002; Benková et al. 2003), it is conceivable that similar feedback regulations may contribute also to the development of roots, cotyledons, leaves, and flower organs.
Discussion
Many aspects of plant development, such as de novo organ formation or tissue regeneration, require the coordinated polarization of individual cells, which in the end leads to the establishment of a new axis of polarity for the whole tissue or organ. The classical canalization hypothesis (Sachs 1981, 1991; Berleth and Sachs 2001) as well as mathematical models of phyllotaxis (de Reuille et al. 2006; Jönsson et al. 2006; Smith et al. 2006) assume that the coordination between polarizing cells occurs via a feedback mechanism, by which auxin influences the directionality of its own transport. Here we reveal a mechanism for a polarizing auxin effect on the directionality of its intercellular flow, which occurs through control of the polar targeting of PIN auxin transport proteins.
Multiple feedback regulations in auxin distribution
Auxin has been shown to regulate the activity of components of directional auxin transport at multiple levels. Auxin can modulate the transcription of multiple PIN proteins through the TIR1-Aux/IAA-ARF pathway in a tissue-specific manner (Schrader et al. 2003; Vieten et al. 2005). Auxin also has been shown to modulate PIN2 protein stability, possibly by a mechanism involving ubiquitination and proteosome activity (Sieberer et al. 2000; Abas et al. 2006). In addition, auxin decreases the internalization rate in subcellular PIN recycling and thus increases PIN levels at the plasma membrane (Paciorek et al. 2005). All these feedbacks were shown to be involved in a complex compensatory mechanism for PIN functional redundancy and attenuating the auxin flow during root gravitropic response (Blilou et al. 2005; Vieten et al. 2005; Abas et al. 2006). However, none of these feedback mechanisms provide any directional information. In contrast, the described effect of auxin on PIN polarity directly influences the direction of intercellular auxin flow and thus provides a so far unidentified mechanism underlying the polarization of tissue as proposed by the canalization hypothesis.
Molecular mechanism of the auxin effect on PIN polarity
The effect of auxin on PIN polar targeting does not strictly require known components of PIN polar targeting such as the Ser/Thr kinase PINOID or PIN subcellular trafficking such as the ARF-GEF GNOM or the Callosin-like protein BIG. Importantly, the effect auxin has on PIN polarity is not linked to auxin-dependent regulation of PIN transcription, as evidenced by unaffected repolarization of PINs expressed under non-auxin-responsive promoters. The effect of auxin on PIN polarity requires the Aux/IAA-ARF-dependent auxin signaling pathway. This pathway so far has been shown to mediate auxin effects by transcriptional regulation (for review, see Parry and Estelle 2006). Therefore, it is likely that some so far unidentified factor, downstream from Aux/ IAA and ARF transcription regulators, interacts with the PIN polar targeting machinery and mediates the effect on PIN polarity. Which components of the Aux/IAAARF signaling pathway act together to regulate PIN polarity seems to depend on the tissue and developmental context, which may reflect differences in protein expression and/or specificity of Aux/IAA-ARF interactions. The importance of cell fate and developmental context for auxin effect on PIN polarity is also obvious from a comparison of our observations to a study based on laser ablation of the quiescent center (QC) (Xu et al. 2006). There, no immediate changes of PIN polarity in provascular cells above the QC were found, but actually a loss of the signal, associated with elevated auxin levels in these cells. The apparent discrepancy between the findings can be easily rationalized by the fact that different cell types and different regions of the root meristem were looked at. It is not surprising that processes such as meristem pattern re-establishment and vascular tissue regeneration are governed by distinct, yet both auxin-dependent, mechanisms.
Auxin acts as a polarizing cue linking tissue and cellular polarity during regeneration and organ formation
The canalization hypothesis (Sachs 1981, 1991; Berleth and Sachs 2001) and other, more recent models (de Reuille et al. 2006; Jönsson et al. 2006; Smith et al. 2006) require an auxin effect on the polarity of its flow as a crucial parameter underlying typical aspects of plant development such as tissue and organ regeneration, de novo organ formation, and positioning as well as embryonic axis formation.
Indeed, we observed auxin-dependent changes in PIN polarity, which precede de novo formation or regeneration of vascular tissue and the establishment of new growth axes in developing primordia during lateral root formation. In vasculature formation, PIN proteins change their subcellular polar localization and gradually establish a new polarity, which precedes the polarity of the newly differentiated vascular strands. This mechanism can even override intrinsic polar cues, as under certain conditions the new polarity can be completely opposite to the original one. Importantly, in both cases— organogenesis and vascular tissue formation—auxin is a sufficient signal to trigger the whole sequence of events (Sachs 1991; Reinhardt et al. 2000).
Auxin-dependent PIN targeting to a specific side of the cell depends on cell type-specific factors, as different cell types display different preferences for auxin-dependent changes in PIN polarity. This could actually resolve a discrepancy between the canalization hypothesis and recent phyllotactic models: While the canalization hypothesis predicts an auxin flow away from a cell with higher auxin content, the phyllotactic models require an orientation toward this cell. Given a cell type-specific decision about PIN orientation in response to auxin, canalization and the more recent models may actually be facets of the same general mechanism by which feedback-driven directional auxin transport eventually leads to self-establishing developmental patterns.
Our data suggest that auxin serves as polarizing signal in a field of respecifying cells during organogenic and regenerative processes. The feedback effect on the polarity of its own flow enables cells to perceive the orientation of neighboring cells and to gradually polarize in a coordinated fashion. This feedback connection between polar targeting at the level of single cells and polar development of the whole tissue allows plants to form new tissues and organs in a correct orientation relative to pre-existing body structures.
Materials and methods
Plant material and growth conditions
PIN2::PIN1:GFP-3 and PIN2::PIN1:HA (Wiśniewska et al. 2006), 35S::PIN1 and DR5::GUS (Benková et al. 2003), SCR::PIN2:GFP (Xu et al. 2006), 35S::PID and pINTAM>>PID (Friml et al. 2004), van7 (Koizumi et al. 2000), umb (Kanyuka et al. 2003), HS::shy2-6 and HS::axr3-1 (Knox et al. 2003), and CYCb::GUS (Ferreira et al. 1994) mutants and transgenic plant lines have been described previously. T-DNA insertion lines SALK_040394 (arf7-394), SALK_009879 (arf19-879), SALK_ 021432 (arf16-432), and SALK_062511 (arf17-511) were crossed, and segregating arf7-394 arf16-432 arf19-879 as well as arf7-394 arf17-511 arf19-879 were scored and confirmed by PCR analysis. arf7-394 and arf19-879 have been described before as nph4-6 and arf19-4, respectively (Wilmoth et al. 2005). Col-0 wild type was used as control. Pea (Pisum sativum L. cv. Vladan) seedlings were grown in perlite soaked with Richter's nutrient solution at a 16 h/8 h day/night photoperiod at 20°C/18°C. Arabidopsis seedlings were grown at a 16 h/8 h day/night photoperiod at 18°C–25°C on 0.5× MS with sucrose as described (Benková et al. 2003).
Immunolocalization and microscopy
Whole-mount immunolocalizations in Arabidopsis roots were performed as described (Friml et al. 2003a). Immunolocalization in pea was performed on 5-mm longitudinal epicotyl sections as established and described for Arabidopsis stem (Friml et al. 2003a). The anti-Arabidopsis-PIN1 antibody also recognizes a polarly localized homologous PIN protein in pea, which we presume is a PIN1 functional ortholog based on the similarity of the expression and localization signal to Arabidopsis. The following antibodies and dilutions were used: anti-PIN1 (1:1000) (Paciorek et al. 2005), anti-PIN2 (1:1000) (Abas et al. 2006), anti-GFP (1:1000, Roche), FITC-and CY3-conjugated anti-rabbit (1:600), or anti-mouse (1:600) secondary antibodies (Dianova). For confocal laser scanning microscopy, a Leica TCS SP2 AOBS was used. Images were processed in Adobe Photoshop.
Wounding and auxin treatments
Five-day-after-germination pea seedlings were used for wounding experiments. Incisions to 70%–80% of stem diameter were made on epicotyls between cotyledons and the first axillary bud as indicated in results; wounded tissue was separated with plastic film. A droplet of 1% IAA in lanoline paste was applied 3 mm below or above the wound as indicated. For each time point and treatment, at least 20 epicotyls from two independent experiments were analyzed. Following the treatments, the epicotyl sections were fixed, embedded in paraffin, and processed for anti-PIN1 immunocytochemistry as described (Friml et al. 2003a). Arabidopsis treatments with auxin or chemicals were done in liquid growth medium at room temperature and continuous lighting. Unless otherwise noted, 10 μM NAA was used for 4 h. Pretreatment with 50 μM MG132 or 100 μM CHX was done for 30 min, then coincubation with 10 μM NAA for 4 h followed.
Analysis of lateral root formation
Seedlings were grown for 9 d and root primordia morphology, cell division activity (using CYCb::GUS), auxin response distribution (using DR5::GUS), and PIN1 localization (using PIN1::PIN1:GFP) were analyzed as described (Benková et al. 2003). For each comparison, at least 15 seelings/50 lateral root primordia per treatment and genotype were analyzed. The reported differences between shy2/iaa3 and wild type were obvious and not statistically evaluated.
Quantitative analysis of PIN relocation
The mean fluorescence intensity of PIN1 signal at the inner lateral and the basal membrane of endodermis cells was measured using ImageJ software (National Institutes of Health, http://rsb.info.nih.gov/ij). The quotients between lateral and basal value were calculated for 10–15 cells of one root and averaged. The averages of at least 15 roots (at least 10 for Fig. 2F) per treatment were subsequently averaged again. In Figure 2F, the values of each genotype were standardized to the corresponding untreated control. P values are from a two-tailed t-test assuming unequal variances. Error bars in all graphs indicate standard error. Actual values are given in Supplementary Table 1.
ACKNOWLEDGMENTS
We dedicate this article to Tsvi Sachs, without whose brilliant and inspiring insights this work would not have been possible. We thank O. Leyser, H. Fukaki, and B. Scheres for sharing material. We thank F. El Kasmi and N. Trunk for technical assistance. We are grateful to T. Berleth, M. Heisler, C. Kuhlemeier, E. Meyerowitz, and B. Scheres for inspiring discussions. This work was supported by the Volkswagenstiftung (J.F., M.S.), the EMBO YIP program (J.F.), the Foundation for Polish Science (J.W.), GACR 522/02/D137 (J.B.), Margarethe von Wrangell Habilitationsprogramm (E.B.), grants from the FWF (to C.L.), and MSMT CR number MSM432100001 (J.B., V.R.).
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.390806.
REFERENCES
- Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wisniewska, J., Moulinier-Anzola, J.C., Sieberer, T., Friml, J., Luschnig, C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- Benjamins, R., Quint, A., Weijers, D., Hooykaas, P., Offringa, R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development. 2001;128:4057–4067. doi: 10.1242/dev.128.20.4057. [DOI] [PubMed] [Google Scholar]
- Benková, E., Michniewicz, M., Sauer, M., Teichmann, M., Seifertová, D., Jürgens, G., Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Berleth, T., Sachs, T. Plant morphogenesis: Long-distance coordination and local patterning. Curr. Opin. Plant Biol. 2001;4:57–62. doi: 10.1016/s1369-5266(00)00136-9. [DOI] [PubMed] [Google Scholar]
- Blilou, I., Xu, J., Wildwater, M., Willemsen, V., Paponov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Christensen, S.K., Dagenais, N., Chory, J., Weigel, D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;100:469–478. doi: 10.1016/s0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- de Reuille, P.B., Bohn-Courseau, I., Ljung, K., Morin, H., Carraro, N., Godin, C., Traas, J. Computer simulations reveal properties of the cell–cell signaling network at the shoot apex in Arabidopsis . Proc. Natl. Acad. Sci. 2006;103:1627–1632. doi: 10.1073/pnas.0510130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri, N., Dharmasiri, S., Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Ferreira, P.C., Hemerly, A.S., Engler, J.D., van Montagu, M., Engler, G., Inze, D. Developmental expression of the Arabidopsis cyclin gene cyc1At . Plant Cell. 1994;6:1763–1774. doi: 10.1105/tpc.6.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml, J., Benková, E., Blilou, I., Wisniewska, J., Hamann, T., Ljung, K., Woody, S., Sandberg, G., Scheres, B., Jürgens, G., et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis . Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml, J., Benková, E., Mayer, U., Palme, K., Muster, G. Automated whole-mount localization techniques for plant seedlings. Plant J. 2003a;34:115–124. doi: 10.1046/j.1365-313x.2003.01705.x. [DOI] [PubMed] [Google Scholar]
- Friml, J., Vieten, A., Sauer, M., Weijers, D., Schwarz, H., Hamann, T., Offringa, R., Jürgens, G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis . Nature. 2003b;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Friml, J., Yang, X., Michniewicz, M., Weijers, D., Quint, A., Tietz, O., Benjamins, R., Ouwerkerk, P.B., Ljung, K., Sandberg, G., et al. A PINOID-dependent binary switch in apical–basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Tameda, S., Masuda, H., Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis . Plant J. 2002;29:153–168. doi: 10.1046/j.0960-7412.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- Geldner, N., Friml, J., Stierhof, Y.D., Jürgens, G., Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Geldner, N., Anders, N., Wolters, H., Keicher, J., Kornberger, W., Muller, P., Delbarre, A., Ueda, T., Nakano, A., Jürgens, G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Geldner, N., Richter, S., Vieten, A., Marquardt, S., Torres-Ruiz, R.A., Mayer, U., Jürgens, G. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis . Development. 2004;131:389–400. doi: 10.1242/dev.00926. [DOI] [PubMed] [Google Scholar]
- Heisler, M.G., Ohno, C., Das, P., Sieber, P., Reddy, G.V., Long, J.A., Meyerowitz, E.M. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Jönsson, H., Heisler, M.G., Shapiro, B.E., Meyerowitz, E.M., Mjolsness, E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. 2006;103:1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyuka, K., Praekelt, U., Franklin, K.A., Billingham, O.E., Hooley, R., Whitelam, G.C., Halliday, K.J. Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J. 2003;35:57–70. doi: 10.1046/j.1365-313x.2003.01779.x. [DOI] [PubMed] [Google Scholar]
- Kepinski, S., Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Knox, K., Grierson, C.S., Leyser, O. AXR3 and SHY2 interact to regulate root hair development. Development. 2003;130:5769–5777. doi: 10.1242/dev.00659. [DOI] [PubMed] [Google Scholar]
- Koizumi, K., Sugiyama, M., Fukuda, H. A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network: Calling the auxin signal flow canalization hypothesis into question. Development. 2000;127:3197–3204. doi: 10.1242/dev.127.15.3197. [DOI] [PubMed] [Google Scholar]
- Morris, D.A. Transmembrane auxin carrier systems—Dynamic regulators of polar auxin transport. Plant Growth Regul. 2000;32:161–172. doi: 10.1023/a:1010701527848. [DOI] [PubMed] [Google Scholar]
- Müller, A., Guan, C., Gälweiler, L., Tänzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., Wisman, E., Palme, K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek, T., Zažímalová, E., Ruthardt, N., Petrášek, J., Stierhof, Y.-D., Kleine-Vehn, J., Morris, D.A., Emans, N., Jürgens, G., Geldner, N., et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- Parry, G., Estelle, M. Auxin receptors: A new role for F-box proteins. Curr. Opin. Cell Biol. 2006;18:152–156. doi: 10.1016/j.ceb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Petrášek, J., Mravec, J., Bouchard, R., Blakeslee, J., Abas, M., Seifertová, D., Wiśniewska, J., Tadele, Z., Kubeš, M., Čovanová, M., et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Reinhardt, D. Phyllotaxis—A new chapter in an old tale about beauty and magic numbers. Curr. Opin. Plant Biol. 2005;8:487–493. doi: 10.1016/j.pbi.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Reinhardt, D., Mandel, T., Kuhlemeier, C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Pesce, E.R., Stieger, P., Mandel, T., Baltensperger, K., Bennett, M., Traas, J., Friml, J., Kuhlemeier, C. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Sachs, T. The control of patterned differentiation of vascular tissues. Adv. Bot. Res. 1981;9:151–262. [Google Scholar]
- Sachs, T. Cell polarity and tissue patterning in plants. Development. 1991;1:83–93. [Google Scholar]
- Scarpella, E., Marcos, D., Friml, J., Berleth, T. Control of leaf vascular patterning by polar auxin transport. Genes & Dev. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader, J., Baba, K., May, S.T., Palme, K., Bennett, M., Bhalerao, R.P., Sandberg, G. Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc. Natl. Acad. Sci. 2003;100:10096–10101. doi: 10.1073/pnas.1633693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell, D.E., Leu, W.M., Gillmor, C.S., Xia, G., Feldmann, K.A., Chua, N.H. EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell. 1994;77:1051–1062. doi: 10.1016/0092-8674(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Sieberer, T., Seifert, G.J., Hauser, M.T., Grisafi, P., Fink, G.R., Luschnig, C. Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Curr. Biol. 2000;10:1595–1598. doi: 10.1016/s0960-9822(00)00861-7. [DOI] [PubMed] [Google Scholar]
- Smith, R.S., Guyomarch, S., Mandel, T., Reinhardt, D., Kuhle-meier, C., Prusinkiewcz, P. A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. 2006;103:1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves, T.A., Sussex, I.M. Patterns in plant development. Cambridge University Press; Cambridge, UK: 1989. [Google Scholar]
- Vieten, A., Vanneste, S., Wisniewska, J., Benková, E., Benjamins, R., Beeckman, T., Luschnig, C., Friml, J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;132:4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- Wilmoth, J.C., Wang, S., Tiwari, S.B., Joshi, A.D., Hagen, G., Guilfoyle, T.J., Alonso, J.M., Ecker, J.R., Reed, J.W. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005;43:118–130. doi: 10.1111/j.1365-313X.2005.02432.x. [DOI] [PubMed] [Google Scholar]
- Wiśniewska, J., Xu, J., Seifertová, D., Brewer, P.B., Růžička, K., Blilou, I., Rouquié, D., Benková, E., Scheres, B., Friml, J. Polar PIN localization directs auxin flow in plants. Science. 2006;312:858–860. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- Woodward, A., Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. (Lond.) 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Hofhuis, H., Heidstra, R., Sauer, M., Friml, J., Scheres, B. A molecular framework for plant regeneration. Science. 2006;311:385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]