Abstract
By using mouse models, it has been shown that Pneumocystis carinii f. sp. muris can be transmitted to immunocompetent mice that are exposed to immunosuppressed mice with active P. carinii pneumonia. We sought to determine whether P. carinii f. sp. muris could be transmitted between normal mice. The rationale for these experiments was to demonstrate whether the normal host could serve as the reservoir of organisms that produce Pcp when the organism is acquired by the immunosuppressed host. Under the conditions of these experiments, normal mice are able to be infected by brief cohousing with P. carinii-infected SCID mice. There was active replication of organisms in the normal host such that the organism could be transmitted to other normal mice, again with active replication. Mice that had seroconverted after exposure to P. carinii-infected SCID mice were more resistant to infection when reexposed. Infection in normal mice was well tolerated with minimal effects on dynamic lung compliance. We speculate, based on these results, that transmission from normal host to normal host, as an asymptomatic or minimally symptomatic infection, could be a way to maintain this opportunistic pathogen in the environment.
Pneumocystis carinii is a ubiquitous organism that causes a life-threatening pneumonitis when it infects an immunosuppressed host. The organism has been isolated from a wide variety of mammalian species. Early studies established a clear relationship between antigenic phenotype of P. carinii and the host of origin (8, 10). Limited genetic analysis of the surface glycoprotein A demonstrated a phylogenetic relationship between organisms that followed the phylogenetic relationships of the hosts from which the organisms were derived (24). A more detailed analysis of the mitochondrial rRNA gene from 18 primate species confirmed this relationship and is consistent with the concept of coevolution between P. carinii and its mammalian hosts (3).
Transmission studies in animal models have established that P. carinii is transmitted by an airborne route and that infection is not transmissible between host species (5, 6, 9, 13). Furthermore, contrary to earlier beliefs, P. carinii can produce a productive infection in both newborn and adult immunologically normal hosts (2, 7, 14, 20). Although there is clearly replication of organisms in these models, the infection is mild and generally occurs without evidence of overt disease in the host. These observations, along with the relationship noted above between host and parasite, suggest that the reservoir for infection of humans may be other humans. Immunocompromised patients not on prophylaxis can have an extremely high attack rate, e.g., 50 to 75% in patients with AIDS (1, 19). Given an attack rate this high, it is hard to envision that transmission occurs solely from immunocompromised human to immunocompromised human.
Recently, it has been shown that pneumocystis can be demonstrated in 14 to 35% of infants dying with a diagnosis of sudden infant death syndrome (16, 21). Although these observations do not establish a cause-and-effect relationship between pneumocystis and sudden infant death syndrome, they are consistent with the hypothesis that the presence of pneumocystis in these infants is a reflection that the organism is circulating in the general population of young children. If this were true, it could help to explain how pneumocystis is maintained in the human population. Since “susceptible” infants are continually being introduced into the population, they could serve as a significant reservoir for the organism to replicate and be passed on. This idea is consistent with the observations of early seroconversion in the first year or two of life (17, 18, 22). However, whether replication of organisms in the normal host reaches the threshold, whereby they can be transmitted to another normal host has not been established.
The series of experiments described here was undertaken to further characterize the course of P. carinii infection in the normal host. We sought to determine whether, under controlled conditions, P. carinii could be serially passaged through the normal host, thereby allowing the normal host to serve as the reservoir of infection for the development of P. carinii pneumonia (Pcp) in the immunocompromised host. Data from these experiments may help us better understand the pathogenesis of Pcp in immunocompromised humans.
MATERIALS AND METHODS
Exposure model.
Six- to eight-week-old BALB/c and CB-17 SCID mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and Taconic (Germantown, N.Y.). Upon arrival in our facility the mice were moved to a common room and housed in filter-top microisolator cages. Exposed mice and appropriate control BALB/c and SCID mice were housed side by side to assure that if transmission occurred it occurred by purposeful exposure and not by accidental exposure from routine maintenance of the mice.
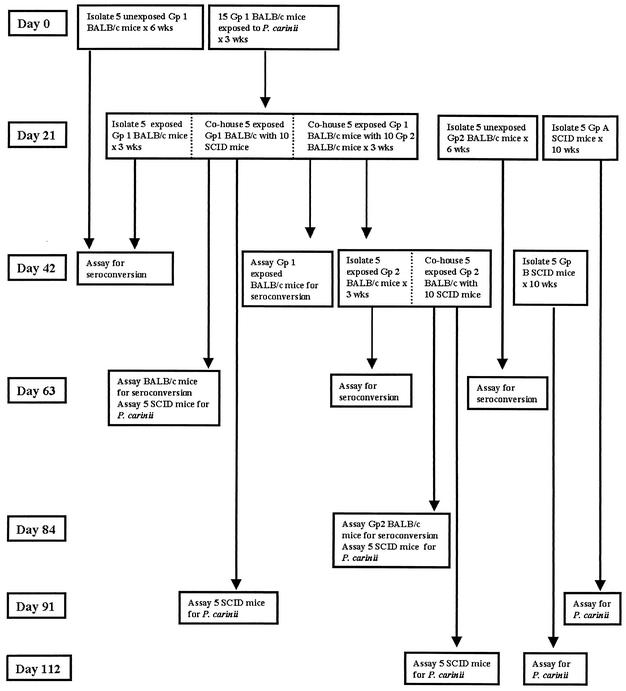
Figure 1 is provided to aid the reader in following the flow of mice through the exposure process. A time line is provided along the left side of the figure. Each box represents a group of similarly exposed mice. Exposures were done in 10-by-19-by-6-in. microisolator cages. Dotted lines within a box are used to indicate manipulations of individual cohorts within a given exposure group. At each time point at which exposed mice were placed in a cage with P. carinii-naive mice, exposed mice were first moved to clean cages, and then the naive mice were introduced into the cage on the following day. The basic design of the experiment was that P. carinii-infected SCID mice were used to expose healthy BALB/c mice (group 1), which were subsequently removed to new cages and used to expose additional groups of BALB/c (group 2) or SCID mice. Group 2 BALB/c mice were then used to expose additional SCID mice to determine whether, after two passages through normal mice, there was sufficient replication of organisms in the normal host to induce Pcp in an immunosuppressed contact.
FIG. 1.
Schematic chart summarizing the contact and sampling periods of the various cohorts of BALB/c and SCID mice followed over the 112 days of the experiment.
A separate experiment was done to determine the effect of prior infection on the outcome of subsequent infection when BALB/c mice were exposed to an airborne challenge with P. carinii. For this experiment, 12 BALB/c mice were cohoused with P. carinii-infected SCID mice for 3 weeks. After 5 weeks (i.e., 8 weeks from start of exposure) mice were assayed for seroconversion. After documentation of the seroconversion, the 12 mice were reexposed to P. carinii-infected SCID mice. At 2, 5, and 7 weeks after we began this second exposure, the mice were examined for the presence of P. carinii by PCR. An additional group of 12 BALB/c mice was similarly exposed and analyzed after primary infection. Plethysmography was done to determine whether there were any changes in lung compliance after primary or secondary exposure to P. carinii.
Documentation of infection and transmission.
Infection in the BALB/c mice was documented by seroconversion and by their ability to transmit organisms to susceptible mice. Seroconversion was demonstrated by enzyme-linked immunosorbent assay by using lectin-isolated surface gpA as the solid-phase antigen (11). Pre- and postexposure serum samples were diluted 1:50 for analysis in the enzyme-linked immunosorbent assay.
Infection of SCID mice was documented by microscopic and PCR analysis of lung homogenates. Homogenates were stained by Gomori methenimine silver to identify cyst forms of P. carinii. Quantitative PCR was performed by using an assay we have developed based on primers specific for the single-copy murine pneumocystis KEX1 gene (15). For this assay lungs were homogenized in 1 ml of sterile phosphate-buffered saline per 150 mg of lung tissue by using an electric tissue homogenizer. The homogenizer was washed three times between samples with 10% sodium hypochlorite and rinsed three times with water to prevent cross-contamination. The homogenates were then boiled for 15 min, vigorously mixed for 2 to 3 min, and then centrifuged for 5 min at 12,000 × g. The supernatant was carefully removed and stored at −80°C for subsequent quantitative PCR analysis by using TaqMan primer-fluorogenic probe chemistry. A primer-probe set specific for a 96-nucleotide region of the mouse P. carinii Kex1 gene was designed by using the Primer Express software (Applied Biosystems, Foster City, Calif.). The sequences of the primers and probe used were as follows: forward primer, 5′-GCACGCATTTATACTACGGATGTT-3′; reverse primer, 5′-GAGCTATAACGCCTGCTGCAA-3′; and fluorogenic probe, 5′-CAGCACTGTACATTCTGGATCTTCTGCTTCC-3′. Real-time quantitation of organism burden was performed on an Applied Biosystems Prism 7000 sequence detection system. The quantitative PCRs consisted of TaqMan universal master mix (Applied Biosystems), a 900 nM concentration of each forward and reverse primer, a 100 nM concentration of Taqman probe, and 2.5 μl of DNA template in a total reaction volume of 25 μl. The thermocycler profile used was 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Quantitation was determined by extrapolation against standard curves constructed from serial dilutions of known copy numbers of plasmid DNA containing the target Kex1 sequence. Data was analyzed by using the ABI Prism 7000 SDS v1.0 software (Applied Biosystems). A more sensitive qualitative PCR based on the multicopy gpA gene (12) was done on selected samples as an additional check for the presence of organisms.
Physiologic assessment of P. carinii infection of normal mice.
To determine whether infection of immunologically intact BALB/c mice was associated with any physiologic abnormalities, we measured lung compliance and analyzed the cellular content of bronchoalveolar lavage in these mice at 2, 5, and 7 weeks after primary or secondary exposure to SCID mice with Pcp. These time points were chosen based on our previous experience with primary infection of normal mice (2).
Dynamic lung compliance of the live mice was measured by using a previously described method with modifications (23). Mice were anesthetized by intraperitoneal injection of 0.13 mg of sodium pentobarbital per g of body weight. A tracheostomy was performed, and a 20-gauge cannula was inserted 3 mm into an anterior nick in the exposed trachea. The thorax was then opened to equalize the airway and transpulmonary pressure. To assure that the mice tolerated the procedure, they were examined for spontaneous respirations before proceeding further. Mice were immediately placed into a plethysmograph designed for anesthetized mice (BUXCO Electronics, Inc., Sharon, Conn.) and then connected to a Harvard rodent ventilator (Harvard Apparatus, Southnatick, Mass.). Mice were ventilated with a tidal volume of 0.01 ml per g of body weight at a rate of 150 breaths per min. Respiratory flow and pressure were measured by using transducers attached to the plethysmograph chamber. Data was collected and analyzed by using the Biosystems XA software package (BUXCO Electronics, Inc.). Dynamic lung compliance was calculated in milliliters/centimeter H2O/ kilogram from the flow and pressure signals.
After dynamic compliance measurements were taken on experimental mice, the chest cavity was surgically opened to expose the lungs and trachea. Four 1-ml volumes of 1× Hanks balanced salt solution was washed through the lungs via the tracheal cannula with recovery of ca. 3.5 ml of lavage fluid. The lavage cells were collected by centrifugation at 200 × g for 5 min. The cells were then resuspended in fresh Hanks balanced salt solution, counted in a hemocytometer, centrifuged onto glass slides, and stained with Diff-Quick (Dade AG, Dudingen, Switzerland) for differential counting.
RESULTS
As expected, normal mice that were cohoused with P. carinii-infected SCID mice for 3 weeks seroconverted when assayed for antibody to P. carinii at day 42 or 63 (experimental day 42 and 63) after the start of cohousing (Table 1 and Fig. 1). Two groups of control unexposed BALB/c mice housed in the same room for 42 days (experimental days 0 to 42 and 21 to 63) did not seroconvert, demonstrating that direct exposure to P. carinii infected mice was necessary for the development of anti-P. carinii antibodies.
TABLE 1.
Seroconversion of BALB/c mice after exposure to P. carinii-infected SCID mice or to other BALB/c mice that had previously been exposed to P. carinii-infected SCID mice
| Time pointa (day) | Exposure cohort | No. of seropositive animals/total no. of animals |
|---|---|---|
| 42 | Unexposed group 1 controls | 0/5 |
| Exposed group 1 mice | 4/5 | |
| Exposed group 1 mice used to expose group 2 BALB/c mice | 5/5 | |
| 63 | Unexposed group 2 control mice | 0/5 |
| Exposed group 1 used to expose SCID mice | 5/5 | |
| Group 2 mice cohoused with group 1 P. carinii-exposed mice | 1/5 | |
| 84 | Group 2 mice cohoused with group 1 P. carinii-exposed mice and used to expose SCID mice | 5/5 |
See Fig. 1 and text.
To determine whether normal BALB/c mice exposed to immunosuppressed mice with active Pcp could transmit organisms to other mice, five “exposed” group 1 BALB/c mice, exposed to P. carinii-infected SCID mice for the preceding 21 days, were cohoused with 10 uninfected SCID mice or 10 normal group 2 BALB/c mice (Fig. 1; day 21). Infection in SCID mice was monitored by homogenizing their lungs and assaying for the presence of organisms. Infection of BALB/c mice was monitored by seroconversion and by assessing their ability to transmit infection to P. carinii-free SCID mice.
P. carinii-free SCID mice cohoused for 6 weeks with BALB/c mice which had had a prior exposure to SCID mice with active Pcp all developed Pcp, as determined by both microscopy and PCR when they were assayed 6 weeks (Fig. 1; experimental day 63) and 10 weeks (Fig. 1; experimental day 91) after the start of cohousing (Table 2). By quantitative PCR the mean KEX1 copy number was determined to be 1.1 × 105/mouse at the 6-week time point and 1.6 × 107/mouse at the 10-week time point. To determine whether P. carinii-infected normal mice could transmit infection to other normal mice, an additional five group 1 BALB/c mice were used to expose ten new BALB/c mice (group 2) (Fig. 1; experimental days 21 to 42) during a 3-week cohousing period. Five of these group 2 BALB/c mice were then isolated for an additional 3 weeks (Fig. 1; experimental days 42 to 63) and then assayed for seroconversion. One of these five mice was seropositive at a serum dilution of 1:50. The other five group 2 mice were cohoused with ten P. carinii-free SCID mice for 6 weeks (Fig. 1; experimental days 42 to 84), and then they were assayed for seroconversion on experimental day 84. All five of these animals demonstrated seroconversion (Table 1).
TABLE 2.
Infection of SCID mice after exposure to BALB/c mice with prior exposure to either P. carinii-infected SCID mice or group 1 exposed BALB/c mice
| Time pointa (day) | Exposure cohort | No. of animals infected/total no. of animals as determined by:
|
|
|---|---|---|---|
| Microscopy | PCR | ||
| 63 | Cohoused with group 1 P. carinii-exposed BALB/c mice (6 wk) | 5/5 | 5/5 |
| 84 | Cohoused with group 2 P. carinii-exposed BALB/c mice (6 wk) | 0/5 | 5/5 |
| 91 | Cohoused with group 1 P. carinii-exposed BALB/c mice (10 wk) | 5/5 | 5/5 |
| Unexposed controls (10 wk) | 0/5 | 0/5 | |
| 112 | Cohoused with group 2 P. carinii-exposed BALB/c mice (10 wk) | 4/4b | 4/4b |
| Unexposed controls (10 wk) | 0/5 | 0/5 | |
See Fig. 1 and text.
One mouse was lost during the observation period.
The SCID mice, which were exposed to the group 2 BALB/c mice, were assayed for the development of Pcp 6 weeks (Fig. 1; experimental day 84) and 10 weeks (Fig. 1; experimental day 112) after the start of the cohousing. At the 6-week time point (experimental day 84), the organisms were not visible by silver staining, but all five were determined to be positive by qualitative PCR. Quantitative PCR verified these results with only one of five mice having detectable organisms with a KEX1 copy number of 6.8 × 104. However, after an additional 28 days (experimental day 112), the organism burden increased to the point where they were easily visible by microscopy and had a mean KEX1 copy number of 1.6 × 106/mouse. Both groups of unexposed SCID mice housed on the same racks with our exposed SCID mice for 10 weeks remained P. carinii-free as judged by both microscopy and qualitative PCR.
Additional experiments were done to assess the outcome in normal mice, which had either a primary or secondary exposure to SCID mice with active Pcp. After primary exposure to P. carinii-infected SCID mice, quantitative PCR was positive in 9 of the 11 normal BALB/c mice (one sample was lost). The mean KEX 1 copy number was 9 × 104 (range, 1.5 × 104 to 3.3 × 105) in the nine mice with detectable P. carinii. In contrast, after a secondary exposure to P. carinii-infected SCID mice none of 12 normal BALB/c mice had detectable P. carinii, as assayed by quantitative PCR. This represents at least a 90% reduction in the organism burden based on estimates of the lower limit of detection by this assay. The qualitative PCR based on the multicopy gpA gene did show faint bands in those of the secondary exposure samples, so a single brief exposure to P. carinii indicated mice did not appear to result in complete immunity to reinfection.
Immunologically normal BALB/c mice with primary P. carinii infection appeared to be well but demonstrated an inflammatory infiltrate in their lungs. To determine whether their primary infection is associated with abnormal lung function, dynamic compliance was performed at 2, 5, and 7 weeks after initiation of infection by cohousing with P. carinii-infected SCID mice. Compliance was reduced by a modest, but statistically significant amount at only the 5-week time point (Table 3). Consistent with the marked decrease in P. carinii numbers, the analysis of compliance after secondary exposure was normal at all three time points (Table 3).
TABLE 3.
Dynamic compliance in BALB/c mice after either primary or secondary exposure to P. carinii-infected SCID mice
| Group | n | Dynamic compliance (mean ml/cm H2O/kg ± SD) |
|---|---|---|
| Primary exposure | ||
| 2 wk | 4 | 2.10 ± 0.18 |
| 5 wk | 4 | 1.85 ± 0.15a |
| 7 wk | 4 | 2.17 ± 0.27 |
| Unexposed controls | 8 | 2.27 ± 0.25 |
| Secondary exposure | ||
| 2 wk | 4 | 1.96 ± 0.29 |
| 5 wk | 4 | 2.01 ± 0.21 |
| 7 wk | 3 | 2.02 ± 0.38 |
| Unexposed controls | 8 | 2.27 ± 0.25 |
Statistically different from unexposed control mice (P < 0.0.5).
DISCUSSION
We were able to demonstrate that under conditions of close approximation pneumocystis can infect a normal host with a resultant productive infection that reaches an organism number sufficient to allow transmission to a subsequent immunologically normal host. This infection occurred without the animals appearing ill and without demonstrable decline in pulmonary compliance. As previously demonstrated (2), this infection elicited an immune response as demonstrated by an inflammatory infiltrate and seroconversion that resulted in the clearance of organisms but not before the mice had the opportunity to transmit the infection. This observation raises the possibility that normal hosts could serve to maintain pneumocystis in the population by passage from individual to individual in the absence of overt disease (pneumonia). A recent study demonstrated that pneumocystis could be transmitted from SCID mice with Pcp to normal mice and then immediately back to SCID mice (4). Our experiments extend this initial observation by demonstrating that pneumocystis can be passaged from normal host to normal host in the absence of clinically evident Pcp. Pcp then results when an immunosuppressed host comes into contact with a pneumocystis-infected but asymptomatic host.
These experiments were designed to determine whether, under natural conditions, P. carinii could be passed through normal hosts. In addition to demonstrating that pneumocystis could be passed from normal host to normal host with a typical pattern of contagion, two other features of the pathogenesis of infection by pneumocystis became apparent. First, there appears to be an inoculum or dose effect that determines how rapidly infection progresses. This was evidenced by the fact that SCID mice exposed to group 1 BALB/c mice had markedly higher organism burdens when examined both at 6 and 10 weeks after the start of exposure compared to SCID mice exposed to group 2 BALB/c mice. Consistent with this observation was the finding that all five group 1 BALB/c mice seroconverted 6 weeks after exposure to SCID mice with Pcp but only one of the five group 2 BALB/c mice demonstrated seroconversion at 6 weeks after exposure to the group 1 BALB/c mice. Second, we were able to observe the effect of immunity on replication of organisms in a normal host. Consistent with our studies of active immunization (12), mice that had seroconverted after initial exposure to pneumocystis-infected SCID mice had far fewer organisms when they were assayed after a second exposure to pneumocystis-infected mice. Thus, the level of immunity induced after exposure was sufficient to protect from a challenge exposure by cohousing.
The rationale for undertaking these experiments was to examine the potential of a normal population to serve as the reservoir for pneumocystis by producing transmissible but subclinical infection. Our results support the possibility that healthy humans could serve as the reservoir of organisms responsible for producing human Pcp. We further hypothesize that infants are likely candidates to serve as a major portion of this reservoir. This may explain why pneumocystis can be found in autopsy specimens of infants dying of causes other than those predisposing for Pcp (16, 21), whereas the incidental finding of pneumocystis in adult lung specimens is a rarity. This is also consistent with the recent observation that most infants are seronegative in the first month of life and then show a steady seroconversion rate to the point where almost all infants are seropositive by 2 years of age (22). Our experimental observation that seroconversion after natural exposure inhibits pneumocystis replication upon subsequent exposure also favors the spread of infection through infants. However, our results do not rule out that normal older children and adults also could be infected, especially if immunity wanes.
In summary, our mouse model of pneumocystis infection demonstrated that pneumocystis can spread from normal host to normal host through a typical pattern of infection, replication of organisms, spread to another susceptible host, and then control of infection by a normal immune response. These results may help to explain how pneumocystis is maintained widely dispersed through the population such that most significantly immunosuppressed individuals will develop Pcp if they do not receive specific antimicrobial prophylaxis with drugs effective against pneumocystis.
Acknowledgments
This work was supported by NIH grants HL59833, AI23302, HL55002, and HL64559.
Editor: T. R. Kozel
REFERENCES
- 1.Centers for Disease Control. 1986. Update: acquired immunodeficiency syndrome: United States. Morb. Mortal. Wkly. Rep. 35:757-766. [PubMed] [Google Scholar]
- 2.Chun, L. A., F. Gigliotti, and A. G. Harmsen. 2003. Exposure of immunocompetent adult mice to Pneumocystis carinii f. sp. muris by cohousing: growth of P. muris and host immune response. Infect. Immun. 71:2065-2070. [DOI] [PMC free article] [PubMed]
- 3.Demanche, C., M. Berethelemy, T. Petit, B. Polack, A. E. Wakefield, E. Dei-Cas, and J. Guillot. 2001. Phylogeny of Pneumocystis carinii from 18 primate species confirms host specificity and suggests coevolution. J. Clin. Microbiol. 39:2126-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumoulin, A., E. Mazars, N. Seguy, D. Gargallo-Viola, S. Vargas, J. Cailliez, E. M. Aliouat, A. E. Wakefield, and E. Dei-Cas. 2000. Transmission of Pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts. Eur. J. Clin. Microbiol. Infect. Dis. 19:671-678. [DOI] [PubMed] [Google Scholar]
- 5.Durand-Joly, I., E. Aliouat, C. Recourt, K. Guyot, N. Francois, M. Wauquier, D. Camus, and E. Dei-Cas. 2002. Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J. Clin. Microbiol. 40:1862-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuta, T., M. Fujita, R. Mukai, I. Sakakibara, T. Sata, K. Miki, M. Hayami, S. Kojima, and Y. Yoshikawa. 1993. Severe pulmonary pneumocystosis in simian acquired immunodeficiency syndrome induced by simian immunodeficiency virus: its characterization by the polymerase-chain-reaction method and failure of experimental transmission to immunodeficient animals. Parasitol. Res. 79:624-628. [DOI] [PubMed] [Google Scholar]
- 7.Garvy, B. A., and A. G. Harmsen. 1996. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect. Immun. 64:3987-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigliotti, F. 1992. Host species-specific antigenic variation of a mannosylated surface glycoprotein of Pneumocystis carinii. J. Infect. Dis. 165:329-336. [DOI] [PubMed] [Google Scholar]
- 9.Gigliotti, F., A. G. Harmsen, C. G. Haidaris, and P. J. Haidaris. 1993. Pneumocystis carinii is not universally transmissible between mammalian species. Infect. Immun. 61:2886-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gigliotti, F., D. C. Stokes, A. B. Cheatham, D. S. Davis, and W. T. Hughes. 1986. Development of murine monoclonal antibodies to Pneumocystis carinii. J. Infect. Dis. 154:315-322. [DOI] [PubMed] [Google Scholar]
- 11.Gigliotti, F., J. A. Wiley, and A. G. Harmsen. 1998. Immunization with Pneumocystis carinii gpA is immunogenic but not protective in a mouse model of P. carinii pneumonia. Infect. Immun. 66:3179-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmsen, A. G., W. Chen, and F. Gigliotti. 1995. Active immunity to Pneumocystis carinii reinfection in T-cell-depleted mice. Infect. Immun. 63:2391-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes, W. T. 1982. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J. Infect. Dis. 145:842-848. [DOI] [PubMed] [Google Scholar]
- 14.Icenhour, C. R., S. L. Rebholz, M. S. Collins, and M. T. Cushion. 2002. Early acquisition of Pneumocystis carinii in neonatal rats as evidenced by PCR and oral swabs. Eukaryot. Cell 1:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, L. H., F. Gigliotti, T. W. Wright, P. J. Simpson-Haidaris, G. A. Weinberg, and C. G. Haidaris. 2000. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene 242:141-150. [DOI] [PubMed] [Google Scholar]
- 16.Morgan, D. J., S. L. Vargas, M. Reyes-Mugica, J. N. Walterspiel, W. Carver, and F. Gigliotti. 2001. Identification of Pneumocystis carinii in the lungs of infants dying of sudden infant death syndrome. Pediatr. Infect. Dis. J. 20:306-309. [DOI] [PubMed] [Google Scholar]
- 17.Peglow, S. L., A. G. Smulian, M. J. Linke, C. L. Pogue, S. Nurre, J. Crisler, J. Phair, J. W. M. Gold, D. Armstrong, and P. D. Walzer. 1990. Serologic responses to Pneumocystis carinii antigens in health and disease. J. Infect. Dis. 161:296-306. [DOI] [PubMed] [Google Scholar]
- 18.Pifer, L. I., W. T. Hughes, S. Stagno, and D. Woods. 1978. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics 61:35-41. [PubMed] [Google Scholar]
- 19.Rogers, M. F., P. A. Thomas, E. T. Starcher, M. C. Noa, T. J. Bush, and H. W. Jaffe. 1987. Acquired immunodeficiency syndrome in children: report of the Centers for Disease Control National Surveillance, 1982 to 1985. Pediatrics 79:1008-1014. [PubMed] [Google Scholar]
- 20.Soulez, B., E. Dei-Cas, P. Charet, G. Mougeot, M. Caillaux, and D. Camus. 1989. The young rabbit: a nonimmunosuppressed model for Pneumocystis carinii pneumonia. J. Infect. Dis. 160:355-356. [DOI] [PubMed] [Google Scholar]
- 21.Vargas, S. L., C. A. Ponce, W. T. Hughes, A. E. Wakefield, J. C. Weitz, S. Donoso, A. V. Ulloa, P. Madrid, S. Gould, J. J. Laterre, R. Avila, S. Benveniste, M. Gallo, J. Belletti, and R. Lopez. 1999. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin. Infect. Dis. 29:1489-1493. [DOI] [PubMed] [Google Scholar]
- 22.Vargas, S. L., W. T. Hughes, M. E. Santolaya, A. V. Ulloa, C. A. Ponce, C. E. Cabrera, F. Cumsille, and F. Gigliotti. 2001. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 32:855-861. [DOI] [PubMed] [Google Scholar]
- 23.Wright, T. W., F. Gigliotti, J. N. Finkelstein, J. T. McBride, C. L. An, and A. G. Harmsen. 1999. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J. Clin. Investig. 104:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright, T. W., F. Gigliotti, C. G. Haidaris, and P. J. Simpson-Haidaris. 1995. Cloning and characterization of a conserved region of human and Rhesus macaque Pneumocystis carinii gpA. Gene 167:185-189. [DOI] [PubMed] [Google Scholar]