Abstract
Individual strains of Staphylococcus aureus have different capacities to become internalized by osteoblasts. Here we report that the levels of σB expressed by S. aureus correlate with the capacity of this bacterium to be internalized by osteoblasts. However, σB is not essential for internalization and does not necessarily account for the differences in the capacities of strains to be internalized.
Staphylococcus aureus is an important human pathogen that causes a range of infections, including those of bone. S. aureus bone infections can be extremely difficult to treat, requiring prolonged antibiotic treatment and surgical intervention (18). We, and others, have shown previously that S. aureus is internalized by the bone-forming cells, osteoblasts (10, 14, 15, 19). The capacity of S. aureus to be internalized by osteoblasts has been proposed as an explanation for the recurrent nature of, and the difficulty in treating, bone infections with this organism.
We have previously shown that osteoblasts employ a receptor-mediated pathway in the uptake of S. aureus (15) and that the expression of fibronectin binding proteins (FnBPs) by the bacterium is essential to the process of internalization (1). However, the capacities of different isolates of S. aureus to be internalized by osteoblasts varied and could not be accounted for by differences in the levels of expression of FnBPs by these strains. In fact, S. aureus strain LS-1, which expressed lower levels of FnBPs, was internalized to a greater extent than strain 8325-4 (1). Although the mechanism underlying the differences in the capacities of these two strains to be internalized is unknown, it is interesting that strain 8235-4 is a derivative of NCTC8325, which is a natural rsbU mutant (13, 17).
In Bacillus subtilis, a network of protein-protein interactions regulates the activity of the alternative sigma factor, σB, posttranslationally. One of the proteins in this network, RsbU, is essential for the activation of σB. It has recently been shown that σB activity in S. aureus also depends on RsbU (13). Derivatives of S. aureus NCTC8325 have an 11-bp deletion in rsbU, resulting in the introduction of a stop codon into this gene, and hence these strains are essentially deficient in σB activity.
In many bacteria the alternative sigma factors of RNA polymerase are important in cell adaptation to environmental stress (20). Association of σB with the core RNA polymerase results in the recognition of a specific subset of promoters and to the initiation of transcription of their genes. To date only one alternative sigma factor has been identified in S. aureus, σB. Although σB has primarily been associated with responses to environmental stress, it has been shown that expression of the global virulence regulator SarA is influenced by σB in S. aureus (8, 9, 12, 17), and it has been suggested that σB functions as a global regulator of virulence genes in this bacterium (16). It has recently been demonstrated that σB expression influences transcription of both the sar and agr loci, and it has been suggested that σB might prolong the production of cell surface proteins such as the FnBPs, while preventing upregulation of secreted exoproteins (4).
Given the apparent importance of σB in the regulation of virulence genes in S. aureus, we have examined the hypothesis that σB activity may influence the capacity of S. aureus to be internalized by osteoblasts and thus account for our previous observation of strain-dependent differences in this capacity (1).
The bacterial strains used in this study are listed in Table 1. Strain BB1591 was obtained by phage 80α-mediated transduction of (ΔrsbUVWsigB)::ermB from IK181 (16) into strain LS-1, selecting for erythromycin resistance. Strain MB258 was obtained by transduction of the reporter construct asp23p::pBTasp23p-luc+ from MB61 (13) into strain LS-1, selecting for tetracycline resistance. The levels of σB activity in S. aureus strains were analyzed during growth by using an asp23 reporter gene system as previously described (13). S. aureus strain BB255 is our laboratory stock of NCTC8325 and carries a mutation in rsbU5. Table 2 shows that the MB33 reporter strain of BB255 expressed very low levels of σB activity. Complementation of this strain with an intact rsbU allele from strain COL, as in strain GP268, resulted in expression of significant levels of σB activity, as indicated by the reporter strain MB49 (Table 2). The level of σB activity in MB49 is similar to that found in other RsbU+ strains of S. aureus (13). Replacement of the σB operon in S. aureus strain BB255 with an erythromycin cassette, as in strain IK181, resulted in no expression of σB activity as shown by the reporter strain MB90 (Table 2). S. aureus strain MB138, BB255 with a point mutation in the anti-sigma factor rsbW, produced high levels of σB activity (Table 2). S. aureus strain LS-1, represented by strain MB258, produced levels of σB activity which were similar to those of strain GP268, as indicated by the reporter strain MB49 (Table 2). Replacement of the σB operon in S. aureus LS-1 with an erythromycin cassette, as in strain BB1591, led to a complete loss of σB activity as indicated by the reporter strain MB259 (Table 2).
TABLE 1.
Strains used in this study
| Strain | Relevant genotype and phenotypea | Reference(s) or source |
|---|---|---|
| BB255 | Laboratory stock of NCTC8325, rsbU | 2 |
| MB33 | BB255 asp23+asp23p::pECasp23p-luc+ Emr | 13 |
| IK181 | BB255 (ΔrsbUVWsigB)::erm(B) Emr | 16 |
| MB61 | RN4220 asp23+asp23p::pBTasp23p-luc+ Tcr | 13 |
| MB90 | IK181 asp23+asp23p::pBTasp23p-luc+ Tcr Emr | 13 |
| GP268 | BB255 (rsbU+V+W+sigB+)-tetL Tcr | 13 |
| MB49 | GP268 asp23+asp23+asp23p::pECasp23p-luc+ Emr Tcr | 13 |
| MB138 | MB33 rsbW7 Emr | 3 |
| LS-1 | 6,7 | |
| MB258 | LS-1-asp23+asp23p::pBTasp23p-luc+ Tcr | This study |
| BB1591 | LS-1 (ΔrsbUVWsigB)::erm(B) Emr | This study |
| MB259 | BB1591 asp23+asp23p::pBTasp23p-luc+ Tcr Emr | This study |
asp23 is the gene for the 23-kDa alkaline shock protein of S. aureus.
TABLE 2.
Strain-dependent differences in σB activity
| Strain | Relevant genotypea | σB activityb |
|---|---|---|
| MB33 | BB255 | 34.0 ± 3.00 |
| MB49 | BB255 rsbU+V+W+sigB+ | 301.3 ± 23.8 |
| MB90 | BB255 ΔrsbUVWsigB | 0.62 ± 0.10 |
| MB138 | BB255 rsbW7 | 1,084.0 ± 46.5 |
| MB258 | LS1 rsbU+V+W+sigB+ | 256.6 ± 17.2 |
| MB259 | LS1 ΔrsbUVWsigB | 0.31 ± 0.14 |
Detailed relevant genotypes and phenotypes are listed in Table 1.
σB transcriptional activity (relative light units) was determined from cells grown to an optical density at 600 nm of 1.5 by measuring the luciferase activity of Luc+, the product of the luc+ reporter gene fused to the σB-dependent promoters of asp23. The values shown are the results of four independent assays.
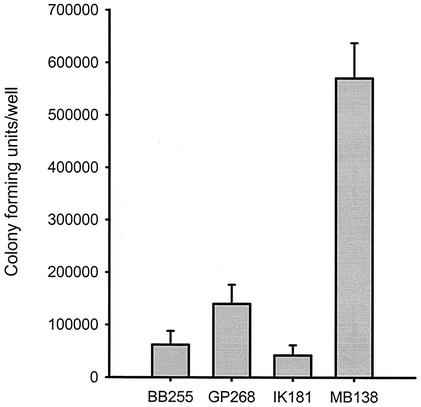
The capacity of S. aureus strains to be internalized by the osteoblastic cell line MG63 was determined as previously described (1) with the modification that overnight cultures of bacteria were diluted 1:20 in 10 ml of fresh brain heart infusion and grown to an optical density at 600 nm of approximately 1 before use. S. aureus strain BB255 was internalized by osteoblasts as shown in Fig. 1. Strain GP268 (BB255 rsbU+) had a twofold-greater capacity to be internalized by osteoblasts than did BB255 (Fig. 1). The capacity of strain IK181 (BB255 ΔrsbUVWsigB) to be internalized by osteoblasts was slightly lower than that of strain BB255, although the difference was not statistically significant (Fig. 1). S. aureus strain MB138 (BB255 rsbW) had a 10-fold-greater capacity to become internalized by osteoblasts than did strain BB255 (Fig. 1). To determine if σB activity had an effect on the ability of S. aureus to grow and/or survive within osteoblasts, cocultures were extended for a period of up to 6 h as previously described (1). There was no significant difference in the percentages of the natural rsbU mutant BB255 and its isogenic mutants GP268 and MB138 recovered, at hourly intervals up to 6 h, from the number recovered after 2 h (data not shown).
FIG. 1.
Capacity of S. aureus strain BB255 and its isogenic mutants to be internalized by osteoblasts. The isogenic strains BB255 (rsbU), GP268 (BB255 rsbU+), IK181 (BB255 ΔrsbUVWsigB), and MB138 (BB255 rsbW) were cocultured with osteoblasts at a multiplicity of infection of 100:1. The figure shows the results from one representative experiment of at least three, and the data are the means and standard deviations of three replicate cultures.
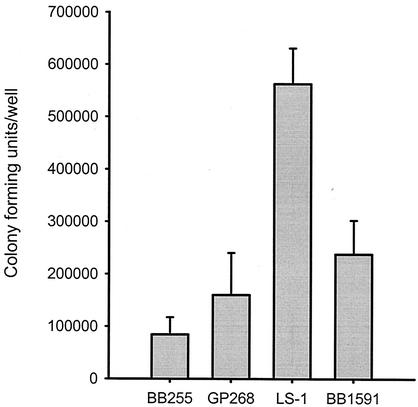
It has previously been shown that S. aureus strain LS-1 has a 10-fold-greater capacity to be internalized by osteoblasts than does the NCTC8325 derivative 8325-4 (1). In this study we compared the capacity of the NCTC8325 derivative BB255 to be internalized by osteoblasts with that of LS-1. Figure 2 shows that S. aureus strain LS-1 had a greater capacity to be internalized than did strain BB255 (sevenfold) or its rsbU+ derivative GP268. Strain BB1591, LS-1 in which the σB operon had been deleted, had a twofold-lower capacity to be internalized by osteoblasts than did its parent LS-1 (Fig. 2).
FIG. 2.
Comparison of the capacities of S. aureus strains BB255 and LS-1 and their respective isogenic mutants to be internalized by osteoblasts. S. aureus strains BB255 (rsbU), GP268 (BB255 rsbUVWsigB+), LS-1, and BB1591 (LS-1 ΔrsbUVWsigB) were cocultured with osteoblasts at a multiplicity of infection of 100:1. The figure shows the results from one representative experiment of at least three, and the data are the means and standard deviations of three replicate cultures.
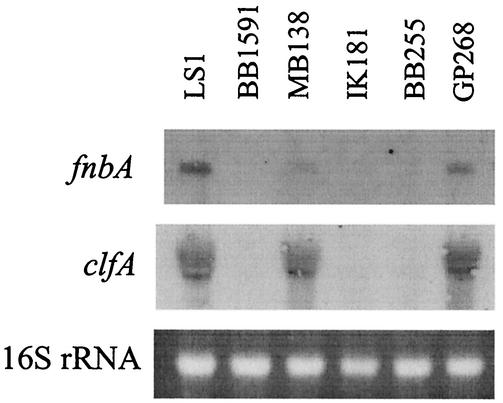
One mechanism by which the σB operon may influence internalization of S. aureus by osteoblasts is alteration of the expression of bacterial cell surface proteins such as the FnBPs. To investigate this possibility, Northern blot analysis for fnbA and clfA expression was performed. Bacterial overnight cultures were diluted to an optical density at 600 nm of 0.1 and grown at 37°C. RNA samples were prepared from cells collected after 1 h and after 8 h. Specific, digoxigenin-labeled fnbA and clfA probes were used for hybridizing 1-h and 8-h RNA extracts (7 μg), respectively. As can be seen in Fig. 3, the levels of expression of fnbA and clfA were highest in strains LS-1, MB138, GP268, and MB79, all of which possessed high σB activity. The capacities of S. aureus strains to bind to fibronectin were determined as previously described (1). The capacity of the rsbU mutant BB1591 to bind fibronectin was reduced to 24% ± 6% (mean ± standard deviation) of that of the wild-type rsbU+ strain LS-1. Similarly the capacity of the natural rsbU mutant BB255 to bind to fibronectin was only 35% ± 9% (mean ± standard deviation) of that of the rsbU-complemented strain GP268.
FIG. 3.
Northern blot analyses of fnbA and clfA expression by S. aureus strains LS1, BB1591 (LS1 ΔrsbUVWsigB), MB138 (BB255 rsbW), IK181 (BB255 ΔrsbUVWsigB), BB255 (rsbU), and GP268 (BB255 rsbU+).
It has been postulated that the capacity of S. aureus to be internalized by mammalian cells may contribute to the pathogenicity of this organism. In particular this capacity could account for the persistent nature of some S. aureus infections. The molecular details of the bacterial-host cell interactions that result in uptake of S. aureus by host cells are beginning to be elucidated. It has become evident that the FnBPs of S. aureus play an important, if not essential, role in the uptake process. However, we have previously reported that strain-dependent differences in the capacity of S. aureus to be internalized by osteoblasts do not necessarily correlate with the levels of expression of the FnBPs, suggesting that other factors are also important in this process (1). Since we were aware that one of the strains used in the previous study was an rsbU mutant that was deficient in σB expression, we have examined the possibility that this may account for strain-dependent differences in the capacity of S. aureus to be internalized by osteoblasts.
Complementation of the natural rsbU mutant BB255 with a functional rsbU gene resulted in the expression of significant levels of σB activity and increased the capacity of this strain to be internalized by osteoblasts by twofold. Deletion of the entire σB operon in BB255 resulted in no expression of σB activity and had little effect on the capacity of this strain to be internalized by osteoblasts. On the other hand, an isogenic strain with a point mutation in rsbW, which resulted in the expression of very high levels of σB activity, had a significantly increased capacity (5- to 10-fold) to be internalized by osteoblasts compared to that of the parental strain. Upon entry into the osteoblast S. aureus is found within intracellular vacuoles, as well as free within the cytosol. If the intracellular vacuoles containing S. aureus fuse with the lysosomal system, then the bacteria may be subject to environmental stresses such as low pH. Since the alternative sigma factor, σB, regulates the expression of general stress response genes (11, 12), it might affect the ability of S. aureus to survive and/or grow within the intracellular environment of the osteoblast. If this were the case, the numbers of staphylococci recovered from within osteoblasts incubated with isogenic strains producing high levels of σB activity would not be a reflection of the capacity of these bacteria to be internalized by osteoblasts. However, our studies show that the levels of σB expressed by isogenic mutants do not affect the ability of S. aureus to survive and/or grow within the intracellular environment of the osteoblasts. These data taken together clearly demonstrate that the level of σB activity expressed by S. aureus affects the capacity of this bacterium to be internalized by osteoblasts.
Given that the level of expression of σB activity in S. aureus affects the capacity of this bacterium to be internalized by osteoblasts, it could account for strain-dependent differences in this capacity. Our previous studies and data presented herein show that S. aureus strain LS-1 has a much higher capacity (7- to 10-fold) to be internalized by osteoblasts than do the NCTC8325 derivatives 8325-4 (1) and BB255. S. aureus strain LS-1 also produces significant amounts of σB activity in comparison to the NCTC8325 derivatives. In fact the levels of σB produced by LS-1 are equivalent to those produced by BB255 complemented with an intact rsbU gene as in GP268. However LS-1 has a three- to fourfold-higher capacity to be internalized by osteoblasts than does strain GP268. Deletion of the σB operon in LS-1 resulted in a complete loss of σB activity but only halved its capacity to be internalized by osteoblasts. These data demonstrate that, while σB activity affects the capacity of S. aureus to be internalized by osteoblasts, it cannot fully account for the strain-dependent differences that have previously been reported (1). Thus, other as-yet-undefined factors must also play a role in the strain-dependent differences in the capacity of S. aureus to be internalized by osteoblasts. In the present study we have also shown that the σB operon affects the level of expression of two microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), fnbA and clfA. Furthermore we have found that the capacities of S. aureus strains to bind to fibronectin correlate with their σB activities. We have previously reported that at least two MSCRAMMs, the FnBPs A and B, play an important role in the process of internalization of S. aureus by osteoblasts. This suggests that one possible mechanism by which the σB regulon may influence the internalization of S. aureus by osteoblasts is through alteration in the level of expression of MSCRAMMs.
Acknowledgments
This study was supported by the Swiss National Science Foundation grant 31-63552.00 to B. Berger-Bächi. S. P. Nair is grateful to the Arthritis Research Campaign for program grant funding (H0600).
Editor: V. J. DiRita
REFERENCES
- 1.Ahmed, S., S. Meghji, R. J. Williams, B. Henderson, J. H. Brock, and S. P. Nair. 2001. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect. Immun. 69:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger-Bächi, B., and M. L. Kohler. 1983. A novel site on the chromosome of Staphylococcus aureus influencing the level of methicillin resistance: genetic mapping. FEMS Microbiol. Lett. 20:305-309. [Google Scholar]
- 3.Bischoff, M., and B. Berger-Bächi. 2001. Teicoplanin stress-selected mutations increasing σB activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff, M., M. Roos, J. Putnik, A. Wada, P. Glanzmann, P. Giachino, P. Vaudaux, and B. Berger-Bächi. 2001. Involvement of multiple genetic loci in Staphylococcus aureus teicoplanin resistance. FEMS Microbiol. Lett. 194:77-82. [DOI] [PubMed] [Google Scholar]
- 6.Bremell, T., A. Abdelnour, and A. Tarkowski. 1992. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect. Immun. 60:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremell, T., S. Lange, L. Svensson, E. Jennische, K. Grondahl, H. Carlsten, and A. Tarkowski. 1990. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 33:1739-1744. [DOI] [PubMed] [Google Scholar]
- 8.Cheung, A. L., Y. T. Chien, and A. S. Bayer. 1999. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect. Immun. 67:1331-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deora, R., T. Tseng, and T. K. Misra. 1997. Alternative transcription factor σSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J. Bacteriol. 179:6355-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellington, J. K., S. S. Reilly, W. K. Ramp, M. S. Smeltzer, J. F. Kellam, and M. C. Hudson. 1999. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb. Pathog. 26:317-323. [DOI] [PubMed] [Google Scholar]
- 11.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 1999. Regulation of σB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261:558-566. [DOI] [PubMed] [Google Scholar]
- 12.Gertz, S., S. Engelmann, R. Schmid, A. K. Ziebandt, K. Tischer, C. Scharf, J. Hacker, and M. Hecker. 2000. Characterization of the σB regulon in Staphylococcus aureus. J. Bacteriol. 182:6983-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson, M. C., W. K. Ramp, N. C. Nicholson, A. S. Williams, and M. T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19:409-419. [DOI] [PubMed] [Google Scholar]
- 15.Jevon, M., C. Guo, B. Ma, N. Mordan, S. P. Nair, M. Harris, B. Henderson, G. Bentley, and S. Meghji. 1999. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect. Immun. 67:2677-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manna, A. C., M. G. Bayer, and A. L. Cheung. 1998. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J. Bacteriol. 180:3828-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair, S. P., R. J. Williams, and B. Henderson. 2000. Advances in our understanding of the bone and joint pathology caused by Staphylococcus aureus infection. Rheumatology 39:821-834. [DOI] [PubMed] [Google Scholar]
- 19.Reilly, S. S., M. C. Hudson, J. F. Kellam, and W. K. Ramp. 2000. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone 26:63-70. [DOI] [PubMed] [Google Scholar]
- 20.Wosten, M. M. 1998. Eubacterial sigma-factors. FEMS Microbiol. Rev. 22:127-150. [DOI] [PubMed] [Google Scholar]