Abstract
Streptolysin O (SLO) and streptolysin S (SLS) are potent cytolytic toxins produced by almost all clinical isolates of group A streptococci (GAS). Allele-replacement mutagenesis was used to construct nonpolar (in-frame) deletion mutations in the slo and sagB genes of the serotype M5 GAS strain Manfredo, producing isogenic single and double SLO- and SLS-defective mutants. In contrast to recent reports on SLS-defective insertion mutants (I. Biswas, P. Germon, K. McDade, and J. Scott, Infect. Immun. 69:7029-7038, 2001; Z. Li, D. Sledjeski, B. Kreikemeyer, A.Podbielski, and M. Boyle, J. Bacteriol. 181:6019-6027, 1999), none of the mutants described here had notable pleiotropic effects on the expression of other virulence factors examined. Comparison of isogenic parent and mutant strains in various virulence models revealed no differences in their abilities to multiply in human blood or in their 50% lethal doses (LD50s) upon intraperitoneal infection of BALB/c mice. A single log unit difference in the LD50s of the parent and SLS-defective mutant strains was observed upon infection by the subcutaneous (s.c.) route. Comparisons over a range of infective doses showed that both SLO and SLS contributed to the early stages of infection and to the induction of necrotic lesions in the murine s.c. model. Individually, each toxin made an incremental contribution to virulence that was not apparent at higher infective doses, although the absence of both toxins reduced virulence over the entire dose range examined. Interestingly, in some cases, the contribution of SLO to virulence was clear only from an analysis of the double-mutant strain, highlighting the value of not confining virulence studies to mutant strains defective in the expression of only single virulence factors.
Streptococcus pyogenes (group A streptococci [GAS]) is a highly versatile pathogen that causes a wide variety of important human diseases (55). These range in severity from self-limiting infections of the pharynx and skin to rapidly fulminating streptococcal toxic shock syndrome or devastating tissue infections, such as rapidly spreading myositis or necrotizing fasciitis (5, 55). GAS infections can also lead to serious poststreptococcal sequelae, such as acute glomerulonephritis or acute rheumatic fever (55). This versatility reflects, in part, differences in the abilities of strains to produce a wide range of known or suspected virulence factors. However, some virulence factors are highly conserved and expressed by almost all GAS strains. These include the potent cytolytic toxins streptolysin O (SLO) and streptolysin S (SLS) (2, 35, 36, 51).
SLO is a 540-amino-acid secreted protein toxin that binds to cholesterol in eukaryotic cell membranes, where it oligomerizes to produce large transmembrane pores leading to cell lysis (40). Sublytic concentrations of SLO have a wide variety of more subtle effects on target cells in vitro (8, 9, 29, 46, 50). In laboratory animals, intravenous (i.v.) administration of small quantities of purified SLO (<0.2 μg in mice) causes death within 2 to 3 min, with cardiotoxicity being the prominent pathophysiological effect (1). Sublethal doses have been reported to have various effects on other tissues, including neurological effects, increased capillary permeability, and dermal necrosis in rabbits (1). Despite these highly potent in vitro and in vivo activities, the first direct studies on the role of SLO during an infection recently revealed that SLO contributed to a surprisingly limited extent to GAS virulence in a murine dermonecrosis-invasive infection model (24). No differences were observed in the abilities of a serotype M3 GAS strain and an isogenic slo mutant derivative to cause weight loss 24 h postinfection or induce necrotic lesions in this model, although SLO did contribute to later stages of infection, producing a significant, albeit small, difference in survival rates (24).
SLS also has very potent cytolytic activity against a wide range of cells in vitro and produces a variety of more subtle effects at sublytic concentrations (1, 16, 17, 37). Early studies showed that SLS is a nonimmunogenic peptide that loses activity upon separation from various “carrier” molecules (17, 58). However, its precise molecular nature remained an enigma until recent analysis of an SLS-defective transposon Tn916 insertion mutant identified an operon of nine SLS-associated genes (sagA to sagI) which resembles operons encoding modified peptide bacteriocins in other species (4, 36, 47). The production of active SLS appears to involve modification and secretion of the 53-amino-acid product of the sagA gene (12) by the products of the other sag genes, which are transcribed at much lower levels due to the presence of a rho-independent “partial” transcriptional terminator between sagA and sagB (36).
Tn916 insertions in the sag operon promoters of serotype M1 and M18 GAS strains abolished SLS activity without apparent effects on the expression of other virulence factors (4). Studies comparing these mutant strains with their isogenic parent strains indicated that SLS contributes markedly to both weight loss at 24 h postinfection and the production of necrotic lesions in the murine dermonecrosis model (4). Subsequently, however, a Tn917 insertion (designated pel-1) in the sag operon promoter of serotype M49 GAS strain CS101 was found to greatly reduce the transcription of multiple virulence genes (sagA, emm, and speB) and the production of extracellular streptokinase (23). These pleiotropic effects were also observed upon transduction of the pel-1 mutation to various serotype M1 GAS strains, including strain MGAS 166, which had been used in the sag::Tn916 mutant studies outlined above; these results indicated that strain differences could not account for the different phenotypes observed (23). It was reported recently that in vivo passage of the pel-1 mutant selected derivatives with restored SLS and M protein expression but not restored expression of the SpeB protease or streptokinase (15). These revertants retained the Tn917 insertion at exactly the same position as the pel-1 mutant, suggesting the involvement of complex regulatory mechanisms that are not understood (15). To complicate matters further, another phenotype, distinct from any of those described above, was observed upon insertion of the strongly polar Ω cassette (44) into the sagA gene of a serotype M6 GAS strain (7). Unlike pel-1, the sagA insertion mutation had no effect on the expression of the emm6 gene but led to the normally antiphagocytic M6 protein being released from the cell surface, resulting in a marked increase in sensitivity to the bactericidal effects of nonimmune human blood (7). The various studies outlined above raise questions about whether SLS contributes directly or indirectly to GAS virulence in infection models.
Here we describe single and double nonpolar deletion mutants which contain mutations in the slo and sagB genes of the serotype M5 GAS strain Manfredo and which lack marked pleiotropic effects on the expression of other virulence factors. Analysis of these mutants in various virulence models indicated that both SLO and SLS contribute directly to the early stages of infection and the induction of necrosis in the murine dermonecrosis model.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and plasmids.
The serotype M5 S. pyogenes strain Manfredo (52) was provided by the late Ed Beachey from the VA Hospital Culture Collection (Memphis, Tenn.), and a derivative (ΔM5) with the emm5 gene replaced by the ΩKm2 cassette (41) was provided by Johnsson et al. (19). SLO plasmid pMK206 was described previously (42). Vector pGh9 (also called pVE6155 or pG+host 9) and Escherichia coli strain TG1-dev were generously provided by E. Maguin (Institut National de la Recherche Agronomique, Jouy en Josas, France). Plasmid pGh9 is a 3.75-kb derivative of cryptic lactococcal plasmid pWV01 (38) that contains a temperature-sensitive mutant repA gene (30), the erythromycin resistance (Emr) determinant from pAMβ1 (31, 53), and the multiple-cloning site from E. coli vector pBluescript SK(+) (E. Maguin, personal communication). E. coli strain TG1-dev was constructed by transducing the chromosomally inserted wild-type copy of pWV01 repA and a linked Kmr marker from strain EC101 (22) into strain TG1 (48), followed by a recA::tet mutation, to facilitate the stable replication of pGh9 recombinant plasmids in E. coli at 37°C (E. Maguin, personal communication). Unless otherwise specified, S. pyogenes and E. coli strains were cultured as described previously (14, 60), except that Todd-Hewitt broth (THB) (Difco, Surrey, United Kingdom) was supplemented with 0.5% (wt/vol) Bacto Yeast Extract (Difco) to produce THYB. For selection in S. pyogenes, 1 to 2 μg of erythromycin/ml was added to the medium. Selection in E. coli was done with antibiotics at the following concentrations: ampicillin at 100 μg/ml, erythromycin at 300 μg/ml, kanamycin at 30 μg/ml, and tetracycline at 20 μg/ml.
General DNA techniques.
Restriction endonucleases, T4 DNA ligase, Taq polymerase, and Pfu polymerase were purchased from standard commercial sources and used according to their manufacturers' instructions. Unless otherwise specified, DNA manipulation and analysis were done by standard procedures described previously (18, 48, 60) or with kits from Qiagen Ltd. (Crawley, West Sussex, United Kingdom) according to the manufacturer's instructions. The Qiagen plasmid miniprep kit was modified as follows to isolate plasmids from S. pyogenes. Cells from 5 to 10 ml of overnight cultures in THYB containing 30 μg of hyaluronidase (Sigma Chemical Company Ltd., Dorset, United Kingdom)/ml and selective antibiotics were resuspended in 180 μl of Qiagen P1 solution. Then, 20 μl (1 U) of mutanolysin solution plus 50 μl (10 μg) of lysozyme solution were added, and the mixture was incubated at 37°C for 30 to 60 min. Next, 250 μl of Qiagen P2 solution was added, and the recommended Qiagen procedure was carried out. For purification of S. pyogenes total cell DNA, Puregene DNA isolation kits (Gentra Systems Inc., Minneapolis, Minn.) were adapted by supplementing the manufacturer's lytic enzyme solution with 1.0 U of mutanolysin plus 10 μg of lysozyme per ml of original culture and extending the incubation at 37°C to 4 h before adding cell lysis solution. PCR primers were synthesized to order by local suppliers and are described in Table 1.
TABLE 1.
Oligonucleotide primers
| Purpose | Primera | Sequence (5′ to 3′)b | Locationc | 5′ Tag site |
|---|---|---|---|---|
| sloΔ1 construction and confirmationd | MF01 (F) | AGCGGGATAACAATTTCACACAGG | pUC18 500 to 478 | |
| MF02 (R) | CGCCAGGGTTTTCCCAGTCACG | pUC18 352 to 373 | ||
| MF03 (R) | gggggtaccGGGTCATTGACCTCAACCGTTGC | slo 637 to 659 | KpnI | |
| MF04 (F) | gggggtaccGAAGGTGATGATTGCAGCATAC | slo 870 to 891 | KpnI | |
| MF05 (F) | GCGCATTATTAGAGAGGCTATGG | slo −126 to −104 | ||
| MF06 (R) | CAAAATTTTCAATGGTTTCACC | slo 382 to 403 | ||
| sagBΔ1 construction and confirmationd | JJ029 (F) | aaggaattcATGTCATTTTTTACAAAGGA | sagB 1 to 20 | EcoRI |
| JJ030 (R) | aagttctcgagTCATTGAGACTCCTTAGTT | sagB 951 to 933 | XhoI | |
| JJ055 (F) | cacaagcttAACGTTAGAGATTTTG | sagB 652 to 667 | HindIII | |
| JJ056 (R) | gcgaagcttGTAACTACTTGAAAAA | sagB 300 to 285 | HindIII | |
| JJ051 (F) | ataaagcttTATACGCCAGGTAAATA | sagB −239 to −223 | HindIII | |
| JJ060 (R) | gagatcgatTTCAAGATTGTAGTCATC | sagB 1138 to 1121 | ClaI | |
| PCR amplification of hybridization probes | sagA (F) | AATTGAGCTAGCCTTGTCCTTG | sagA −107 to −85 | |
| sagA (R) | TTTACCTGGCGTATAACTTCC | sagA 159 to 139 | ||
| emm5 (F) | AGGCCCTTAATGAACTCTTG | emm5 476 to 495 | ||
| emm5 (R) | CCTGCTTGTGGTGCTTGAC | emm5 1217 to 1198 | ||
| ska (F) | TGATCGAAACGGCAAGGTCTA | ska 408 to 428 | ||
| ska (R) | ACGGTTATGATACGGTTGGTG | ska 1178 to 1158 | ||
| speB (F) | CTTATGCTGGTACCGCTGAGA | speB 410 to 430 | ||
| speB (R) | TCCGCCTACTTTACCGACACC | speB 1017 to 997 | ||
| recA (F) | TCTTCCGGTAAAACGACTGTG | recA 88 to 108 | ||
| recA (R) | GGCGGAGCGACCTTGTTTTTA | recA 656 to 636 | ||
| 39-M5 (F) | GGTTCTGATAATACAGGACC | sagA −430 to −411 | ||
| 40-M5 (R) | GATTAATATGTAAACCCTTTC | sagA −166 to −186 |
F, forward; R, reverse.
Some primers were synthesized with additional 5′ sequence tags (lowercase letters) to incorporate particular restriction endonuclease cleavage sites (underlining) (as specified in the last column).
For MF001 and MF002, which correspond to pUC18 vector lacZ sequences flanking the cloned slo gene in plasmid pMK206 (42), positions indicated are relative to nucleotide 1 of the pUC18 vector sequence (62). For all other primers, positions indicated are relative to the translation start sites of the specified GAS genes, with negative numbers indicating flanking upstream sequences. GAS gene sequences were obtained from serotype M5 strain Manfredo genome sequence data published at The Sanger Centre website (www.sanger.ac.uk/Projects/S_pyogenes/).
Only primers specified in the text are shown. Additional sequencing primers were omitted to save space.
RNA isolation and blotting.
RNA was isolated from late-log-phase cultures of S. pyogenes (optical density at 600 nm, 0.9) as described by Cheung et al. (13) with an RNA FastPrep kit purchased from Bio 101 (Vista, Calif.). DNA probes were amplified by PCR from S. pyogenes chromosomal DNA and labeled with a fluorescein gene images kit (Amersham Life Science Ltd., Amersham, United Kingdom). With a dot blotting manifold, twofold serial dilutions of RNA in water were applied to Hybond N+ blotting membranes (Amersham); the filters were baked, hybridized with denatured probe, and washed essentially as described elsewhere (48). Fluorescein-labeled targets were detected by using a gene images CDP-Star detection module (Amersham). Treatment of RNA with RNase-free DNase did not reduce hybridization signals, and in control blots with a probe corresponding to untranscribed sequences upstream from sagA (amplified by using primers 39-M5 and 40-M5; Table 1), no hybridization signals were detected; these results confirmed the absence of DNA contamination. Experiments were repeated with RNAs isolated on at least two independent occasions.
Allele replacement mutagenesis.
Following electrotransformation (54), Emr S. pyogenes harboring recombinant pGh9 with the desired mutant alleles was selected at 28°C on blood agar containing erythromycin. Wild-type sequences in the chromosome were then replaced in two steps. First, integration of the temperature-sensitive plasmid via a single crossover with homologous chromosomal sequences was selected for by growth at 37°C in the presence of erythromycin. Next, after purification of the desired cointegrants, plasmid excision via a second crossover between flanking homologous sequences was stimulated by growth at 28°C in the absence of erythromycin essentially as described by Biswas et al. (6), followed by growth at 37°C to ensure loss of the excised plasmid. The resulting erythromycin-sensitive colonies were screened by PCR to identify the desired mutants.
SLO and SLS assays.
To maximize the sensitivity of SLO detection, strains were grown in morpholinepropanesulfonic acid (MOPS)-buffered THB and monitored and maintained at pH 6.8 to pH 7.4 throughout growth, and SLO was precipitated from cell-free culture supernatants with ammonium sulfate (45 to 60% saturation) as described by Canalias et al. (11). Twofold serial dilutions in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4 [pH 7.4]) containing 5.0 mM dithiothreitol and 0.5% (wt/vol) bovine serum albumin were assayed for hemolytic activity in the presence and absence of 25 μg of trypan blue (a specific inhibitor of SLS)/ml or 0.5 mg of cholesterol (a specific inhibitor of SLO)/ml as described previously (42). Polypeptides from concentrated culture supernatants were separated on sodium dodecyl sulfate-12.5% (wt/vol) polyacrylamide gels, electrotransferred to nitrocellulose membranes, and subjected to immunoblotting with rabbit anti-SLO sera essentially as described previously (43).
The production of SLS was routinely detected as zones of beta-hemolysis surrounding S. pyogenes colonies on blood agar plates grown under aerobic conditions. SLS was quantified by hemolytic titration assays in the presence and absence of 25 μg of trypan blue/ml or 0.5 mg of cholesterol/ml essentially as described elsewhere (25).
Assays for other virulence factors.
SpeB-derived cysteine protease was assayed by measuring the sizes of clear zones around colonies on casein plates as outlined in a modification (61) of the method of Martley et al. (33). Hyaluronic acid was quantified as described elsewhere (49) with human umbilical hyaluronic acid (Sigma) as a standard. Streptokinase production by colonies on plates was determined by a plate overlay method (32), and levels of streptokinase in cell-free culture supernatants were determined by assaying the conversion of human plasminogen to plasmin as described elsewhere (57) with purified streptokinase (Sigma) as a standard.
Bactericidal assay.
The survival of S. pyogenes in fresh human blood from nonimmune donors was assayed essentially as described elsewhere (21, 34). Anti-SLO (ASO) titers were determined by using a standard ASO assay kit (Difco) according to the manufacturer's instructions.
Mouse virulence assays.
Female BALB/c mice were purchased from Harlan Olac (Blackthorn, United Kingdom) and rested for a minimum of 2 weeks before experiments. Five groups of five mice (each ca. 20 g) were inoculated intraperitoneally (i.p.) with 200 μl of 10-fold serial dilutions of GAS in PBS and monitored closely (a minimum of twice daily) for 14 days. The 50% lethal dose (LD50) was calculated by the method of Reed and Muench (45). For invasive soft tissue infections, five groups of five mice were inoculated subcutaneously (s.c.) in the scruff of the neck with 200 μl of 10-fold serial dilutions of GAS in PBS by using a 1-ml tuberculin syringe. Mice were weighed immediately before and at 24 h after inoculation and were monitored closely (a minimum of three times daily and more frequently as appropriate) for 14 days. Sizes of necrotic lesions were estimated as described by Bunce et al. (10) by using the following equation: A = [Π(L × W)]/2; in this equation, A is area, L is the longest axis, and W is the shortest axis.
Statistical analyses.
Statistical analyses were performed by using standard analysis of variance (ANOVA) methods with the aid of the Prism software package (GraphPad Software Inc., San Diego, Calif.). With two variables (different infective doses and different strains), overall comparisons were made by using two-way ANOVA methods to determine whether each variable contributed to the observed differences. At each infective dose, comparisons of parent and mutant strains (single variables) were made with one-way ANOVA and Tukey post hoc tests (GraphPad Software).
RESULTS
Construction of SLO- and SLS-defective mutants of serotype M5 GAS strain Manfredo by allele replacement mutagenesis.
To avoid any potential complications from polar effects, the mutagenesis strategy was designed to introduce in-frame deletions into the target genes. Large (>200-bp) in-frame deletions of the slo and sagB genes in the temperature-sensitive shuttle vector pGh9 were first constructed in E. coli to produce recombinant plasmids pMF002 and pJJ120, respectively (Fig. 1). These deletions were then introduced into the chromosome of M5 GAS strain Manfredo by allele replacement mutagenesis to produce SLO-negative and SLS-negative mutants, designated sloΔ1 and sagBΔ1, respectively. An sloΔ1 sagBΔ1 double mutant was then constructed by using the sloΔ1 mutant as the host for a second round of allele replacement with pJJ120. Each pair of parent and mutant strains was compared in a series of Southern blotting or PCR experiments to confirm the structures of the regions spanning the junctions of the sequences that had been cloned in pGh9 plasmid sequences and flanking chromosomal sequences. Mutant alleles were then reisolated by PCR from each of the mutant strains, and their precise structures were confirmed by DNA sequencing.
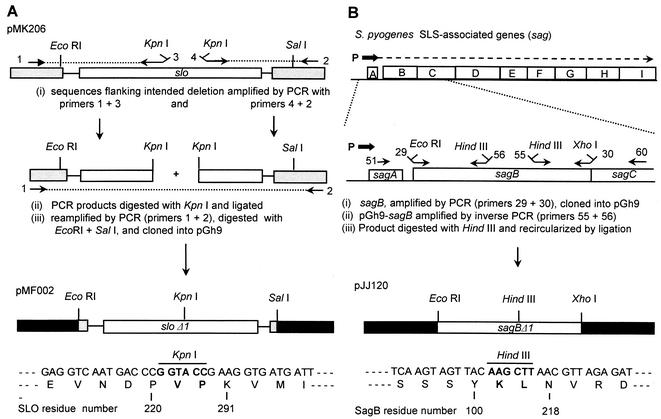
FIG. 1.
Construction and structures of sloΔ1 and sagBΔ1 mutant alleles. Outlined is the initial construction in allele replacement vector pGh9 of in-frame deletion mutations in the slo (A) and sagB (B) genes. Unshaded boxes and lines represent GAS sequences, and shaded boxes represent flanking vector plasmid sequences; grey shading corresponds to pUC18 vector sequences in recombinant plasmid pMK206 (42), and black shading represents pGh9 vector sequences. In the upper half of panel B, the arrows above the sag genes indicate transcription from the sag operon promoter (P) and its marked reduction by the partial transcriptional terminator between sagA and sagB (36). The numbered arrows indicate the locations of the PCR primers listed in Table 1. Sequences spanning the religated deletion end points of the mutants are shown below the pMF002 sloΔ1 and pJJ120 sagBΔ1 diagrams, with the numbers indicating the corresponding residues of the wild-type, parent gene products.
Initial characterization of mutants.
Under conditions where over 2 million hemolytic units of SLO could be detected in concentrated culture supernatants from the parent strain, no SLO activity was detected in corresponding concentrates from the sloΔ1 mutant (Table 2). Moreover, immunoblots with anti-SLO antibodies readily detected wild-type SLO in concentrates from parent strain cultures but failed to detect a truncated SLO polypeptide in concentrates from mutant sloΔ1 cultures (data not shown), suggesting that the latter may be rapidly degraded. There was no difference between the parent and the sloΔ1 mutant strains in either the sizes of beta-hemolysis zones surrounding colonies on the surfaces of blood agar plates (which were due predominantly to SLS) or the amounts of cell-associated SLS detected in quantitative assays (Table 2). In contrast, the sagBΔ1 mutant strain produced no detectable SLS activity, whereas it produced amounts of SLO similar to those produced by the parent strain. The production of SLO or SLS by the sloΔ1 sagBΔ1 double mutant could not be detected.
TABLE 2.
Phenotypic characteristics of mutants
| GAS | Hemolytic units of the following toxin/ml of culture
|
Hyaluronic acid (fg/CFU) | Streptokinase (U/ml of culture) | Protease (mm)c | Multiplicity index in human blood from donord
|
||||
|---|---|---|---|---|---|---|---|---|---|
| SLOa
|
SLSb
|
A | B | ||||||
| TB | CH | TB | CH | ||||||
| Wild type | >1,000 | 0 | 0 | 136 | 16.1 ± 3.4 | 9.8 ± 0.7 | 8.8 ± 1.3 | 21.8 | 24.1 |
| sloΔ1 mutant | 0 | 0 | 0 | 136 | 16.9 ± 1.1 | 9.5 ± 0.6 | 8.6 ± 1.0 | 21.7 | 37.8 |
| sagBΔ1 mutant | >1,000 | 0 | 0 | 0 | 18.4 ± 2.9 | 10.0 ± 0.7 | 6.6 ± 1.5 | 45.3 | 47.1 |
| sloΔ1 sagBΔ1 mutant | 0 | 0 | 0 | 0 | 16.0 ± 2.0 | 9.9 ± 0.7 | 6.4 ± 1.9 | 27.9 | 32.3 |
Precipitated from cell-free supernatants of pH-controlled late-log-phase cultures. Tested in the presence of trypan blue (TB) and cholesterol (CH).
Cell-bound activity determined for cells from 50-ml early-stationary-phase cultures. Tested as described for SLO.
Diameters of cleared zones on casein plates, determined as described by Wheeler et al. (61).
The multiplicity index is the output CFU after 3 h of rotation in fresh human blood divided by the input CFU at time zero. In these assays, an M protein-deficient mutant of strain Manfredo had an index of <1.0.
Growth in vitro (optical density readings obtained at 600 nm at regular intervals for >12 h) of wild-type strain Manfredo and its mutant derivatives in either THB or THYB at 37°C revealed no differences in either growth rates or maximum growth levels achieved (data not shown).
Expression of other virulence factors.
It was reported that a Tn917 insertion mutation (designated pel-1) in the sag operon promoter of serotype M49 GAS strain CS101 abolished detectable transcription of the sagA (pel), emm (encoding the cell surface M protein), and speB (encoding a secreted cysteine protease) genes and greatly reduced the levels of secreted streptokinase, suggesting a posttranscriptional effect on the expression of this virulence factor (23). To determine whether the sagBΔ1 deletion had similar pleiotropic effects in M5 GAS strain Manfredo, RNA was isolated from late-log-phase cultures of parent strain Manfredo and the isogenic sagBΔ1 mutant, and hybridization assays were carried out to compare the levels of transcription of the sagA, emm5, speB, ska, and recA (internal control) genes in the parent and mutant strains. In contrast to the reported pleiotropic effects of the pel-1 mutation, transcription for all of the genes examined was readily detected in the sagBΔ1 mutant strain (data not shown). Image analysis (pixel density normalized with respect to the recA control) confirmed that in most cases, quantitative differences in the signals obtained from corresponding samples from the parent and mutant strains were <1.5-fold, although a slightly larger (2.6-fold) difference was observed for speB in two separate experiments, suggesting that this gene might be expressed at a slightly lower level in the mutant strain.
The levels of hyaluronic acid, streptokinase, and protease expressed by the parent and mutant strains are shown in Table 2. No differences were detected in the levels of hyaluronic acid produced, consistent with previous reports that insertion mutations in the sag operon promoter have no effect on the expression of the hyaluronic acid capsule (4, 23). However, the finding that the parent and all three isogenic mutants also produced very similar levels of streptokinase (Table 2) differs sharply from the dramatic reduction in streptokinase production reported for the serotype M49 pel-1 mutant (23). In addition, both immunoblotting with anti-M5 protein sera (data not shown) and the bactericidal assays described below showed that the sagBΔ1 mutation did not result in a significant loss of the antiphagocytic cell surface M5 protein in strain Manfredo. The parent and all three mutants also produced readily detectable quantities of SpeB protease, although a small reduction in the amount of the protease produced was observed for the two mutants containing the sagBΔ1 mutation (Table 2), possibly reflecting the slightly reduced transcription of the speB gene described above.
Effects of mutations on survival in human blood.
To determine whether the potent cytotoxic activities of SLO and/or SLS contribute to the survival of serotype M5 strain Manfredo in human blood, the parent and mutant strains were compared in standard 3-h bactericidal assays with fresh human blood from five different nonimmune donors lacking readily detectable anti-SLO antibodies (ASO titers, <12). Table 2 includes typical results for two donors. Perhaps surprisingly, all three mutants, including the mutant lacking both SLO and SLS, grew as effectively as the parent in fresh human blood.
Effects of mutations on lethality.
The LD50s of the parent and mutant strains were determined upon infection by the i.p. and s.c. routes. Upon i.p. infection, the LD50s were remarkably similar for the strains (e.g., log10 CFUs of 8.8 and 8.6 for the parent and the sloΔ1 sagBΔ1 mutant, respectively). Passage in vivo, which can increase the virulence of GAS for mice (21), decreased the LD50s of both parent strain Manfredo and the sloΔ1 sagBΔ1 double-mutant strain, but there was still no significant difference (log10 CFUs of 7.0 and 6.9, respectively). Upon infection by the s.c. route, there was no apparent difference in LD50s between the parent (log10 CFU of 7.0) and sloΔ1 mutant (log10 CFU of 6.9) strains, and there was only a small difference between these strains and the sagBΔ1mutant strain (log10 CFU of 8.0); however, it is interesting that in two separate experiments, the difference was slightly larger (1.5 log units) for the sloΔ1 sagBΔ1 double mutant. In the above experiments, it was also noted that, irrespective of the route of infection, the average time to death at each lethal dose was extended for the mutant strains (data not shown).
Virulence in a murine dermonecrosis model.
In the experiments described here, disease progression in mice inoculated s.c. with wild-type strain Manfredo generally followed a course similar to that described by others for different serotypes of GAS (3, 4, 10, 24). Briefly, depending on the infective dose, by 24 h postinfection, mice displayed clear weight losses, and raised abscesses were apparent at the inoculation sites. Over the following days, depending on the infective dose, abscesses developed necrotic centers, followed by sunken necrotic lesions. Mice either developed bacteremia and died or recovered fully following the development of necrotic lesions.
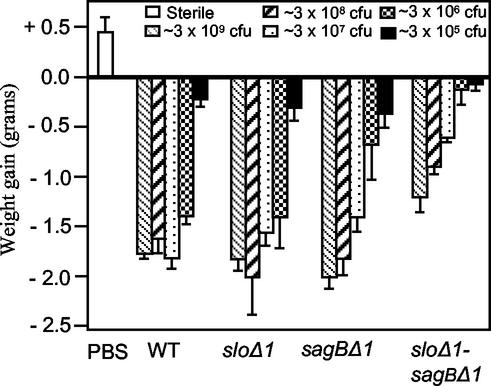
The relative contributions of SLO and SLS to the early stages of infection in this model were assessed by determining weight gain or loss at 24 h postinfection in groups of five mice inoculated over a range of 10-fold dilutions (in PBS) with the parent or mutant strains. ANOVA analysis confirmed that both differences between strains and differences in infective doses made significant (P < 0.0001 in both cases) contributions to the observed variance. A group of control mice inoculated in an identical manner with PBS alone displayed a clear weight gain (0.45 ± 0.2 g [mean and standard deviation]) by 24 h postinoculation, whereas all mice infected at the dose range shown in Fig. 2 either failed to gain weight or displayed clear weight losses by 24 h postinfection. This range was selected following preliminary experiments which showed that mice inoculated with lower doses (approximately 104 CFU) of wild-type parent strain Manfredo displayed weight gains similar to those of PBS-inoculated control mice and did not develop abscesses or lesions at the site of inoculation. For all of the experiments described here, very similar results were obtained over the entire dose range on at least two separate occasions, with the exception of the highest (∼3 × 109 CFU), most severe dose shown in Fig. 2, which was omitted from subsequent experiments to minimize distress to animals.
FIG. 2.
Mean weight losses in animals 24 h after infection (s.c.) with wild-type (WT) M5 GAS parent strain Manfredo and its isogenic mutant derivatives. A control group inoculated in an identical manner with sterile PBS is also shown. Different groups of mice were inoculated with various 10-fold serial dilutions of strains in PBS; the corresponding groups (dilutions) for each strain are indicated by shading, and the approximate inoculum doses are shown at the top of the diagram. Since precise viable counts for inocula were not available until the day after infection, some variations between strains in the precise infective doses administered at comparative dilutions were unavoidable. However, these variations were small, ranging from 2.4 × 109 CFU to 3.4 × 109 CFU for the highest inoculum doses administered in the experiment depicted here. Error bars indicate standard deviations.
As shown in Fig. 2, at an inoculum dose of ∼3 × 106 CFU, mice infected with the sagBΔ1 single-mutant strain did not lose as much weight as mice infected with parent strain Manfredo (P < 0.01), but at higher or lower infective doses, differences between these strains were not significant (Fig. 2) (the P value was >0.05 in all cases). A comparison of mice infected with parent strain Manfredo and mice infected with the sloΔ1 single-mutant strain revealed no significant differences at any of the infective doses examined (the P value was >0.05 in all cases). However, when both mutations were combined in the sloΔ1 sagBΔ1 double-mutant strain, highly significant (P < 0.01) differences in the amounts of weight lost compared to the data for mice infected with the parent strain were observed at infective doses ranging from ∼3 × 106 to ∼3 × 108, and moderately significant differences were observed at the highest infective dose of ∼3 × 109 (P < 0.05). Moreover, mice infected with the double mutant also did not lose as much weight (P < 0.01) as mice infected with the corresponding doses of either the sagBΔ1 single mutant or the sloΔ1 single mutant. It is clear from these data that both SLO and SLS contribute to the early stages of infection in this model.
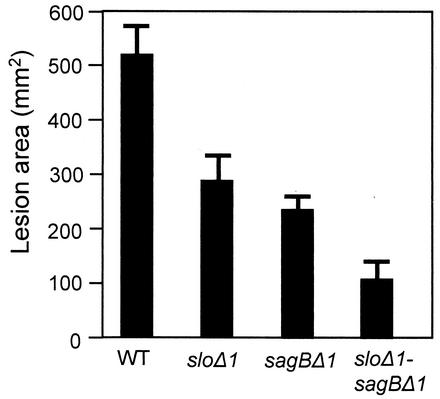
Table 3 and Fig. 3 illustrate the effects of the mutations on the development of necrosis. Table 3 summarizes the numbers of mice that displayed visible signs of necrosis, irrespective of severity, on or before two selected times during the experiment (middle and end). At high infective doses, all mice, including mice infected with the sloΔ1 sagBΔ1 double mutant, developed necrosis, but at lower infective doses, there were clear differences between the parent and mutant strains. It is interesting that, as reported above, comparing the parent and single-mutant strains illustrated the contribution of SLS to the development of necrosis more readily than that of SLO, which was clear only when both mutations were combined in the sloΔ1 sagBΔ1 double mutant. However, the contribution of SLO, as well as SLS, to necrosis was observed more directly when the maximum sizes of the necrotic lesions that developed in mice surviving the first few days of infection were compared. Figure 3 shows the maximum sizes of the lesions that had developed by day 7 in mice infected with ∼3 × 108 CFU of the parent or mutant strains. At this dose, two of five mice infected with the wild-type parent strain became moribund and were killed within the first few days of the experiment, before necrotic lesions had developed fully; consequently, data for these mice were excluded from the data presented. The three surviving mice in this group developed large necrotic lesions by day 7, before becoming moribund and being killed. Significantly smaller lesions were produced by both the sloΔ1 and the sagBΔ1 mutants, with the latter producing slightly smaller lesions than the former. Again, as was observed for the other virulence measures, the double mutant proved to be less virulent that either of the two single mutants (Fig. 3).
TABLE 3.
Numbers of mice per group (n = 5) that developed necrosis by days 7 and 14
| Infective dosea | No. of mice that developed necrosis after infection with:
|
||||
|---|---|---|---|---|---|
| Day post- infection | Wild-type M5 GAS | sloΔ1 mutant | sagBΔ1 mutant | sloΔ1 sagBΔ1 mutant | |
| ∼3 × 107 | 7 | 4 | 5 | 5 | 4 |
| 14 | 5 | 5 | 5 | 5 | |
| ∼3 × 106 | 7 | 3 | 5 | 3 | 0 |
| 14 | 5 | 5 | 3 | 1 | |
| ∼3 × 105 | 7 | 1 | 0 | 0 | 0 |
| 14 | 1 | 2 | 0 | 0 | |
At higher infective doses, all mice developed necrosis by day 7.
FIG. 3.
Severity (area) of necrotic lesions at day 7 in mice infected with approximately 3 × 108 CFU (Fig. 2) of wild-type (WT) M5 GAS parent strain Manfredo and its isogenic mutant derivatives. Error bars indicate standard deviations.
DISCUSSION
The allele replacement mutagenesis procedure used in this study provided an efficient means for introducing in-frame deletion mutations into the GAS chromosome. This approach avoids potential complications from polar effects of inserted sequences, which may have contributed to apparent conflicts in published data concerning the effects of SLS-defective insertion mutations. It also enables mutations in two or more distinct vir genes to be combined easily in a single GAS strain, without the need to use multiple selective markers or retain any inserted sequences. As described here, this strategy can reveal contributions to virulence of particular products which might not be as readily apparent from studies with single mutations alone. In the present study, the application of this approach to the construction of both single and double mutations in the slo and sagB genes of serotype M5 GAS strain Manfredo has provided further insights into the contributions of SLO and SLS in a variety GAS virulence models.
As outlined in the introduction, apparent conflicts in the recent literature concerning the effects of SLS-defective insertion mutants on the expression of other virulence factors raised questions concerning the role of SLS in virulence. Some of the reported phenotypic differences may be attributable to differences in GAS strains used in different studies (7), but others are more difficult to understand (23). In the study reported here, we made no attempt to address the reasons for these directly. Instead, we adopted an alternative approach to the construction of SLS-deficient mutants which was designed to avoid any potential complications from polar effects arising from inserted sequences. The sagBΔ1 mutant described here lacked detectable SLS activity but, unlike SLS-defective insertion mutants described previously (7, 23), it had little or no effect on the transcription of sagA, which appears to encode the SLS precursor (12). Others have shown that a transcriptional terminator between sagA and sagB limits normal expression of the downstream sag genes to such an extent that the corresponding transcripts could be detected only by highly sensitive reverse transcription-PCRs (36). No attempt was made in this study to quantify this effect directly, but we have observed that the introduction of a recombinant plasmid carrying an intact sagB gene alone is sufficient to restore SLS activity to the sagBΔ1 mutant (J. J. Lee and M. A. Kehoe, unpublished data), confirming that the mutation has not resulted in a significant reduction in the expression of downstream genes that are known to be essential for SLS production (36). Since previous studies used insertional mutagenesis to inactivate sag genes (36), it may be worth noting that the nonpolar sagB mutation described here provides the first direct evidence that the sagB gene product, in addition to other sag gene products, is required for the production of active SLS.
Possibly because of the absence of polar effects on the expression of other sag genes, the sagBΔ1 mutation did not result in significant alterations in the expression of other virulence factors examined. This finding was demonstrated independently by both hybridization dot blot and phenotypic assays. No significant differences were detected in the levels of the emm5 gene transcript produced by the sagBΔ1 mutant and isogenic parent strains, and both immunoblotting with anti-M5 protein sera (data not shown) and the ability of the sagBΔ1 mutant to multiply as effectively as parent strain Manfredo in fresh human blood confirmed that the antiphagocytic M5 protein was both secreted and associated in a fully functional manner with the cell surface. Similarly, no differences were detected between the parent and mutant strains in the levels of hyaluronic acid produced, consistent with previous studies (4, 23), or in the levels of either the ska transcript or extracellular streptokinase, in contrast to the reported phenotype of the sag::Tn917 pel-1 mutation (23). Both the speB transcript and the secreted SpeB protease were also readily detected in the mutant strain as well as the parent strain; however, in two independent experiments, a small (<3-fold) reduction was observed in the level of the speB transcript produced by the mutant strain, and this effect was reflected in slightly smaller zones of proteolysis on casein assay plates. Previous studies showed that, depending on the GAS strain studied, complete inactivation of speB either had no significant effect on virulence in a murine dermonecrosis model or resulted in reduced lesion severity and lethality (3, 20, 26-28). The reason for the small reduction in protease production by the sagBΔ1 mutant described here is not understood, and the possibility that it contributes to some extent to the reduced virulence of the sagBΔ1 mutant has not been excluded. However, in view of the small quantitative differences involved, it seems unlikely that such an effect, if any, would be large and, for reasons of animal welfare, it was not possible to justify additional infection experiments to test this notion directly in the present study. Phenotypic assays detected no differences between the parent and sloΔ1 mutant strains in the expression of other virulence factors or between the sagBΔ1 mutant described above and the sloΔ1 sagBΔ1 double mutant.
Previous in vitro studies of purified SLO and SLS established that both toxins are highly potent cytolysins and that sublytic concentrations have other effects against a range of mammalian cells, including, for example, inhibition of various phagocytic cell functions (1, 37). The study reported here revealed no evidence that these activities contribute to the ability of GAS to survive in fresh human blood. All three mutants, including the sloΔ1 sagBΔ1 double mutant, were found to multiply as effectively as the wild-type parent strain in fresh human blood lacking neutralizing anti-SLO antibodies detectable by the standard ASO test. SLS is nonimmunogenic, and cholesterol in the form that circulates in blood does not inhibit SLO in in vitro assays (1). It is possible that the relatively small numbers of GAS cells used in the standard GAS bactericidal assay (21) simply do not produce sufficient quantities of toxins to have significant effects in the in vitro assay or that other components in fresh human blood inhibit these activities.
In view of previous accounts describing the potent toxic properties of purified or partially purified SLO or SLS in various small animal models (for reviews, see references 1, 17, and 59), it is perhaps surprising that these toxins do not appear to have very dramatic effects on the lethality of GAS infections in mice. There are only two previous reports in the literature describing in vivo virulence studies of SLS-defective mutants of GAS. One early study was compromised by use of a poorly defined, chemically induced SLS-defective mutant that differed from the parent strain in its in vitro, as well as in vivo, growth properties (39). The more recent and reliable study of Betschel et al. (4) used a single infective dose to examine genetically defined sag::Tn916 mutants in a nonlethal infection model and did not assess the extent to which SLS contributes to lethality. The single log unit difference reported here in the LD50s of serotype M5 parent strain Manfredo and its isogenic sagBΔ1 derivative in the murine s.c. infection model provides the first direct evidence that SLS contributes to lethality in a GAS infection model. However, it appears to do so to a remarkably limited extent for such a potent cytolysin (1, 59). The only account in the literature of in vivo virulence studies of an SLO-deficient GAS mutant reached a very similar conclusion regarding the contribution of SLO to GAS lethality in the mouse s.c. infection model (24). Here we detected no significant difference in the LD50s of M5 parent strain Manfredo and its isogenic sloΔ1derivative in either i.p or s.c. infections. Taking into account the different strains and different measures of survival used (LD50 versus Kaplan-Meier plots), the studies described here and those of Limbago et al. (24) are remarkably consistent in demonstrating that SLO contributes to GAS lethality in this model to a much lesser extent than might have been predicted from the properties of the purified toxin.
As noted previously by others (4), lethality in murine models may be a relatively insensitive measure of GAS virulence, and alternative measures have the potential to reveal more subtle differences between strains. In this study, the extent of weight loss at 24 h after infection by the s.c. route and the development of necrotic lesions over the following days revealed clearer differences between parent and mutant strains than LD50 assays. Since the nonpolar sagBΔ1 mutant of strain Manfredo did not have marked effects on the expression of other virulence factors, our findings that it reduced the weight loss and the severity of necrotic lesions produced by serotype M5 GAS parent strain Manfredo strongly support the original conclusions of Betschel et al. (4) concerning the importance of SLS in the murine dermonecrosis model. Betschel et al. (4) compared parent and sag::Tn916 mutant strains by using a single infective dose selected to produce nonlethal infections. From the study reported here, it is interesting that the extent to which SLS affected weight loss clearly depended on the infective dose, suggesting that SLS makes an incremental rather than a critical contribution to virulence and may be redundant at higher infective doses. It is also interesting that the contribution of SLO to weight loss at 24 h after infection was not at all clear from studies of the sloΔ1 mutant alone, consistent with the previous report of Limbago et al. (24). However, when both mutations were combined in the same strain, the effect of the sloΔ1 mutation was seen to be highly significant. This result suggests that, like SLS, SLO also makes an incremental rather than a critical contribution to virulence in this model. Moreover, the ability of the double mutant lacking both toxins to cause weight loss, albeit at a significantly reduced level, indicates that SLO and SLS are by no means the only virulence factors that contribute to the early stages of infection in the murine s.c. infection model.
In general, the effects of the mutations on the development of necrotic lesions paralleled their effects on weight loss. At higher infective doses, all mice, including those that had been infected with the sloΔ1 sagBΔ1 double mutant, developed necrotic lesions, demonstrating that SLO and SLS are not the only factors that contribute to necrosis in this model. As described above, at lower infective doses, the contribution of SLO was not readily apparent from the numbers of mice that developed necrotic lesions following infection with the sloΔ1 single mutant, but the double mutant was less virulent than either of the single mutants, indicating that both toxins contributed to necrosis. The contribution of SLO, as well as SLS, to necrosis was seen more directly when the maximum sizes of the lesions that developed by day 7 of the experiment were compared. With this measure, a clear reduction in the severity of the lesions induced by both the SLO- and the SLS-deficient mutant strains compared to the parent strain was observed. Paralleling the results obtained with other measures, the double mutant displayed a further clear reduction in the severity of the lesions produced, again highlighting the contributions of both toxins.
In conclusion, the study reported here shows clearly that both SLO and SLS contribute to the early stages of infection and induction of necrosis in a murine GAS s.c. infection model but that they are by no means the only virulence factors involved. The data suggest that both toxins display some degree of functional redundancy, with each making incremental rather than critical contributions to virulence in the models examined here. Unlike many GAS virulence factors, both SLO and SLS are expressed by almost all GAS isolates, and both are encoded by sequences that appear to be highly conserved among distinct M serotypes of GAS (36, 56). These data could imply that both toxins play particularly important roles in the ability of GAS to survive in its natural habitat, the human host, without excluding the possibility that under certain circumstances, either toxin alone may be dispensable.
Acknowledgments
J.J.L. and M.C.F. contributed equally to this work and are listed alphabetically.
This work was supported in part by grants from The Wellcome Trust and HBLB, and M.C.F. was supported by a UK BBSRC research studentship.
We are indebted to E. Maguin (INRA, Jouy en Josas, France) for very generously providing plasmid pG+host 9 and E. coli strain TG1-dev and for helpful advice on their use and to Gunner Lindahl (University of Lund, Lund, Sweden) for providing the ΔM5 mutant of GAS strain Manfredo.
Editor: V. J. DiRita
REFERENCES
- 1.Alouf, J. 1980. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol. Ther. 11:661-717. [DOI] [PubMed]
- 2.Alouf, J., and C. Geoffroy. 1991. The family of antigenically-related cholesterol-binding (′sulphydryl-activated') cytolytic toxins, p. 146-186. In J. Alouf and J. Freer (ed.), Sourcebook of bacterial protein toxins. Academic Press Ltd., London, England.
- 3.Ashbaugh, C., H. Warren, V. Carey, and M. Wessels. 1998. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Investig. 102:550-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betschel, S., S. Borgia, N. Barg, D. Low, and J. De Azavedo. 1998. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect. Immun. 66:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisno, A., and D. Stevens. 1996. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 334:240-245. [DOI] [PubMed] [Google Scholar]
- 6.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas, I., P. Germon, K. McDade, and J. Scott. 2001. Generation and surface localization of intact M protein in Streptococcus pyogenes are dependent on sagA. Infect. Immun. 69:7029-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremm, K., W. Konig, P. Pfeiffer, I. Rauschen, K. Theobald, M. Thelestam, and J. Alouf. 1985. Effects of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect. Immun. 50:844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant, A., M. A. Kehoe, and D. L. Stevens. 1992. Streptococcal pyrogenic exotoxin A and streptolysin O enhance polymorphonuclear leukocyte binding to gelatin matrixes. J. Infect. Dis. 166:165-169. [DOI] [PubMed] [Google Scholar]
- 10.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canalias, F., J. Viver, J. Beleta, F. Gonzalez-Sastre, and F. Gella. 1992. Purification and characterization of streptolysin O from Streptococcus pyogenes. Int. J. Biochem. 24:1073-1079. [DOI] [PubMed] [Google Scholar]
- 12.Carr, A., D. Sledjeski, A. Podbielski, M. Boyle, and B. Kreikemeyer. 2001. Similarities between complement-mediated and streptolysin S-mediated haemolysis. J. Biol. Chem. 276:41790-41796. [DOI] [PubMed] [Google Scholar]
- 13.Cheung, A. L., K. J. Eberhardt, and V. A. Fischetti. 1994. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal. Biochem. 222:511-514. [DOI] [PubMed] [Google Scholar]
- 14.Degnan, B., M. Fontaine, A. Doebereiner, J. Lee, P. Mastroeni, G. Dougan, J. Goodacre, and M. Kehoe. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 68:2441-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberhard, T., D. Sledjeski, and M. D. P. Boyle. 2001. Mouse skin passage of a Streptococcus pyogenes Tn917 mutant of sagA/pel restores virulence, beta-hemolysis and sagA/pel expression without altering the position or sequence of the transposon. BMC Microbiol. [Online.] http://www.biomedcentral.com/1471-2180/1/33. [DOI] [PMC free article] [PubMed]
- 16.Ginsberg, I. 1999. Is streptolysin S of group A streptococci a virulence factor? Acta Pathol. Microbiol. Immunol. Scand. 107:1051-1059. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg, I. 1972. Streptolysin S, p. 99-171. In T. Montie, S. Kadis, and S. Ajl (ed.), Microbial toxins: bacterial protein toxins, vol. 3. Academic Press, Inc., New York, N.Y.
- 18.Ish-Horowicz, D., and J. F. Burke. 1981. Rapid and efficient cosmid cloning. Nucleic Acids Res. 9:2989-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnsson, E., K. Berggard, H. Kotarsky, J. Hellwage, P. Zipfel, U. Sjobring, and G. Lindahl. 1998. Role of hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J. Immunol. 161:4894-4901. [PubMed] [Google Scholar]
- 20.Kuo, C., J. Wu, K. Lin, P. Tsai, S. Lee, Y. Jin, H. Lei, and Y. Lin. 1998. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect. Immun. 66:3931-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancefield, R. 1962. Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 89:307-313. [PubMed] [Google Scholar]
- 22.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Z., D. Sledjeski, B. Kreikemeyer, A. Podbielski, and M. Boyle. 1999. Identification of pel, a Streptococcus pyogenes locus that affects both surface and secreted proteins. J. Bacteriol. 181:6019-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limbago, B., V. Penumalli, B. Weinrick, and J. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococal disease. Infect. Immun. 68:6384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, S., S. Sela, G. Cohen, J. Jadoun, A. Cheung, and I. Ofek. 1997. Insertional inactivation of streptolysin S expression is associated with altered riboflavin metabolism in Streptococcus pyogenes. Microb. Pathog. 22:227-234. [DOI] [PubMed] [Google Scholar]
- 26.Lukomski, S., E. Burns, P. Wyde, A. Podbielski, J. Rurangirwa, D. Moore-Poveda, and J. Musser. 1998. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect. Immun. 66:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukomski, S., C. Montgomery, J. Rurngirwa, R. Geske, J. Barrish, G. Adams, and J. Musser. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukomski, S., S. Sreevatsan, C. Amberg, W. Reichardt, M. Woischnik, A. Podbielski, and J. Musser. 1997. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of sreotype M3 and M49 strains. J. Clin. Investig. 99:2574-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madden, J., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of Type III secretion in Gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 30.Maguin, E., P. Duwat, T. Hege, D. Ehrlich, and A. Gruss. 1992. New thermosensitive plasmid for gram-positive bacteria. J. Bacteriol. 174:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguin, E., H. Prevost, S. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malke, H., and J. Ferretti. 1984. Streptokinase: cloning, expression, and excretion by Escherichia coli. Proc. Natl. Acad. Sci. USA 81:3557-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martley, F., A. Jarvis, D. Bacon, and R. Lawrence. 1970. Typing of coagulase-positive staphylococci by proteolytic activity on buffered caseinate agar with special reference to bacteriophage nontypeable strains. Infect. Immun. 2:439-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miles, A., S. Misra, and J. Irwin. 1938. The estimation of the bactericidal power of the blood. J. Hyg. (Cambridge) 38:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Alouf, H., C. Greoffroy, P. Geslin, A. Bouvert, A. Felton, E. Gunther, J.-H. Ozegowski, W. Reichardt, and J. Alouf. 1997. Serotype, biotype, pyrogenic exotoxin, streptolysin O, and exoenzyme patterns of invasive Streptococcus pyogenes isolates from patients with toxin shock syndrome, bactermia and other severe infections. Adv. Exp. Med. Biol. 418:241-243. [DOI] [PubMed] [Google Scholar]
- 36.Nizet, V., B. Beall, D. J. Bast, V. Datta, L. Kilburn, D. E. Low, and J. C. De Azavedo. 2000. Genetic locus for streptolysin S production by group A streptococcus. Infect. Immun. 68:4245-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ofek, I., S. Bergner-Rabinowitz, and I. Ginsberg. 1972. Oxygen-stable hemolysis of group A streptococci. VIII. Leukotoxic and antiphagocytic effects of streptolysins S and O. Infect. Immun. 6:459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otto, R., W. de Vos, and J. Gravieli. 1982. Plasmid DNA in Streptococcus cremoris Wg2: influence of pH on selection of a variant lacking a protease plasmid. Appl. Environ. Microbiol. 43:1272-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens, W., F. Henley, and B. Baridge. 1978. Hemolytic mutants of group A Streptococcus pyogenes. J. Clin. Microbiol. 7:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer, M., I. Vulicevic, P. Saweljew, A. Valeva, M. Kehoe, and S. Bhakdi. 1998. Streptolysin O: a proposed model of allosteric interaction between a pore-forming protein and its target lipid bilayer. Biochemistry 37:2378-2383. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Casal, J., M. Caparon, and J. Scott. 1991. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J. Bacteriol. 173:2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinkney, M., E. Beachey, and M. Kehoe. 1989. The thiol-activated toxin streptolysin O does not require a thiol group for cytolytic activity. Infect. Immun. 57:2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinkney, M., V. Kapur, J. Smith, U. Weller, M. Palmer, M. Glanville, M. Messner, J. Musser, S. Bhakdi, and M. Kehoe. 1995. Different forms of streptolysin O produced by Streptococcus pyogenes and by Escherichia coli expressing recombinant toxin: cleavage by streptococcal cysteine protease. Infect. Immun. 63:2776-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentki, P., and H. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 45.Reed, L., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 46.Ruiz, N., B. Wang, A. Pentland, and M. Caparon. 1998. Streptolysin O and adherence modulate proinflammatory responses of keratinpcytes. Mol. Microbiol. 27:337-346. [DOI] [PubMed] [Google Scholar]
- 47.Sahl, H.-G., and G. Bierbaum. 1998. LANTIBIOTICS: biosynthesis and biological activities of uniquely modified peptides from Gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schrager, H., J. Rheinwald, and M. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanley, T., P. Schrier, V. Kapur, M. Kehoe, J. Musser, and P. Ward. 1996. Streptococcal cysteine protease augments lung injury induced by products of group A streptococci. Infect. Immun. 64:870-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiseki, M., K. Miwa, Y. Nemoto, H. Kato, J. Suzuki, K. Sekiya, T. Murai, T. Kikuchi, N. Yamashita, K. Totsuka, K. Ooe, Y. Shimizu, and T. Uchiyama. 1999. Comparison of pathogenic factors expressed by group A streptococci isolated from patients with streptococcal toxic shock syndrome and scarlet fever. Microb. Pathog. 27:243-252. [DOI] [PubMed] [Google Scholar]
- 52.Siegel, A., E. Johnson, and G. Stollerman. 1961. Controlled studies of streptococcal pharyngitis in a pediatric population. 1. Factors related to the attack rate of rheumatic fever. N. Engl. J. Med. 265:559-566. [Google Scholar]
- 53.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-566. [DOI] [PubMed] [Google Scholar]
- 54.Simon, D., and J. Ferretti. 1991. Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol. Lett. 82:219-224. [DOI] [PubMed] [Google Scholar]
- 55.Stevens, D., and E. Kaplan. 2000. Streptococcal infections. Clinical aspects, microbiology, and molecular pathogenesis. Oxford University Press, New York, N.Y.
- 56.Suvorov, A., Y. Tang, C. Yu, and J. Ferretti. 1992. PCR analysis of the streptolysin O (slo) gene of Streptococcus pyogenes, p. 223-224. In G. Orefici (ed.), New perspectives on streptococci and streptococcal diseases. Gustav Fischer Verlag, Stuttgart, Germany.
- 57.Tewodros, W., M. Norgren, and G. Kronvall. 1995. Streptokinase activity among group A streptococci in relation to streptokinase genotype, plasminogen binding and disease manifestations. Microb. Pathog. 18:53-65. [DOI] [PubMed] [Google Scholar]
- 58.Theodore, T., and G. Calandra. 1981. Streptolysin S activation by lipoteichoic acid. Infect. Immun. 33:326-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wannamaker, L. 1983. Streptococcal toxins. Rev. Infect. Dis. 5:S723-S732. [DOI] [PubMed] [Google Scholar]
- 60.Whatmore, A., and M. Kehoe. 1994. Horizontal gene transfer in the evolution of group A streptococcal emm-like genes: gene mosaics and variation in vir regulons. Mol. Microbiol. 11:363-374. [DOI] [PubMed] [Google Scholar]
- 61.Wheeler, M., M. Roe, E. Kaplan, P. Schlievert, and J. Todd. 1991. Outbreak of group A Streptococcus septicemia in children. JAMA 266:533-537. [PubMed] [Google Scholar]
- 62.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]