Abstract
The antimicrobial peptides human β-defensin-1 (hBD1), hBD2, hBD3, and CAP18 expressed by keratinocytes have been implicated in mediation of the innate defense against bacterial infection. To gain insight into Staphylococcus aureus infection, the susceptibility of S. aureus, including methicillin-resistant S. aureus (MRSA), to these antimicrobial peptides was examined. Based on quantitative PCR, expression of hBD2 mRNA by human keratinocytes was significantly induced by contact with S. aureus, and expression of hBD3 and CAP18 mRNA was slightly induced, while hBD1 mRNA was constitutively expressed irrespective of the presence of S. aureus. Ten clinical S. aureus isolates, including five MRSA isolates, induced various levels of expression of hBD2, hBD3, and CAP18 mRNA by human kertinocytes. The activities of hBD3 and CAP18 against S. aureus were found to be greater than those of hBD1 and hBD2. A total of 44 S. aureus clinical isolates, including 22 MRSA strains, were tested for susceptibility to hBD3 and CAP18. Twelve (55%) and 13 (59%) of the MRSA strains exhibited more than 20% survival in the presence of hBD3 (1 μg/ml) and CAP18 (0.5 μg/ml), respectively. However, only three (13%) and two (9%) of the methicillin-sensitive S. aureus isolates exhibited more than 20% survival with hBD3 and CAP18, respectively, suggesting that MRSA is more resistant to these peptides. A synergistic antimicrobial effect between suboptimal doses of methicillin and either hBD3 or CAP18 was observed with 10 MRSA strains. Furthermore, of several genes associated with methicillin resistance, inactivation of the fmtC gene in MRSA strain COL increased susceptibility to the antimicrobial effect mediated by hBD3 or CAP18.
Staphylococcus aureus is one of the pathogenic bacteria that cause suppurative diseases, food poisoning, and staphylococcal scaled skin syndrome. Chemotherapy is an effective treatment for these diseases, and various agents have been used, such as β-lactam antibiotics, aminoglycosides, and quinolones. β-Lactam antibiotics are often employed, but the emergence of methicillin-resistant S. aureus (MRSA) has become a serious problem in chemotherapy (30). MRSA exhibit resistance to β-lactam antibiotics because of acquisition of the mecA gene, which encodes PBP2′ (PBP2A), which exhibits low affinity for penicillin (37, 46). Also, recent clinical isolates have exhibited multiple resistances to various antibiotics, making control of S. aureus infections by chemotherapy more difficult. Moreover, recent reports have suggested that there has been emergence of MRSA strains that exhibit low susceptibility to glycopeptide antibiotics, such as vancomycin and teicoplanin, which are considered the only effective antibiotics for use against multiply resistant MRSA (5, 16, 31, 43, 45). Therefore, it is becoming more difficult to treat MRSA infections with the current antimicrobial agents, and the demand for new chemotherapeutic agents that can be used against these microorganisms is increasing.
For the most part human antimicrobial peptides are classified into three types: defensins, cathelicidins, and histatins (2, 9, 12, 28, 48, 51). Defensins are cysteine-rich, cationic peptides and are divided into α- and β-defensins on the basis of structure and the cells that produce them (17, 18, 51). α-Defensins (human neutrophil α-defensin 1 [HNP1] to HNP4) are produced by neutrophils (9, 42), and α-defensin-5 and α-defensin-6 are found in Paneth cells in the gastrointestinal tract (20, 36). β-Defensins (human β-defensin-1 [hBD1] to hBD3) are produced in the epithelium (7, 13, 14, 38, 47). Recently, hBD4 was also identified as an inducible and salt-sensitive peptide (10). CAP18 (LL37) is the only human antimicrobial peptide that has been identified in the cathelicidin family, and it is produced in the epithelium and in neutrophils (27). Histatins belong to a family of histidine-rich peptides found in human saliva (11, 12, 48). These peptides have activities against some microorganisms, and some of them have been reported to play a role as chemotactic factors for T cells or dendritic cells (50, 51). Also, it has been demonstrated that CAP18 binds and neutralizes lipopolysaccharide and lipoteichoic acid, which are considered mediators of inflammation (27, 40). Since β-defensins and CAP18 are produced by epithelial cells that are the first line of defense against pathogens (1), it has been suggested that these peptides are responsible for innate defense against microorganisms in the skin epithelium. Therefore, defensins and CAP18 could be new chemotherapeutic agents for use against bacterial infections, including S. aureus infections. However, little is known about the interaction of these peptides and S. aureus cells in the context of the skin epithelium. In this study, we demonstrated the patterns of hBD1 to hBD3 and CAP18 mRNA expression in human skin keratinocytes in response to exposure to heat-killed S. aureus. The activities of these peptides against S. aureus, including MRSA, were also evaluated along with the combined effects of peptides and antibiotics.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus COL and clinical isolates, including methicillin-sensitive S. aureus (MSSA) and MRSA strains, were grown in Trypticase soy broth (TSB) at 37°C. When necessary, a chemically defined medium for staphylococci was used (15). The chemically defined medium consisted of five solutions, as follows. Solution 1 contained 20.1 g of Na2HPO4 · 12H2O, 3 g of KH2PO4, 150 mg of l-aspartic acid, 150 mg of l-gultamic acid, 150 mg of l-isoleucine, 150 mg of l-leucine, 150 mg of l-proline, 150 mg of l-threonine, 150 mg of l-valine, 100 mg of l-alanine, 100 mg of l-arginine, 100 mg of glycine, 100 mg of l-histidine, 100 mg of l-lysine, 100 mg of l-methionine, 100 mg of l-phenylalanine, 100 mg of l-serine, 100 mg of l-tryptophan, 100 mg of l-tyrosine, and 50 mg of l-cystine dissolved in 700 ml of distilled water, and the pH was adjusted to 7.2. Solution 2 contained 0.1 mg of biotin, 2 mg of nicotinic acid, 2 mg of d-pantothenic acid, 4 mg of pyridoxal, 4 mg of pyridoxamine dihydrochloride, 2 mg of riboflavin, and 2 mg of thiamine hydrochloride dissolved in 100 ml of distilled water. Solution 3 contained 20 mg of adenine sulfate and 20 mg of guanine hydrochloride dissolved in 0.1 M HCl, and the volume was adjusted to 50 ml with distilled water. Solution 4 contained 10 mg of CaCl2 · 6H2O, 5 mg of MnSO4, and 3 mg of (NH4)2SO4 · FeSO4 · 6H2O dissolved in 10 ml of 0.1 M HCl. Solution 5 contained 10 g of glucose and 500 mg of MgSO4 · 7H2O dissolved in 100 ml of distilled water. Solutions 1 to 4 were mixed, and the volume was adjusted to 900 ml with distilled water. The mixed solution and solution 5 were autoclaved separately, and the two solutions were mixed prior to use.
Keratinocyte culture.
Normal human skin obtained from plastic surgery was incubated with 250 U of dispase (Sigma-Aldrich, Tokyo, Japan) per ml overnight at 4°C. After separation of the epidermis from the dermis, the epidermal sheets were incubated in a 0.25% trypsin solution (Sigma-Aldrich) for 10 min at 37°C. After centrifugation, normal human keratinocytes were cultured in MCDB153 medium supplemented with insulin (5 μg/ml), hydrocortisone (5 × 10−7 M), ethanolamine (0.1 mM), phosphoethanolamine (0.1 mM), and bovine hypothalamic extract (100 μg/ml). When necessary, kanamycin (50 μg/ml) was added to the medium. Confluent third- or fourth-passage cells were used in this study.
Coculture of keratinocytes with heat-killed S. aureus.
Overnight cultures of S. aureus cells grown in TSB at 37°C were harvested and washed with phosphate-buffered saline (PBS) twice. Then the S. aureus cells were suspended in PBS and incubated at 68°C for 30 min to kill them. The cells were subjected to mild sonication, and dissociation of clumps was confirmed by microscopic observation. At 12 h prior to bacterial contact, confluent keratinocytes in MCDB medium with supplements was replaced with MCDB without supplements, and this was followed by addition of heat-killed bacteria (final concentration, 108 cells/ml) to the medium. The culture was incubated for an appropriate time up to 24 h. This experiment was carried out three times independently, and then the following reverse transcriptase PCR (RT- PCR) and real-time PCR experiments were performed.
RNA extraction and RT-PCR.
After exposure to bacterial cells, total RNA was extracted from the keratinocytes with ISOGEN (Nippon Gene, Tokyo, Japan) used according to the manufacturer's protocol. RT-PCR was performed as follows. Two micrograms of total RNA was subjected to cDNA synthesis with a first-strand cDNA synthesis kit (Roche, Tokyo, Japan) in a 20-μl (total volume) mixture. For first-strand synthesis, each antisense primer listed in Table 1 was used. Then one-tenth of each cDNA preparation generated was used as the template DNA for a subsequent PCR performed with the High Fidelity Expand system (Roche). A battery of sense and antisense primer sets, including primers for β-defensins, CAP18, and cytokines, are listed in Table 1. The housekeeping gene β-actin was also amplified as a control.
TABLE 1.
Primers used in this study
| Primer | Sequence |
|---|---|
| RT-PCR primers | |
| hBD1-1 | CTC TGT CAG CTC AGC CTC |
| hBD1-2 | CTT GCA GCA CTT GGC CTT CCC |
| hBD2-1 | CCA GCC ATC AGC CAT GAG GGT |
| hBD2-2 | GGA GCC CTT TCT GAA TCC GCA |
| hBD3-1 | AGC CTA GCA GCT ATG AGG ATC |
| hBD3-2 | CTT CGG CAG CAT TTT GCG CCA |
| CAP18-1 | GGC CAT GAA GAC CCA AAG |
| CAP18-2 | CTA GGA CTC TGT CCT GGG TAC |
| IL-1β-1 | AAA CAG ATG AAG TGC TCC TTC CAG G |
| IL-1β-2 | TGG AGA ACA CCA CTT GTT GCT CCA |
| IL-6-1 | ATG AAC TCC TTC TCC ACA AGC GC |
| IL-6-2 | GAA GAG CCC TCA GGC TGG ACT G |
| IL-8-1 | ATG ACT TCC AAG CTG GCC GTG GCT |
| IL-8-2 | TCT CAG CCC TCT TCA AAA ACT TCT C |
| TNF-α-1 | CGG GAC GTG GAG CTG GCC GAG GAG |
| TNF-α-2 | CAC CAG CTG GTT ATC TCT CAG CTC |
| Real-time PCR primers | |
| hBD1-67Fa | TCG CCA TGA GAA CTT CCT ACC T |
| hBD1-195Rb | CTC CAC TGC TGA CGC AAT TGT A |
| hBD1-136Tc | CCA CCT GAG GCC ATC TCA GAC AAA AGT AAG |
| hBD2-16F | TGA AGC TCC CAG CCA TCA G |
| hBD2-144R | GGC TCC ACT CTT CCA AAG GA |
| hBD2-107T | CAC CAA AAA CAC CTG GAA GAG GCA TCA |
| hBD3-346F | TCA GCT GCC TTC CAA AGG A |
| hBD3-414R | TTC TTC GGC AGC ATT TTC G |
| hBD3-367T | AAC AGA TCG GCA AGT GCT CGA CGC |
| CAP18-284F | CAC AGC AGT CAC CAG AGG ATT G |
| CAP18-366R | GGC CTG GTT GAG GGT CAC T |
| CAP18-341T | ATA CAC CGC TTC ACC AGC CCG TCC |
Sense primer.
Antisense primer.
TagMan probe.
Quantitative (real-time) PCR.
Real-time PCR was performed with the ABI 7700 system (Applied Biosystems, Tokyo, Japan). By using a Core Reagent kit (Applied Biosystems), reactions were carried out according to the manufacturer's protocol. The TaqMan probe, sense primers, and antisense primers used for detection of β-defensins and CAP18 are listed in Table 1. The primer set for glyceraldehyde-3-phosphate dehydrogenase (GADPH) is commercially available (Applied Biosystems).
Synthetic peptides.
To examine the effects of antimicrobial peptides on S. aureus, all of the peptides listed in Table 2 were synthesized with a Shimazu peptide synthesizer. Then the peptides were purified by reversed-phase high-performance liquid chromatography with an octadecyl-4PW column (Tosoh, Tokyo, Japan). Separation was performed with a linear gradient of aqueous 0.05% trifluoroacetic acid to 100% acetonitrile containing 0.05% trifluoroacetic acid at a flow rate of 1 ml/min for 30 min. Major peak fractions (as determined by absorbance at 230 nm) were collected and lyophilized to remove the organic solvent completely. To confirm the purity and the quality of the peptides, matrix-assisted laser desorption ionization-time of flight mass spectrometry was performed with Voyager (PerSeptive Biosystems, Framingham, Mass.). The time of flight mass spectrometry analysis revealed that the molecular masses of hBD1, hBD2, hBD3, and CAP18 were 4,532.6, 4,227.7, 5,151.3, and 4,173.1 Da, respectively. The molecular mass of synthetic CAP18 was identical to the molecular mass calculated from the primary sequence, while the molecular mass of each β-defensin (hBD1, hBD2, and hBD3) was 6 Da less than the value expected from the primary sequence. Native β-defensins have three disulfide bonds involving six cysteine residues and form three β-sheets and one α-helix (17, 18). Our molecular mass data suggested that each synthetic β-defensin had three disulfide bonds.
TABLE 2.
Synthetic peptides used in this study
| Peptide | Amino acid sequence | Mer | Mol wta |
|---|---|---|---|
| hBD-1 | GLGHRSDHYNCVSSGGQCLYSACPIFTKIQGTCYR GKAKCCK | 42 | 4,539 |
| hBD-2 | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGT KCCKK | 40 | 4,234 |
| hBD-3 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCST RGRKCCRRKK | 45 | 5,157 |
| CAP18 | LLGDDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPR | 34 | 4,173 |
The molecular weight was calculated from the amino acid sequence without disulfide bonds.
Antibacterial assay.
Overnight cultures of S. aureus strains were harvested, washed with PBS, and suspended in 10 mM sodium phosphate buffer (NaPi) (pH 6.8). Each bacterial suspension was subjected to mild sonication to disperse clumping cells and diluted to a concentration of 107 cells/ml with NaPi (pH 6.8). Ten microliters of a bacterial suspension (105 cells) was inoculated into 200 μl of NaPi with or without various concentrations of the antibacterial peptides and incubated for 2 h at 37°C. An appropriate dilution of the reaction mixture (100 μl) was plated on Trypticase soy agar and then incubated at 37°C overnight. The number of CFU was determined from the number of colonies on each plate. The antibacterial effect was estimated by determining the rate of survival of cells in comparison with the total number of cells. The same experiment was carried out three times. To evaluate the effect of NaCl, 10 mM NaPi (pH 6.8) containing various concentrations of NaCl (10 to 300 mM) was used in the antibacterial assay described above.
Electron microscopy.
Thin-section electron microscopy was performed to observe the influence of each antimicrobial peptide on cultured S. aureus. Overnight cultures of S. aureus 2PF-18, which was isolated from S. aureus 209P as a protein A-deficient mutant (49), were harvested, washed with 10 mM NaPi (pH 6.8), and suspended in the same buffer. Antimicrobial peptides were added at a final concentration of 100 μg/ml and incubated for 2 h at 37°C. S. aureus cells were washed with Dulbecco's PBS, and then the cells were doubly fixed with 2.5% glutaraldehyde. The samples were dehydrated in a series of ethanol concentrations and then embedded in Spurr's Epon. Thin sections were cut with an ultramicrotome with a diamond knife and were examined with a JOEL JEM-2000 EX II electron microscope at 80 kV.
Effects of combinations of antibacterial peptides with antibiotics on S. aureus cells.
Since more than two antibiotics are sometimes used for chemotherapy, we evaluated the effects of combinations of antimicrobial peptides with antibiotics. The following two methods were employed.
(i) Checkerboard titration method.
The effects of combinations of antibacterial peptides (hBD3 and CAP18) with antibiotics (methicillin, imipenem, vancomycin, gentamicin, chloramphenicol, and ofloxacin) were analyzed by a checkerboard titration method by using a 96-well titer plate (Nalge Nunc International, Tokyo, Japan) as described elsewhere (24). This procedure was performed at 30°C to decrease the growth rate, because peptides had a greater effect on nongrowing or slowly growing cells than on rapidly growing cells. Since the antibacterial activities of the peptides were influenced by NaCl, a chemically defined medium without NaCl was used in this assay. Test strains grown overnight at 37°C in 10 ml of TSB were diluted to a concentration of 106 CFU/ml and incubated in TSB containing serially twofold diluted antibacterial peptides and antibiotics at 30°C. After 24 h, MICs were determined by determining the minimum concentration at which no growth was visible. The fractional inhibitory concentration (FIC) was calculated as follows: FIC = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone). The minimal FIC of corresponding FICs was defined as the FIC index. Synergy was defined as an FIC index of ≤0.5; an additive effect was defined as 0.5 < FIC index < 1; indifference was defined as an FIC index of 1 to 2; and antagonism was defined as an FIC index of >2.
(ii) Effect of pretreatment with methicillin.
S. aureus cells grown in the presence or absence of methicillin were used for the antibacterial assay described above. Small portions of an overnight culture grown without antibiotics were inoculated into fresh TSB containing 1/16 MIC of methicillin and then incubated for 3 h at 37°C. Then the cells were harvested and used for the assay. Bactericidal activity was estimated by determining the rate of survival of cells in comparison with the total number of cells. Finally, the ratio of bactericidal activity in methicillin-treated cells to bactericidal activity in control cells was calculated.
Effects of combinations of antibacterial peptides on S. aureus cells.
Epithelial cells produce several antimicrobial peptides. Therefore, we investigated the effects of combinations of antimicrobial peptides on S. aureus. To evaluate the combined effects of peptides, the antibacterial effect of each paired set of peptides (1 μg/ml) or each peptide alone (1 and 2 μg/ml) was measured by the method described above.
Susceptibilities to antimicrobial peptides of MRSA mutants that exhibited reduced methicillin resistance.
A number of genes have been reported to be associated with resistance to methicillin in S. aureus (4, 6, 19, 22, 23). We found that methicillin resistance in MRSA is affected by the fmtC gene (22), which turned out to be the same gene as mprF, which affects the susceptibility to defensins (34). Therefore, we investigated the susceptibilities of mutants to hBD3 and CAP18. Mutants that exhibited reduced methicillin resistance (femA, fmtB, fmtC, and femD mutants) (4, 19, 22, 23) and a vancomycin-resistant mutant (21) were employed. All mutants were derived from the MRSA COL strain. The susceptibilities of the mutants to hBD3 and CAP18 were determined by the method described above.
RESULTS
Detection of antimicrobial peptide transcripts in keratinocytes.
To evaluate the expression of antimicrobial peptides, RT-PCR was performed with total RNA extracted from human keratinocytes derived from skin. Using primers specific for hBD1, hBD2, hBD3, and CAP18, we found PCR products of the expected sizes in each reaction mixture (data not shown). We found no PCR products (no RT reaction) when total RNA was used as the template (data not shown). We cloned the products into the pGEM-T Easy vector, a PCR cloning vector, and determined their DNA sequences, which exhibited perfect matches with sequences reported in a database, including the sequences of CAP18 (accession no. U19970), hBD1 (U73945), hBD2 (NM_004942), and hBD3 (NM_018661).
Expression of antimicrobial peptides and cytokines by keratinocytes in contact with S. aureus.
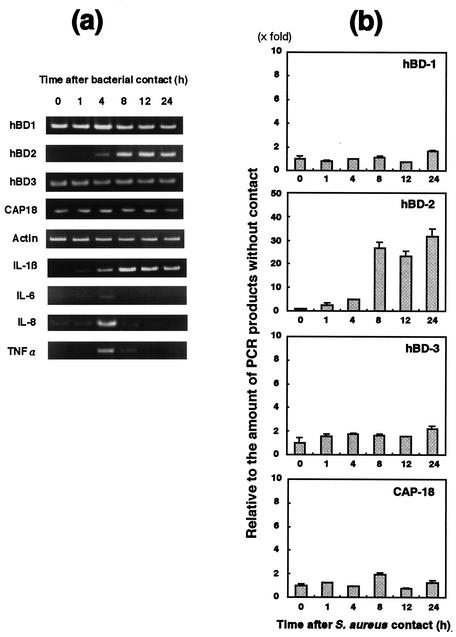
We investigated the levels of expression of antimicrobial peptides in keratinocytes incubated with heat-killed S. aureus in a kinetic experiment (Fig. 1a and 2b). The amount of hBD1 mRNA was relatively large compared to the amounts of other peptide mRNA, whereas hBD2 mRNA was not clearly found in keratinocytes without any bacterial contact. The amount of hBD2 mRNA gradually increased during the first 4 h following exposure of keratinocytes to S. aureus COL (the amount was twofold larger). After 8 h, the amount of hBD2 mRNA was significantly larger (25-fold larger), and the level of expression was constant up to 24 h. The amount of hBD3 mRNA was larger after 4 h (twofold larger), and this level was maintained for 24 h. The amount of CAP18 mRNA was larger only at 8 h (twofold larger) after bacterial exposure. On the other hand, the amount of hBD1 mRNA was not affected by exposure to S. aureus.
FIG. 1.
Expression of β-defensins, CAP18, and cytokine mRNA by human keratinocytes in contact with heat-inactivated S. aureus 209P cells. Total RNA was extracted from cells after contact with S. aureus for various times and then used for RT-PCR (a) and real-time PCR (b) performed by using the methods described in Materials and Methods. The results of the real-time PCR are expressed as a ratio in comparison to the value at zero time and are means ± standard deviations from three independent experiments.
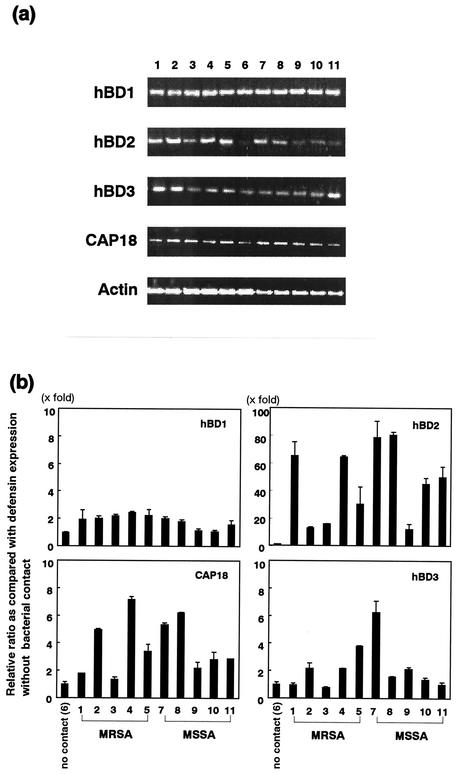
FIG. 2.
Expression of hBD1, hBD2, hBD3, and CAP18 mRNA by human keratinocytes in contact with heat-inactivated clinically isolated S. aureus cells. Total RNA was extracted from cells in contact with S. aureus for 8 h and used for RT-PCR (a) and real-time PCR (b) performed by using the methods described in Materials and Methods. (a) Lanes 1 to 5, MRSA strains; lane 6, no bacterial contact; lanes 7 to 11, MSSA strains. (b) Ratio of defensin expression in comparison to the value obtained when there was no contact with S. aureus cells. Bars 1 to 5, MRSA strains; bar 6, no bacterial contact; bars 7 to 11, MSSA strains. The results of the real-time PCR are means ± standard deviations from three independent experiments.
Also, we investigated the expression of cytokines, including interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), in keratinocytes exposed to S. aureus cells (Fig. 1a). The amount of IL-1β mRNA increased gradually until 8 h after the initial bacterial contact and then decreased gradually. IL-8 and TNF-α mRNA were found after 4 h of bacterial contact, and both mRNA disappeared after 8 h of bacterial contact. IL-6 mRNA was not observed during bacterial contact.
Both MRSA and MSSA induce expression of β-defensin and CAP18 mRNA by keratinocytes.
All 10 clinical isolates of S. aureus induced expression of hBD2 mRNA (10- to 80-fold), while 5 of 10 strains induced expression of hBD3 mRNA (2- to 6-fold) and 9 of 10 strains induced CAP18 mRNA expression (2- to 7-fold) (Fig. 2). Expression of hBD1 mRNA was less induced or not induced (one- to twofold) by bacterial exposure. Altogether, both MRSA and MSSA induced production of hBD2, hBD3, and CAP18 by keratinocytes, and the levels of expression of these peptides were different for different strains of S. aureus used to stimulate keratinocytes.
Activities of peptides against S. aureus.
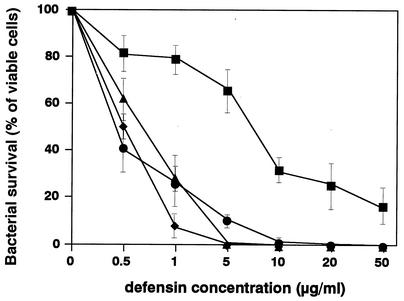
The activities of four peptides against S. aureus COL were analyzed (Fig. 3). The antibacterial activity of hBD1 was weaker than those of the other peptides. The concentrations that killed 100% of the bacteria were 5 μg/ml for both hBD3 and CAP18 and 10 μg/ml for hBD2, while hBD1 killed only 82% of the bacteria even at the highest dose used in this experiment (50 μg/ml).
FIG. 3.
Antibacterial activities of β-defensins and CAP18. Each peptide was incubated for 4 h at 37°C in 200 μl of 10 mM NaPi (pH 6.8) containing 105 bacterial cells. Then serial dilutions were plated on Trypticase soy agar, and colony counts were obtained after 24 h of incubation at 37°C. Bacterial survival is expressed as a percentage (number of cells that survived in the presence of peptides compared to the number of cells that survived without peptide). The data are means ± standard deviations from three independent experiments. Symbols: ▪, hBD1; •, hBD2; ▴, hBD3; ⧫, CAP18.
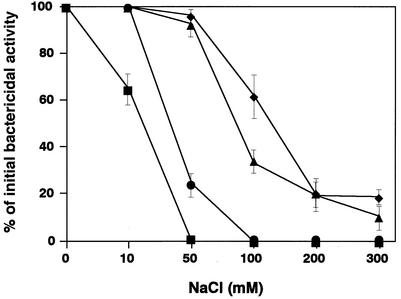
The effect of NaCl on the antimicrobial activities of the peptides is shown in Fig. 4. hBD1 and hBD2 were more sensitive to NaCl than hBD3 or CAP18. The antibacterial activities of hBD1 and hBD2 were completely inhibited by 50 and 100 mM NaCl, respectively. The activities of hBD3 and CAP18 were greatly suppressed (80% suppression of CAP18, 90% suppression of hBD3), but both of these peptides maintained antimicrobial activity, in contrast to hBD1 and hBD2, which were completely inactivated at the same concentration of NaCl.
FIG. 4.
Salt sensitivity of antimicrobial peptides. Various concentrations of NaCl (0 to 300 mM) were added to 10 mM NaPi (pH 6.8), and each peptide (50 μg/ml) was reacted with S. aureus cells by using the method described in Materials and Methods. The data are means ± standard deviations from three independent experiments. Symbols: ▪, hBD1; •, hBD2; ▴, hBD3; ⧫, CAP18.
Electron microscopic features of antimicrobial peptide-treated S. aureus cells.
As shown in Fig. 5, the microscopic features of S. aureus 2PF-18 cells treated with hBD1, hBD2, hBD3, or CAP18 were quite similar with regard to perforation of the peripheral cell wall, which led to release of the cytoplasmic contents. Prior to bacteriolysis, morphological changes were observed along with different degrees of cytoplasmic disintegration.
FIG. 5.
Thin sections of S. aureus 2PF-18 exposed to antimicrobial peptides. S. aureus cells were reacted with no peptide (panel 1), with 200 μg of hBD1 per ml (panels 2a and 2b), with 200 μg of hBD2 per ml (panels 3a and 3b), with 200 μg of hBD3 per ml (panels 4a and 4b), or with 200 μg of CAP18 per ml (panels 5a and 5b). Bars, 100 nm.
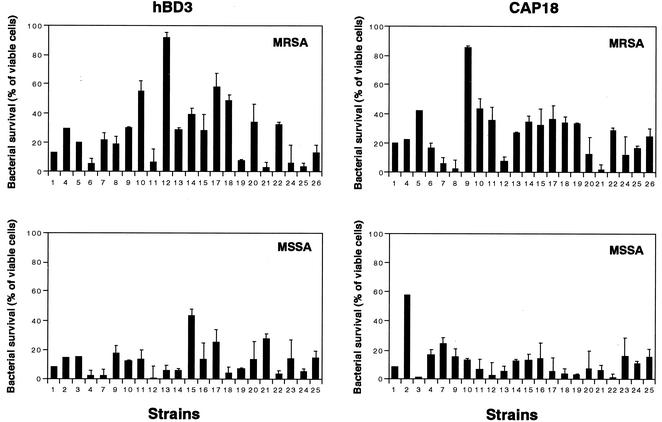
Susceptibilities of S. aureus clinical isolates to hBD3 and CAP18.
Figure 6 shows the antibacterial activities of hBD3 and CAP18 for 44 S. aureus clinical isolates, including 22 MRSA strains. The susceptibilities to these two peptides varied among strains. Interestingly, the number of MRSA strains that exhibited resistance to hBD3 or CAP18 was greater than the number of MSSA strains that exhibited resistance to these peptides. Twelve (55%) of the MRSA strains had a cutoff level at a survival rate of >20% in the presence of hBD3 (1 μg/ml), whereas only three MSSA strains (9%) had survival rates of > 20% at the same cutoff level. In the presence of CAP18 (0.5 μg/ml), 13 MRSA strains (59%) and two MSSA strains (9%) had survival rates of >20%.
FIG. 6.
Activities of hBD3 and CAP18 against S. aureus clinical isolates. The susceptibilities of 22 MSSA strains and 22 MRSA strains to hBD3 (1 μg/ml) and CAP18 (0.5 μg/ml) were analyzed as described in Materials and Methods. Bacterial survival is expressed as a percentage of the viable cells. The data are means ± standard deviations from three independent experiments.
Susceptibilities of S. aureus clinical strains to combinations of antibacterial peptides with antibiotics.
Using a checkerboard titration method, we observed synergistic effects of combinations of antibacterial peptides with β-lactam antibiotics but not with other antibiotics (data not shown). We then investigated the effects of combinations of methicillin with either hBD3 or CAP18 on each of 10 MRSA clinically isolated strains. For all MRSA strains we observed a synergistic effect (FIC index, <0.5) with a combination of methicillin and hBD3 or with a combination of methicillin and CAP18 (Table 3). Also, S. aureus cells pretreated with methicillin were more sensitive to hBD3 or CAP18 than nontreated S. aureus cells were, although the degree of synergy varied among strains (Table 3).
TABLE 3.
Effects of combinations of methicillin and defensins on survival of S. aureus strains
| Strain | FIC indexa
|
Bactercidal activity (ratio)b
|
||
|---|---|---|---|---|
| hBD3 | CAP18 | hBD3 | CAP18 | |
| MRSA-1 | 0.45 | 0.45 | 1.2 | 1.2 |
| MRSA-4 | 0.26 | 0.84 | 8.8 | 1.3 |
| MRSA-8 | 0.16 | 0.45 | 4.8 | 2.1 |
| MRSA-9 | 0.11 | 0.09 | 1.8 | 1.3 |
| MRSA-10 | 0.26 | 0.26 | 1.1 | 1.6 |
| MRSA-11 | 0.09 | 0.16 | 1.2 | 1.1 |
| MRSA-12 | 0.19 | 0.19 | 2.7 | 8.0 |
| MRSA-13 | 0.22 | 0.15 | 1.5 | 2.6 |
| MRSA-14 | 0.32 | 0.32 | 15.4 | 1.7 |
| MRSA-22 | 0.15 | 0.12 | 1.1 | 1.6 |
The FIC index was calculated as described in Materials and Methods.
The ratio of bactericidal activity was determined as follows: bactericidal activity for methicillin-treated cells/bactericidal activity for nontreated cells.
Susceptibilities of S. aureus clinical isolates to combinations of antibacterial peptides.
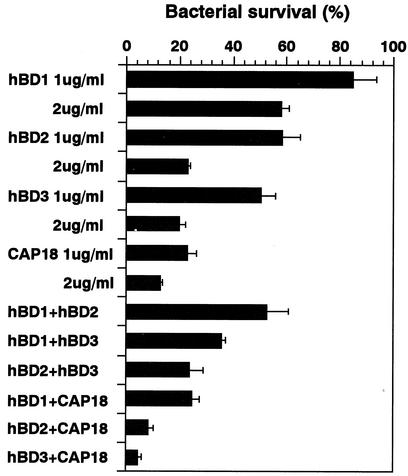
Since a combination of CAP18 (1 μg/ml) with hBD2 (1 μg/ml) or hBD3 (1 μg/ml) exhibited greater bactericidal activity than each peptide alone exhibited at a twofold-higher concentration (2 μg/ml), these combinations had synergistic effects on MRSA strain COL (Fig. 7). In contrast, combinations of β-defensins (hBD1, hBD2, and hBD3) or a combination of CAP18 and hBD1 had an additive effect.
FIG. 7.
Effects of individual antimicrobial peptides and combinations of antimicrobial peptides on S. aureus survival. Peptides alone (1 or 2 μg/ml) or combinations of two peptides (1 μg/ml each) were used to determine the susceptibilities of the S. aureus COL strain as described in Materials and Methods. The data are means ± standard deviations from three independent experiments.
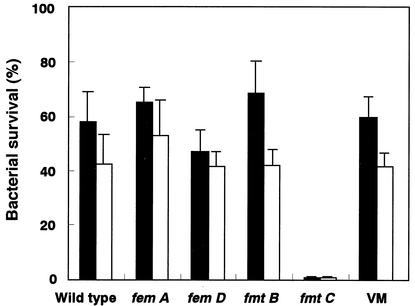
Susceptibilities of S. aureus mutants to the antibacterial peptides.
As shown above, MRSA clinical isolates were more resistant to antimicrobial peptides than MSSA clinical isolates (Fig. 6), and a combination of methicillin and a microbial peptide (either hBD3 or CAP18) had a synergistic effect for killing clinical isolates of MRSA (Table 3). Therefore, we investigated whether any gene that is associated with resistance to methicillin can influence the susceptibility to antimicrobial peptides (Fig. 8). The fmtC mutant showed significantly increased susceptibility to hBD3 and CAP18 compared to the susceptibility of other mutants (femA,femD,fmtA, andfmtB) or the wild type, as well as the vancomycin-resistant mutant.
FIG. 8.
Activities of hBD3 and CAP18 against S. aureus wild type and mutants. The susceptibilities of S. aureus COL, femA, femD, fmtB, and fmtC mutants, and a vancomycin-resistant mutant (VM) to hBD3 (1 μg/ml) and CAP18 (0.5 μg/ml) were determined by the method described in Materials and Methods. Solid bars, hBD3; open bars, CAP18. The data are means ± standard deviations from three independent experiments.
DISCUSSION
Inducible expression of hBD2, hBD3, and CAP18 has been found in various inflamed regions in vivo (8, 29, 33, 44) and also in cultured keratinocytes in contact with bacteria in vitro (13, 14, 25, 26). However, coordinate expression of these peptides in human keratinocytes was first demonstrated in this study. Exposure of keratinocytes to S. aureus resulted in upregulation of hBD2, hBD3, and CAP18 production, whereas hBD1 was constitutively expressed. Interestingly, the mode of expression was different for different peptides (Fig. 1), suggesting that different signals are associated with expression of each peptide. Several reports have indicated that activation of NF-κBsignaling by binding of lipopolysaccharide or a lipopolysaccharide-CD14 (lipopolysaccharide-binding protein) complex to Toll-like receptor 4 (TLR4) induces hBD2 expression in various regions of the epithelium (3, 25, 38, 41). Lipoteichoic acid or cell wall peptidoglycan in gram-positive bacteria has been demonstrated to stimulate NF-κB activation through binding to TLR2 (39), suggesting that TLR2 binding molecules expressed by S. aureus may be responsible for expression of some of these antimicrobial peptides. However, a variety of expression patterns also suggest that there are different pathways for expression of each peptide. Contact of S. aureus with keratinocytes also induced expression of IL-1β, IL-8, and TNF-α (Fig. 1a). Some cytokines have been implicated in defensin production; these include TNF-α for hBD3 production (13), IL-1β and TNF-α for hBD2 production (32, 38, 44), and IL-6 for CAP18 production (33). Therefore, the autocrine secretion of these proinflammatory cytokines may also induce expression of β-defensins and CAP18 after exposure of S. aureus to keratinocytes. Taken together, these data suggest that several pathways mediate the production of each peptide. Therefore, it is plausible that more than one ligand and/or signaling molecule of S. aureus is involved in induction of expression of these peptides.
hBD1 exhibited weaker bactericidal activity than the other three peptides exhibited, and the activity of hBD2 was comparable to the activities of hBD3 and CAP18 (Fig. 3). Previous reports have shown that hBD1 and hBD2 exhibit less activity against gram-positive bacteria, including S. aureus, than against gram-negative bacteria, and both peptides are salt sensitive (14, 44). In particular, hBD2 had no bactericidal effect on S. aureus and only a bacteriostatic effect even at concentrations greater than 100 μg/ml (14). Our study supported the salt sensitivity of these antimicrobial peptides, but our results for activity against gram-positive bacteria are inconsistent with the results of previous reports. This discrepancy might have been due to the different assay systems used to measure antimicrobial activity. In previous studies, the assay was performed with 10 mM phosphate buffer containing 1% (vol/vol) TSB or Luria-Bertani medium (13, 14, 44), while we used NaPi without culture medium. We also measured the antibacterial activities of β-defensins using authentic peptides (Peptide Institute, Inc., Osaka, Japan) with our assay and confirmed their activities against S. aureus cells (data not shown). Furthermore, when we measured the MICs of the peptides using TSB, we failed to determine the MICs of hBD1 and hBD2 (>1,000 μg/ml), while the MICs of hBD3 and CAP18 were both 100 μg/ml, suggesting that the presence of culture media in antimicrobial assay mixtures profoundly affects the antimicrobial activities of β-defensins.
It is noteworthy that a greater number of MRSA strains than of MSSA strains exhibited low levels of susceptibility to hBD3 and CAP18 (Fig. 6), suggesting that MRSA strains have greater resistance to the components of innate immunity. Two factors, dlt and mprF, which are associated with the susceptibility to antimicrobial peptides, have been described previously (34, 35). A dlt mutant lacks d-alanine incorporation into teichoic acid, and an mprF mutant exhibits a defect in the incorporation of lysine into phosphatidylglycerol, a component of the cell membrane. Therefore, inactivation of dlt or mprF increases the negative charge of the bacterial cell surface, consequently increasing the affinity of the cell membrane for cationic peptides. The factors responsible for low levels of susceptibility remain unknown, but they are thought to decrease the negative charge of S. aureus cells.
The fact that MRSA is relatively resistant to the peptides investigated may argue against clinical use of these peptides. However, these compounds continue to be candidates for chemotherapeutic agents, because the approximately fivefold-higher concentrations tested in this study (1 μg of hBD3 per ml, 0.5 μg of CAP18 per ml) had bactericidal effects on more than 90% of clinical isolates of both MSSA and MRSA. Furthermore, synergistic effects of defensins with methicillin and of β-defensins with CAP18 were observed for MRSA (Fig. 7 and Table 3). Exposure of S. aureus to concentrations of methicillin less than the MIC decreased the cross-linking of cell wall peptidoglycan and resulted in alteration of the composition of the cell surface. This alteration may contribute to the increased susceptibilities to antimicrobial peptides. Also, the synergistic effects of β-defensins and CAP18 indicate that the modes of action of the two peptides against S. aureus are not identical.
fmtC has been identified previously as the factor which affects methicillin resistance in MRSA (22). Peschel et al. demonstrated that the mprF gene, which is identical to fmtC, is associated with defensin susceptibility and designated it mprF (34). In this study, of five genes that are associated with resistance to methicillin, only the fmtC gene mutation affected the susceptibilities to β-defensins and CAP18 (Fig. 8). Since fmtC inactivation leads to increased susceptibility to both methicillin and antimicrobial peptides, this gene could be a potential target for a new chemotherapeutic agent.
The present study demonstrated that exposure of human skin keratinocytes to S. aureus triggered the production of β-defensins and CAP18. These peptides exhibited activity against S. aureus, although the levels of this activity were different for all clinical isolates. MRSA strains had higher survival rates than MSSA strains in response to hBD3 and CAP18. A combination of β-defensins with CAP18 or with β-lactam antibiotics and hBD3 or CAP18 had a synergistic effect on S. aureus, including MRSA strains. These results demonstrated that susceptibility to antimicrobial peptides plays a crucial role in S. aureus infection and suggested that these peptides may be useful clinically.
Acknowledgments
Part of this study was carried out in the Research Facility of Hiroshima University Faculty of Dentistry and the Center for Molecular Medicine, Hiroshima University Faculty of Medicine.
This work was supported by a grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan, by Health and Labour Sciences research grants from the Ministry of Health and Welfare of Japan, and by NIH grants DE03420 and DE14551-01 from the National Institute of Dental and Craniofacial Research.
Editor: V. J. DiRita
REFERENCES
- 1.Bals, R. 2000. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 1:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals, R., D. J. Weiner, A. D. Moscioni, R. L. Meegalla, and J. M. Wilson. 1999. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 67:6084-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, M. N., G. Diamond, M. W. Verghese, and S. H. Randell. 2000. CD14-dependent lipopolysaccharide-induced β-defensin-2 expression in human tracheobronchial epithelium. J. Biol. Chem. 275:29731-29736. [DOI] [PubMed] [Google Scholar]
- 4.Berger-Bächi, B., L. Barberis-Maino, A. Strässle, and F. H. Kayser. 1989. femA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol. Gen. Genet. 219:263-269. [DOI] [PubMed] [Google Scholar]
- 5.Bobin-Dubreux, S., M.-E. Reverdy, C. Nervi, M. Rougier, A. Bolmstrom, F. Vandenesch, and J. Etienne. 2001. Clinical isolate of vancomycin-heterointermediate Staphylococcus aureus susceptible to methicillin and in vitro selection of a vancomycin-resistant derivative. Antimicrob. Agents Chemother. 45:349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lancastre, H., B. L. M. de Jonge, P. M. Matthews, and A. Tomasz. 1994. Molecular aspects of methicillin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 33:7-24. [DOI] [PubMed] [Google Scholar]
- 7.Diamond, G., V. Kaiser, J. Rhodes, J. P. Russell, and C. L. Bevins. 2000. Transcriptional regulation of β-defensin gene expression in tracheal epithelial cells. Infect. Immun. 68:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocyte during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 9.Ganz, T., M. E. Selsted, D. Szklarek, S. S. Harwig, K. Daher, D. F. Bainton, and R. I. Lehrer. 1985. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 76:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia, J. R., A. Krause, S. Schulz, F. J. Rodriguez-Jimenez, E. Kluver, K. Adermann, U. Forssmann, A. Frimpong-Boateng, R. Bals, and W. G. Forssmann. 2001. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 15:1819-1821. [PubMed] [Google Scholar]
- 11.Gusman, H., J. Travis, E. J. Helmerhorst, J. Potempa, R. F. Troxler, and F. G. Oppenheim. 2001. Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect. Immun. 69:1402-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyurko, C., U. Lendenmann, R. F. Troxler, and F. G. Oppenheim. 2000. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob. Agents Chemother. 44:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harder, J., J. Bartels, E. Christophers, and J.-M. Schroder. 2001. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 14.Harder, J., J. Bartels, E. Christophers, and J.-M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 15.Hassain, M., M. H. Wilcox, P. J. White, M. K. Faulkner, and R. C. Spencer. 1992. Importance of medium and atmosphere type to both slime production and adherence by coagulase-negative staphylococci. J. Hosp. Infect. 20:173-184. [DOI] [PubMed] [Google Scholar]
- 16.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 17.Hoover, D. M., O. Chertov, and J. Lubkowski. 2001. The structure of human β-defensin-1. J. Biol. Chem. 276:39021-39026. [DOI] [PubMed] [Google Scholar]
- 18.Hoover, D. M., K. R. Rajashankar, R. Blumenthal, A. Puri, J. J. Oppenheim, O. Chertov, and J. Lubkowski. 2000. The structure of human β-defensin-2 shows evidence of higher order oligomerization. J. Biol. Chem. 275:32911-32918. [DOI] [PubMed] [Google Scholar]
- 19.Jolly, L., S. Wu, J. van Heijenoort, H. de Lencastre, D. Mengin-Lecreulx, and A. Tomasz. 1997. The femR315 gene from Staphylococcus aureus, the interruption of which results in reduced methicillin resistance, encodes a phosphoglucosamine mutase. J. Bacteriol. 179:5321-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, D. E., and C. L. Bevins. 1993. Defensin-6 mRNA in human paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315:187-192. [DOI] [PubMed] [Google Scholar]
- 21.Komatsuzawa, H., K. Ohta, S. Yamada, K. Ehlert, H. Labischinski, J. Kajimura, T. Fujiwara, and M. Sugai. 2002. Increased glycan chain length distribution and decreased susceptibility to moenomycin in a vancomycin-resistant Staphylococcus aureus mutant. Antimicrob. Agents Chemother. 46:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komatsuzawa, H., K. Ohta, T. Fujiwara, G. H. Choi, H. Labischinski, and M. Sugai. 2001. Cloning and sequencing of the gene, fmtC, which affects oxacillin resistance in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 203:49-54. [DOI] [PubMed] [Google Scholar]
- 23.Komatsuzawa, H., K. Ohta, M. Sugai, T. Fujiwara, P. Glanzzmann, B. Berger-Bachi, and H. Suginaka. 2000. Tn551 mediated insertional inactivation of the fmtB gene encoding a cell-wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 45:421-431. [DOI] [PubMed] [Google Scholar]
- 24.Komatsuzawa, H., J. Suzuki, M. Sugai, Y. Miyake, and H. Suginaka. 1994. Effect of combination of oxacillin and non-β-lactam antibiotics on methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 33:1155-1163. [DOI] [PubMed] [Google Scholar]
- 25.Krisanaprakornkit, S., J. R. Kimball, A. Weinberg, R. P. Darveau, B. W. Bainbridge, and B. A. Dale. 2000. Inducible expression of human β-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 68:2907-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krisanaprakornkit, S., A. Weinberg, C. N. Perezz, and B. A. Dale. 1998. Expression of the peptide antibiotic human β-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect. Immun. 66:4222-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larrick, J. M., M. Hirata, R. F. Balint, J. Lee, J. Zhong, and S. C. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defense. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 29.Malm, J., O. Sorensen, T. Persson, M. Frohm-Nilsson, B. Johansson, A. Bjartell, H. Lilja, M. Stahle-Backdahl, N. Borregaard, and A. Egesten. 2000. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 68:4297-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maple, P. C., J. M. Hamilton-Miller, and W. Brumfitt. 1989. Worldwide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet ii:537-540. [DOI] [PubMed] [Google Scholar]
- 31.Marchese, A., G. Balistreri, E. Tonoli, E. A. Debbia, and G. C. Schito. 2000. Heterogeneous vancomycin resistance in methicillin-resistant Staphylococcus aureus strains isolated in a large Italian hospital. J. Clin. Microbiol. 38:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathews, M., H. P. Jia, J. M. Guthmiller, G. Losh, S. Graham, G. K. Johnson, B. F. Tack, and P. B. McCray Jr. 1999. Production of β-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect. Immun. 67:2740-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson, M. F., B. Sandstedt, O. Sorensen, G. Weber, N. Borregaard, and M. Stahle-Backdahl. 1999. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 67:2561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. M. van Kessel, and J. A. G. Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor mprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peschel, A., M. Otto, R. W. Jack, H. Kaibacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 36.Quayle, A. J., E. M. Porter, A. A. Nussbaum, Y. M. Wang, C. Brabec, K. P. Yip, and S. C. Mok. 1998. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 152:1247-1258. [PMC free article] [PubMed] [Google Scholar]
- 37.Renolds, P. E., and C. Fuller. 1986. Methicillin resistant strains of Staphylococcus aureus; presence of an identical additional penicillin-binding protein in all strains examined. FEMS Microbiol. Lett. 33:251-254. [Google Scholar]
- 38.Schroder, J.-M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 39.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 40.Scott, M. G., M. R. Gold, and R. E. W. Hancock. 1999. Interaction of cationic peptides with lipoteichoic acid and gram-positive bacteria. Infect. Immun. 67:6445-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott, M. G., A. C. E. Vreugdenhil, W. A. Buurman, R. E. W. Hancock, and M. R. Gold. 2000. Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 164:549-553. [DOI] [PubMed] [Google Scholar]
- 42.Selsted, M. E., S. S. Harwig, T. Ganz, J. W. Schilling, and R. I. Lehrer. 1985. Primary structures of three human neutrophil defensins. J. Clin. Investig. 76:1436-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517-523. [DOI] [PubMed] [Google Scholar]
- 44.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B.-A. D. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of β-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. Tenover, M. J. Zervos, J. D. Band, E. White, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 46.Ubukata, K., R. Nogorochi, M. Matsuhashi, and M. Konno. 1989. Expression and inducibility in Staphylococcus aureus of the mecA gene which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J. Bacteriol. 171:2882-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valore, E. V., C. H. Park, A. J. Quayle, K. R. Wiles, P. B. McCray, Jr., and T. Ganz. 1998. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Investig. 101:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu, Y., I. Ambudkar, H. Yamagishi, W. Swaim, T. J. Walsh, and B. C. O'Connell. 1999. Histatin 3-mediated killing of Candida albicans: effect of extracellular salt concentration on binding and internalization. Antimicrob. Agents Chemother. 43:2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada, S., M. Sugai, H. Komatsuzawa, S. Nakashima, T. Oshida, A. Matsumoto, and H. Suginaka. 1996. An autolysin ring associated with cell separation of Staphylococcus aureus. J. Bacteriol. 178:1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, M. Z. Howard, and J. J. Oppenheim. 1999. β-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]
- 51.Yang, D., O. Chertov, and J. J. Oppenheim. 2001. Participation of mammalian defensins and cathelicidins in antimicrobial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J. Leukoc. Biol. 69:691-697. [PubMed] [Google Scholar]