Abstract
In the present study, we evaluated whether motility of Kaposi’s sarcoma (KS) cells induced by platelet-activating factor (PAF) is dependent on the regulation of adherens junctions components. The results obtained indicate that PAF dose and time dependently reduced the endogenous expression of the main components of the adherens junctions: VE-cadherin, α-catenin, and β-catenin. In addition, PAF initiated events that directly or indirectly up-regulated both the tyrosine and serine/threonine phosphorylation pathways, and both types of phosphorylation of β-catenin were involved in the motility of KS cells. This motility was abrogated by addition of the tyrosine kinase inhibitor genistein, suggesting that this phosphorylation is an important signal responsible for breaking down the adherens junctions and diminishing the ability of neighboring cells to interact. Furthermore, immunofluorescence analysis showed that β-catenin and VE-cadherin staining changed from a uniform distribution along the membrane of controls to a diffuse pattern with gap formation in PAF-treated KS cells. In conclusion, the data presented here indicate that PAF induces tumor cell motility by altering cell-cell adhesion through β-catenin phosphorylation.
Platelet-activating factor (PAF), a phospholipid mediator of cell-to-cell communication belongs to the structurally related family of acetylated phosphoglycerides possessing dual actions in regulating cell growth and oncogenic transformation.1,2 Indeed PAF is a powerful mediator of tumor growth and dissemination in CHO cells3 and promotes cell motility in Kaposi’s sarcoma (KS) cells.4 These properties render PAF a suitable model to study the molecular mechanisms of tumor diffusion.
Tumor invasion and metastasis primarily depend on the interaction among the tumor cells, the extracellular matrix, and the blood vessels surrounding the tumor. In addition, malignant progression requires a decreased affinity between tumor cells and the extracellular matrix facilitating their release from the primary tumor mass. Within a primary tumor mass, cell-to-cell adhesion involves a variety of molecules, including the cadherin-catenin complex and the immunoglobulin superfamily member platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31). Cadherins are transmembrane glycoproteins that mediate homophilic, adherens calcium-dependent adhesion and are specifically associated with the junction region.5 The classic members of this protein family (E-cadherin, N-cadherin, P-cadherin, and C-cadherin) are characterized by five, highly similar, Ig-like extracellular CAD-domains6,7 followed by a transmembrane region and a cytoplasmic domain. The highly conserved cytoplasmic domain interacts with β-catenin or γ-catenin (also called plakoglobin) in a mutually exclusive manner.8,9 β- or γ-Catenins also interact with α-catenin which links the cadherin-adhesion complex either directly or indirectly to the actin cytoskeleton.10,11 Disruption of the normal cell-to-cell adhesion in transformed cells may contribute to tumor cell-enhanced migration and proliferation, leading to invasion and metastasis. This disruption can be achieved by down-regulating the expression of cadherin or catenin family members or by activation of signaling pathways that prevent the assembly of adherens junctions (AJs).12 However, several observations indicate that the loss of E-cad expression causes disruption of cell adhesion and is associated with the transition from adenoma to carcinoma and acquisition of metastatic capacity.13–16 By contrast, the re-establishment of AJs in these cells by restoration of cadherin expression exerts tumor-suppressive effects, including decreased proliferation, motility, and invasiveness.17–20 It descends that the loss of expression or function of E-cadherin is an important factor in tumorigenic progression.13,21,22 In addition, the integrity of AJs appears to be dynamically regulated by tyrosine phosphorylation.23 Tyrosine phosphorylation of β-catenin well correlates with inhibition of cadherin-mediated adhesion.24–28 It is therefore conceivable that the rapid changes in the phosphorylation patterns of cadherins and catenins in these junctions mediated by protein tyrosine kinases and phosphatases might represent one of the main mecha-nisms at the posttranslational level.
The aim of the present study was to evaluate the molecular mechanism of motility induced by PAF in KS; we have investigated the total expression of the AJ components VE-cadherin, α-catenin, and β-catenin, phosphorylation of β-catenin, and cellular distribution of VE-cadherin and β-catenin during migration of KS cells. It was found that PAF down-regulates the expression of the main components of the AJs and triggers the β-catenin phosphorylation, events that may facilitate tumor diffusion.
Materials and Methods
Antibodies and Reagents
The following antibodies were used for immunoblotting and immunocytochemistry according to standard protocols: anti-α-catenin (Chemicon Int., Temecula, CA), anti-phospho-β-catenin (Ser33/37/Thr41) and anti-phospho-tyrosine (P-Tyr-100; Cell Signaling Technology, Beverly, MA). Antibodies against VE-cadherin and β-catenin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). For immunolabeling, fluorescein isothiocyanate-conjugated antibodies against rabbit or mouse immunoglobulin (Santa Cruz Biotechnology) were used.
Culture media and supplements were purchased from Life Technologies (Paisley, UK) and fetal bovine serum was from Hyclone (Logan, UT). Synthetic C16 PAF (1-hexadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine) was obtained from Sigma-Aldrich Chemie (GmbH, Germany). Genistein was from Alexis Biochemicals (c/o Vinci-Biochem, Italy). CV-3988 and BN-52021 (Ginkgolide B) were purchased from Biomol (Plymouth Meeting, PA).
Cell Culture and Treatments
The KS cell line derived from a HIV-1 patient and spontaneously immortalized was propagated as previously described.29 Briefly, cells were grown as a monolayer in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 100 U/ml of penicillin, and 100 mg/ml of streptomycin at 37°C in a atmosphere of 5% CO2. For all experiments cells were grown as subconfluent cultures by maintaining cells in complete medium for at least 24 to 48 hours, serum-starved for 8 to 12 hours, and then washed three times with phosphate-buffered saline (PBS). In each experiment the cells were incubated with RPMI medium containing 0.25% bovine serum albumin (BSA) with the appropriate amounts of agonists, depending on the experimental protocol.
Immunoprecipitation
KS cells were plated on 100-mm dishes and cultured for 24 hours. Subconfluent cells were serum-starved for 12 hours and then incubated with RPMI medium containing 0.25% BSA and the appropriate agonists. For cell extract preparation, the cells were washed twice with PBS and lysed on ice for 30 minutes with 300 μl of lysis buffer (50 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 1 mmol/L ethylenediamine tetraacetic acid, 10% glycerol, 20 mmol/L pyrophosphate, 1% Triton X-100, 100 mmol/L NaF, 2 mmol/L phenylmethyl sulfonyl fluoride, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 0.7 μg/ml pepstatin, 0.2 mmol/L ammonium molybdate, 200 μmol/L NaF, and 2 mmol/L sodium orthovanadate). The lysates were centrifuged at 12,000 × g for 5 minutes at 4°C and the supernatant was stored at −80°C until used. The protein concentration was measured by the Bradford method. Equal amounts of protein (300 μg) for each sample were incubated with the specific antibody against β-catenin (2 μg) for 1 hour at 4°C; protein A-Sepharose beads were added and incubation continued overnight at 4°C. The precipitates were washed three times with ice-cold PBS, and the beads were resuspended in sodium dodecyl sulfate (SDS) sample buffer for subsequent Western blotting analysis.
Western Blotting Analysis
The protein from above the immunoprecipitation or the supernatant of whole cell lysates were boiled for 5 minutes and then subjected to SDS/polyacrylamide gel electrophoresis (PAGE) (7.5%) under reducing (5% β-mercaptoethanol) conditions. After the transfer of proteins onto nitrocellulose, the blots were blocked with 5% nonfat dry milk in 20 mmol/L Tris/HCl, pH 7.5, 500 mmol/L NaCl, plus 0,1% Tween 20 (TBS-T), except the antiphosphotyrosine immunoblot, which was incubated in 2% BSA in TBS-T. The membranes were subsequently incubated in agitation at 4°C overnight in 1% BSA-TBS-T buffer containing the specific antibody against phosphotyrosine, VE-cadherin, β-catenin, α-catenin, or phospho-β-catenin (Ser33/37/Thr41) protein. After washing four times with TBS-T the blots were incubated for 1 hour at room temperature with the second antibody conjugated to peroxidase, washed four times with TBS-T, developed with enhanced chemiluminescence detection reagents (Amersham) for 1 minute and exposed to X-Omat film (Eastman Kodak Co., Rochester, NY). The densitometric measurements were performed using the gel image system Fluor-S equipped with the analysis software Quantity One (Bio-Rad).
Ubiquitination of β-Catenin
Subconfluent KS cells were serum-starved for 12 hours and then stimulated with appropriate agonists for different times. At the time of the assay, cells were washed three times with PBS and cell proteins were extracted as described above. For the determination of β-catenin ubiquitination, the supernatants were subjected to immunoprecipitation with the specific antibody against β-catenin. β-Catenin was precipitated from 300 μg of cell lysate using 5 μg of antibody for 12 hours at 4°C and 50 μl of protein A-Sepharose [1:1 (w/v) suspension in PBS] for 12 hours at 4°C. Immunoprecipitated samples were washed four times with lysis buffer, boiled in 20 μl of Laemmli buffer for 5 minutes, and electrophoresed on SDS/10%-PAGE. Proteins were then electroblotted and probed with the anti-ubiquitin rabbit antiserum. The specific bands for ubiquitin were detected with anti-rabbit conjugated to peroxidase and subsequent enhanced chemiluminescence detection.
Immunofluorescence Microscopy
KS cells were cultured on glass coverslips with appropriate media. Twenty-four hours later, cells were washed twice with PBS, fixed for 20 minutes at room temperature in 3.7% formaldehyde in PBS and permeabilized with 0.1% Triton X-100 for 10 minutes. After washing with PBS, cells were blocked with 1% bovine serum albumin in PBS for 30 minutes and then incubated with the primary rabbit polyclonal anti-β-catenin antibody (1:100) or mouse monoclonal anti-VE-cadherin (1:100) for 1 hour at 37°C. After incubation with the corresponding secondary antibodies (1:500) conjugated with fluorescein isothiocyanate, the cells were washed, mounted in anti-fade medium, and examined under a Nikon microscope. Images were processed using Adobe Photoshop.
In Vitro Cell Migration
Cells (105) were plated in each well from essentially nondividing confluent cultures. Cells were rested for 12 hours with RPMI medium without fetal bovine serum and were then washed three times with PBS and incubated with RPMI medium containing 0.25% BSA and the appropriate agonists. Cells did not begin to divide to any significant degree during the experiments. Motility was studied throughout a 20-hour period under a Nikon Eclipsed TE 300 microscope with a 40× phase-contrast objective in an attached, hermetically sealed Plexiglas Nikon NP-2 incubator at 37°C. Cell motility was recorded using a COHU high-performance CCD camera. Image analysis was performed with a MicroImage analysis system Lucia G (Laboratories Imaging s.r.o., Praha) and an IBM-compatible system equipped with a video card (S3 VIRGE-DX/GXPCI 375/385) by digitally saving images at 30-minute intervals. Migration tracks were generated by marking the positions of the nuclei of individual cells on each image. The net migratory speed (velocity straight line) was calculated by the MicroImage software based on the straight-line distance between the starting and ending points divided by the time of observation. Migration of at least 30 cells was analyzed for each experimental condition. Values are given as mean ± SD. All migration assays were performed in triplicate. Tyrosine kinases were inhibited by genistein (100 μmol/L) added 1 hour before the addition of PAF.
Results
Effect of PAF on Cadherin-Catenin Complex in KS Cells
We have previously shown that PAF is a powerful inducer of tumor cell motility,3,4 which is related to the tumor invasiveness and metastatic process. Cell motility essentially depends by the regulation of the AJ components. For these reasons we examined variations of VE-cadherin, β-catenin, and α-catenin levels in KS cells after PAF treatments.
The amount of VE-cadherin in the cells was evaluated by Western blotting using a human VE-cadherin-specific monoclonal antibody that detected a band of 130 kd in extracts of KS cells that are known to express VE-cadherin.30,31 Time-course experiments were performed to detect the protein level in KS cells stimulated with PAF 50 ng/ml; as reported in Figure 1A, a slight decrease in VE-cadherin level was observed 30 minutes after PAF addition while a longer incubation time (1 to 4 hours) produced a stronger effect (lanes 3 to 5) that reached a maximum at 12 hours (lane 6). This effect was dose-dependent as reported in Figure 1B and a strong decrease of VE-cadherin was evidenced on incubation for 4 hours with 50 ng/ml of PAF. A further confirmation of the PAF effect on VE-cadherin expression in KS cells came from the experiments reported in Figure 2 showing that the addition of the specific PAF receptor antagonists, Ginkgolide B or CV 398832–34 completely abrogated the effect of PAF.
Figure 1.
The regulation of total protein expression of VE-cadherin in KS cells by PAF. A: Time course of 50 ng/ml of PAF on VE-cadherin expression. Cells were incubated with vehicle alone or with PAF at the indicated concentration for varying time points up to 12 hours; this is summarized by densitometric analysis (bottom) showing the decrease of its expression in total cell lysate. B: Dose response of PAF on VE-cadherin expression; cells were stimulated for 4 hours with or without various concentration of PAF (1 to 50 ng/ml), which is summarized graphically (bottom). C, cells incubated with vehicle alone. The data shown are representative of three different experiments.
Figure 2.
The inhibitory effect of specific PAF receptor antagonists Ginkgolide B or CV 3988 on VE-cadherin expression. Cells were incubated with PAF (10 ng/ml) in the presence or absence of 0.34 μmol/L Ginkgolide B (G) or 3 μmol/L CV 3988 (CV) for 4 hours. C, cells incubated with the vehicle alone. The data shown are representative of three different experiments.
Next we analyzed whether changes in VE-cadherin levels were accompanied by variations in expression patterns of molecules of α- and β-catenins. The amounts of these catenins were assessed by Western blotting using monoclonal anti-α- and -β-catenins antibodies. As shown in Figure 3, in PAF-treated (10 ng/ml) KS cells, the β-catenin level was markedly reduced from 30 minutes to 12 hours in a dose-dependent manner. A similar finding was obtained for α-catenin when KS cells were stimulated with PAF at the same concentration (Figure 4). These findings were further confirmed by the observation that the addition of PAF-receptor antagonists CV 3988 or Ginkgolide B prevented the β- and α-catenins down-regulation induced by PAF (see the last two lanes of Figures 3 and 4).
Figure 3.
The regulation of total protein expression of β-catenin in KS cells by PAF. Cells were stimulated with the vehicle alone (C) or with PAF (10 ng/ml) or PAF plus PAF receptor antagonists Ginkgolide B (G; 0.34 μmol/L) or CV 3988 (CV; 3 μmol/L) for time ranging from 30 minutes to 12 hours. Top: The blot after immunoprobing with a polyclonal β-catenin antibody. Bottom: The quantitative measurements of the band intensities. The data shown are representative of three different experiments.
Figure 4.
PAF regulates total protein expression of α-catenin in KS cells as assessed by Western blotting. Cells were stimulated with vehicle alone (C) or with PAF (10 ng/ml) or PAF plus PAF receptor antagonists Ginkgolide B (G; 0.34 μmol/L) or CV 3988 (CV; 3 μmol/L) at the indicated times. Top: The blot after immunoprobing with a polyclonal α-catenin antibody, which is summarized in the bottom quantitative graph. The data shown are representative of three different experiments.
PAF Induces Phosphorylation of β-Catenin in KS Cells
The disruption of cadherin-catenin cell-cell adhesion and the increase of cellular migration have been correlated with a high phosphorylation levels of junction components.28,35–37 With this in mind, we investigated the effect of PAF on β-catenin phosphorylation in KS cells. To determine changes in tyrosine phosphorylation of β-catenin, whole cell lysates at various times after PAF stimulation were collected and immunoprecipitated with the specific anti-β-catenin antibody. Levels of phosphotyrosine-β-catenin (PY-β-catenin) in the precipitates were analyzed by Western blotting with an antibody anti-phosphotyrosine. As shown in Figure 5A, PY-β-catenin levels were already evident 15 minutes after the treatment with 50 ng/ml of PAF, whereas cells stimulated with 10 ng/ml of PAF showed a significant increase of PY-β-catenin after 1 hour with a maximum at 2 hours of incubation. As can be seen from the Figure 5, PAF stimulates the β-catenin phosphorylation in a dose-dependent manner. The addition of receptor antagonists Ginkgolide B (0.34 μmol/L) or CV 3988 (3 μmol/L) entirely inhibits the PAF-mediated phosphorylation reaction.
Figure 5.
PAF stimulates phosphorylation of β-catenin in KS cells. Subconfluent cultures of KS cells were starved for 12 hours and then incubated with PAF (10 and 50 ng/ml) at the indicated times. Cells were also treated with the vehicle alone (C), or with PAF (50 ng/ml) plus PAF-receptor antagonists Ginkgolide B (G; 0.34 μmol/L) or CV 3988 (CV; 3 μmol/L) for 1 hour. A: Cells extracts were subject to immunoprecipitation with antibody against β-catenin. Precipitated proteins were analyzed by SDS-PAGE followed by immunoblotting with an antibody to phosphotyrosine. PY-β-catenin, tyrosine phosphorylated β-catenin. B: Western blotting analysis using the anti-phospho-S33/S37/T41 antibody to β-catenin. The data shown are representative of three different experiments.
To reveal whether PAF mediates phosphorylation at residues known to be targets of glycogen synthase kinase 3β (GSK3β) (S33, S37, T41), cell lysates were collected at various times after stimulation and the levels of β-catenin phosphorylation were analyzed by Western blotting with an antibody that specifically recognized the protein phosphorylated at S33/S37/T41. It was observed that the behavior of serine/threonine phosphorylation is similar to that observed for tyrosine phosphorylation. In fact, as shown in Figure 5B, a band of phospho-β-catenin is visible at 30 minutes and increased 1 hour after PAF stimulation in a dose-dependent manner.
Ubiquitination of β-Catenin in KS Cells by PAF
Since we found that the expression of β-catenin is down-regulated by PAF in KS cells, we investigated whether this down-regulation proceeds via ubiquitination and, consequently, through proteasome-dependent degradation. As evaluated by Western blotting for ubiquitin after immunoprecipitation of β-catenin (Figure 6) we found that 10 ng/ml of PAF induced an increase in the ubiquitination of β-catenin after 30 minutes with a maximum at 2 hours of incubation. Prolonged incubation times showed a decrease in the ubiquitin staining due to the protein degradation as also found after the antibody-mediated β-catenin staining (Figure 3).
Figure 6.
Effects of PAF on ubiquitination of β-catenin. KS cells were cultured for the indicated times in the absence (C) or presence of 10 ng/ml of PAF. Then cellular proteins were extracted and immunoprecipitated with an anti-β-catenin antibody as described in the Materials and Methods section. The immunoprecipitate was subsequently run on SDS/PAGE and immunoblotted for ubiquitin. The experiment is representative of at least three different experiments giving similar results. Ub β-cat, ubiquitinated β-catenin.
Distribution of Cadherins in KS Cells after PAF Stimulation
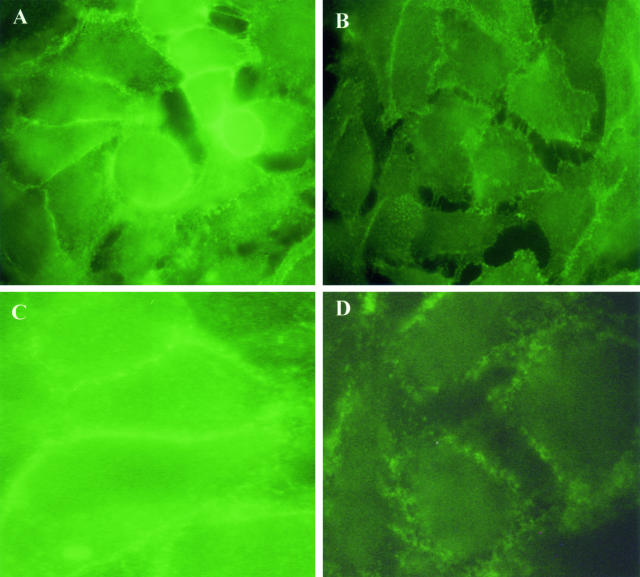
To further examine the significance of PAF action on cadherin-associated junctions, the localization of cadherins and catenins was examined by immunofluorescent labeling. Subconfluent KS cells were exposed to PAF and then subjected to immunocytochemistry to detect changes in β-catenin or VE-cadherin localization. In control cells, β-catenin was uniformly dispersed around the periphery of a tightly confluent monolayer (Figure 7A). When the cells were treated with PAF (10 ng/ml) for 2 hours it a widespread gap formation became evident as the cells retracted with a concomitant loss of β-catenin staining in areas where individual cells have lost contact with neighboring cells (Figure 7B).
Figure 7.
Effect of PAF on the junctional localization of β-catenin and VE-cadherin in KS cells. Cells were grown to subconfluence and treated with 10 ng/ml of PAF for 2 hours. A and C represent control staining for β-catenin and VE-cadherin, respectively; B and D represent β-catenin and VE-cadherin staining after PAF treatment. In B and D, it is evident the gap formation and absence of β-catenin and VE-cadherin in areas where the cells have separated. PAF also promoted a zigzag pattern distribution of β-catenin (B) and a diffuse staining pattern for VE-cadherin (D) different from controls displaying a linear pattern distribution localized at cell-cell contact. Three experiments were performed with similar results. Original magnifications, ×400.
Interestingly, VE-cadherin staining appeared as a continuous linear labeling at the cell adhesion in control cell monolayers (Figure 7C). By contrast, 2 hours of incubation with 10 ng/ml of PAF produced a widespread gap formation and a diffuse staining pattern for VE-cadherin (Figure 7D) due to the presence of a lesser amount of VE-cadherin in the areas of cell-cell contacts. This was consistent with our previous study that indicates that PAF modified the normal distribution of actin stress fibers and induced shape changes in KS cells.4
Cell Motility of KS Cells after PAF Stimulation
When KS cells were stimulated with PAF (10 ng/ml) a high increase in cell motility was observed within 1 hour of stimulation (Figure 8), the cell speed average decreased after 1 hour still remaining higher than control throughout the observation period (20 hours). Cells, preincubated for 1 hour with the tyrosine kinase inhibitor genistein (100 μmol/L), fully reversed the effects of PAF on KS motility. Furthermore, no increase of cell motility was observed when cells were incubated with PAF in presence of the PAF receptor antagonist CV 3988 (3 μmol/L).
Figure 8.
Effect of tyrosine kinase inhibitor genistein on PAF-induced KS motility. Cells were plated on 35 × 10-mm tissue culture dishes in RPMI medium with 0.25% BSA and then were incubated with vehicle alone (C) or with 10 ng/ml of PAF or with PAF plus genistein (100 μmol/L) or with PAF plus the PAF-receptor antagonist CV 3988 (CV; 3 μmol/L). For inhibition studies, genistein was added 1 hour before the addition of PAF. Cell motility was monitored with a time-lapse cinematography system as described in the Materials and Methods section. The values are the average of triplicate cultures ± SD. Inset: Cell treated with PAF plus genistein (100 μmol/L) and stained for β-catenin.
Discussion
The involvement of PAF in the tumor biology is supported by a series of observations: PAF displays a dual action in regulating cell growth and oncogenic transformation2 and it is also involved in the metastatic process because the PAF receptor blockade reduces B16 melanoma dissemination in the lung.38,39
As already reported11 we have shown that PAF triggers cell motility in KS cells, in the present study we have investigated some cellular and biochemical mechanisms potentially responsible for this effect. KS is a hemoangiosarcoma characterized in early stages by an excessive proliferation of typical spindle-shaped cells mixed with endothelial cell, fibroblasts, and inflammatory cells, paralleled by new blood vessel formation (neoangiogenesis).40–44
The loss of epithelial differentiation in carcinomas, which is accompanied by higher mobility and invasiveness of the tumor cells, is often a consequence of reduced intercellular adhesion. The down-regulation of cadherins favors the separation of neighboring tumor cells that thus acquire a motile and invasive phenotype; this behavior requires, besides the abrogation of the cell-cell contacts, the remodeling of the extracellular matrix and of the cell-matrix interaction followed, finally, by the movement of the cell mediated by the actin cytoskeleton.45 On the other hand, in KS cells, PAF induces up-regulation of β1 integrins and down-regulation of αvβ3 integrins, modifying the normal distribution of actin-containing stress fibers that tend to condense axially, retract, and fuse.4
In this study we report evidence that PAF is involved in KS cell motility by regulating the endogenous expression of AJ components: VE-cadherin, β-catenin, and α-catenin. In fact, PAF at physiological concentrations,13 reduces the levels of these proteins as shown in Figures 1, 3, and 4 and this decrease occurred in a dose- and time-dependent manner. Moreover, the exposure of KS cells to different PAF receptor-specific antagonists, inhibits the decrease of AJ component expression (Figures 2, 3, and 4). It is conceivable, however, that the reduced amount of AJ components we observed could be the result of an increased phosphorylation rate induced by PAF. It is known, in fact, that the adhesive function of cadherin requires the attachment to the actin cytoskeleton, an association mediated by catenins. On the other hand, β-catenin is considered to be a tyrosine phosphorylation-sensitive component of the adhesion complexes and its modification disrupts these junctions dissociating cadherins from the cytoskeleton.27,28 Furthermore the phosphorylation of β-catenin in serine/threonine residues commits the protein for its degradation through the ubiquitin/proteasome pathway.46,47 On the basis of these indications we verified if the phosphorylation of β-catenin took place after PAF stimulation of KS cells. As shown in Figure 5, β-catenin was phosphorylated in tyrosine as well as in serine/threonine residues in a time- and dose-dependent manner. Interestingly the phosphorylation events occurred at different times with the tyrosine phosphorylation coming first (Figure 5A). Moreover we also found that after PAF stimulation a degradation of β-catenin occurred via ubiquitin/proteasome pathway as shown in Figure 6. Our findings suggest that on PAF stimulation the first event occurring in the KS cell is the β-catenin phosphorylation at level of tyrosine residue, this modification is known to impair cadherin-catenin binding48 thus breaking down the AJ. The free β-catenin can be next phosphorylated in serine/threonine cluster by glycogen synthase kinase 3β and labeled for degradation by ubiquitin/proteasome pathway. Indeed, together with β-catenin we also observed a VE-cadherin and α-catenin decrease (Figures 1 and 4) indicating that the proteins involved in the AJ complex are degradated once the complex is disassembled. However we did not investigate the degradation pathway of these two proteins, it requires further investigations that will be the object of successive studies.
A further demonstration of the involvement of the cadherin-catenin complex in PAF effects came from immunocytochemistry studies (Figure 7) that showed a modification in the distribution pattern of cadherins after PAF stimulation. When incubated with low doses of PAF, KS cells displayed a reduced β-catenin-VE-cadherin staining with the widespread gap formation.
Recent studies indicate that the down-regulation or the loss of β-catenin expression is associated with tumor progression,49,50 in agreement with these studies our data indicate that β-catenin is down-regulated by PAF in KS cells, furthermore, tumor progression is related to the increase of cell motility. Actually we observed an increase in motility when KS cells were stimulated with PAF (Figure 8) but it was fully abolished by treatment with genistein, a protein tyrosine kinase inhibitor.51 Moreover, cells incubated with the PAF receptor antagonist CV 3988 did not show any increase in motility, revealing that this activity is directly mediated by PAF. It seems conceivable that the cell motility increase occurs through the β-catenin phosphorylation, which is an important signal responsible for breaking down the AJ and diminishing the ability of neighboring cells to interact.
However, it has been shown that genistein may exert multiple inhibitory effects on cancer cells through regulation of several cell signal transduction pathways including apoptosis. In our case, as reported by others,52 neither necrosis nor apoptosis was observed at least for the concentration and time of stimulation used for genistein (data not shown). Furthermore no difference was observed in the β-catenin staining when KS cells were incubated with PAF in presence of genistein (Figure 8, inset).
Taken together, these results suggest the following molecular events. To allow KS cells to migrate after treatment with PAF, cells must detach from neighboring cells to form gaps. Therefore, at initial stages of cell-cell adhesion, β-catenin is strongly phosphorylated on tyrosine, which decreases the affinity with VE-cadherin diminishing the reciprocal ability of cells to interact. Later on, there is a decrease in the complex components. On the other hand, PAF induces disappearance of actin stress fibers that coincides with the breakdown of AJ and increases motility.3,4 In conclusion, the data presented here indicate that PAF induces tumor cell motility in vitro by altering cell-to-cell adhesion through β-catenin phosphorylation.
Footnotes
Address reprint requests to L. Quagliuolo, Dipartimento di Biochimica e Biofisica, Seconda Università di Napoli, Via Costantinopoli, 16, I-80138 Napoli, Italy. E-mail: lucio.quagliuolo@unina2.it.
Supported by a research grant from Provincia di Salerno, project “Alimentazione e prevenzione malattie” (to C.B.).
References
- Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem. 1990;265:17381–17384. [PubMed] [Google Scholar]
- Kume K, Shimizu T. Platelet-activating factor (PAF) induces growth stimulation, inhibition, and suppression of oncogenic transformation in NRK cells overexpressing the PAF receptor. J Biol Chem. 1997;272:22898–22904. doi: 10.1074/jbc.272.36.22898. [DOI] [PubMed] [Google Scholar]
- Boccellino M, Biancone L, Cantaluppi V, Ye RD, Camussi G. Effect of platelet-activating factor receptor expression on CHO cell motility. J Cell Physiol. 2000;183:254–264. doi: 10.1002/(SICI)1097-4652(200005)183:2<254::AID-JCP12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Biancone L, Cantaluppi V, Boccellino M, Bussolati B, Del Sorbo L, Conaldi PG, Albini A, Toniolo A, Camussi G. Motility induced by human immunodeficiency virus-1 Tat on Kaposi’s sarcoma cells requires platelet-activating factor synthesis. Am J Pathol. 1999;155:1731–1739. doi: 10.1016/S0002-9440(10)65488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Bork P, Downing AK, Kieffer B, Campbell ID. Structure and distribution of modules in extracellular proteins. Q Rev Biophys. 1996;29:119–167. doi: 10.1017/s0033583500005783. [DOI] [PubMed] [Google Scholar]
- Schultz J, Copley RR, Doerks T, Ponting CP, Bork P. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Proteins associated with the cytoplasmic surface of adhesion molecules. Neuron. 1993;11:551–564. doi: 10.1016/0896-6273(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W, Hulsken J, Behrens J. E-cadherin as an invasion suppressor. Ciba Found Symp. 1995;189:124–136. doi: 10.1002/9780470514719.ch10. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001;276:27424–27431. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen UH, Nagamine Y. Stimulation of urokinase-type plasminogen activator expression by blockage of E-cadherin-dependent cell-cell adhesion. Cancer Res. 1993;53:3618–3623. [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76. doi: 10.1016/s0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. Tyrosine phosphorylation and cadherin/catenin function. Bioessays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Muller T, Choidas A, Reichmann E, Ullrich A. Phosphorylation and free pool of beta-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem. 1999;274:10173–10183. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia DH, Dunach M. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Albini A, Paglieri I, Orengo G, Carlone S, Aluigi MG, DeMarchi R, Matteucci C, Mantovani A, Carozzi F, Donini S, Benelli R. The beta-core fragment of human chorionic gonadotrophin inhibits growth of Kaposi’s sarcoma-derived cells and a new immortalized Kaposi’s sarcoma cell line. AIDS. 1997;11:713–721. doi: 10.1097/00002030-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Uccini S, Ruco LP, Monardo F, Stoppacciaro A, Dejana E, La Parola IL, Cerimele D, Baroni CD. Co-expression of endothelial cell and macrophage antigens in Kaposi’s sarcoma cells. J Pathol. 1994;173:23–31. doi: 10.1002/path.1711730105. [DOI] [PubMed] [Google Scholar]
- Uccini S, Sirianni MC, Vincenzi L, Topino S, Stoppacciaro A, Lesnoni LP, Capuano MI, Masini C, Cerimele D, Cella M, Lanzavecchia A, Allavena P, Mantovani A, Baroni CD, Ruco LP. Kaposi’s sarcoma cells express the macrophage-associated antigen mannose receptor and develop in peripheral blood cultures of Kaposi’s sarcoma patients. Am J Pathol. 1997;150:929–938. [PMC free article] [PubMed] [Google Scholar]
- Heuer HO, Casals-Stenzel J, Muacevic G, Weber KH. Pharmacologic activity of bepafant (WEB 2170), a new and selective hetrazepinoic antagonist of platelet activating factor. J Pharmacol Exp Ther. 1990;255:962–968. [PubMed] [Google Scholar]
- Terashita Z, Tsushima S, Yoshioka Y, Nomura H, Inada Y, Nishikawa K. CV-3. Life Sci. 1983;32:1975–1982. doi: 10.1016/0024-3205(83)90049-8. [DOI] [PubMed] [Google Scholar]
- Vercellotti GM, Yin HQ, Gustafson KS, Nelson RD, Jacob HS. Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood. 1988;71:1100–1107. [PubMed] [Google Scholar]
- Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and beta catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitton C, Lanson M, Besson P, Fetissoff F, Lansac J, Benveniste J, Bougnoux P. Presence of PAF-acether in human breast carcinoma: relation to axillary lymph node metastasis. J Natl Cancer Inst. 1989;81:1298–1302. doi: 10.1093/jnci/81.17.1298. [DOI] [PubMed] [Google Scholar]
- Im SY, Ko HM, Kim JW, Lee HK, Ha TY, Lee HB, Oh SJ, Bai S, Chung KC, Lee YB, Kang HS, Chun SB. Augmentation of tumor metastasis by platelet-activating factor. Cancer Res. 1996;56:2662–2665. [PubMed] [Google Scholar]
- Masood R, Cai J, Law R, Gill P. AIDS-associated Kaposi’s sarcoma pathogenesis, clinical features, and treatment. Curr Opin Oncol. 1993;5:831–834. doi: 10.1097/00001622-199309000-00010. [DOI] [PubMed] [Google Scholar]
- Regezi JA, MacPhail LA, Daniels TE, DeSouza YG, Greenspan JS, Greenspan D. Human immunodeficiency virus-associated oral Kaposi’s sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am J Pathol. 1993;143:240–249. [PMC free article] [PubMed] [Google Scholar]
- McNutt NS, Fletcher V, Conant MA. Early lesions of Kaposi’s sarcoma in homosexual men. An ultrastructural comparison with other vascular proliferations in skin. Am J Pathol. 1983;111:62–77. [PMC free article] [PubMed] [Google Scholar]
- Sirianni MC, Vincenzi L, Fiorelli V, Topino S, Scala E, Uccini S, Angeloni A, Faggioni A, Cerimele D, Cottoni F, Aiuti F, Ensoli B. Gamma-interferon production in peripheral blood mononuclear cells and tumor infiltrating lymphocytes from Kaposi’s sarcoma patients: correlation with the presence of human herpesvirus-8 in peripheral blood mononuclear cells and lesional macrophages. Blood. 1998;91:968–976. [PubMed] [Google Scholar]
- Rabkin CS, Janz S, Lash A, Coleman AE, Musaba E, Liotta L, Biggar RJ, Zhuang Z. Monoclonal origin of multicentric Kaposi’s sarcoma lesions. N Engl J Med. 1997;336:988–993. doi: 10.1056/NEJM199704033361403. [DOI] [PubMed] [Google Scholar]
- Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Morin PJ. Beta-catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J Biol Chem. 2001;276:20436–20443. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Zhurinsky J, Ben Ze’ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelandsmo GM, Holm R, Nesland JM, Fodstad O, Florenes VA. Reduced beta-catenin expression in the cytoplasm of advanced-stage superficial spreading malignant melanoma. Clin Cancer Res. 2003;9:3383–3388. [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Meyer TN, Hunt J, Schwesinger C, Denker BM. Galpha12 regulates epithelial cell junctions through Src tyrosine kinases. Am J Physiol Cell Physiol. 2003;285:C1281–C1293. doi: 10.1152/ajpcell.00548.2002. [DOI] [PubMed] [Google Scholar]