Plague, caused by Yersinia pestis, is recognized as the most devastating acute infectious disease experienced by humankind. This notoriety is based upon the high rate of mortality, the rapid onset, and the appalling pathology associated with both the bubonic and pneumonic forms of the infection. Until recently, these extraordinary symptoms prompted investigators to describe the pestilence as a frontal assault upon the host that overwhelms innate mechanisms of defense, inevitably resulting in death caused by septic shock (10, 19). It is now established, however, that the invading organisms inhibit the innate immune response, at least during the late stages of infection, thereby avoiding containment within granulomas (9). The first purpose of this review is to present two seemingly exclusive traditions that purport to define the process whereby yersiniae inhibit the generic inflammatory response. An attempt is then made to integrate these concepts.
Of the 11 species of Yersinia (genus XI of the Enterobacteriaceae), those pathogenic to humans are Y. pestis and enteropathogenic Y. pseudotuberculosis and Y. enterocolitica. These three organisms share an approximately 70-kb plasmid, termed pCD in Y. pestis and pYV in the enteropathogenic species, that encodes at least one determinant required for the downregulation of inflammation (9, 75). Despite this common feature, the diseases caused by the two enteropathogenic yersiniae are chronic in nature and otherwise markedly distinct from plague (e.g., Y. pseudotuberculosis usually causes a modest mesenteric lymphadenitis in humans, and Y. enterocolitica typically promotes limited gastrointestinal disease). It is therefore remarkable that less than 20,000 years has elapsed since Y. pestis evolved from Y. pseudotuberculosis; divergence of the latter from Y. enterocolitica occurred about 1 million years earlier (1). The close relationship between Y. pestis and Y. pseudotuberculosis provides an excellent system for defining the hereditary events required for the evolution of acute disease. The known significant changes largely reflect lateral acquisition by Y. pestis of genes permitting infection by fleabite followed by dissemination through interstitial spaces and the lymphatics. In addition, the mutational loss by Y. pestis of functions required by enteropathogenic yersiniae for invasion via the small intestine may further contribute to the severity of plague (9, 75). Additional modifications of the Y. pestis genome that minimize inflammation as a mediator of innate immunity will also be addressed in this report.
INNATE IMMUNITY AND THE GENERIC INFLAMMATORY RESPONSE
Introduction of bacteria into the mammalian host results in prompt upregulation of major proinflammatory cytokines that initiate innate or nonspecific processes of defense. The resulting inflammatory response promoted by these cytokines may alone be sufficient to eliminate invading organisms without the necessity of invoking explicit countermeasures. If it is not, the consequences of the initial upregulation of proinflammatory cytokines generally buy time for the occurrence of clonal selection and the amplification of lymphocytes, which in turn mediate specific immunity (38, 57, 104). The expression of proinflammatory cytokines following infection is initially directed by host Toll-like receptors (TLRs) that interact individually or in concert with molecules specific to microbial pathogens. Salient to this review are TLR-2 and TLR-4, which recognize lipoprotein or peptidoglycan components and lipopolysaccharide (LPS), respectively (100, 104, 106, 108). After interacting with their substrates, all TLRs initiate specific intracellular signaling that eventually activates the transcription factor NF-κB. LPS-induced cascades are dependent upon the high-affinity LPS receptor CD14, known to be strongly expressed on monocytes and macrophages but not on neutrophils.
NF-κB is normally associated with IκB in its inactive form within the host cell cytosol, and its activation is dependent on any one of a number of mitogen-activated protein (MAP) kinases. The binding of TLRs to their specific targets initiates convergent MyD88-dependent cascades resulting in the phosphorylation of IκB via an activated IκB kinase complex. Once phosphorylated, IκB undergoes degradation, exposing a translocation site on NF-κB permitting its migration into the nucleus, where it functions as a universal transcriptional activator of genes encoding proinflammatory cytokines as well as a plethora of allied effectors (42, 104). Following initiation by TLRs, inflammation can be amplified by end products of the cascade, such as interleukin 1 (IL-1). Studies first undertaken with IL-1 demonstrated that the bound receptor associates with an accessory protein (IL-1RacP) and the adapter protein MyD88 prior to interaction with IL-1R-activated kinase. The latter is a serine/threonine kinase that undergoes autophosphorylation before binding to TRAF6, which in turn activates the IκB kinase complex by associating with NF-κB-inducing kinase. Probably all TLRs share this MyD88-dependent cascade after they bind their bacterial targets (50, 58, 66, 104, 106, 109). However, downstream signaling of TLR-2 and TLR-4 (but not the remaining TLRs) by this pathway is modulated by the adapter protein TIRAP (108). Further consideration of alternative MyD88-independent cascades leading to NF-κB or other MAP kinase-mediated pathways is beyond the scope of this review.
Once expressed, the major proinflammatory cytokines initiate a series of events that provide prompt protection against infection and, if necessary, lead to specific immunity. Immediate actions include the promotion of fever accompanied by the withdrawal of extracellular iron (IL-1 and tumor necrosis factor alpha [TNF-α]), increased endothelial-cell stickiness (IL-1 and TNF-α), mobilization and activation of professional phagocytes (IL-8), recruitment of neutrophils from marrow (IL-1), expression of reactive oxygen intermediates (gamma interferon [IFN-γ] and TNF-α), enhanced production of Fc receptors (IFN-γ), and initiation of granuloma formation (IL-1, IFN-γ, and TNF-α). Granulomas are packets of inflammatory cells (primarily monocytes and macrophages) that surround infectious foci within host organs. Once formed, their internal environment becomes anaerobic and otherwise altered to prevent further microbial growth. This strategy is the last resort of the host in stemming systemic invasion by facultative intracellular parasites such as yersiniae, and it is utterly dependent upon expression of the generic inflammatory response. This innate mechanism of defense can take up to a week for full development in the immunologically naïve mouse. However, granulomas form almost immediately after the infection of mice already capable of expressing delayed-type hypersensitivity, thus again emphasizing the close relationship between inflammation and specific immunity (42, 51).
The generic inflammatory response is eventually abolished by anti-inflammatory cytokines, especially IL-10. This normal regulatory process ensures homeostasis within the host and provides tolerance to septic shock (101, 104). The mechanisms utilized by IL-10 to reverse inflammation are cosmopolitan in that they include inhibiting NF-κB activation and TLR synthesis (thereby preventing the expansion of proinflammatory cytokine synthesis), deactivating professional phagocytes, and inducing a macrophage-like cell that limits ongoing immune responses while preventing further inflammation (62, 105). Indeed, the message provided by IL-10 to the host is that tissue damage has been controlled, the afflicted tissue is now sterile, and healing may begin. This announcement is essential to initiate recovery from trauma. However, as noted below, it is entirely inappropriate for a host infected with plague bacilli.
LCRV AND THE PLAGUE TRADITION
Theobald Smith first stated that it is faulty strategy for a parasite to seriously debilitate its host because this act both jeopardizes the only natural niche available to that parasite and inhibits its dispersal in nature (94). Diseases caused by enteropathogenic yersiniae are contracted by fecal contamination and clearly obey this classical tenet. However, maintenance of plague among rodents in nature depends upon the ability of the organisms to promptly kill their current hosts, thereby forcing resident fleas to disembark in search of new victims. This lifestyle is contrary to the traditional epidemiological dogma of T. Smith and is more akin to predator-prey relationships or those established between parasitoids and their insect larval hosts (which never survive infestation). Y. pestis has therefore achieved its remarkable degree of virulence as a consequence of selective pressure rewarding the lethality associated with pronounced terminal septicemia. Terminal septicemia occurs as a function of bacterial spillover from visceral organs, which serve as favored niches for vegetative growth. Fleas typically consume blood meals of about 0.1 μl but must ingest ∼104 yersiniae to ensure their colonization (J. Hinnebusch, personal communication). Accordingly, a terminal concentration of ∼108 bacteria per ml of blood is required to ensure that the final meal partaken by the flea vector is infectious. In the mouse, this population reflects an observed terminal whole-body burden of ≥109 yersiniae (9), which may contain sufficient endotoxin to exceed the lethal limit. Plague bacilli are therefore markedly distinct from the enteropathogenic yersiniae in that they are required to kill their host in order to survive in nature (but not too soon, or they may fail to board the lifeboats).
As noted above, important features accounting for acute disease were acquired by lateral transfer during the recent evolution of Y. pestis. These include unique ∼10- and ∼100-kb plasmids termed pPCP and pMT, respectively (9, 75). Both of these genetic elements mediate functions that favor infection by fleabite versus by ingestion. That is, pPCP encodes a potent plasminogen activator (Pla) that facilitates bacterial metastasis, thereby permitting prompt access by plague bacilli to the viscera (52, 96). This capability ensures a mouse a 50% lethal dose of ≤10 bacteria via subcutaneous or intradermal injection, a value that increases by a millionfold upon the mutational loss of Pla (11). Similarly high values also occur for Y. pseudotuberculosis following injection by these peripheral routes, but it is important to note that this species is nearly as virulent as Y. pestis in mice following intravenous injection (50% lethal dose, ∼100 bacteria). Biotype 1b strains of Y. enterocolitica (capable of expressing and utilizing the plague siderophore yersiniabactin) are also highly infectious in mice via the intravenous, but not peripheral, method of injection. The efficient filtration of the vascular system by the capillary network and the attendant fixed-macrophage system of the liver and spleen probably account for these route-dependent differences in virulence (48).
pMT encodes a protein capsular antigen termed fraction 1 (Caf1) plus a multifunctional exotoxin that is lethal to Muridae and also facilitates colonization of the flea (46). Loss mutations that occurred during the emergence of Y. pestis from Y. pseudotuberculosis include removal of the fibrillar adhesin YadA and the cell invasin termed Inv (9, 40, 75). YadA and Inv enable the enteropathogenic yersiniae to traverse the small intestine but are replaced by the tissue invasin Pla in Y. pestis. The rationale for their removal in Y. pestis is uncertain, but one possibility is that they would otherwise serve as anchors to retard dissemination from peripheral sites infected by fleabite (9). Between the two organisms, there are additional differences that primarily reflect the loss in Y. pestis of enzymes of intermediary metabolism (8, 9). Some of these lesions radically alter the flow of metabolic carbon, causing obvious loss of fitness in vitro (37, 63). Further study will be required to determine if these blocks provide a selective advantage in vivo and, if so, the role they play in promoting acute disease.
Early studies concerned with the interaction of plague bacilli and professional phagocytes defined three critical tenets. First, expression of Caf1 renders wild-type organisms resistant to uptake by professional phagocytes (16). Second, phagocytic cells obtained from mice infected with Y. pestis are dysfunctional in that they are unable to engulf Caf1-deficient mutants or any other bacterium that would readily be ingested following its harvest from uninfected control mice (18). Third, plague bacilli are largely resistant to intracellular killing even under conditions chosen to permit their ingestion by professional phagocytes (22, 49). These landmark observations provided the basis for a tradition embraced by early investigators stating that phagocytosis plays little or no role in controlling plague. Straley and Harmon (99) subsequently verified that even avirulent mutants of Y. pestis lacking pCD are intrinsically resistant to intracellular killing by mouse peritoneal macrophages.
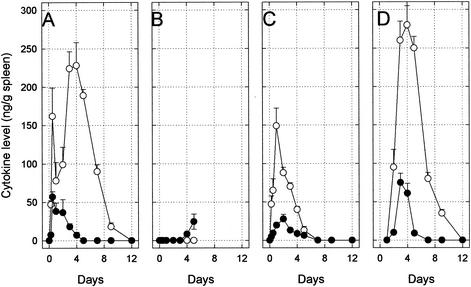
The results of more-recent experiments suggested that the generalized yersinia-induced paralysis of professional phagocytes that occurs in vivo (18) is caused by the systemic downregulation of inflammation (Fig. 1). Mice infected with avirulent pCD− cells of Y. pestis exhibited a generic inflammatory response characterized by marked upregulation of IFN-γ and TNF-α in spleen (Fig. 1A). This phenomenon is consistent with the occurrence of TLR-initiated and then proinflammatory cytokine-initiated cascades leading to free nuclear NF-κB, followed by eventual downregulation of IFN-γ and TNF-α by homeostatic anti-inflammatory mechanisms, including the induction of IL-10. In contrast, mice infected with virulent pCD+ yersiniae failed to express any detectable IFN-γ, although a modest spike of TNF-α appeared immediately before death (Fig. 1B). This dramatic downregulation of IFN-γ and TNF-α is the hallmark of plague. A more typical inflammatory response (associated with 100% survival) occurred if the injected mice were primed by concomitant administration of trace levels of IFN-γ and TNF-α (Fig. 1C), thereby initiating cascades leading to free nuclear NF-κB. Finally, passive immunization with anti-LcrV (V antigen), a secreted pCD or pYV-encoded 37.3-kDa pure monomeric protein, restored the full generic inflammatory response, thus ensuring complete survival (Fig. 1D). These results demonstrated that LcrV directly or indirectly inhibits the expression of IFN-γ and TNF-α and showed that this blockade is prevented by specific antiserum (67, 68).
FIG. 1.
Expression of the proinflammatory cytokines IFN-γ (○) and TNF-α (•) in C57BL/6 mouse spleen following intravenous challenge with 106 pCD− cells of Y. pestis KIM10 (A), 102 pCD+ cells of Y. pestis KIM10 (B), 102 pCD+ cells of Y. pestis KIM10 after the cells were primed with 20 μg of IFN-γ and 20 ng of TNF-α (C), and 102 pCD+ cells of Y. pestis KIM10 by passive immunization on postinfection day 1 with 100 μg of polyclonal rabbit anti-LcrV (D). All untreated mice challenged with pCD+ yersiniae died by postinfection day 6 (B), whereas the remainder survived. This figure is redrawn from the work of Nakajima and Brubaker (67) and Nakajima et al. (68).
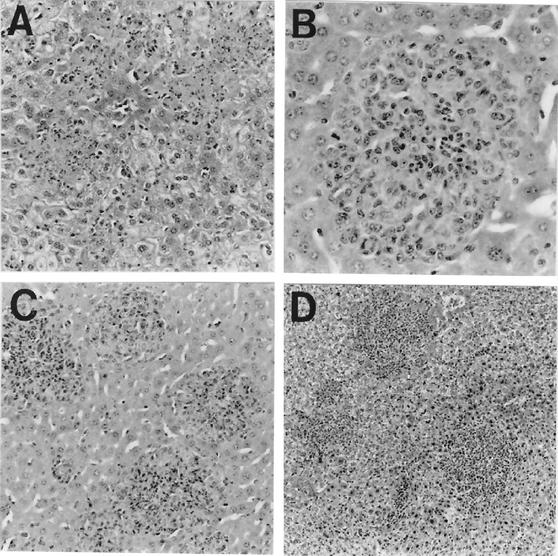
LcrV was assumed to be a protective antigen (12, 15, 17, 54, 102) shortly after its discovery by T. W. Burrows in 1956 (14). Formal proof of this notion awaited demonstration by Motin et al. (64) of significant passive immunity mediated by antibodies directed against at least one internal epitope located within the LcrV moiety of a cloned staphylococcal protein A-LcrV fusion protein (PAV). Injected PAV prevented upregulation of IFN-γ and TNF-α by LcrV-deficient mutants, providing further evidence that this protein blocks the generic inflammatory response (68). Additional study verified earlier findings (103) showing that pCD+ yersiniae growing in mouse liver formed stark focal necrotic lesions that failed to attract inflammatory cells. These foci progressively increased in size, coalesced with similar neighboring foci, and eventually caused loss of organ function (Fig. 2A). In contrast, similar infection with pCD− yersiniae was characterized by the prompt formation of granulomas and the loss of bacterial viability (Fig. 2B). Mice immunized either passively with anti-PAV (Fig. 2C) or actively with PAV (Fig. 2D) also contained intravenously injected pCD+ yersiniae within granulomas and thereby survived infection (68). Finally, a second-generation hexahistidine-tagged LcrV was engineered, expressed in Escherichia coli, and purified to near homogeneity by nickel affinity chromatography in an LPS-free and biologically active form (65). Second-generation hexahistidine-tagged LcrV immunized mice against intravenous challenge with 107 pCD+ plague bacilli (65) and provided immediate resistance to LPS-mediated killing by downregulating IFN-γ and TNF-α. This anti-inflammatory effect was found to reflect the prompt upregulation of IL-10 (69).
FIG. 2.
Characteristic histopathological changes in liver caused by Y. pestis KIM10 on postinfection day 3 (hematoxylin and eosin stain). (A) Control mouse infected with pCD+ yersiniae showing multiple necrotic focal lesions without an inflammatory cell response (magnification, ×140); (B) control mouse infected with pCD− yersiniae exhibiting granuloma formation (magnification, ×280); (C) mouse actively immunized with PAV and then infected with pCD+ yersiniae showing protective granulomatous lesions (magnification, ×140); (D) mouse passively immunized with polyclonal rabbit anti-PAV and infected with pCD+ yersiniae showing pregranulomatous lesions prompting infiltration of inflammatory (mononuclear) cells (magnification, ×70). This figure was reprinted from the work of Nakijima et al. (68).
Considered together, these findings suggest that cells of Y. pestis both rely upon Pla to rapidly access the livers and spleens of infected rodents and are indifferent to phagocytosis (but not containment within granulomas). Due to the anti-inflammatory activity of LcrV, sufficient vegetative growth of yersiniae occurs within these privileged niches to ensure spillover into the vascular system and infection of resident fleas, which then depart the moribund host and perpetuate the disease in nature. This scenario largely ignores the type III secretion engine of pCD and therefore differs radically from models defining chronic disease caused by enteropathogenic yersiniae. The nature of the distinctions between these two traditions is considered below.
YOPS AND THE ENTEROPATHOGENIC TRADITION
Most pYV-deficient isolates of Y. enterocolitica, regardless of biovar, are killed following uptake by macrophages. On the other hand, at least some strains of Y. pseudotuberculosis evidently acquired pYV-independent resistance to intracellular killing during their million years of evolution following their divergence from Y. enterocolitica. However, not all strains of Y. pseudotuberculosis possess innate resistance to intracellular killing. For example, pYV− mutants of the well-studied YpIII strain are readily killed by macrophages (J. Bliska, personal communication). The innate sensitivity of pYV− isolates of Y. enterocolitica and the atypical Y. pseudotuberculosis strain YpIII persuaded investigators of enteropathogenic disease that the ability of pYV to protect against phagocytosis is its primary physiological function. This logical assumption led to intensive study of pYV as an “antihost” genome (31) capable of disabling professional phagocytes by translocation of virulence effectors, termed Yersinia outer proteins (Yops), via type III secretion.
The important features of the type III secretion process are its requirement for intimate physical contact between the host cell and docked bacteria, its independence from the ubiquitous Sec system, its reliance upon chaperones or noncoding mRNA sequences for priming, its utilization of an injectisome traversing the entire gram-negative envelope, and its widespread distribution among pathogenic species. The type III system is especially effective in subverting individual target cells but is unable to cause systemic intoxication, as occurs with AB subunit toxins released extracellularly by the type II secretion processes. A corollary is that virulence effectors delivered by type III secretion machines lack the receptor site domains required by AB subunit toxins for host cell penetration and therefore are inert if released in free form by the bacterium (20, 23, 32, 41, 47, 55, 56).
Carriage of pCD or pYV at 37°C in the absence of Ca2+ promotes bacteriostasis in vitro due to as yet incompletely understood lesions in bioenergetics that are especially pronounced in Y. pestis. This environment, however, enables full induction of Yops and LcrV (although the addition of Ca2+ causes their downregulation with concomitant vegetative growth). This phenomenon, termed the low-calcium response, has received intensive study resulting in considerable understanding of its regulation and mechanics. Especially helpful in this context was the analysis of “Ca2+-blind” mutants that remain growth restricted and express Yops and LcrV at 37°C in the presence of Ca2+. Also invaluable were “Ca2+-independent” mutants, especially those with mutations in lcrV, capable of vegetative growth without Yop production at 37°C in Ca2+-deficient medium (9, 75). Landmark findings in defining type III secretions in yersiniae were the discovery of a pCD/pYV-encoded transcriptional activator termed LcrF (VirF in Y. enterocolitica) for linked structural genes (53) and the demonstration that temperature dependency reflects the melting of the DNA encoding lcrF and virF (81). The significance of the LCR phenotype was further clarified by the important discovery that its growth with host cells in tissue culture media (necessarily) containing Ca2+ permitted both the upregulation of LcrF/VirF-activated genes and the subsequent translocation of Yops (79, 82). These events and the subsequent progress in defining the pYV-encoded type III secretion engine and virulence effectors noted directly below have been exhaustively reviewed (25-33). Evidence favoring the posttranscriptional regulation of Yop expression via 5′ untranslated mRNA has recently appeared (21).
Six established virulence effectors termed YopE, YopT, YopO (YpkA in Y. pseudotuberculosis and Y. pestis), YopH, YopM, and YopP (YopJ in Y. pseudotuberculosis and Y. pestis) are secreted via this system. YopE, -T, -O, and -H function primarily by disrupting the host cell actin cytoskeleton and the attendant scaffolding, although YopH, like YopP/YopJ, possesses anti-inflammatory activity; the function of YopM remains uncertain. YopH is a potent phosphotyrosine phosphatase (6, 44, 112) that dephosphorylates macrophage protein p130cas, thereby disrupting the focal adhesions involved in phagocytosis (4, 45, 77). This activity also dephosphorylates the protein scaffolding in macrophages (45), an event possibly accounting for the ability of YopH to block the oxidative burst of macrophages (5) and inhibit calcium signaling in neutrophils (2). The discovery that YopH can downregulate the costimulatory molecule B7.2 and thereby modulate a wide variety of T- and B-cell-mediated responses bears directly on inflammation (110) and possibly accounts for its ability to repress monocyte chemotactic protein 1 (87). YopJ/YopP downregulates multiple inflammatory modulators, including proinflammatory cytokines, intercellular adhesin molecule 1, and E selectin (7, 35, 72, 73, 90). This inhibition essentially involves blocking MAP kinase signaling, thereby maintaining NF-κB in inactive form (70, 71, 111), an event found to modulate at least 37 macrophage genes (88). A major consequence of this interaction is macrophage-specific apoptosis (60, 61, 83) occurring through classical Bid proteolysis, cytochrome c release, and caspase cleavage (34).
Based on this information, YopJ/YopP and YopH (rather than LcrV) were logically assigned primary roles in inhibiting the innate immune processes initially described with Y. pestis (67, 68). This assessment, however, failed to explain the dramatic role observed for anti-LcrV in restoring the generic inflammatory response (67, 68) and protecting against lethality (64, 65, 68). One alternative to the concept that anti-LcrV directly prevents anti-inflammatory activity was the possibility that LcrV instead facilitates secretion of Yops and that anti-LcrV blocks this process (by preventing translocation of anti-inflammatory YopH and YopJ/YopP). The precedent for this prospect was the finding that LcrV is encoded on pCD or pYV within an lcrGVH-yopBD operon (3, 76, 80), where YopB and YopD serve as integral components of the translocation machinery (24, 27-29, 32). Mutational analysis of this operon failed to clarify the issue because nonpolar loss of lcrV results in a Ca2+-independent phenotype where all LcrF/VirF-activated genes are constitutively repressed (3, 80). Nevertheless, LcrV was assigned roles as either an antibody-sensitive structural component of the injectisome (78) or a tool required for its maturation (86). Furthermore, an LcrV homolog termed PcrV was discovered in nosocomial isolates of Pseudomonas aeruginosa. PcrV facilitated type III translocation of exoenzyme S but lacked detectable anti-inflammatory activity, which further suggested that LcrV functions solely as a mediator of Yop translocation (89, 107). Although anti-PcrV protected against infection with Pseudomonas by blocking type III secretions (89), results of similar experiments with yersiniae remain controversial in that translocation of YopE by yersiniae was blocked by anti-LcrV in one study (78) but not in another (39).
In conclusion, the enteropathogenic tradition states that cells of Y. pseudotuberculosis and Y. enterocolitica rely upon pYV to avoid killing by phagocytosis. Anti-inflammatory activity is largely accomplished by YopH and YopJ/YopP rather than LcrV, which functions as a regulator or mediator of type III secretions. Accommodation of this model with the plague tradition can be accomplished by evaluating the differences between acute and infectious disease. That is, cells of the enteropathogenic species remain localized at the small intestine and adjacent lymphatic tissue and seldom breach the barriers protecting visceral organs. Indeed, doing so would risk initiating acute (lethal) disease, thereby jeopardizing further dissemination of these cells in natural environments. Some discrepancies between the plague and enteropathogenic traditions are summarized in Table 1. One take-home lesson to be gained by comparing these features is the recognition that attempts to define enteropathogenic disease in terms of the plague tradition is presumptuous; another is that the reciprocal is naïve. Nevertheless, evaluation of the two models in the light of new or additional information provides a basis for reconciliation.
TABLE 1.
Major distinctions between the plague and enteropothogenic traditions of yersiniology
| Tradition | Most effective innate mechanism of control | Consequence of uptake by macrophages | Host cell invasin(s) | Tissue invasin | Host cell adhesion | Typical route of transmission | Major anti-inflammatory activities | Favored in vivo nicheb | Primary role of Yops | Function performed by anti-LcrV to facilitate specific immunity |
|---|---|---|---|---|---|---|---|---|---|---|
| Plague | Formation of granulomas | None | Aila | Pla | Unknown | Flea | LcrV | Visceral organs | Facilitates tissue necrosis | Prevents systemic upregulation of IL-10, thereby permitting inflammation |
| Enteropathogenic | Phagocytosis | Death | Inv, Ail | None | YadA | Fecal contamination | YopP/YopJ, YopH | Lymphatic tissue or small intestine | Prevents phagocytosis | Inhibits Yop translocation, thereby permitting normal phagocytosis |
INTEGRATION OF TRADITIONS
Initial skepticism regarding the discovery of IL-10 upregulation by LcrV (69) is understandable because this mechanism of virulence was largely without precedent, ligand fusion protein technology was in its infancy, LcrV is extremely unstable, and the phenomenon resembled LPS tolerance. IL-10 upregulation by LcrV has now been verified with histidine-tagged LcrV from Y. enterocolitica by Sing et al. (91), who further showed that IL-10 knockout mice both express the generic inflammatory response and exhibit marked resistance to infection. Related studies demonstrated that this induction is dependent upon the CD14 receptor and reflects the physical binding of TLR-2, thereby subverting the cascade to free NF-κB (92). These reports also emphasized that the PcrV of Pseudomonas lacks a sequence associated with the anti-inflammatory activity of LcrV and further noted that mice deficient in TLR-2 were less sensitive to infection than normal controls (92). Further study will be necessary to determine the precise mechanism of IL-10 upregulation. Nevertheless, these studies have legitimized LcrV as a potent anti-inflammatory agent and major determinant of virulence.
The conclusion that YopH and YopJ/YopP minimize inflammation is not, of course, discredited by the studies of Sing et al. (91, 92) verifying that LcrV performs the same function. As noted by those authors, the two anti-inflammatory Yops likely serve as short-distance weapons in the trenches, whereas LcrV may function as a long-range missile capable of systemic immunosuppression. Two additional pieces of information relating to acute disease bear directly on this possibility. First, it was established upon its discovery that YopJ is not essential for the virulence of Y. pestis (97); YopJ-deficient mutants retain the ability to block the generic inflammatory response in a manner identical to that shown in Fig. 1B for wild-type yersiniae (R. R. Brubaker, unpublished observations). Another difference between the two activities is that YopJ/YopP functions by preventing the activation of MAP kinases (thereby preventing the activation of NF-κB), whereas LcrV, by upregulating IL-10, ensures the inactivation of preformed NF-κB. Collectively, these observations suggest that LcrV replaces YopJ in downregulating inflammation during the rapid and lethal course of plague (where peripheral inflammation and macrophage apoptosis are moot).
Second, the type III secretion machine of Y. pestis is clearly distinct from those of the enteropathogenic species. For example, the efficiency of Yop translocation in Y. pseudotuberculosis and Y. enterocolitica is dependent upon close bacterium-host cell contact mediated by YadA, Inv, and Ail (40, 43, 84). As already noted, these surface structures are either absent or obscured by Caf1 in Y. pestis. Another difference in secretion is that plague bacilli fail to express Yops in vitro in a Ca2+-deficient environment found to promote excellent expression by enteropathogenic species (98). This difference is due to the presence of Pla in Y. pestis, which catalyzes the prompt posttranslational degradation of Yops but does not destroy LcrV (59, 85, 95). This finding does not mean that Yops are redundant in plague bacilli; indeed, mutational loss of any such effector other than YopJ causes significant loss of virulence (9, 75, 97). It is nevertheless highly significant that Pla does not degrade LcrV, which is produced abundantly and, unlike Yops, released extracellularly in low-calcium environments. (13, 54, 98). Also unlike Yops, LcrV accumulates copiously within the tissues of experimental animals suffering from plague (93).
The following events may account for the terminal vegetative phase of plague in rodents. Although Yops are degraded by Y. pestis in vitro, these proteins undoubtedly undergo normal delivery following docking to host cells. The bulk of the vegetative growth observed during the course of plague occurs within nonvascularized focal lesions composed of cytoplasm released from lysed host cells of the visceral organs (Fig. 2A). This nutrient-rich fluid lacks significant Ca2+ and can thus support the synthesis of both Yops and LcrV. Yops synthesized by unattached plague bacilli in these lesions cannot be delivered and thus undergo Pla-mediated degradation (and thus not accumulate and diffuse in inert form as potential mediators of inflammation). However, Yops synthesized by docked yersiniae undergo translocation, thus ensuring continued necrosis and enlargement of the lesions. LcrV released by both docked and free bacilli within these necrotic lesions provide an essential anti-inflammatory umbrella blocking the infiltration of inflammatory cells and the subsequent formation of protective granulomas.
A corollary of this model is that the enteropathogenic yersiniae do not breach barriers protecting visceral organs since this act results in lethal disease (an epidemiological dead end for these species). The cells of Y. pseudotuberculosis and biotype 1b Y. enterocolitica are highly infectious upon artificial introduction into mouse liver and spleen by intravenous injection and thereafter cause lethal diseases similar to plague. It is not yet clear if the normal reluctance of enteropathogenic yersiniae to invade the viscera following ingestion merely reflects the absence of Pla or if active steps are taken to avoid the invasion of the liver and spleen (perhaps by permitting the occurrence of a modest degree of inflammation). Indeed, even Y. pestis generates initial inflammation, as judged from bubo formation during the course of human plague.
Further work will be required to decide many of these issues. Especially important will be the resolution of the innate pCD-independent mechanism of resistance to intracellular killing exhibited by Y. pestis and typical isolates of Y. pseudotuberculosis. Elucidation of the precise mechanism whereby LcrV upregulates IL-10 is of equal importance, as is characterization of its mechanism of secretion and its role in acute versus chronic disease. An explanation of how cells of Y. pestis adhere to host cells during the translocation of Yops may also be required for a full understanding of plague versus enteropathogenic infection. Full recognition of how far the anti-inflammatory activities of YopH, YopJ/YopP, and LcrV extend is also critical. Regardless of the mechanism involved, it is now evident that inactivation of NK-κB accounts for the absence of proinflammatory cytokines during the course of acute disease. This lesion has many other consequences; it also represses chemokines (e.g., IL-8, MIP-1α, MCP1, Rantes, and eotaxin), host adhesins (e.g., ICAM, VCAM, and E selectin), acute-phase proteins, and even the inducible stress effectors INOS and COX-2 (42). Full resolution of the mode of action of LcrV will better define the nature of events required for the expression of acute infectious disease. Additional study may also facilitate the development of novel therapeutic procedures and improved management of noninfectious inflammatory processes.
Editor: D. A. Portnoy
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, K., K.-E. Magnussen, M. Majeed, O. Stendahl, and M. Fällman. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman, T., S. Håkansson, Å. Forsberg, L. Norlander, A. Macellaro, A. Bäckman, I. Bölin, and H. Wolf-Watz. 1991. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, D. S., A. Marie-Cardine, B. Schraven, and J. B. Bliska. 2000. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell Microbiol. 2:401-414. [DOI] [PubMed] [Google Scholar]
- 5.Bliska, J. B., and D. S. Black. 1995. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliska, J. B., K. Guan, J. E. Dixon, and S. Falkow. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA 88:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland, A., and G. R. Cornelis. 1998. Role of YopP in supression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker, R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4:309-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brubaker, R. R. 2000. Yersinia pestis and bubonic plague. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackelbrandt (ed.), The prokaryotes, an evolving electronic resource for the microbiological community. Springer-Verlag. [Online.] http://www.link.springer.de. Accessed: 8 September 2000.
- 10.Brubaker, R. R. 1972. Yersinia: biochemistry and genetics of virulence. Curr. Top. Microbiol. Immunol. 57:111-158. [DOI] [PubMed] [Google Scholar]
- 11.Brubaker, R. R., E. D. Beesley, and M. J. Surgalla. 1965. Pasteurella pestis: role of pesticin I and iron in experimental plague. Science 149:422-424. [DOI] [PubMed] [Google Scholar]
- 12.Brubaker, R. R., A. K. Sample, D.-Z. Yu, R. J. Zahorchak, P. C. Hu, and J. M. Fowler. 1987. Proteolysis of V antigen from Yersinia pestis. Microb. Pathog. 2:49-62. [DOI] [PubMed] [Google Scholar]
- 13.Brubaker, R. R., and M. J. Surgalla. 1964. The effect of Ca++ and Mg++ on lysis, growth, and production of virulence antigens by Pasteurella pestis. J. Infect. Dis. 114: 13-25. [DOI] [PubMed] [Google Scholar]
- 14.Burrows, T. W. 1956. An antigen determining virulence in Pasteurella pestis. Nature 177:426-427. [DOI] [PubMed] [Google Scholar]
- 15.Burrows, T. W. 1960. Biochemical properties of virulent and avirulent strains of bacteria: Salmonella typhosa and Pasteurella pestis. Ann. N. Y. Acad. Sci. 88:1125-1135. [DOI] [PubMed] [Google Scholar]
- 16.Burrows, T. W. 1963. Virulence of Pasteurella pestis and immunity to plague. Ergeb. Mikrobiol. 37:59-113. [DOI] [PubMed] [Google Scholar]
- 17.Burrows, T. W., and G. A. Bacon. 1958. The effects of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br. J. Exp. Pathol. 39:278-291. [PMC free article] [PubMed] [Google Scholar]
- 18.Burrows, T. W., and G. W. Bacon. 1954. The basis of virulence in Pasteurella pestis: comparative behavior of virulent and avirulent strains in vivo. Br. J. Exp. Pathol. 35:134-143. [PMC free article] [PubMed] [Google Scholar]
- 19.Butler, T. 1983. Plague and other Yersinia infections. Plenum Press, New York, N.Y.
- 20.Büttner, D., and U. Bonas. 2002. Port of entry—the type III secretion translocon. Trends Microbiol. 10:186-192. [DOI] [PubMed] [Google Scholar]
- 21.Cambronne, E. D., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: yscM1 and yscM2 regulate yop gene expression by a posttranscriptional mechanism that targets the 5′ untranslated region of yop mRNA. J. Bacteriol. 184:5880-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of fleaborne plague. J. Immunol. 85:348-363. [PubMed] [Google Scholar]
- 23.Collmer, A., M. Lindeberg, T. Petniki-Ocwieja, D. J. Schneider, and J. R. Alfano. 2002. Genomic mining type III secretion system effectors in Pseudomonas syringae yields new picks for all TTSS prospectors. Trends Microbiol. 10:462-469. [DOI] [PubMed] [Google Scholar]
- 24.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97:8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornelis, G. R. 1998. The Yersinia deadly kiss. J. Bacteriol. 180:5495-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornelis, G. R. 1994. Yersinia pathogenicity factors. Curr. Top. Microbiol. Immunol. 192:243-263. [DOI] [PubMed] [Google Scholar]
- 27.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornelis, G. R. 2002. The Yersinia Ysc-Yop ′type III' weaponry. Nat. Rev. Mol. Cell Biol. 3:742-752. [DOI] [PubMed] [Google Scholar]
- 29.Cornelis, G. R. 2002. The Yersinia Ysc-Yop virulence apparatus. Int. J. Med. Microbiol. 291:455-462. [DOI] [PubMed] [Google Scholar]
- 30.Cornelis, G. R. 1992. Yersiniae, finely tuned pathogens. SGM Symp. 49:231-265. [Google Scholar]
- 31.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 33.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 34.Denecker, G., W. Declercq, C. A. Geuijen, R. Benabdillah, M. van Gurp, M. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of Bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 35.Denecker, G., S. Tötemeyer, L. J. Mota, P. Troisfontaines, I. Lambermont, C. Youta, I. Stainier, M. Ackermann, and G. R. Cornelis. 2002. Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect. Immun. 70:3510-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreyfus, L. A., and R. R. Brubaker. 1978. Consequences of aspartase deficiency in Yersinia pestis. J. Bacteriol. 136:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 39.Fields, K. A., M. L. Nilles, C. Cowan, and S. C. Straley. 1999. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67:5395-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finlay, R. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galán, J. E., and A. Collemer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 43.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan, K., and J. E. Dixon. 1990. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science 249:553-556. [DOI] [PubMed] [Google Scholar]
- 45.Hamid, N., A. Gustavsson, K. Andersson, K. McGee, C. Persson, C. E. Rudd, and M. Fallman. 1999. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb. Pathog. 27:231-242. [DOI] [PubMed] [Google Scholar]
- 46.Hinnebusch, J., P. Cherepanov, Y. Du, A. Rudolph, J. D. Dixon, T. Schwan, and Å. Forsberg. 1999. Murine toxin of Yersinia pestis shows phospholipase D activity but is not required for virulence in mice. Int. J. Med. Microbiol. 290:483-487. [DOI] [PubMed] [Google Scholar]
- 47.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janssen, W. A., G. W. Fukui, and M. J. Surgalla. 1958. A study of the fare of Pasteurella pestis following intracardial injection into guinea pigs. J. Infect. Dis. 103: 183-187. [DOI] [PubMed] [Google Scholar]
- 49.Janssen, W. A., and M. J. Surgalla. 1969. Plague bacillus: survival within host phagocytes. Science 163:950-952. [DOI] [PubMed] [Google Scholar]
- 50.Kirschning, C. J., H. Wesche, T. Merrill Ayres, and M. Rothe. 1998. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi, K., K. Kaneda, and T. Kasama. 2001. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 52: 241-245. [DOI] [PubMed] [Google Scholar]
- 52.Lähteenmäki, K., R. Virkola, A. Sarén, L. Emödy, and T. K. Korhonen. 1998. Expression of plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect. Immun. 66:5755-5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambert de Rouvroit, C., C. Sluiters, and G. R. Cornelis. 1992. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 6:395-409. [PubMed] [Google Scholar]
- 54.Lawton, W. D., R. L. Erdman, and M. J. Surgalla. 1963. Biosynthesis and purification of V and W antigen in Pasteurella pestis. J. Immunol. 91:179-184. [DOI] [PubMed] [Google Scholar]
- 55.Lee, V. T., and O. Schneewind. 2002. Protein secretion and the pathogenesis of bacterial infections. Genes Dev. 15:1725-1752. [DOI] [PubMed] [Google Scholar]
- 56.Lory, S. 1998. Secretion of proteins and assembly of bacterial surface organelles: shared pathways of extracellular protein targeting. Curr. Opin. Microbiol. 1:27-35. [DOI] [PubMed] [Google Scholar]
- 57.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 58.Medzhitov, R., P. Preston-Hurlbert, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adapter protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253-258. [DOI] [PubMed] [Google Scholar]
- 59.Mehigh, R. J., and R. R. Brubaker. 1993. Major stable peptides of Yersinia pestis synthesized during the low-calcium response. Infect. Immun. 61:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mills, D. S., A. Boland, M.-P. Sory, P. Van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 63.Mortlock, R. P., and R. R. Brubaker. 1962. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase activities of Pasteurella pestis and Pasteurella pseudotuberculosis. J. Bacteriol. 84:1122-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motin, V. L., R. Nakajima, G. B. Smirvnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Motin, V. L., Y. A. Nedialkov, and R. R. Brubaker. 1996. V antigen-polyhistidine fusion peptide: binding to LcrH and active immunity against plague. Infect. Immun. 64:4313-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muzio, M., S. Natoli, S. Saccani, M. Levrero, and A. Mantovani. 1998. The human Toll signaling pathway: divergence of nuclear factor κB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6). J. Exp. Med. 187:2097-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nedialkov, Y. A., V. L. Motin, and R. R. Brubaker. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orth, K., Z. Xu, M. B. Mudgett, Z. Q. Bao, L. E. Palmer, J. B. Bliska, W. F. Mangel, B. Staskawicz, and J. E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protease. Science 290:1594-1597. [DOI] [PubMed] [Google Scholar]
- 71.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 72.Palmer, L. E., S. Hobbie, J. E. Galán, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downstream regulation of the MAP kinase p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 73.Palmer, L. E., A. R. Pancetti, S. Greenberg, and J. B. Bliska. 1999. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect. Immun. 67:708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. G. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Chercher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeño-Tárraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. F. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 75.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perry, R. D., P. A. Harmon, W. S. Bowmer, and S. C. Straley. 1986. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect. Immun. 54:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Persson, C., R. Nordfelth, K. Andersson, Å. Forsberg, H. Wolf-Watz, and M. Fallman. 1999. Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33:828-838. [DOI] [PubMed] [Google Scholar]
- 78.Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von-Euler-Matell, E. Carlsson, R. Titball, Å. Forsberg, and H. Wolf-Watz. 1999. The V antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 79.Pettersson, J., R. Nordfeith, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 80.Price, S. B., C. Cowan, R. D. Perry, and S. C. Straley. 1991. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J. Bacteriol. 173:2649-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohde, J. R., X.-S. Luan, H. Rohde, J. M. Fox, and S. A. Minnich. 1999. The Yersinia enterocolitica pYV virulence plasmid contains multiple intrinsic DNA bends which melt at 37°C. J. Bacteriol. 181:4198-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruckdeschel, K., A. Roggenkamp, S. Schubert, and J. Heesemann. 1996. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect. Immun. 64:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sample, A. K., and R. R. Brubaker. 1987. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb. Pathog. 3:239-248. [DOI] [PubMed] [Google Scholar]
- 86.Sarker, M. R., C. Neyt, E. Stainier, and G. R. Cornelis. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sauvonnet, N., I. Lambermont, P. Van der Bruggen, and G. R. Cornelis. 1997. YopH prevents monocyte chemoattractant protein 1 expression in macrophages and T cell proliferation through fractionation of the phosphatidylinositol 3-kinase pathway. Mol. Microbiol. 45:805-815. [DOI] [PubMed] [Google Scholar]
- 88.Sauvonnet, N., B. Predet-Balede, J. A. Garcia-Sanz, and G. R. Cornelis. 2002. Regulation of mRNA expression in macrophages following Yersinia enterocolitica infection: role of different Yop effectors. J. Biol. Chem. 277:25133-25142. [DOI] [PubMed] [Google Scholar]
- 89.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 90.Schesser, K., A. K. Spiik, J. M. Dukuzumuremyl, M. F. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The YopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067-1079. [DOI] [PubMed] [Google Scholar]
- 91.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 92.Sing, A., D. Rost, N. Tvardovaskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith, H., J. Keppie, E. C. Cocking, and K. Witt. 1960. The chemical basis of the virulence of Pasteurella pestis. I. The isolation and the aggressive properties of Past. pestis and its products from infected guinea pigs. Br. J. Exp. Pathol. 41:452-459. [PMC free article] [PubMed] [Google Scholar]
- 94.Smith, T. 1934. Parasitism and disease. Princeton University Press, Princeton, N.J.
- 95.Sodeinde, O. A., A. K. Sample, R. R. Brubaker, and J. D. Goguen. 1988. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect. Immun. 56:2749-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sodeinde, O. A., Y. V. B. K. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 258:1004-1007. [DOI] [PubMed] [Google Scholar]
- 97.Straley, S. C., and W. S. Bowmer. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Straley, S. C., and R. R. Brubaker. 1981. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc. Natl. Acad. Sci. USA 78:1224-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Straley, S. C., and P. A. Harmon. 1984. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect. Immun. 45:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 101.Tato, C. M., and C. A. Hunter. 2002. Host-pathogen interactions: subversion and utilization of the NF-κB pathway during infection. Infect. Immun. 70:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Une, T., and R. R. Brubaker. 1984. Roles of V antigen in promoting virulence and immunity in yersiniae. J. Immunol. 133:2226-2230. [PubMed] [Google Scholar]
- 103.Une, T., R. Nakajima, and R. R. Brubaker. 1986. Roles of V antigen in promoting virulence in Yersinia. Contrib. Microbiol. Immunol. 9:179-185. [PubMed] [Google Scholar]
- 104.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. M. Billah. 1995. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 270:9558-9563. [DOI] [PubMed] [Google Scholar]
- 106.Wang, Q., R. Dziarski, C. J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect. Immun. 69:2270-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamamoto, K., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, K. Takeda, and S. Akira. 2002. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420:324-328. [DOI] [PubMed] [Google Scholar]
- 109.Yang, R. B., G. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowksi. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 110.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chein. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, YopH. J. Exp. Med. 190:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoon, S., Z. Liu, Y. Eyobo, and K. Orth. 2003. Yersinia effector YopJ inhibits yeast MAPK signaling pathways by an evolutionarily conserved mechanism. J. Biol. Chem. 278:2131-2135. [DOI] [PubMed] [Google Scholar]
- 112.Zhang, Z. Y., J. C. Clemens, H. L. Schubert, J. A. Stuckey, M. W. Fischer, D. M. Hume, M. A. Saper, and J. E. Dixon. 1992. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 267:23759-23766. [PubMed] [Google Scholar]