Abstract
Nontyphoidal salmonellae are enteric pathogens that cause acute gastroenteritis and colonize the intestinal tract for prolonged periods. In the intestinal epithelia, these bacteria induce secretion of proinflammatory cytokines, such as interleukin-8 (IL-8), which leads to a profound inflammatory response through recruitment of polymorphonuclear leukocytes. Production of IL-8 induced by Salmonella spp. is due to the activation of the transcription factors nuclear factor κB (NF-κB) and activator protein-1 (AP-1). This work demonstrates that Salmonella enterica serovar Typhimurium can downmodulate IL-8 production after invasion of intestinal epithelial cells. The Salmonella translocated effector proteins SspH1 and SptP participate in this process. SspH1 is a member of the bacterial LPX repeat protein family that localizes to the mammalian nucleus and inhibits NF-κB-dependent gene expression. A Shigella flexneri translocated effector, IpaH9.8, which has a similar structure and subcellular localization in mammalian cells, also inhibits NF-κB-dependent gene expression. We propose that suppression of inflammatory responses by intracellular S. enterica serovar Typhimurium, and perhaps Shigella flexneri, contributes to bacterial colonization of host tissues and pathogenesis.
Salmonellae are gram-negative bacterial pathogens that can infect a wide variety of animal hosts through ingestion of contaminated food or water (25). Typhoidal strains, such as Salmonella enterica serovars Typhi and Paratyphi, infect only humans, which often results in a life-threatening systemic illness termed enteric fever. In contrast, nontyphoidal strains, such as S. enterica serovars Typhimurium and Enteridis, have a broad host range and usually cause acute gastroenteritis. In humans, nontyphoidal Salmonella serotypes induce an early inflammatory response in the intestinal epithelium, leading to infiltration of polymorphonuclear leukocytes into the intestinal lumen and diarrhea. Production of the potent polymorphonuclear leukocyte chemokine interleukin-8 (IL-8) by the infected epithelia appears to be important for this process. Although the disease is typically self-limiting in immunocompetent adults, the bacterium can persist in the intestinal tract for prolonged periods (3). In addition, infection of the elderly, neonates, and immunocompromised individuals can result in a severe and even life-threatening disease (25).
Essential to Salmonella pathogenesis is a specialized apparatus called the type III secretion system (TTSS), which mediates the transfer of bacterial virulence proteins, called effectors, from the bacterial cell into the host cell cytoplasm (13). Once inside the host cell, these effectors are capable of altering such host cellular functions as cytoskeletal architecture, membrane trafficking, signal transduction, and cytokine gene expression in order to promote bacterial survival and colonization. Salmonellae encode two distinct virulence-associated TTSS within Salmonella pathogenicity islands 1 and 2 (SPI1 and SPI2, respectively) that function at different times during infection. While the SPI1-encoded TTSS is active upon contact with the host cell and translocates bacterial proteins across the plasma membrane, the SPI2 TTSS is expressed after the bacterium has been internalized by the host cell and translocates effectors across the vacuolar membrane.
The SPI1 TTSS is involved in invasion of epithelial cells and induction of the intestinal inflammatory response. SPI1 effectors, such as SopE, SopE2, and SopB, activate the Rho GTPases Rac-1 and Cdc42, which leads to actin cytoskeletal reorganization and eventually bacterial internalization (2, 15, 28, 38). Stimulation of these GTPases also triggers several mitogen-activated protein kinase pathways, including Erk, Jnk, and p38, resulting in the activation of the transcription factors activator protein-1 (AP-1) and nuclear factor κB (NF-κB) (18). Salmonella enterica serovar Typhimurium-induced production of proinflammatory cytokines, such as IL-8, has been shown to be due to the activation of these transcription factors. Interestingly, shortly after bacterial invasion the actin cytoskeleton regains its normal architecture (30). SptP, an SPI1 effector, has been shown to participate in this reversal process via its GTPase-activating protein activity toward Rac-1 and Cdc42 (12). Although StpP has also been reported to downmodulate activation of Erk via its tyrosine phosphatase activity (23), its effect on the Salmonella-induced host inflammatory response has not been explored. This manuscript presents evidence that S. enterica serovar Typhimurium can downmodulate Salmonella-induced secretion of IL-8 in intestinal epithelial cells. SptP and SspH1, an S. enterica serovar Typhimurium effector that can be translocated into mammalian cells by both the SPI1 and the SPI2 TTSS, participate in this process.
SspH1 belongs to a family of TTSS effector proteins that share a subtype of the leucine-rich repeat motif called the LPX repeat (22). While leucine-rich repeats are typically found in eukaryotic proteins and are thought to function as protein-binding domains, the LPX subtype has only been detected in putative bacterial TTSS effectors in Salmonella, Shigella, Yersinia, Edwardsiella, Rhizobium, and Bradyrhizobium species (8, 9, 14, 22, 34, 36). Although the role of these proteins during infection is not clear, two LPX repeat protein family members, YopM of Yersinia pestis and IpaH9.8 of Shigella flexneri, have been shown to localize to the eukaryotic cell nucleus (27, 32). This work demonstrates that SspH1 is also transported into the mammalian nucleus after translocation by the TTSS. In addition, we show that SspH1 and IpaH9.8 both inhibit NF-κB-dependent gene expression, while two other S. enterica serovar Typhimurium LPX repeat proteins, SlrP and SspH2, which do not localize to the mammalian nucleus, do not. This suggests that inhibition of NF-κB may be a common property of nuclear LPX repeat proteins, which in turn may function to attenuate the host's inflammatory response.
MATERIALS AND METHODS
Bacterial strains, mammalian cell lines, and culture conditions.
The wild-type S. enterica serovar Typhimurium strain CS401 and its mutant derivatives with nonpolar deletion of sspH1 (EM124) or sptP (MJH2362) have been described previously (22, 33). The details of strain JAF119, which is CS401 harboring the SseJ-HA-HA-expressing plasmid pJAF111, have also been published (10). AH58 is CS401 transformed with plasmid pAH41, while AH74 is EM124 carrying pAH43. For infection of mammalian cells, bacterial strains were grown in Luria-Bertani broth under conditions that promote expression of the SPI1 TTSS. Briefly, overnight cultures were diluted 1:50, grown for 3 h at 37°C with aeration, and used immediately. Human Intestine-407 cells were obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Chinese hamster ovary (CHO-K1) cells and the mouse macrophage-like Raw-TT10 cell line, which stably expresses the tetracycline transactivator (35), were provided by David Underhill (Institute for Systems Biology, Seattle, Wash.) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Plasmid construction.
For complementation, the open reading frame of sspH1 plus 718 bp of sequence upstream of the start codon was amplified by PCR from S. enterica serovar Typhimurium CS401 genomic DNA, and the PCR product was cloned into pCAS61 (11) in frame with the coding sequence of the hemagglutinin (HA) epitope at the SspH1 carboxy terminus to yield pAH43. For immunofluorescent detection, pAH41 was constructed by cloning the sspH1 open reading frame, the sseJ promoter (1 kb upstream of the sseJ start codon) upstream of sspH1, and one additional HA tag in frame with the coding sequence of sspH1 at its carboxy terminus into pCAS61. For transfections, C-terminally HA-tagged slrP, sspH1, or sspH2 was cloned into the expression vector pTIGZ (35), resulting in pAH8, pAH9, and pAH10, respectively. The sspH1-nonexpressing control plasmid pAH40 was generated by cloning sspH1-HA into pTIGZ in the reverse orientation relative to the plasmid promoter.
Immunofluorescence microscopy.
Intestine-407 cells were seeded onto 12-mm glass coverslips in antibiotic-free culture medium and infected with S. enterica serovar Typhimurium at a multiplicity of infection of 20. After 1 h, the infected cells were washed three times with phosphate-buffered saline and incubated with culture medium containing 10 μg of gentamicin per ml for an additional 16 h. CHO-K1, Intestine-407, and Raw-TT10 cells transfected by electroporation with the appropriate expression plasmid and pNeo/Tak, expressing the tetracycline transactivator (35) (CHO-K1 and Intestine-407 cells only), were seeded onto glass coverslips 24 h before immunofluorescence staining. Infected or transfected cells were then washed three times with phosphate-buffered saline and fixed with 10% formyl saline for 15 min. Cells were washed again and then permeabilized with ice-cold acetone for 20 s. After repeated washing and blocking for 1 h with 10% goat serum in phosphate-buffered saline, samples were probed with primary and secondary antibodies for 1 h each with washes afterwards.
The HA epitope tag was detected with mouse monoclonal antibody HA.11 (Covance), while Salmonella strains were probed with rabbit polyclonal antilipopolysaccharide (anti-LPS) antibody (Difco). Secondary staining was performed with anti-mouse immunoglobulin G-tetramethylrhodamine isothiocyanate and anti-rabbit immunoglobulin G-fluorescein isothiocyanate conjugates (Sigma). Cell nuclei were visualized by staining with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma). All antibodies were used at a 1:1,000 dilution, while DAPI was diluted 1:10,000. Stained coverslips were mounted onto microscope slides with Vectashield (Vector Laboratories, Inc.) and examined with a Zeiss Axiovert S100 TV microscope with a 63× or a 100× lens. Images were acquired with softWoRx imaging software (Applied Precision).
IL-8 assay.
Intestine-407 cells seeded into 96-well plates in antibiotic-free culture medium were infected with the indicated strains of S. enterica serovar Typhimurium at a multiplicity of infection of 50. After 1 h the cells were washed three times with phosphate-buffered saline and incubated with medium containing 10 μg of gentamicin per ml with or without 50 ng of tumor necrosis factor alpha (TNF-α; Endogen) per ml for another 5 h. Culture supernatants were collected and stored at −80°C overnight. IL-8 immunodetection was performed by enzyme-linked immunosorbent assay (ELISA) as described previously (6).
Luciferase assay.
For measuring NF-κB-dependent gene expression, 5 × 106 to 107 CHO-K1 cells were transiently transfected by electroporation with the following plasmids: 3 μg of pELAM-1-fluc, expressing firefly luciferase from the ELAM-1 promoter (26); 0.3 μg of pRL-TK, expressing Renilla luciferase from the herpes simplex virus thymidine kinase promoter (Promega); 5 μg of pNeo/Tak; 5 μg of pCDNA3.1/Zeo/mCD14, expressing mouse CD14 (35); and 5 μg of the appropriate expression plasmid. For measuring NF-κB-independent gene expression, 0.1 μg of pUC18L-RSV, expressing firefly luciferase from the Rous sarcoma virus promoter (gift from Cheryl Carlson, University of Washington, Seattle, Wash.) was used instead of pELAM-1-fluc. For measuring dose dependence, cells were transfected with increasing concentrations of pAH9 (0, 2.5, 5, and 7.5 μg), while the amount of introduced DNA was kept constant by the addition of pTIGZ. For the IpaH9.8 transfection, pFlag-IpaH9.8 and the control pMEsf-neo plasmids were used (32). Cells were pulsed at 260 V and 960 μF in a Bio-Rad Gene Pulser and then seeded into 24-well plates. After 24 h, the transfected cells were incubated with 100 ng of Escherichia coli LPS per ml (Sigma) or fresh medium for 5 h, and luciferase activity was measured with the Dual Luciferase Reporter Assay System (Promega) in a Berthold LB9501 luminometer. Firefly luciferase activity was normalized to the activity of Renilla luciferase.
RESULTS
SspH1 is localized to the mammalian cell nucleus.
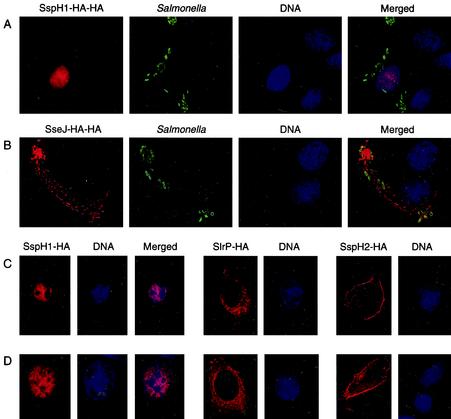
Since SspH1 is a TTSS effector and is translocated into host cells during infection, identification of its subcellular localization in mammalian cells could provide clues regarding its intracellular function. To localize SspH1, immunofluorescent microscopy analysis of Intestine-407 cells infected with S. enterica serovar Typhimurium expressing HA-tagged SspH1 was employed. Because HA-tagged SspH1 was only weakly detectable by immunostaining when its expression was driven by its native promoter, sspH1 was cloned behind the higher-expressing, SPI2-inducible sseJ promoter (21). Intestine-407 cells were infected with wild-type S. enterica serovar Typhimurium harboring a low-copy plasmid that encodes SspH1-HA-HA, and 17 h later the cells were fixed, permeabilized, and stained for SspH1-HA-HA, internalized bacteria, and DNA. As shown in Fig. 1A, HA-tagged SspH1 localized predominantly to the nucleus, with a weak signal also visible in the cytoplasm. This is similar to the subcellular localization of two other LPX repeat protein family members, YopM of Yersinia pestis (27) and IpaH9.8 of Shigella flexneri (32). As shown in Fig. 1B, the HA-tagged S. enterica serovar Typhimurium SPI2 effector SseJ, which was used as a TTSS control, was not detected in the nucleus but was targeted to Salmonella-induced filaments, as described previously (10).
FIG. 1.
SspH1 is localized to the nucleus in mammalian cells. Intestine-407 cells were infected with S. enterica serovar Typhimurium expressing SspH1-HA-HA (A) or SseJ-HA-HA (B). Cells were fixed, permeabilized, and immunostained for HA (red) and Salmonella LPS (green). Cell nuclei were visualized by staining with DAPI (blue). Raw-TT10 (C) and CHO-K1 (D) cells were transiently transfected with constructs expressing SspH1-HA, SlrP-HA, or SspH2-HA. HA-tagged proteins were detected by immunofluorescent staining (red), while DNA was stained with DAPI (blue).
The nuclear localization of SspH1 was further demonstrated in Raw-TT10 (Fig. 1C) and CHO-K1 cells (Fig. 1D) transiently transfected with a construct expressing HA-tagged SspH1. Two other HA-tagged LPX repeat proteins, SlrP-HA and SspH2-HA, which served as reference TTSS effectors, were not detected in the nucleus. SlrP-HA was evenly distributed in the cytoplasm, while SspH2-HA was enriched at the periphery of the cell, specifically in areas of active actin polymerization (20). These results indicate that, unlike SseJ, SlrP, and SspH2, SspH1 contains sequences that direct localization into the mammalian nucleus. In addition, the observation that SspH1 is nuclear even in the absence of bacterial translocation suggests that no other bacterial factors are required for its subcellular localization in mammalian cells.
SspH1 inhibits NF-κB-dependent reporter gene expression and activity.
Since NF-κB is a central regulator in response to various stress signals, including bacterial infection, the effect of SspH1 on host NF-κB signaling was examined. To determine whether expression of SspH1 would activate the NF-κB pathway, CHO-K1 cells were transiently transfected with two reporter constructs in which the endothelial cell-leukocyte adhesion molecule (ELAM-1; E-selectin) promoter controls expression of firefly luciferase and the herpes simplex virus thymidine kinase promoter drives Renilla luciferase. The ELAM-1 promoter is NF-κB dependent (26); thus, measurement of firefly luciferase activity reflects NF-κB activation, while the constitutive thymidine kinase promoter provides low to moderate levels of Renilla expression for normalizing transfection efficiency. In addition, cells were also transfected with an SspH1-expressing or empty plasmid.
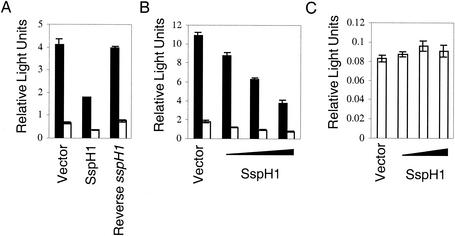
SspH1 was found not to activate NF-κB (Fig. 2A). However, when transfected CHO-K1 cells, which express endogenous Toll-like receptor 4 and exogenous CD14, were stimulated with LPS, SspH1 was found to inhibit activation of NF-κB. As shown in Fig. 2A, cells expressing SspH1 exhibited a marked decrease in NF-κB-dependent reporter gene expression and activity compared to cells transfected with the empty vector. In addition, this effect was dose dependent, because NF-κB-dependent gene expression decreased with increasing amounts of SspH1 expression plasmid (Fig. 2B).
FIG. 2.
SspH1 inhibits NF-κB-dependent reporter gene expression. CHO-K1 cells were transiently transfected with the indicated constructs: (A) a plasmid in which expression of firefly luciferase is under the control of the ELAM-1 promoter, a construct in which Renilla luciferase is expressed from the herpes simplex virus thymidine kinase promoter, vectors expressing mouse CD14 and the tetracycline transctivator, plus an empty, SspH1-expressing, or SspH1-nonexpressing vector (Reverse sspH1); (B and C) a plasmid in which expression of firefly luciferase is under the control of either ELAM-1 (B) or the Rous sarcoma virus promoter (C), a construct in which Renilla luciferase is expressed from the herpes simplex virus thymidine kinase promoter, vectors expressing mouse CD14 and the tetracycline transctivator, plus increasing concentrations of SspH1 expression vector (0, 2.5, 5, and 7.5 μg), while the amount of introduced DNA was kept constant by the addition of empty vector. After 24 h, the cells were incubated with E. coli LPS (solid bars) or fresh medium (open bars) for 5 h, and luciferase activity was measured. Firefly luciferase activity was normalized to the activity of Renilla luciferase. Data are represented as the mean ± standard deviation of triplicate samples.
To demonstrate that inhibition of the transcriptional activator function of NF-κB was not because the plasmid-introduced sspH1 provided a DNA binding partner for NF-κB and titrated NF-κB away from the ELAM-1 promoter, another control plasmid containing the sspH1 sequence but not expressing the SspH1 protein was also assayed. In this construct, the sspH1 open reading frame was in the reverse orientation relative to the plasmid promoter. As shown in Fig. 2A, this plasmid had no effect on NF-κB-dependent reporter gene expression and activity. In addition, SspH1 did not function to generally inhibit transcription, because firefly luciferase activity did not change when it was expressed from the NF-κB-independent Rous sarcoma virus promoter (5) (Fig. 2C). These results indicate that SspH1 expression in CHO-K1 cells results in specific inhibition of NF-κB-dependent gene expression after LPS stimulation.
The Shigella LPX repeat protein IpaH9.8 also inhibits NF-κB-dependent gene expression.
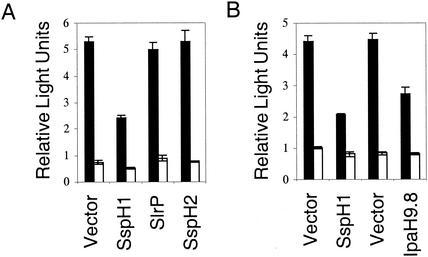
To determine whether inhibition of the NF-κB pathway is a common property of the bacterial LPX repeat family of proteins, CHO-K1 cells expressing S. enterica serovar Typhimurium SlrP or SspH2 were assayed for NF-κB-dependent luciferase gene expression and activity. Despite the high similarity of SlrP and SspH2 to SspH1 37% and 62% identity to SspH1, respectively, cells expressing either SlrP or SspH2 did not behave like cells expressing SspH1 but looked identical to cells transfected with the control plasmid (Fig. 3A). Since the subcellular localizations of SlrP and SspH2 in mammalian cells differ from that of SspH1, another nuclear LPX repeat protein, Shigella IpaH9.8, was tested for its effect on NF-κB-dependent reporter gene expression. As shown in Fig. 3B, cells expressing IpaH9.8 also had reduced firefly luciferase activity, similar to cells expressing SspH1. This suggests that inhibition of NF-κB may be a common property of LPX repeat effector proteins with a similar structure and nuclear localization.
FIG. 3.
The Shigella LPX repeat protein IpaH9.8 also inhibits NF-κB-dependent reporter gene expression. CHO-K1 cells were transiently transfected with pELAM-1-fluc, pRL-TK, pCDNA3.1/Zeo/mCD14, pNeo/Tak, and an empty vector or a construct expressing one of the following LPX repeat proteins: SspH1 (A and B), SlrP (A), SspH2 (A), or IpaH9.8 (B). After 24 h, the cells were incubated with E. coli LPS (solid bars) or fresh medium (open bars) for 5 h, and luciferase activity was measured. Firefly luciferase activity was normalized to the activity of Renilla luciferase. Data are represented as the mean ± standard deviation of triplicate samples. Each LPX repeat protein-expressing vector is diagramed to the right of its corresponding empty vector.
S. enterica serovar Typhimurium inhibits IL-8 production by TNF-α-activated Intestine-407 cells.
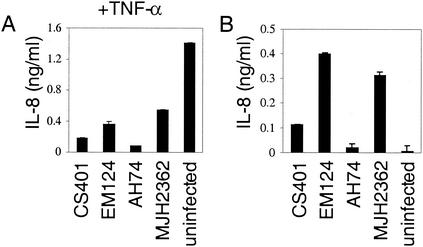
The observation that SspH1 inhibits NF-κB-dependent gene expression suggested that S. enterica serovar Typhimurium could attenuate cytokine production by the infected intestinal epithelia. To test this hypothesis, production of the proinflammatory cytokine IL-8, whose expression is in part NF-κB regulated, was examined with S. enterica serovar Typhimurium-infected and uninfected Intestine-407 cells. In order to assess the effect of Salmonella infection on IL-8 production, infected and uninfected cells were stimulated with the proinflammatory cytokine TNF-α 1 h postinfection or post-medium change, respectively. It is a physiological stimulus, since macrophages infected with Salmonella produce TNF-α in the intestinal tract (1). The levels of secreted IL-8 were measured in the culture supernatants by ELISA at 6 h after bacterial infection, which allowed time for the full delivery of SspH1 by the SPI1 and the SPI2 TTSS. As shown in Fig. 4A, cells infected with S. enterica serovar Typhimurium had greatly reduced IL-8 production compared to uninfected cells, which suggests that repression of inflammatory responses induced by proinflammatory stimuli occurs after Salmonella enter the epithelia.
FIG. 4.
S. enterica serovar Typhimurium expressing SspH1 inhibits IL-8 production by Intestine-407 cells. Cells were infected with wild-type (CS401), ΔsspH1 (EM124), ΔsspH1 complemented with a plasmid expressing SspH1 (AH74), or ΔsptP (MJH2362) S. enterica serovar Typhimurium strains at a multiplicity of infection of 50. After 1 h, the cells were washed and incubated with medium containing gentamicin plus TNF-α (A) or only gentamicin (B) for another 5 h. Culture supernatants were collected, and the levels of secreted IL-8 were measured by ELISA. Data are represented as the mean ± standard deviation of quadruplicate samples.
SspH1 contributes to repression of IL-8 production by S. enterica serovar Typhimurium.
To determine whether downmodulation of cytokine production by S. enterica serovar Typhimurium is mediated by SspH1, the levels of IL-8 secreted by Intestine-407 cells infected with wild-type or sspH1 deletion mutant S. enterica serovar Typhimurium strains were compared. As shown in Fig. 4B, cells infected with wild-type S. enterica serovar Typhimurium produced significantly less IL-8 than cells infected with the sspH1 mutant strain. To verify that the reduced cytokine secretion was due to the presence of SspH1, the deletion mutant was complemented with a low-copy plasmid expressing sspH1 from its native promoter. Expression of SspH1 by this strain was confirmed by Western blotting with antibody to the carboxy-terminal HA epitope tag (data not shown). As shown in Fig. 4B, the complemented strain behaved similarly to wild-type S. enterica serovar Typhimurium, inducing less IL-8 than the mutant strain, which indicates that SspH1 interferes with Salmonella-induced IL-8 production.
However, when cells were also stimulated with TNF-α, cells infected with the sspH1 mutant strain still secreted less IL-8 than uninfected cells, suggesting that there are other S. enterica serovar Typhimurium factors capable of blocking secretion of proinflammatory cytokines (Fig. 4A). Since the SPI1 effector SptP has been shown previously to downmodulate Erk activation, which is required for Salmonella-induced IL-8 production in Intestine-407 cells (23), an S. enterica serovar Typhimurium sptP deletion strain was tested in our assay. As shown in Fig. 4A and 4B, this strain behaved similarly to the sspH1 mutant, indicating that at least two S. enterica serovar Typhimurium effectors are involved in inhibiting IL-8 production by intestinal epithelial cells.
DISCUSSION
S. enterica serovar Typhimurium infection induces intestinal inflammation through the activation of the NF-κB signaling pathway (18). The SPI1 TTSS has been shown to play a central role in this process. SopE, SopE2, and SopB activate the Rho GTPases Rac-1 and Cdc42, which induces cytoskeletal changes and membrane ruffling but also triggers several mitogen-activated protein kinase pathways, resulting in the activation of NF-κB (2, 15, 18, 28, 38). SipB activates caspase-1 and induces macrophage toxicity, followed by the release of the proinflammatory cytokines IL-1β and IL-18 (17). In addition, recognition of pathogen-associated molecular patterns, such as LPS, lipoprotein, and flagella, by host Toll-like receptors also contributes to amplification of the NF-κB response (29). Much of this host response occurs on bacterial contact with mammalian cells.
This work demonstrates that S. enterica serovar Typhimurium can downmodulate Salmonella-induced secretion of the proinflammatory cytokine IL-8 after invasion of epithelial cells. SspH1, a TTSS effector that localizes to the mammalian nucleus, participates in this process by inhibiting NF-κB-dependent gene expression. These results also show that SspH1 is not the only TTSS effector involved in attenuation of the host's inflammatory response. Since SptP has been shown previously to reverse the SPI1-induced Rho GTPase and mitogen-activated protein kinase activation via its GTPase-activating protein and tyrosine phosphatase activities (12, 23), it was not surprising to find that it also downmodulated Salmonella-induced IL-8 production. It is possible that additional effectors also contribute to the anti-inflammatory response of S. enterica serovar Typhimurium. Another candidate is AvrA, an SPI1 TTSS effector, which has recently been reported to participate in inhibition of the NF-κB signaling pathway by S. enterica serovar Pullorum, an avian-adapted Salmonella serovar that is nonpathogenic for humans (4). Thus, this report demonstrates that, similar to the recovery from the dramatic actin cytoskeleton rearrangement after bacterial invasion (12, 30), the bacteria actively participate in the reversal of the Salmonella-induced inflammatory response.
The ability of salmonellae to interfere with the host's inflammatory response could promote bacterial pathogenesis. This is best illustrated by studies demonstrating the protective role of proinflammatory cytokines in host defense against S. enterica serovar Typhimurium. For example, TNF-α receptor-deficient mice are highly susceptible to S. enterica serovar Typhimurium infection (7). Similarly, administration of neutralizing anti-TNF-α antibodies to mice infected with a sublethal dose of S. enterica serovar Typhimurium results in increased bacterial replication and, subsequently, decreased survival of the host (24). In humans, S. enterica serovar Typhimurium induces an early intestinal inflammatory response, which in immunocompetent adults resolves on its own; however, the bacterium continues to persist in the intestinal tract for prolonged periods without inducing a significant host response. We speculate that the anti-inflammatory function of SspH1, SptP, and possibly AvrA contributes to the ability of S. enterica serovar Typhimurium to survive and to colonize the intestinal tract. The presence of multiple translocated effectors with anti-inflammatory properties could also explain why deletion of a single effector has only a modest or no effect on S. enterica serovar Typhimurium virulence in BALB/c mice and calves (16, 19, 22, 33, 37).
Downmodulation of inflammation is not unique to salmonellae and has contributed to the pathogenicity of a variety of bacterial species. For example, Yersinia pseudotuberculosis, Yersinia enterocolitica, uropathogenic Escherichia coli, and Mycobacterium ulcerans have all developed strategies to interfere with the activation of NF-κB (31). Therefore, it is not surprising that Shigella flexneri, which is a proinflammatory enteric pathogen similar to S. enterica serovar Typhimurium, would also employ a TTSS effector to attenuate the NF-κB pathway and, subsequently, the host's inflammatory response. Since IpaH9.8 and SspH1 have similar leucine-rich repeat and carboxy-terminal domains and both are localized to the mammalian nucleus, it is likely that they interact with the same host factor and elicit their function by the same mechanism.
Acknowledgments
This work was supported by Public Health Service National Research Service Award T32 GM07270 from the National Institute of General Medical Sciences (A.H.) and by grant RO1 AI48683 from the National Institutes of Health (S.I.M.).
We thank Stanley Fields for use of the Zeiss Axiovert S100 TV microscope, Steve Moseley for use of the Bio-Rad Gene Pulser, and David Underhill, Chihiro Sasakawa, and Cheryl Carlson for generously providing reagents.
Editor: V. J. DiRita
REFERENCES
- 1.Arnold, J. W., D. W. Niesel, C. R. Annable, C. B. Hess, M. Asuncion, Y. J. Cho, J. W. Peterson, and G. R. Klimpel. 1993. Tumor necrosis factor-alpha mediates the early pathology in Salmonella infection of the gastrointestinal tract. Microb. Pathog. 14:217-227. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi, C. S., V. P. Singh, M. W. Wood, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2000. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol. 182:2341-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchwald, D. S., and M. J. Blaser. 1984. A review of human salmonellosis. II. Duration of excretion following infection with non-typhi Salmonella. Rev. Infect. Dis. 6:345-356. [DOI] [PubMed] [Google Scholar]
- 4.Collier-Hyams, L. S., H. Zeng, J. Sun, A. D. Tomlinson, Z. Q. Bao, H. Chen, J. L. Madara, K. Orth, and A. S. Neish. 2002. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-κB pathway. J. Immunol. 169:2846-2850. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, J. T., D. M. Stroka, C. Brostjan, A. Palmetshofer, F. H. Bach, and C. Ferran. 1996. A20 blocks endothelial cell activation through a NF-κB-dependent mechanism. J. Biol. Chem. 271:18068-18073. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 7.Everest, P., M. Roberts, and G. Dougan. 1998. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect. Immun. 66:3355-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez, D. H., L. Pittman-Cooley, and R. L. Thune. 2001. Sequencing and analysis of the Edwardsiella ictaluri plasmids. Plasmid 45:52-56. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa-Bossi, N., S. Uzzau, D. Maloriol, and L. Bossi. 2001. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol. Microbiol. 39:260-271. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, J. A., M. E. Ohl, and S. I. Miller. 2003. The Salmonella enterica serovar Typhimurium translocated effectors SseJ and SifB are targeted to the Salmonella-containing vacuole. Infect. Immun. 71:418-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman, J. A., C. Rappl, V. Kuhle, M. Hensel, and S. I. Miller. 2002. SpiC is required for translocation of Salmonella pathogenicity island 2 effectors and secretion of translocon proteins SseB and SseC. J. Bacteriol. 184:4971-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu, Y., and J. E. Galan. 1999. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401:293-297. [DOI] [PubMed] [Google Scholar]
- 13.Galan, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 14.Gottfert, M., S. Rothlisberger, C. Kundig, C. Beck, R. Marty, and H. Hennecke. 2001. Potential symbiosis-specific genes uncovered by sequencing a 410-kilobase DNA region of the Bradyrhizobium japonicum chromosome. J. Bacteriol. 183:1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 16.Hardt, W. D., and J. E. Galan. 1997. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl. Acad. Sci. USA 94:9887-9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 19.Kaniga, K., J. Uralil, J. B. Bliska, and J. E. Galan. 1996. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21:633-641. [DOI] [PubMed] [Google Scholar]
- 20.Miao, E. A., M. Brittnacher, A. Haraga, R. L. Jeng, M. D. Welch, and S. I. Miller. 2003. Salmonella effectors translocated across the vacuolar membrane interact with the actin cytoskeleton. Mol. Microbiol. 48:401-415. [DOI] [PubMed] [Google Scholar]
- 21.Miao, E. A., and S. I. Miller. 2000. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 97:7539-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao, E. A., C. A. Scherer, R. M. Tsolis, R. A. Kingsley, L. G. Adams, A. J. Baumler, and S. I. Miller. 1999. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 34:850-864. [DOI] [PubMed] [Google Scholar]
- 23.Murli, S., R. O. Watson, and J. E. Galan. 2001. Role of tyrosine kinases and the tyrosine phosphatase SptP in the interaction of Salmonella with host cells. Cell. Microbiol. 3:795-810. [DOI] [PubMed] [Google Scholar]
- 24.Nauciel, C., and F. Espinasse-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegues, D. A., M. E. Ohl, and S. I. Miller. 2002. Salmonella, including Salmonella typhi, p. 669-697. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract, 2nd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 26.Schindler, U., and V. R. Baichwal. 1994. Three NF-κB binding sites in the human E-selectin gene required for maximal tumor necrosis factor alpha-induced expression. Mol. Cell. Biol. 14:5820-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skrzypek, E., C. Cowan, and S. C. Straley. 1998. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30:1051-1065. [DOI] [PubMed] [Google Scholar]
- 28.Stender, S., A. Friebel, S. Linder, M. Rohde, S. Mirold, and W. D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 29.Takeda, K., and S. Akira. 2001. Roles of Toll-like receptors in innate immune responses. Genes Cells 6:733-742. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi, A. 1967. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109-136. [PMC free article] [PubMed] [Google Scholar]
- 31.Tato, C. M., and C. A. Hunter. 2002. Host-pathogen interactions: subversion and utilization of the NF-κB pathway during infection. Infect. Immun. 70:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyotome, T., T. Suzuki, A. Kuwae, T. Nonaka, H. Fukuda, S. Imajoh-Ohmi, T. Toyofuku, M. Hori, and C. Sasakawa. 2001. Shigella protein IpaH(9.8) is secreted from bacteria within mammalian cells and transported to the nucleus. J. Biol. Chem. 276:32071-32079. [DOI] [PubMed] [Google Scholar]
- 33.Tsolis, R. M., L. G. Adams, M. J. Hantman, C. A. Scherer, T. Kimbrough, R. A. Kingsley, T. A. Ficht, S. I. Miller, and A. J. Baumler. 2000. SspA is required for lethal Salmonella enterica serovar Typhimurium infections in calves but is not essential for diarrhea. Infect. Immun. 68:3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsolis, R. M., S. M. Townsend, E. A. Miao, S. I. Miller, T. A. Ficht, L. G. Adams, and A. J. Baumler. 1999. Identification of a putative Salmonella enterica serotype Typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect. Immun. 67:6385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 36.Venkatesan, M. M., M. B. Goldberg, D. J. Rose, E. J. Grotbeck, V. Burland, and F. R. Blattner. 2001. Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect. Immun. 69:3271-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W. D. Hardt, A. J. Baumler, and L. G. Adams. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou, D., L. M. Chen, L. Hernandez, S. B. Shears, and J. E. Galan. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248-259. [DOI] [PubMed] [Google Scholar]