Abstract
Dirofilaria immitis polyproteins (DiAgs) are found as 15-kDa monomeric and 30-kDa dimeric forms in exceretory-secretory products of the adult worm. We evaluated the ability of various types of recombinant DiAg (rDiAg; V1 and V2 as monomers and V1V2, V2V1, V1V1, and V2V2 as dimers) to influence Th1/Th2 immune responses. V1-, V1Vx- and V2-, V2Vx-driven nonspecific immunoglobulin E (IgE) production peaked at 21 and 14 days after administration, respectively. Dimer-induced IgE response was an interesting biphasic pattern with the second peaks on days 35 (V2Vx) or 42 (V1Vx). Absolute amounts of nonspecific IgE production induced with monomers were larger than those observed with dimers at the first peak. The magnitude of cell expansion and interleukin-10 (IL-10) production in mesenteric lymph node (MLN) B-cell induced with rDiAgs was linked to the levels of the first IgE peak in vivo and IgE produced by rDiAg plus IL-4-stimulated B cells in vitro. All rDiAgs failed to augment IgG2c production. V2 and V2Vx elicited IL-4 production by MLN cells more rapidly than V1 and V1Vx. The inhibitory effect of rDiAg on gamma interferon (IFN-γ) production was stronger in monomers than in dimers. Neutralization of IL-10 restored IFN-γ production, whereas the expression of IL-4 and IgE was partly prevented by depletion of IL-10. These results indicate that monomer rather than dimer is an efficient form of DiAg and suggest that the difference of IgE-inducing capacity among these DiAgs is closely associated with the pattern of both B-cell activation and IL-4 production.
Parasitic nematode infections are well known as a potent inducer of a Th2 phenotype, as characterized by elevated levels of immunoglobulin E (IgE) antibody and interleukin-4 (IL-4), and by depressed levels of gamma interferon (IFN-γ). Most of the IgE antibody produced in nematode-infected hosts is antigen-nonspecific polyclonal IgE, which does not react to parasitic antigens (8). Although parasitic nematodes secrete large amounts of proteins as excretory-secretory (ES) products, their organisms can survive for many years in an immunocompetent host. Therefore, the longevity may be attributed to immunosuppression induced by some factor in ES products. The nonspecific IgE antibody may be involved in a survival of invading parasites from the host immune system (21, 29). IL-4, the canonical Th2-type cytokine, induces antibody class switching to IgE and suppresses Th1 cells to produce IFN-γ, which inhibits IL-4-dependent IgE production (2, 24). Therefore, nonspecific IgE production seen during nematode infections appears to be dependent on parasite-derived products and IL-4. However, the mechanisms by which parasitic products (especially molecularly defined products) induce nonspecific IgE synthesis in infected hosts remain to be clarified completely.
Nematode polyprotein allergen (NPA) is a <15-kDa polypeptide seen in both somatic and ES products of several parasitic nematodes (19). The biosynthesis of NPAs is very unique. The gene encoding NPAs is composed of tandem repeat units (10 to 50 repeating units). After transcription, NPAs are first synthesized as a large precursor polyprotein that possesses the cleavage site (Arg-Arg-Lys-Arg) of subtilisin serine protease at the C terminus of each unit. The precursor is then proteolytically digested into <15-kDa polypeptides (the size of monomer), thereby yielding multiple copies of identical or similar polypeptides. NPAs are detected not only as monomers but also as several oligomers in living worms. For example, the canine filarial parasite Dirofilaria immitis adult worms produce various sizes (ranging from monomer to 50-mer) of NPA (D. immitis polyprotein [DiAg]) as a cuticular component and secrete only two sizes of DiAg (approximately 15-kDa [monomer] and 30-kDa [dimer] proteins) in ES (5, 27). DiAg dimers are due to a dimeric tandem repeat that is composed of two units but are not due to the aggregation of monomers. It has been shown that the DiAg monomer is distinguished into two distinct classes (designated as V1 and V2) from difference between sequences in C-terminal half of monomers and that the precursor of DiAg is composed of repeating structure of V1V2 (5, 25, 27, 28). We have previously shown that DiAg (V1) preferentially induces nonspecific IgE synthesis by polyclonally expansion and IL-10 synthesis in B cells (33). We here tested whether another monomer (V2), heterodimers (V1V2 and V2V1), or homodimers (V1V1 and V2V2) of DiAg possess the immunomodulatory properties as V1.
MATERIALS AND METHODS
Amplification, expression, and purification of rDiAgs.
Recombinant DiAgs (rDiAgs; V1, V2, V1V2, and V2V1) were prepared basically according to the procedure as described previously (33). To prepare monomers, the cDNAs encoding these DiAgs were prepared from pDi6, which encodes repeat units of DiAg (V1V2V1), by PCR with specific primers as follows: a 5′ primer, which included a restriction site for NdeI and an initiating codon (5′-GCATATGAATGATCATAATTTAGAAAGC-3′; for V1 and V2), and 3′ primers, which included a restriction site for BamHI and a stop codon (5′-CTAAAGGATCCTATCACCGCTTACGCCGTTCATTCATTG-3′ [for V1] and 5′-CTAAAGGATCCTATCACCGCTTACGCCTTTCATGTATCA-3′ [for V2]). PCR amplifications (ca. 400 bp) were digested with NdeI and BamHI and inserted into NdeI/BamHI-digested pET3a expression vector (Novagen, Madison, Wis.). These pET3a constructs were designated pDP5 (encoding V1) and pDP4 (encoding V2). DiAg dimers were prepared as follows: pDi6 was first digested with NspV (Nippon gene, Osaka, Japan) to obtain fragments including V1 or V2 regions. To prepare V1V2 and V1V1, the fragment including V1 was ligated into NspV-digested pDP4 and pDP5, respectively. Similarly, the fragment including V2 was inserted into NspV-digested pDP5 and pDP4 to prepare V2V1 and V2V2, respectively. The nucleotide sequences of DiAg in these constructs were checked by DNA sequencing analysis. Soluble recombinant proteins were produced in Escherichia coli strain HMS174(DE3) (Novagen) for high-level expression and purified by using a Superdex 200 column (Pharmacia Biotech, Uppsala, Sweden). Recombinant control protein (rCont; N-terminal half [amino acid residues 1 to 67] of Ascaris NPA [ABA-1]) was prepared by the same procedure as rDiAgs (33). These recombinant proteins were passed through a column of polymyxin B-immobilized beads to remove contaminating endotoxins. The preparations used in the present study had a negligible endotoxin concentration (<2 pg of endotoxin/mg of protein) as measured by Endotoxin Test-D (Seikagaku, Tokyo, Japan). The N-terminal amino acid sequences of the recombinant proteins were checked by the peptide sequencing analysis, and whole amino acid sequences were deduced from nucleotide sequences (see Fig. 1A). The purity of rDiAgs (15.2 and 30.4 kDa) was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie brilliant blue and Western blotting (see Fig. 1B) with anti-DiAg rabbit serum (33) as described below.
FIG. 1.
(A) Alignment of rDiAg V1 and V2 amino acid sequences. Asterisks and dots designate amino acid identity and similarity, respectively. Gaps were introduced in sequences for optimal alignment. DiAg (D. immitis; GenBank accession no. D88757). (B) Map and expression of rDiAgs used to evaluate immune responses. Recombinant proteins were separated by SDS-14% PAGE and detected by staining with Coomassie brilliant blue and immunoblotting with rabbit anti-DiAg antiserum.
Protease digestion of DiAg dimer.
A total of 25 μg of DiAg dimers (in 25 μl of phosphate-buffered saline [PBS]) was digested with 1 μg of trypsin or proteinase K (in 1 μl of PBS; both from Sigma) for 15 or 30 min at 37°C. The reaction (25.1 μl) was stopped by heat treatment (100°C, 5 min).
Western blotting.
Immunoblotting was performed as previously described (33). In brief, intact and digested rDiAgs were separated by SDS-PAGE in a 14% gel (Tefco, Tokyo, Japan) under reducing conditions and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Bedford, United Kingdom). The protein blots were blocked with Block Ace (Dainihon Pharm, Osaka, Japan) and were then examined for DiAg by immunostaining with mouse (diluted 1:500; on each IgE peak) or rabbit (diluted 1:1,000) serum. The blots were developed by using horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG(H+L) (Jackson Immunoresearch Laboratories, West Grove, Pa.), HRP-conjugated goat anti-mouse IgE (diluted 1:2,000; GAM/IGE [Fc] PO; Nordic Immunological Laboratories B.V., Tilburg, The Netherlands), or goat anti-mouse IgG (diluted 1:2,000; Bethyl Laboratories, Inc., Montogomery, Tex.) and the POD immunostain set (Wako Pure Chemicals, Osaka, Japan).
Dot blotting.
One microgram of each DiAg was blotted onto nitrocellulose membrane (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). The protein blots were dried prior to treatment with Block Ace (Dainihon Pharm) for 30 min and dried completely. The membrane was treated with serum from mice or rabbits (diluted 1:200) for 1 h at 37°C. After being washed, the blots were incubated with HRP-conjugated anti-mouse IgG (diluted 1:750; Bethyl Laboratories) or anti-rabbit IgG (diluted 1:750; Jackson Immunoresearch Laboratories) for 1 h at 37°C. Bolts were developed by 4-chloro-1-naphthol substrate (Sigma; 0.4 mg/ml in 16% ethanol in PBS [pH 7.4] containing 0.015% hydrogen peroxide) for 5 min, and the colorimetric reaction was stopped with ice-cold distilled water.
Mice.
Female C57BL/6 and C3H/HeJ mice were purchased from Clea Japan (Tokyo, Japan). These mice were rested for a week after arrival at the facility and used in experiments at 8 to 12 weeks of age.
In vivo treatment.
The microosmotic pump (Alza 1002; Alza Co., Palo Alto, Calif.) filled with each rDiAg (100 μg/100 μl in PBS) was subcutaneously implanted in the dorsal flank of mice according to the manufacturer's instructions. rDiAg was released at a pumping rate of 0.25 μg/h for 14 days. After implantation, plasma and serum were collected weekly for up to 9 weeks. For some experiments, the IgG fraction in serum was depleted by using a protein A-IgG purification system (MAPSII; Bio-Rad, Richmond, Calf.) as previously described (33).
Assay for cell proliferation.
B cells were freshly prepared from the mesenteric lymph nodes (MLN) of naive mice as described previously (33). Briefly, MLN cells (pooled from four 8-week-old mice) were treated with the combination of rabbit anti-mouse Thy1.2 antibody and Low-Tox-M rabbit complement (both from Cedarlane Laboratories, Hornby, Ontario, Canada). Thy1.2+ cell-depleted fractions were labeled with rat anti-mouse B220 monoclonal antibody (MAb) conjugated with magnetic beads (RA3-6B2; Miltenyi Biotec, Auburn, Calif.), and B220+ cells were then purified by magnetic cell sorting (Miltenyi Biotec). The purity of cells was >95% B220+. MLN B cells (2 × 106/ml) were cultured with various concentrations of rDiAgs, rCont, hamster anti-mouse CD40 MAb (HM40-3; Pharmingen, San Diego, Calif.), or lipopolysaccharide (LPS; from E. coli serotype O55 B5; Sigma Chemical Co., St. Louis, Mo.) for 48 h in a total volume of 100 μl in 96-well flat-bottom culture plates (Corning, Cambridge, Mass.). Pretreatment of rDiAg with polymyxin B sulfate (10 μg/ml; Sigma) was performed at 37°C for 1 h, and the mixture was then applied to the cell cultures. Cell proliferation was measured by using a bromodeoxyuridine (BrdU) incorporation assay (BrdU enzyme-linked immunosorbent assay [ELISA]; Roche Diagnostics, Mannheim, Germany). Cell injury was determined by a lactate dehydrogenase (LDH) cytotoxicity test (Wako) according to the manufacturer's instructions.
Assay for immunoglobulin production.
Whole MLN cells and MLN B cells (2 × 106/ml) from naive mice were cultured with various rDiAgs (3.3 μg/ml), rCont (3.3 μg/ml), or anti-CD40 MAb (1 μg/ml) for 8 days in the absence or presence of IL-4 (200 U; Chemicon International, Inc., Temecula, Calif.) in a total volume of 100 μl in 96-well culture plates (Corning). Total and specific IgE levels in plasma (both untreated and IgG depleted) or culture supernatants were determined by using enzyme-linked ELISA as described previously (33). Total IgG2c and specific IgG levels were measured according to the manufacturer's instructions by ELISA kit (Bethyl Laboratories, Inc., Montgomery, Tex.).
Assay for cytokine production.
MLN cells (5 × 106/ml) or MLN B cells (2 × 106/ml) from naive mice were cultured with various rDiAgs (3.3 μg/ml) in the absence or presence of concanavalin A (ConA; 1 μg/ml; Sigma) for various periods in a total volume of 100 μl in 96-well culture plates (Corning), and culture supernatants were collected. IL-4, IL-10, and IFN-γ levels secreted in the supernatants were determined by using both the cytokine ELISA kit (Endogen, Woburn, Mass.) and the immunoassay kit (BioSource International, Camarillo, Calif.). For neutralization studies, rat anti-mouse IL-10 MAb (5 μg/ml; JES5-2A5; Genzyme-Techne, Cambridge, Mass.) or an IgG1 isotype-matched control MAb (5 μg/ml; R3-34; Pharmingen) were used.
Statistical analysis.
The statistical significance of the values obtained were evaluated by using the Student t test. P values <0.05 were considered significant.
RESULTS
DiAg monomers induce nonspecific IgE synthesis.
We confirmed that rDiAg monomers are classified into two types (V1 and V2) as reported previously (5, 25, 27, 28). The amino acid sequences between V1 and V2 were completely consistent in N-terminal half (amino acids 1 to 61, 100% identical) but dramatically differ in C-terminal half (amino acids 62 to 129, 56.9% identical and 19.4% similar) (Fig. 1A). We have previously found that DiAg (V1) preferentially induces nonspecific IgE production in BALB/c mice (33). To define the effect of V2, another DiAg monomer, on the IgE responses, C57BL/6 mice were treated with rDiAgs, and IgE levels in plasma from their mice were measured. As shown in our earlier study, V1-driven IgE production peaked 21 days after implantation (Fig. 2A). V2-induced IgE synthesis reached a peak at 14 days. At each single prominent peak, V2 induced much higher IgE production than did V1. Mice given PBS only produced <50 ng of IgE antibody/ml throughout the experimental period. Control protein (α-lactoalbumin [Sigma]), a molecule of similar size to the monomer, and rCont, a control recombinant protein prepared in the same procedure as rDiAg, had no effect on the IgE synthesis (data not shown). As expected, DiAg-specific IgE was not detected in plasma from monomer-treated mice throughout the experimental period (data not shown). These results indicate that both DiAg monomers have the ability to induce nonspecific IgE synthesis, but the IgE-inducing activity differs distinctly between V1 and V2.
FIG. 2.
Time course of immunoglobulin synthesis in rDiAg-treated mice. Mice were administrated several types of DiAg, plasmas were collected weekly. Total IgE (A to C) and IgG2c (D) levels were quantitated by ELISA. The data represent the mean values and standard deviations (error bars) for five mice.
Effect of DiAg dimer on IgE synthesis.
It has been shown that ES products from D. immitis adult worms contain not only monomer but also dimer of DiAg (5, 27). However, there has been no report that the NPA dimer has physiological and immunological properties. To define whether the DiAg dimer has the ability to induce IgE synthesis, mice were treated with V1V2 or V2V1. Interestingly, dimers elicited IgE responses with two peaks for up to 63 days (Fig. 2B). Two peaks induced by V1V2 were detected 21 and 42 days after implantation. In contrast, V2V1-driven IgE production peaked at 14 and 35 days. Absolute amounts of IgE were lower in dimer treatment than in monomer treatment. No control protein (flavoprotein [Wako]) of that molecular size is comparable to the size of the dimer induced the IgE synthesis (data not shown). These results indicate that monomers rather than dimers have the ability to trigger IgE responses.
Effect of mutagenically modified DiAg homodimers on IgE synthesis.
From these results, we hypothesized that the position of V1 or V2 on the dimer is responsible for the difference in dimer-driven IgE responses. To define this, we prepared two homodimeric mutant proteins, V1V1 and V2V2. As expected, mice given homodimers also showed biphasic IgE responses. V1V1 induced first minor (on day 21) and second major (on day 42) peaks of IgE, whereas V2V2 elicited first major (on day 14) and second minor peaks (on day 35) (Fig. 2C). Although IgE level at the first peak was significantly higher in V2V2 treatment than in V1V1 treatment, V1V1-treated mice produced IgE larger than did V2V2 at the second peak. The timing of peak responses induced with V1V1 and V2V2 were similar to those observed with V1V2 and V2V1, respectively. Taken together, these results indicate that V2Vx causes an earlier increase in IgE synthesis than does V1Vx and suggest that the kinetics of IgE synthesis induced with dimers are influenced by the nature of N-terminal unit on the dimer.
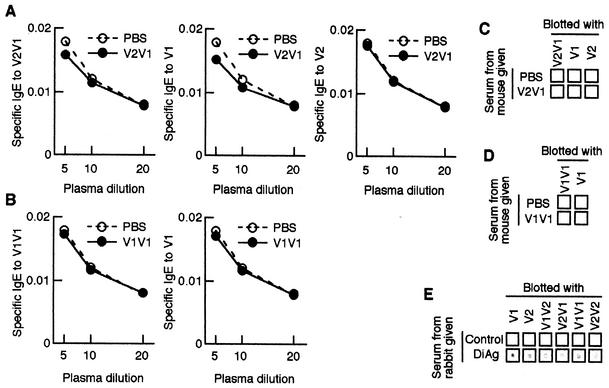
Like monomers, dimer-specific IgE was not detected in the first peaks induced with any dimers (data not shown). We examined by using a general ELISA system the question of whether specific IgE antibody is contained in the dimer (V2V1 [as a heterodimer] or V1V1 [as a homodimer])-induced second peaks. Dimer-specific IgE was not detected in plasma from mice given the same dimer (Fig. 3A and B). We next tested whether dimer-treated mice produce specific IgE antibody against each monomer that composes dimer molecule. Monomer-specific IgE levels were less in dimer-treated mice than in control mice (Fig. 3A and B).
FIG. 3.
rDiAg dimers do not induce specific IgE antibody. Mice were administered rDiAg dimers (V2V1 or V1V1), and plasma samples were collected weekly. At second peaks (V2V1 [on day 35] and V1V1 [on day 42]), specific IgE antibodies to dimer (V2V1 and V1V1) and monomer (V1 and V2) were detected by ELISA (A and B) and dot blot analysis (C to E). rDiAg-blotted membranes were treated with serum from mice or rabbits and then reacted with HRP-conjugated anti-mouse IgG (C and E) or anti-rabbit IgG (E). The data represent the mean values for five mice (A and B). The data represent one typical experiment out of five (C to E).
It is possible that, since the binding of specific IgE to rDiAg immobilized on ELISA plate is competitively inhibited by large amounts of specific other isotype antibodies to rDiAg (blocking antibodies), the specific IgE is not detected. To assess this, we first performed dot blot analysis for rDiAg-specific IgG. rDiAg-specific IgG was never detected (Fig. 3C and D). Also, the specific IgG was not detected by ELISA and Western blot analysis (data not shown). To further clarify this, each serum was incubated with protein A-immobilized Sepharose beads to deplete IgG fractions, and IgE levels were measured by ELISA. As expected, specific IgE was not detected after depletion of IgG (data not shown). Similar results were obtained in V1V2- or V2V2-treated mice (data not shown). These results indicate that undetectable levels of specific IgE are not responsible for the presence of blocking antibodies and suggest that specific antibodies to DiAg are not present in plasma or serum from mice given DiAg.
DiAgs do not induce IgG2c synthesis.
We next determined the levels of IgG2c, a Th1-associated immunoglobulin isotype (23), in rDiAg-treated mice. IgG2c levels were lower in DiAg-treated mice than in PBS-treated control mice throughout the experimental period (Fig. 2D). Control proteins also failed to promote IgG2c synthesis (data not shown). These results indicate that DiAgs, including both monomers and dimers, preferentially prime nonspecific IgE synthesis.
DiAgs induce B-cell activation.
We have previously shown that DiAg (V1) acts as a mitogen of murine B cells (33). To determine whether other DiAgs have the mitogenic activity, MLN B cells from naive mice were cultured with each rDiAg for 48 h. Titration of the filarial proteins showed that an effective concentration was 3.3 μg/ml (data not shown). B-cell proliferation by V2 was slightly higher than that by V1 and was nearly comparable to anti-CD40 MAb (1 μg/ml) and LPS (2 μg/ml) (Fig. 4A). In contrast, dimers showed less B-cell mitogenicity than do monomers. As expected, rDiAgs failed to induce the proliferation of T cells and macrophages from MLN (data not shown).
FIG. 4.
rDiAgs induce B-cell activation. MLN B cells (2 × 106/ml) from naive C57BL/6 (A and C) and C3H/HeJ (B) mice were cultured with each rDiAg (3.3 μg/ml), rCont (3.3 μg/ml), anti-CD40 MAb (1 μg/ml), or LPS (2 μg/ml) for 48 h. Cell proliferation (A and B) and IL-10 production (C) were measured by the BrdU incorporation assay and ELISA, respectively. The data represent the mean values and standard deviations (error bars) from five experiments. ∗∗ and ∗, P < 0.01 and P < 0.05, respectively, compared to the use of medium alone.
To exclude the possibility that the activities of rDiAgs may be mediated by the contamination of endotoxins or CpG nucleotides (known inducers of B-cell expansion), B cells were cultured with each rDiAg plus polymyxin B, an inhibitor of LPS, or B cells were cultured with rCont prepared in the same way as the rDiAgs. B-cell proliferative responses induced with rDiAgs were not prevented by polymyxin B (Fig. 4A), and rDiAgs normally expanded B cells from LPS-hyporesponsive C3H/HeJ mice (Fig. 4B). In addition, rCont failed to induce B-cell expansion (Fig. 4A and B). These results suggest that monomers are more efficient as a B-cell mitogen than dimers and indicate that LPS or CpG contamination is not responsible for the proliferative response.
Previously, we have reported that DiAg (V1) elicits the producton of IL-10, a Th2-type cytokine, by B cells (33). We next measured DiAg-induced IL-10 production by MLN B cells. All rDiAgs elicited IL-10 production as early as 12 h after stimulation, and these levels drastically increased for up to 48 h (Fig. 4C). Similar to the B-cell expansion, the IL-10 level was higher in monomer treatments than in dimer treatments. These results indicate that the IL-10-stimulatory activity of DiAg is concomitant with a B-cell proliferative response.
DiAgs elicit IL-4 production.
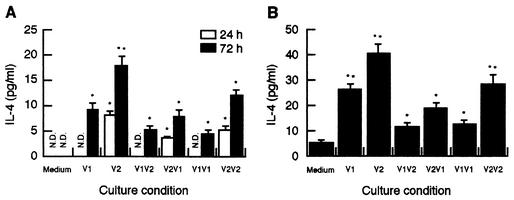
In filarial nematode-infected mice, IL-4 produced promptly after infection is known to play a crucial role in a Th2-dominant immune response (3). To further define the basis for the preferential Th2-type response bias observed with DiAg, the ability of DiAgs to influence the early cytokine profile was evaluated in MLN cells from naive mice. At 24 h after treatment with rDiAgs, the rapid production of IL-4 was detected in V2-, V2V1-, and V2V2-treated MLN cells, whereas V1, V1V2, and V1V1 failed to elicit IL-4 production (Fig. 5A). IL-4 production by V1, V1V2, and V1V1 was observed 72 h after stimulation but at low levels compared to IL-4 production by V2, V2V1, and V2V2, respectively. Similarly, rDiAgs strikingly enhanced IL-4 production by MLN cells stimulated with ConA, a polyclonal stimulus (Fig. 5B). These results indicate that V2 or V2Vx induce IL-4 more strongly than do V1 or V1Vx and suggest that the timing of IgE class switching may be dependent on the IL-4-inducing capacity of DiAg.
FIG. 5.
rDiAgs upregulate IL-4 production. Whole MLN cells (5 × 106/ml) from naive mice were cultured with each rDiAg (3.3 μg/ml) in the absence (A) or presence (B) of ConA (1 μg/ml) for 24 (A) or 72 h (A and B). IL-4 levels in the culture supernatants were quantitated by ELISA. The data represent the mean values and standard deviations (error bars) from five experiments. ∗∗ and ∗, P < 0.01 and P < 0.05, respectively, compared to the use of medium alone.
DiAgs prevent IFN-γ production.
The signature Th1-type cytokine IFN-γ has been shown to inhibit IgE synthesis (2, 24). We investigated the effect of DiAgs on IFN-γ production by MLN cells. As expected, rDiAgs induced decreased IFN-γ levels compared to unstimulated control cells; however, the prevention of spontaneous IFN-γ production by MLN cells indicated no significant differences among rDiAgs (Fig. 6A). To further address the inhibitory effect of DiAgs on IFN-γ synthesis, MLN cells were cultured with various rDiAgs in the presence of ConA. Monomers were even more effective than dimers at inhibiting ConA-driven ongoing IFN-γ production by their cells (Fig. 6B). In a comparison of monomers, V2 inhibited the IFN-γ production more strongly than did V1. These inhibitory effects were not due to necrotic cell death as measured by the release of LDH from the cells (Fig. 6C and D). These results suggest that DiAg monomers have a potent inhibitory effect on IFN-γ synthesis and predominantly induce the development of Th2-type cells.
FIG. 6.
rDiAgs downregulate IFN-γ production. Whole MLN cells (5 × 106/ml) from naive mice were cultured with each rDiAg (3.3 μg/ml) in the absence (A and C) or presence (B and D) of ConA (1 μg/ml) for 72 h. IFN-γ levels in the culture supernatants were quantitated by ELISA (A and B). The data represent the mean values and standard deviations (error bars) from five experiments. ∗∗ and ∗, P < 0.01 and P < 0.05, respectively, compared to the use of medium alone. Cell injury was estimated by LDH assay, and the data represent the relative ratio, which is equal to the mean optical density value of rDiAg-treated cells divided by the mean value of untreated cells and standard deviations (error bars) from five experiments (C and D).
DiAgs favor polyclonal IgE synthesis by MLN B cells.
We have previously shown that DiAg (V1) induces nonspecific polyclonal IgE production by splenic B cells in the presence of IL-4 in vitro (33). To assess whether absolute amounts of IgE at the first peak are associated with the magnitude of B-cell proliferative responses, MLN B cells were cultured with each DiAg in the absence or presence of IL-4 for 8 days. All rDiAgs tested induced IgE production by IL-4-stimulated MLN B cells (Table 1), and the IgE levels were in parallel with both cell expansion and IL-10 synthesis in B cells. The combination of anti-CD40 MAb and IL-4 induced a significant level of IgE (42.5 ± 3.6 ng/ml). rDiAg alone or IL-4 alone had no effect on IgE synthesis (data not shown). These results indicate that DiAgs synergize IL-4 in IgE class switching, suggesting that the amount of IgE is involved in the B-cell mitogenic and cytokine-stimulatory properties of DiAgs.
TABLE 1.
Effect of rDiAgs on IgE production by MLN B cells in vitroa
| Medium | Mean IgE production (ng/ml)b ± SD in:
|
||||||
|---|---|---|---|---|---|---|---|
| Medium | V1 | V2 | V1V2 | V2V1 | V1V1 | V2V2 | |
| Medium alone | 3.5 ± 0.2 | 24.6 ± 2.4* | 32.8 ± 3.6* | 10.2 ± 1.4* | 14.1 ± 2.1* | 10.7 ± 1.8* | 20.7 ± 1.5* |
| Control MAb | 3.0 ± 0.3 | 22.4 ± 2.4 | 30.7 ± 3.2 | 9.8 ± 0.8 | 13.1 ± 1.7 | 10.0 ± 1.2 | 19.7 ± 2.1 |
| αIL-10 MAb | 2.9 ± 0.4 | 16.5 ± 1.8** | 21.9 ± 2.6** | 6.8 ± 0.7** | 9.4 ± 1.1** | 7.2 ± 0.8** | 13.9 ± 1.5** |
Naive B cells (2 × 106/ml) were cultured with the combination of each rDiAg (3.3 μg/ml) plus IL-4 (200 U/ml) in the absence or presence of anti-IL-10 MAb or isotype-matched control MAb (5 μg/ml) for 8 days. IgE levels in culture supernatants were measured by ELISA. The mean and standard deviations are from five experiments.
Statistical significance: *, P < 0.05 compared to medium alone; **, P < 0.05 compared to control MAb.
Effect of IL-10 on DiAg-driven cell responses.
It is possible that DiAgs upregulate Th2-type immune responses via early IL-10 secreted by B cells. To define the role of early IL-10 production by B cells on DiAg-induced immune responses, we cultured MLN cells with a neutralizing anti-IL-10 MAb or isotype control MAb in the presence of each DiAg. The production of IL-4 and IgE was partly prevented by the depletion of IL-10 (Fig. 7A and Table 1). In contrast, treatment of MLN cells with anti-IL-10 MAb restored the production of IFN-γ (Fig. 7B), and the effect was greater in monomer treatments than in dimer treatments. The anti-IL-10 MAb did not affect IgG2c production (Table 2). Together, these data imply that Th2-type immune responses induced by DiAgs are due to the downregulation of IFN-γ (rather than to the upregulation of IL-4) by early IL-10 from B cells.
FIG. 7.
Effect of endogenous IL-10 on DiAg-induced immnue responses. MLN cells were cultured with anti-IL-10 MAb or isotype-mached control MAb (5 μg/ml) in the presence of each rDiAg (3.3 μg/ml) for 72 h. IL-4 (A) and IFN-γ (B) levels were measured by ELISA. The data represent the mean values and standard deviations (error bars) from five experiments. ∗∗ and ∗, P < 0.01 and P < 0.05, respectively, compared to the use of control MAb.
TABLE 2.
Effect of rDiAgs on IgG2c production by MLN cells in vitroa
| Mean IgG2c production (ng/ml) ± SD in:
|
|||||||
|---|---|---|---|---|---|---|---|
| Medium | V1 | V2 | V1V2 | V2V1 | V1V1 | V2V2 | |
| Control MAb | 4.1 ± 0.8 | 3.5 ± 0.2 | 3.3 ± 0.4 | 3.7 ± 0.4 | 3.6 ± 0.6 | 3.8 ± 0.5 | 3.5 ± 0.7 |
| αIL-10 MAb | 4.3 ± 0.5 | 3.8 ± 0.4 | 3.9 ± 0.5 | 4.1 ± 0.6 | 3.9 ± 0.5 | 4.1 ± 0.5 | 3.9 ± 0.4 |
Naive MLN cells (2 × 106/ml) were cultured with each rDiAg (3.3 μg/ml) in the presence of anti-IL-10 MAb or isotype-matched control MAb (5 μg/ml) for 8 days. IgG2c levels in culture supernatants were measured by ELISA. The mean and standard deviations are from five experiments.
DiAg monomer is an efficient form for IgE synthesis.
We found here that only mice given DiAg dimer showed a biphasic pattern in IgE production. It has been shown that DiAg polyprotein precursor is readily digested up to stable monomer unit by treatment with proteinase K or trypsin (28). Therefore, it is possible that the second IgE peak seen in mice given DiAg dimer molecule is not due to dimer itself. To first clarify whether dimer is cleaved into monomer, V1V1 (which induces most marked IgE levels at second peak) was digested with trypsin, and the V1 in the digests was detected by Western blotting. As shown in Fig. 8A, when V1V1 was digested with trypsin for 15 min, a shift (from 30.4 to 15.2 kDa) in the size of the band was observed. The treatment for 30 min caused complete digestion of the dimer to generate protease-resistant monomer. To define whether monomer derived from dimer has the ability to induce IgE synthesis, MLN cells were cultured with each digest (containing V1 and/or V1V1) in the presence of IL-4 and polymyxin B for 8 days. Monomer derived from digested dimer induced higher IgE production by MLN cells than intact dimer (Fig. 8B). Heat-inactivated trypsin has no effect on the IgE synthesis (data not shown). Similar results were observed when all dimers used in the present study were digested with trypsin or proteinase K (data not shown). These results suggest that the second peak may be due to monomer derived from proteolytically cleaved dimer in vivo.
FIG. 8.
Effect of cleaved DiAg dimer on IgE synthesis. (A) A total of 25 μg of DiAg dimer (25 μl) was treated with 1 μg of native or heat-inactivated trypsin (1 μl) for 15 or 30 min. Immunoblot of trypsin-digested DiAg dimer (V1V1) probed with anti-DiAg antiserum. The data represent one typical experiment out of five. (B) MLN B cells were cultured with 10 μl of each trypsin-treated V1V1 in the presence of IL-4 (200 U/ml) and polymyxin B (10 μg/ml) for 8 days. IgE levels in culture supernatants were measured by ELISA. The data represent the relative ratio, which is equal to the mean value of rDiAg-treated cells divided by the mean value of untreated cells, and standard deviations (error bars) from five experiments. ∗∗ and ∗, P < 0.01 and P < 0.05, respectively, compared to the use of heat-inactivated trypsin.
DISCUSSION
D. immitis adult worms secrete a 15-kDa monomer and a 30-kDa dimer of NPA in their environment (5, 27). DiAg monomer, the dominant ES product of the worm, can be divided into two types based on the difference of sequence on C-terminal half. We have previously found that DiAg monomer (either native [including V1 and V2] or recombinant [V1]) has the ability to induce nonspecific IgE synthesis (10, 17, 18, 33, 40). However, little is known about biological activities of another monomer (V2) and dimers. In the present study, we investigated the role of these rDiAgs in the induction of Th2-type immune responses.
We here demonstrated that monomers can induce higher absolute amounts of IgE than dimers and that these rDiAgs fail to enhance IgG2c responses. V1- and V2-driven IgE responses showed monophasic patterns, which reached a peak at 21 and 14 days, respectively. These time scales are in agreement with the reported kinetics of nonspecific IgE production seen in the early phase (on days 14 to 21) of the gastrointestinal nematode Nippostrongylus brasiliensis infections (13). rDiAg monomers had more powerful B-cell mitogenic and IL-10-stimulatory activities than dimers. The magnitude of these B-cell responses was in parallel with nonspecific IgE levels in vivo (at the first peak) and in vitro. These results suggest that the active form of DiAg is a monomer rather than a dimer and support our previous finding that DiAg polyclonally stimulates B cells to produce nonspecific IgE antibody. The half-life of IgE antibody in the circulation is known to be as short as 12 h in mice (16). Noticeably, although the release of DiAg into mice is for up to 14 days (using the osmotic pump), moderately elevated levels of IgE persist for a long time, being in the region of 250 ng/ml 63 days after implantation. The finding is consistent with a report that IgE production induced by nematode infections is maintained for long periods even after the worm has been expelled (13). It has been shown that, in N. brasiliensis-infected rats, the long-standing IgE response is due to the generation of radioresistant long-lived IgE-forming cells (38). IL-10 is known to selectively promote IL-4 plus anti-CD40 MAb-driven polyclonal IgE synthesis and to maintain B-cell viability (11, 22). These findings suggest that parasitic nematode-derived products preferentially may cause polyclonal cell expansion and IgE class switching in B cells and that IL-10 appears to sustain the survival of IgE-secreting B cells in an autocrine or paracrine manner.
In general, parasitic nematode-derived products predominantly induce Th2-type cytokine synthesis (6, 15, 33, 35, 36, 39, 40). However, there is no report that a parasitic nematode-derived single molecule induces IL-4 production by naive immune cells in vitro. We have previously found that native DiAg (the mixture of V1 and V2) induces IL-4 production by human spleen cells in vitro (40). We found here that all rDiAgs used in the present study induced IL-4 production (within 72 h after stimulation) by murine MLN cells. However, IL-4 production was not observed when purified T cells or T-cell-depleted MLN cell fracctions were stimulated with rDiAg (data not shown), suggesting that DiAg indirectly acts on IL-4 production by T cells. This possibility is supported by a report that IL-4 production by CD4+ T cells is preferentially induced by mediating B cells rather than monocytes (32). IL-10 production by B cells was induced within 12 h after rDiAg stimulation. The inhibitory effect of DiAgs on the synthesis of IFN-γ was due to the early IL-10 production by B cells, whereas the blockade of IL-10 depressed the production of IL-4 and IgE. We have previously shown that, although DiAg directly induces the production of IL-12, an inducer of IFN-γ, by THP-1 cells, the production was significantly prevented by pretreatment with IL-10 (18). These findings indicate that DiAg prevents Th1-type immune responses via an IL-10-dependent mechanism, thereby upregulating Th2-type responses. rDiAg monomer induces nonspecific IgE synthesis in two genetic backgrounds: BALB/c (Th2-dominant strain) and C57BL/6 (Th1-dominant strain) mice (18, 33). In addition, diabetes (which is mediated by Th1 cells) in nonobese diabetic mice can be completely prevented by treatment with rDiAg monomer (17), indicating that the parasitic molecule can preferentially trigger a Th2-dominant response independent of the genetic background of hosts.
In contrast to monomers, DiAg dimers induced biphasic IgE patterns. The first peak of nonspecific IgE was detectable 14 to 21 days, and the second rise of IgE was 35 to 42 days after implantation. In each peak, specific IgE antibody was not detected. These observations raise the question of why only dimer can elicit two IgE peaks. Since a protease-sensitive linker (including Arg-Arg-Lys-Arg) is located near the center of the dimer molecule (28), one molecule of the dimer may generate two molecules of monomer by enzymatic digestion. D. immitis-derived precursor polyprotein has been known to be completely digested up to protease-resistant monomer units by treatment with proteinase K or trypsin (28) and to be spontaneously cleaved in a time-dependent manner, as measured by a metabolic labeling study (27). Furthermore, DiAg monomer is extremely stable in response to heat, acid-alkali, or periodate treatment other than enzymatic digestion (9). Indeed, we also found that monomer was generated from dimer treated with proteinase K or trypsin and that the monomer could induce IgE synthesis. These findings suggest that, in mice given the DiAg dimer, the first IgE peak is due to the dimer itself, whereas the second peak is induced by monomers derived from dimer cleaved with ubiquitous proteases. Therefore, we consider that long-standing IgE production may be due to the prolonged existence of stable DiAg monomers (from dimers) in vivo, as well as the generation of long-lived IgE-secreting B cells.
Another interesting finding of the present study was that the immunological properties of DiAgs are dependent on their structure. As described above, IgE synthesis and B-cell activation (including cell expansion and early IL-10 production) were higher with the monomer treatment than with the dimer treatment. When the monomers were compared, V2 was found to induce significantly higher levels of IL-4 and IgE than did V1. In comparisons between the dimers, the induction of IL-4 and IgE by V2Vx was significantly earlier and higher than that by V1Vx. From these results, we speculate that (i) monomer, but not dimer, appears to be an efficient form in the induction of absolute IgE levels, B-cell activation, and IL-4 synthesis; (ii) the differences of these activities between DiAg monomers may be dependent on the sequence of C-terminal half on the molecule; (iii) IL-4- and IgE-inducing activities of DiAg dimers seem to be influenced by the property of N-terminal unit on the dimer; and (iv) the bioactive moiety on monomer may be partly masked by dimerization.
Parasitic nematodes have adapted a variety of strategies for evading or modifying the host immune response. One of their strategies is the production of molecules that have homologies to host immunoregulatory molecules, such as cytokines (4, 7, 12, 26). We have recently shown that DiAg monomers functionally bind to CD40 (18, 34). Since DiAg dimers failed to induce cell expansion, IL-10 production, and IgE synthesis in B cells from CD40-deficient mice (data not shown), CD40 also appears to mediate DiAg dimer signaling. Indeed, although D. immitis secretes large quantities of DiAg, the molecule is not detected by serum from hosts infected naturally with the worm (1, 31), suggesting that the filarial molecule mimics a host molecule. Some parasitic nematodes possess B-cell mitogen-like molecules (14, 20, 37) and selective inducers of IL-10 (15, 36). Polyclonal activation (including nonspecific IgE synthesis) and anti-inflammatory cytokine production (e.g., IL-10) may be associated with an immunosuppression in terms of the prevention of specific responses (21, 29, 30). Interestingly, Th2-dominant immune responses are also considered to be associated with parasite longevity (41). Although such parasitic molecules may contribute to parasite persistence, they may also benefit the host by preventing harmful pathology mediated by excessive anti-parasite immune responses.
In conclusion, we found that DiAg is a polyclonal stimulus of B cells, as well as a selective inducer of Th2-type responses, and that the absolute amount and timing of IgE production at the first peak appear to be dependent on the pattern of B-cell activation and IL-4 production, respectively. DiAg monomer rather than dimer is an active form in the induction of these responses. In this respect, the behavior of the filarial molecule appears to be like a hormone that biologically active molecule generates by processing of inactive precursor. Thus, D. immitis may employ the system that can continuously secrete molecules, such as DiAg, to favor the survival of the organism in hosts.
Acknowledgments
We thank Makoto Owhashi (University of Tokushima, Tokushima, Japan) for the gift of pDi6 and helpful comments and Miki Takeishi (Urayasu Public Library, Chiba, Japan) for assistance with manuscript preparation.
Editor: J. M. Mansfield
REFERENCES
- 1.Akao, N., K. Kondo, and K. Fujita. 1991. Immunoblot analysis of Dirofilaria immitis recognized by infected humans. Ann. Trop. Med. Parasitol. 85:455-460. [DOI] [PubMed] [Google Scholar]
- 2.Bacharier, L. B., H. Jabara, and R. S. Geha. 1998. Molecular mechanisms of immunogloblin E regulation. Int. Arch. Allergy Immunol. 115:257-269. [DOI] [PubMed] [Google Scholar]
- 3.Balmer, P., and E. Devaney. 2002. NKT cells are a source of early interleukin-4 following infection with third-stage larvae of the filarial nematode Brugia pahangi. Infect. Immun. 70:2215-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuellar, C., M. J. Perteguer, and M. Rodero. 2001. Presence of IL-4-like molecules in larval exceretory-secretory and crude extracts from Anisakis simplex. Scand. J. Immunol. 53:483-488. [DOI] [PubMed] [Google Scholar]
- 5.Culpepper, J., R. B. Grieve, L. Friedman, M. Mika-Grieve, G. R. Frank, and B. Dale. 1992. Molecular characterization of a Dirofilaria immitis cDNA encoding a highly immunoreactive antigen. Mol. Biochem. Parasitol. 54:51-62. [DOI] [PubMed] [Google Scholar]
- 6.Ehigiator, H. N., A. W. Stadnyk, and T. D. G. Lee. 2000. Extract of Nippostrongylus brasiliensis stimulates polyclonal type-2 immunoglobulin response by inducing de novo class switch. Infect. Immun. 68:4913-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcone, F. H., A. G. Rossi, R. Sharkey, A. P. Brown, D. I. Pritchard, and R. M. Maizels. 2001. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infect. Immun. 69:4007-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 9.Fujita, K. T. Ikeda, and S. Tsukidate. 1979. Immunological and physicochemical properties of a highly purified allergen from Dirofilaria immitis. Int. Arch. Allergy Appl. Immun. 60:121-131. [DOI] [PubMed] [Google Scholar]
- 10.Furuhashi, Y., S. Imai, H. Tezuka, and K. Fujita. 2001. Recombinant Dirofilaria immitis-derived antigen can suppress passive cutaneous anaphylaxis reactions. Int. Arch. Allergy Immunol. 125:144-151. [DOI] [PubMed] [Google Scholar]
- 11.Go, N. F., B. E. Castle, R. Barrett, R. Kastelein, W. Dang, T. R. Mosmann, K. W. Moore, and M. Howard. 1990. Interleukin 10, a novel B-cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J. Exp. Med. 172:1625-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Escobar, W. F. Gregory, and R. M. Maizels. 2000. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor β, expressed in microfilarial and adult stages of Brugia malayi. Infect. Immun. 63:6402-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarrett, E. E. E., and H. R. P. Miller. 1982. Production and activities of IgE in helminth infection, p. 178-233. In K. Ishizaka, P. Kallos, B. H. Waksman, and A. L. de Weck (ed.), Progress in allergy, vol. 31. Karger, Basel, Switzerland. [PubMed]
- 14.Harnett, W., M. R. Deehan, K. M. Houston, and M. M. Harnett. 1999. Immunomoduratory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 21:601-608. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann. S., B. Kyewski, B. Sonnenburg, and R. Lucius. 1997. A filarial cysteine protease inhibitor downregulates T-cell proliferation and enhances interleukin-10 production. Eur. J. Immunol. 27:2253-2260. [DOI] [PubMed] [Google Scholar]
- 16.Hirano, T., C. Hom., and Z. Ovary. 1983. Half-life of murine IgE antibodies in the mouse. Int. Arch. Allergy Immunol. 71:182-184. [DOI] [PubMed] [Google Scholar]
- 17.Imai, S., H. Tezuka, and K. Fujita. 2001. A factor of inducing IgE from a filarial parasite prevents insulin-dependent diabetes mellitus in nonobese diabetic mice. Biochem. Biophys. Res. Commun. 286:1051-1058. [DOI] [PubMed] [Google Scholar]
- 18.Imai, S., H. Tezuka, R. Muto, Y. Furuhashi, and K. Fujita. 2001. A factor of inducing IgE from a filarial parasite is an agonist of human CD40. J. Biol. Chem. 276:46118-46124. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy, M. W. 2000. The nematode polyprotein allergens/antigens. Parasitol. Today 16:373-380. [DOI] [PubMed] [Google Scholar]
- 20.Lee, T. D. G., and C. Y. Xie. 1995. IgE regulation by nematodes: the fluid of Ascaris contains a B-cell mitogen. J. Allergy Clin. Immunol. 95:1246-1254. [DOI] [PubMed] [Google Scholar]
- 21.Maizels, R. M., D. A. P. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 22.Marcelletti, J. F., and D. H. Katz. 1996. IL-10 stimulates murine antigen-deriven antibody responses in vitro by regulating helper cell subset participation. Cell. Immunol. 167:86-98. [DOI] [PubMed] [Google Scholar]
- 23.Martin, R. M., J. L. Brady, and A. M. Lew. 1998. The need for IgG2c antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187-192. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2, and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 25.Owhashi, M., S. Futaki, K. Kitagawa, Y. Horii, H. Maruyama, H. Hayashi, and Y. Nawa. 1993. Molecular cloning and characterization of a novel neutrophil chemotactic factor from filarial parasite. Mol. Immunol. 30:1315-1320. [DOI] [PubMed] [Google Scholar]
- 26.Pastrana, D. V., N. Raghavan, P. Fitzgerald, S. W. Eisinger, C. metz, R. Bucala, R. P. Schleimer, C. Bickel, and A. L. Scott. 1998. Filarial nematode parasites secrete a homologue of the human cytokine migration inhibitory factor. Infect. Immun. 66:5955-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole, C. B., A. G. Grandea III, C. V. Maina, R. E. Jenkins, M. E. Selkirk, and L. A. McReynolds. 1992. Cloning of a cuticular antigen that contains multiple tandem repeats from filarial parasite Dirofilaria immitis. Proc. Natl. Acad. Sci. USA 89:5886-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole, C. B., L. J. Hornstra, J. S. Benner, J. R. Fink, and L. A. McReynolds. 1996. Carboxyl-terminal sequence divergence and processing of the polyprotein antigen from Dirofilaria immitis. Mol. Biochem. Parasitol. 82:51-65. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard, D. I. 1993. Immunity to helminths: is too much IgE parasite- rather than host-protective? Parasite Immunol. 15:5-9. [DOI] [PubMed] [Google Scholar]
- 30.Reina-San-Martin, B., A. Cosson, and P. Minoprio. 2000. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol. Today 16:62-67. [DOI] [PubMed] [Google Scholar]
- 31.Scott, A. L., C. Diala, D. A. Moraga, S. Ibrahim, L. Redding, and W. K. Tamashiro. 1988. Dirofilaria immitis: biochemical and immunological characterization of the surface antigens from adult parasites. Exp. Parasitol. 67:307-323. [DOI] [PubMed] [Google Scholar]
- 32.Secrist, H., R. H. DeKruyff, and D. T. Umetsu. 1995. Interleukin 4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J. Exp. Med. 181:1081-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tezuka, H., S. Imai, R. Muto, Y. Furuhashi, and K. Fujita. 2002. Recombinant Dirofilaria immitis polyprotein that stimulates B cells to produce nonspecific polyclonal immunoglobulin E antibody. Infect. Immun. 70:1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tezuka, H., S. Imai, S. Tsukidate, and K. Fujita. 2002. A Dirofilaria immitis polyprotein upregulates nitric oxide production. Infect. Immun. 70:5283-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchikawa, R., S. Matsuda, and N. Arizono. 2000. Suppression of gamma interferon transcription and production by nematode exceretory-secretory antigen during polyclonal stimulation of rat lymph node T cells. Infect. Immun. 68:6233-6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velupillai, P., W. E. Secor, A. M. Horaut, and D. A. Harn. 1997. B-1 cell (CD5+ B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J. Immunol. 158:338-344. [PubMed] [Google Scholar]
- 37.Wang, M. Q., H. J. Jiang, H. Inoue, M. Myozaki, and U. Yamashita. 1995. B-cell mitogenic activity of Toxocara canis adult worm antigens. Parasite Immunol. 17:609-615. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe, N., and A. Kobayashi. 1989. Nippostrongylus brasiliensis: radioresistant IgE antibody-forming cells in infected rats. Exp. Parasitol. 68:216-222. [DOI] [PubMed] [Google Scholar]
- 39.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453-6460. [DOI] [PubMed] [Google Scholar]
- 40.Yamaoka, K. A., J.-P. Kolb, N. Miyasaka, G. Inuo, and K. Fujita. 1994. Purified exceretory-secretory component of filarial parasite enhances FcɛRII/CD23 expression on human splenic B and T cells and type 2-related cytokine generation from T cells. Immunology 81:507-512. [PMC free article] [PubMed] [Google Scholar]
- 41.Yazdanbakhsh, M., W. A. Paxton, Y. C. Kruize, E. Sartono, A. Kurniawan, A. van het Wout, M. E. Selkirk, F. Partono, and R. M. Maizels. 1993. T-cell responsiveness correlates differentially with antibody isotype levels in clinical and asymptomatic filariasis. J. Infect. Dis. 167:925-931. [DOI] [PubMed] [Google Scholar]