Abstract
In this study, we compared Escherichia coli isolates from chickens with avian cellulitis with those from feces of healthy chickens. Cellulitis-derived strains presented phenotypic and genotypic characteristics of greater virulence than did the fecal isolates. Phylogenetic analysis by repetitive extragenic palindromic-PCR showed that, in agreement with their virulence characteristics, the cellulitis isolates form two clonal groups distinct from the fecal isolates.
Escherichia coli causes a variety of diseases in poultry, including respiratory tract infection, omphalitis, swollen-head syndrome, enteritis, septicemia, and cellulitis (3, 7), and these diseases are responsible for major economic losses in the chicken industry. Cellulitis lesions cause carcass downgrading and condemnation losses estimated at $40 million annually (7) in the United States. In Brazil, cellulitis is responsible for 45.2% of the broiler carcasses condemned for skin lesions (2), and the economic losses are estimated at $10 million annually.
Some clones of E. coli may be more effective in causing cellulitis, since experimental inoculation of isolates from cellulitis lesions reproduced this disease with a significantly greater frequency (100%) than did inoculation of isolates from airsacculitis lesions (42%) or inoculation of fecal isolates (8%) (9). However, E. coli strains isolated from cellulitis lesions expressed many virulence-associated factors similar to those presented by strains isolated from other colibacillosis lesions and from feces (6, 8), which shows that the expression of these virulence factors by themselves cannot explain the differences in pathogenicity presented by these isolates.
Since avian colibacillosis in its different forms occurs worldwide, we can gain a better understanding of its pathogenesis by a phylogenetic analysis of the clonal relations among E. coli isolates in several regions and in different countries. In this study, we used phenotypic and genotypic methods to examine the presence of virulence factors in E. coli isolates obtained in Southern Brazil from broiler chickens and determined by repetitive extragenic palindromic (REP)-PCR the genetic relationship among these isolates and avian fecal isolates.
Fifty-two broiler chickens presenting cellulitis were collected from 52 different flocks in Southern Brazil, and from each animal one E. coli strain was isolated from pure culture and maintained by standard procedures. Twelve E. coli strains were obtained from the feces of healthy chickens. Isolates were grown on brain heart infusion agar (Difco) for 18 h at 37°C for phenotypic or genotypic analysis.
The following phenotypic properties of the E. coli isolates were evaluated by standard methods (1, 9, 11): antibiotic resistance, pathogenicity to 1-day-old chickens, motility, ability to experimentally reproduce cellulitis (applied to 20 isolates), production of hemolysins, presence of K1 capsule, hemagglutination, production of aerobactin, resistance to chicken serum, and production of cytotoxins to Vero cells. All isolates were examined by PCR (4) for the presence of genes related to these phenotypes and for the presence of DNA sequences related to serum resistance (iss and traT).
The E. coli isolates from cellulitis lesions presented some distinctive phenotypic and genotypic characteristics. Thus, none of the fecal isolates presented resistance to chicken serum, whereas 63% of the cellulitis isolates were resistant; correspondingly, DNA sequences related to the iss locus associated with serum resistance were not found in fecal isolates but were found in 83% of the cellulitis isolates. None of the fecal isolates produced aerobactin or carried the iutA gene responsible for the aerobactin receptor, whereas 43 (83%) isolates from cellulitis lesions produced aerobactin and 48 (92%) carried the iutA gene. Most (88%) of the cellulitis isolates were motile, whereas only one (8%) of the fecal isolates was motile. Furthermore, most of the cellulitis isolates were pathogenic (67% of these isolates killed ≥90% of the 1-day-old chickens tested), whereas the fecal isolates were either apathogenic or presented low pathogenicity. The cellulitis isolates also presented a greater capability to reproduce cellulitis than did the fecal isolates (P < 0.05; n = 20). The main results are shown in Table 1.
TABLE 1.
Virulence factors in E. coli isolates from chickens with cellulitis and from feces of healthy chickens
| Virulence factor or gene detected by PCR | No. (%) of isolates with factor or gene from:
|
|
|---|---|---|
| Healthy chickens (n = 12) | Chickens with cellulitis (n = 52) | |
| Colicin | 5 (42)a | 44 (85) |
| Colicin V | 3 (25) | 25 (48) |
| Serum resistance | 0 (0)a | 33 (63) |
| Motility | 1 (8)a | 46 (88) |
| Aerobactin | 0 (0)a | 43 (83) |
| Mannose-sensitive hemagglutination | 3 (25)a | 34 (65) |
| Toxin Stx1 (stx1) | 0 (0) | 3 (6) |
| Hemaglutinin Tsh (tsh) | 0 (0) | 10 (19) |
| Type 1 pili (fimH) | 11 (92) | 48 (92) |
| Fimbria P (papC and papG) | 0 (0) | 10 (19) |
| Fimbria F11 (felA) | 0 (0) | 10 (19) |
| Aerobactin receptor (iutA) | 0 (0)a | 48 (92) |
| Colicin V (cvaC) | 4 (33) | 25 (48) |
| TraT (traT) | 11 (92)a | 31 (60) |
| Capsule K1 or K5 (kpsII) | 0 (0)a | 16 (31) |
| Serum resistance (iss) | 0 (0)a | 43 (83) |
Significantly different from value obtained with isolates from chickens with cellulitis (P ≤ 0.05, Fisher's exact probability test).
Overall, the E. coli isolates from cellulitis lesions and from feces shared some phenotypic properties, such as antibiotic resistance, type 1 pili, colicin V production, and the presence of the TraT protein, and analysis by PCR confirmed that both kinds of isolates frequently carried the genes fimH, cvaC, and traT, in agreement with the results of previous studies (6, 8). Thus, both kinds of isolates presented high levels of resistance (57.4 to 98.4%) to nitrofurantoin, sulfamethoxazole, tetracycline, streptomycin, and nalidixic acid, although the cellulitis-derived isolates presented significantly greater (P < 0.05) resistance to quinolone and to nalidixic acid than did the fecal isolates. Gene fimH, which encodes type 1 pili, was very often detected in both kinds of isolates, but the corresponding phenotype of mannose-sensitive hemagglutination was significantly less often detected among the fecal isolates. This can probably be attributed either to an insufficiency of the growth conditions for the expression of type 1 pili or to in vitro selection of mutants that do not express those pili. Similarly, none of the isolates presented K1 capsule, as indicated by a specific bacteriophage assay, but 16 (31%) of the cellulitis-derived isolates carried the kpsII gene, which can also be attributed either to an insufficiency of the growth conditions for the expression of K1 capsule or to the in vitro selection of nonencapsulated mutants due to the instability of the genes encoding K1 encapsulation. Both kinds of isolates did not express some putative virulence factors, such as hemolysins and cytotoxins, and the genes for these virulence factors were not detected in the isolates; these negative data are important because they further delimitate the virulence factors that might be characteristic of E. coli strains that cause cellulitis. These data show, therefore, that the virulence-associated factors common to both kinds of isolates cannot be responsible for the ability of some E. coli strains to cause cellulitis, although they still might be required for infection.
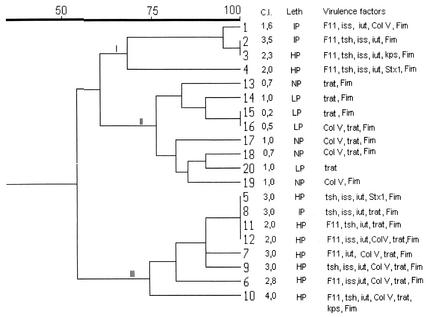
Fingerprinting with primer pair REP-PCR (5) generated distinct amplification bands ranging in size from 100 bp to 2.0 kb, and the patterns of bands were reproduced in independent experiments performed on three different days. The 20 selected isolates gave 15 different patterns, ranging from 4 to 12 bands, by REP-PCR. Phylogenetic analysis and clustering were conducted with simple matching coefficients, which were calculated by the unweighted pair group method, with arithmetic averages generating a dendrogram by the sequential, agglomerative, hierarchical, and nested clustering methods (NTSYS-PC program, version 1.7; Applied Biostatistics, Inc., Setauket, N.Y.). The strains were grouped into three clusters (I, II, and III) with 60% similarity (Fig. 1). Clusters I and III included cellulitis-derived isolates that presented high or intermediate pathogenicity and a high cellulitis index (1.6 to 4.0) and possessed F11 fimbriae, Tsh hemagglutinin, aerobactin production, and serum resistance. The majority of strains from cluster III were highly virulent and presented the highest cellulitis index. In contrast, cluster II comprised fecal isolates that were nonpathogenic or presented low pathogenicity, that presented a cellulitis index from 0.2 to 1.0, and that carried genes which are commonly present in commensal, clinical, avian, and nonavian isolates (fimH, traT, and cvaC) but did not carry any of the other putative virulence genes harbored by strains from clusters I and III (iutA, iss, tsh, felA, and kpsII). While genes felA and tsh were not detected in any of the fecal isolates, they were detected in 19% of the cellulitis isolates, but this difference was not statistically significant and probably can be attributed to insufficient sample size. These genes cannot be ruled out as virulence factors, however, for avian pathogenic E. coli strains harbor multiple virulence traits and can possibly exploit several alternative paths to colonize and invade their hosts. These results show, therefore, that strains with similar pathogenicities possessed similar patterns of virulence traits and suggest that cellulitis-derived E. coli isolates belong to specific clones showing association among some or all of the genes iutA, iss, tsh, felA, and kpsII.
FIG. 1.
Dendrogram of E. coli isolates from chickens with cellulitis (strains 1 to 12) and from feces of healthy chickens (strains 13 to 20) based on simple matching similarity coefficients, calculated from REP-PCR analysis data. The virulence factors, lethality (Leth), and cellulitis index (C.I.) of each isolate are also shown. NP, not pathogenic; LP, low pathogenicity; IP, intermediate pathogenicity; HP, high pathogenicity.
Some strains from clusters I and III showed 100% similarity, although they were isolated from different farms, which is in contrast to previous data showing that endemic clones occurred only in the same farm (10). The results of this study show, therefore, that cellulitis is caused by pathogenic clones of E. coli which possess a characteristic set of virulence factors and that some of these virulent clones may be endemically disseminated in a region.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Editor: V. J. DiRita
REFERENCES
- 1.Delicato, E. R., B. G. de Brito, A. P. Konopatzki, L. C. J. Gaziri, and M. C. Vidotto. 2002. Occurrence of the temperature-sensitive hemagglutinin (Tsh) among avian Escherichia coli. Avian Dis. 46:594-601. [DOI] [PubMed] [Google Scholar]
- 2.Fallavena, L. C. B., H. L. S. Moraes, C. T. P. Salle, A. B. da Silva, R. S. Vargas, V. P. do Nascimento, and C. W. Canal. 2000. Diagnosis of skin lesions in condemned or downgraded broiler carcasses—amicroscopic and macroscopic study. Avian Pathol. 29:557-562. [DOI] [PubMed] [Google Scholar]
- 3.Gross, W. B. 1994. Diseases due to Escherichia coli in poultry, p. 237-259. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Wallingford, United Kingdom.
- 4.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 5.Moura, A. C., K. Irino, and M. C. Vidotto. 2001. Genetic variability of avian Escherichia coli strains evaluated by enterobacterial repetitive intergenic consensus and repetitive extragenic palindromic polymerase chain reaction. Avian Dis. 45:173-181. [PubMed] [Google Scholar]
- 6.Ngeleka, M., J. K. Kwaga, D. G. White, T. S. Whittam, C. Riddell, R. Goodhope, A. A. Potter, and B. Allan. 1996. Escherichia coli cellulitis in broiler chickens: clonal relationships among strains and analysis of virulence-associated factors of isolates from diseased birds. Infect. Immun. 64:3118-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton, R. A. 1997. Avian cellulitis. World's Poult. Sci. J. 53:337-349. [Google Scholar]
- 8.Peighambari, S. M., J. P. Vaillancourt, R. A. Wilson, and C. L. Gyles. 1995. Characteristics of Escherichia coli isolates from avian cellulitis. Avian Dis. 39:116-124. [PubMed] [Google Scholar]
- 9.Peighambari, S. M., R. J. Julian, J. P. Vaillancourt, and C. L. Gyles. 1995. Escherichia coli cellulitis: experimental infections in broiler chickens. Avian Dis. 39:125-134. [PubMed] [Google Scholar]
- 10.Singer, R. S., J. S. Jeffrey, T. E. Carpenter, C. L. Cooke, E. R. Atwill, W. O. Johnson, and D. C. Hirsh. 2000. Persistence of cellulitis-associated Escherichia coli DNA fingerprints in successive broiler chicken flocks. Vet. Microbiol. 75:59-71. [DOI] [PubMed] [Google Scholar]
- 11.Vidotto, M. C., E. E. Müller, J. C. de Freitas, A. A. Alfieri, I. G. Guimarães, and D. S. Santos. 1990. Virulence factors of avian Escherichia coli. Avian Dis. 34:531-538. [PubMed] [Google Scholar]