Abstract
Gender-specific and parity-dependent acquired antibody recognition is characteristic of variant surface antigens (VSA) expressed by chondroitin sulfate A (CSA)-adherent Plasmodium falciparum involved in pregnancy-associated malaria (PAM). However, antibody recognition of recombinant products of a specific VSA gene (2O2var1) implicated in PAM and transcribed by a CSA-adhering parasite line did not have these characteristics. Furthermore, we could not demonstrate preferential transcription of 2O2var1 in the CSA-adhering line versus the unselected, parental isolate. Our data call for circumspection regarding the molecular identity of the parasite ligand mediating adhesion to CSA in PAM.
Individuals living in areas with high-intensity transmission of Plasmodium falciparum parasites acquire protective immunity against malaria during childhood, and severe clinical disease is consequently rare among adults. An increasing body of evidence suggests that antibodies specific for parasite-encoded variant surface antigens (VSA) on the surface of the infected erythrocytes (IE) mediate this protection. An important function of VSA is to mediate IE adhesion to specific host receptors, and the ability of parasites to switch between different VSA with different receptor specificities, thus enabling IE to sequester themselves in various tissues and avoid splenic clearance, is considered a central element in the parasites' attempt to evade host immunity (reviewed in references 5 and 15).
Pregnant women constitute a striking exception to the rule that malaria is mainly a childhood disease in areas where it is highly endemic, as previously clinically immune women become highly susceptible to pregnancy-associated malaria (PAM) when they become pregnant for the first time (3). Together with the fact that women become less and less susceptible to PAM with increasing parity in such areas, this finding suggest that the parasites causing PAM are antigenically distinct from other parasites and that protective immunity to PAM can be developed after only a few PAM episodes, pointing to a relatively conserved target antigen (reviewed in reference 26).
PAM is characterized by placental accumulation of large numbers of parasites that use VSA to adhere to low-sulfated chondroitin sulfate proteoglycans in the placental intervillous space and that can adhere to chondroitin sulfate A (CSA) in vitro (1, 9). As expected, women from areas with intense P. falciparum transmission often have high levels of antibodies with specificity for these particular VSA (VSAPAM), with average levels increasing with increasing parity (11, 20, 27). However, it is a consistent finding that sympatric adult males, children, and nulligravidae never possess VSAPAM-specific antibodies despite high levels of antibodies to VSA expressed by parasites not involved in PAM. In short, antibody recognition of VSAPAM, but not other VSA, can be said to be gender specific (20, 27), supporting the hypothesis that VSAPAM are antigenically unique and are only expressed by parasites sequestered in the placenta.
As for P. falciparum malaria in general, available evidence suggests that VSA-specific antibodies mediate protection from PAM as well. VSAPAM-specific antibodies can block adhesion to CSA of placental and in vitro-selected parasite isolates (11, 20), and there is an inverse relationship between VSAPAM-specific immunoglobulin G (IgG) levels on the one hand and (i) placental parasitemia, (ii) maternal anemia, and (iii) the birth weight of the offspring on the other (27; T. Staalsoe et al., unpublished data). Finally, levels of VSAPAM IgG mirror the parity-dependent acquisition of protection against PAM remarkably well (20, 27).
P. falciparum erythrocyte membrane protein 1 (PfEMP1) is the collective term for the best-characterized family of VSA that is encoded by the var gene family, with approximately 60 members per haploid parasite (12, 15). The DBLγ domain of the PfEMP1 protein encoded by a particular var gene, FCR3varCSA, can adhere to CSA and is being promoted as a candidate antigen for the development of a vaccine against PAM (4, 8). FCR3varCSA belongs to a subfamily (var1) of the var gene family. The var1 subfamily is composed of var genes with high intergenomic conservation over the entire gene sequence (10, 22, 23), which fits the epidemiologically based assumption that the antigen mediating immunological protection against PAM is relatively conserved (11).
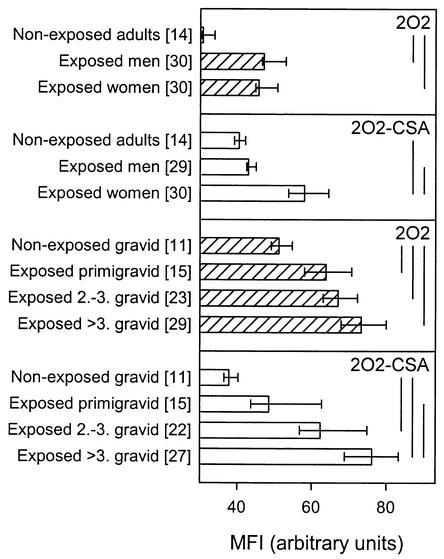
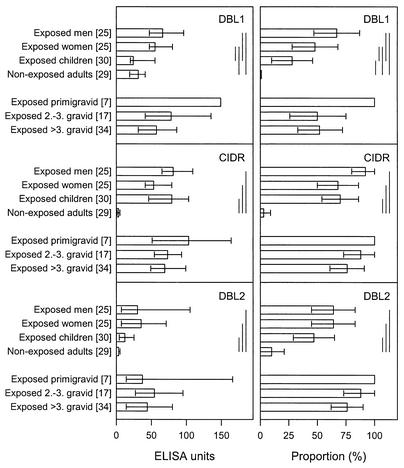
Here we have used the parasite line 2O2 and a genotypically identical CSA-adhering subline of 2O2 (2O2-CSA) to investigate the hypothesis that the product of the var1-type gene 2O2var1 (2O2VAR1) is the target on the surface of intact 2O2-CSA IE that is recognized by IgG in a manner characteristic of VSAPAM. VSA-specific IgG recognition of 2O2-CSA was both gender specific (P < 0.001, Mann-Whitney rank sum test) and parity dependent (P < 0.001, Spearman rank order correlation), in contrast to that of the parental line 2O2 (P > 0.05 in both cases) (Fig. 1). To test our hypothesis, we subcloned the DBL1α, DBL2β, and CIDR1α domains of 2O2var1 into the pGEX-4T1 (Amersham Pharmacia Biotech, Hoersholm, Denmark) vector by PCR with domain-specific oligonucleotide primers DBL1α-Fw (5′-CGGAATTCTATGATGATGTGAATTTGAGA-3′), DBL1α-Rv (5′-ATTTGCGGCCGCCTTGTTGATTCCCTAACCAAAC-3′), DBL2β-Fw (5′-CGGAATTCGAGGAAGCACGAACTCGTGG-3′), DBL2β-Rv (5′-ATTTGCGGCCGCCAGACATTTGTGCTTGTTCA-3′), CIDR1α-Fw 5′-CGGAATTCACTGACTGTTCGACTAAATGC-3′, and CIDR1α-Rv (5′-ATTTGCGGCCGCGAGGTTTAACACACGG-3′) (EcoRI sites are underlined, and NotI sites are underlined and in italics). The proteins were expressed as fusion proteins at the carboxy terminus of glutathione S-transferase from Schistosoma japonicum (24) and purified by affinity chromatography on glutathione Sepharose 4B (Amersham Pharmacia Biotech). The levels of antibodies to each of the three recombinant domains depended significantly on the donor category (P < 0.001 in all cases, Kruskal-Wallis one-way analysis of variance) and were significantly higher in plasma from malaria-exposed donors than in plasma from nonexposed donors (P < 0.05 in all cases, Dunn's post hoc test) (Fig. 2, left). Virtually identical results were obtained when the proportions of responders (i.e., with antibody levels greater than the mean level plus 2 standard deviations of plasma samples from 29 unexposed Danish control donors) were analyzed by χ2 test with appropriate correction for multiple analyses (Fig. 2, right). These results indicate that the recombinant proteins reproduced genuine P. falciparum products (Fig. 2, left). In further support of this, antibody reactivity appeared to reflect the degree of parasite exposure, as levels were generally higher in adults than in children (Fig. 2). However, in no case was there any significant difference in reactivity between plasma samples from malaria-exposed males and females, and there was no evidence of parity dependency of any of the domain-specific antibody reactivities (P > 0.05 in all cases, Dunn's post hoc test or χ2 test, as appropriate) (Fig. 2). Analysis by Western blotting confirmed that enzyme-linked immunosorbent assay-positive malaria-exposed men and women recognized the recombinant proteins equally well (data not shown). When the recombinant proteins were used to immunize BALB/c mice (5 μg given subcutaneously in Freund's complete adjuvant, followed by two 5-μg booster injections in Freund's incomplete adjuvant), the resulting immune sera (but not prevaccination sera) reacted with the immunizing antigen when tested by Western blotting as previously described (14) (data not shown). However, no murine antibodies with specificity for antigens expressed on the surface of 2O2-CSA IE could be detected by flow cytometry (data not shown). Finally, quantitative real-time PCR analysis of RNAs prepared from 2O2 and 2O2-CSA did not produce evidence of preferential transcription of 2O2var1 in 2O2-CSA versus 2O2, regardless of whether the 5′ domain CIDR1α (transcription level relative to actin, 1.41 in 2O2 versus 1.44 in 2O2-CSA) or the 3′ domain DBL6β (1.48 versus 1.56) of 2O2var1 exon 1 was targeted. For the latter experiments, isolated RNA (RNAaquous isolation kit; Ambion, Austin, Tex.) was treated with DNase I (InVitrogen; Taastrup, Denmark) until free of DNA, annealed by random hexamer primers, and extended with Superscript II reverse transcriptase (InVitrogen) at 42°C for 50 min. The following specific primers were designed by using the Primer3 website (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi): Actin-Fw (5′-AGCAGCAGGAATCCACACA-3′), Actin-Rv (5′-TGATGGTGCAAGGGTTGTAA-3′), CIDR1α-Fw (5′-AACGGATGGTCACCCACTTA-3′), CIDR1α-Rv (5′-GCTTGGTGTTTTGGGTCAGT-3′), DBL6β-Fw (5′-AGACAAATGGGACTGTAAAGAACG-3′), and DBL6β-Rv (5′-GCTGTACGCAATACTTTTCTGAAC-3′). Quantitative real-time PCRs were completed with a Rotorgene thermal cycler system (Corbett Research, Motlake, Australia) and 20-μl volumes with QuantiTect SYBR Green PCR master mix and 0.5 μM primers (Qiagen, Merck Eurolab, Albertslund, Denmark). Cycling conditions optimized for P. falciparum cDNA were 95°C for 15 min, followed by 45 cycles of 94°C for 30 s, 55°C for 60 s, and 70°C for 60 s with a final extension at 68°C for 10 min, with data acquisition at the end of elongation in each cycle. Amplification specificity was ascertained by melting curve analysis, and quantification was done with the Rotorgene software, version 4.4.
FIG. 1.
Levels in plasma of antibodies specifically recognizing parasite-encoded variant erythrocyte surface antigens expressed by nonselected (2O2; shaded bars) and CSA-selected (2O2-CSA; open bars) P. falciparum in different categories of individuals, as indicated. Antibody levels were measured by flow cytometry (25), and results are shown as median reactivity and 95% confidence intervals. Vertical bars to the right indicate statistically significant intergroup differences (P < 0.05, Kruskal-Wallis one-way analysis of variance on ranks). Values in brackets are numbers of donors tested. MFI, mean fluorescence intensity.
FIG. 2.
Plasma antibody reactivity to recombinant domains of 2O2VAR1 in different categories of individuals, as indicated. Reactivity was measured by enzyme-linked immunosorbent assay, and the results are shown as antibody levels in plasma (left panels) and as proportions of individuals with a positive response (right panels). Data are otherwise presented as in Fig. 1, except that intergroup differences in proportions were analyzed by χ2 test. Values in brackets are numbers of donors tested.
Gender-specific and parity-dependent plasma antibody recognition is a striking and exclusive characteristic of VSA expressed by placental parasites and parasites selected for strong CSA adhesion in vitro (Fig. 1) (20, 27). The lack of gender specificity and parity dependency of the recombinant constructs from 2O2var1, despite gender-specific and parity-dependent antibody recognition of native VSAPAM expressed by 2O2-CSA, can be explained in at least three ways.
One possibility is that 2O2var1 and other var1-type genes, such as FCR3varCSA (23), indeed encode VSAPAM expressed by placenta-adhering parasites causing PAM. In that case, it follows that all of the observed recombinant antigen-specific antibody reactivity in the plasma of exposed males must be directed against cross-reactive epitopes present in many PfEMP1 molecules expressed by non-PAM parasites and that these epitopes are not accessible in the intact, surface-expressed 2O2VAR1 protein (as plasma antibodies from men cannot recognize VSAPAM on the surface of 2O2-CSA IE). The most likely candidates are epitopes encoded by conserved sequences, such as the LARSFADIG box found in all of the var genes described to date. However, recent evidence suggests that recognition of variant, rather than conserved, epitopes dominates among plasma antibodies from exposed individuals (18; our unpublished results), and if so, a gender-specific recognition pattern would be expected (but was not seen). This explanation furthermore requires that 2O2VAR1 expression must be regulated posttranscriptionally (17), as levels of 2O2var1 mRNA in unselected 2O2 and CSA-selected 2O2-CSA were similar, as has also been observed in several other recent studies (10, 16, 22). We have recently identified another conserved var gene subfamily (var2) (23). In contrast to var1, members of var2 are markedly upregulated in response to selection for adhesiveness to CSA in vitro and are highly transcribed among placental isolates.
Alternatively, 2O2var1 is transcribed but not expressed on the surface of erythrocytes infected by 2O2-CSA (and perhaps not on the surface of those infected by 2O2 either), and for this reason, it cannot be the VSA involved in the pathogenesis of PAM. In that case, all of the antibody reactivity seen in the plasma of exposed individuals, regardless of gender, must be due to cross-reactivity with epitopes in PfEMP1 molecules expressed by certain non-PAM parasites. The bottom line is that there is no hard evidence on the basis of which to distinguish between the above two possibilities.
Finally, we cannot exclude the possibility that the basis of the conflicting evidence from our experiments with recombinant constructs and intact IE is related to the conformation of the recombinant proteins used here.
It is the identification of PfEMP1 species with affinity for CSA that has led to the assumption that placental parasite accumulation in PAM is mediated by PfEMP1 encoded by var1-type genes (4, 19). However, the parasite lines used in both of the cited studies bind CSA-glycosylated thrombomodulin (2, 13) that is expressed constitutively on the endothelium throughout the vasculature. The marked and sudden increase in susceptibility to P. falciparum malaria in otherwise clinically immune women during their first pregnancy is generally explained as the availability of a novel adhesion ligand in the newly formed placenta (9), and the general availability of CSA on thrombomodulin in the circulation is at odds with this hypothesis. Indeed, CSA-adhering parasites have been observed in nonpregnant individuals (6, 21), and single parasite genomes can contain multiple var genes that encode PfEMP1 domains with measurable affinity for CSA (7).
In conclusion, our data suggest that caution is advisable before concluding that PfEMP1 molecules encoded by var1-type genes are the mediators of the placental parasite sequestration that is the central element in the pathogenesis of PAM. The gender-specific and parity-dependent recognition pattern characteristic of VSAPAM expressed by placental parasites and some CSA-adherent parasite lines remains the most unequivocal VSA marker of relevance in terms of PAM. Affinity for CSA in itself appears to be an insufficient criterion.
Acknowledgments
This study received financial support from the Enhancement of Research Capacity in Developing Countries program of the Danish International Development Assistance (grant 104.Dan.8.L.306) and the Commission of the European Communities (grant QLK2-CT-2001-01302). A.S. is funded by a grant from the Bill and Melinda Gates Foundation.
Kirsten Pihl and Jimmy Weng are thanked for excellent technical assistance.
Editor: J. M. Mansfield
REFERENCES
- 1.Achur, R. N., M. Valiyaveettil, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Beeson, J. G., W. Chai, S. J. Rogerson, A. M. Lawson, and G. V. Brown. 1998. Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A. Infect. Immun. 66:3397-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 4.Buffet, P. A., B. Gamain, C. Scheidig, D. Baruch, J. D. Smith, R. Hernandez-Rivas, B. Pouvelle, S. Oishi, N. Fujii, T. Fusai, D. Parzy, L. H. Miller, J. Gysin, and A. Scherf. 1999. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl. Acad. Sci. USA 96:12743-12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull, P. C., and K. Marsh. 2002. The role of antibodies to Plasmodium falciparum-infected erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 10:55-58. [DOI] [PubMed] [Google Scholar]
- 6.Chaiyaroj, S. C., P. Angkasekwinai, A. Buranakiti, S. Looareesuwan, S. J. Rogerson, and G. V. Brown. 1996. Cytoadherence characteristics of Plasmodium falciparum isolates from Thailand: evidence for chondroitin sulfate A as a cytoadherence receptor. Am. J. Trop. Med. Hyg. 55:76-80. [DOI] [PubMed] [Google Scholar]
- 7.Degen, R., N. Weiss, and H. P. Beck. 2000. Plasmodium falciparum: cloned and expressed CIDR domains of PfEMP1 bind to chondroitin sulfate A. Exp. Parasitol. 95:113-121. [DOI] [PubMed] [Google Scholar]
- 8.Douki, L. J. B., B. Traore, F. T. M. Costa, T. Fusai, B. Pouvelle, Y. Sterkers, A. Scherf, and J. Gysin. 2002. Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood 100:1478-1483. [DOI] [PubMed] [Google Scholar]
- 9.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulphate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 10.Fried, M., and P. E. Duffy. 2002. Two DBLγ subtypes are commonly expressed by placental isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 122:201-210. [DOI] [PubMed] [Google Scholar]
- 11.Fried, M., F. Nosten, A. Brockman, B. T. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 12.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gysin, J., B. Pouvelle, M. Le Tonquèze, L. Edelman, and M. C. Boffa. 1997. Chondroitin sulfate of thrombomodulin is an adhesion receptor for Plasmodium falciparum-infected erythrocytes. Mol. Biochem. Parasitol. 88:267-271. [DOI] [PubMed] [Google Scholar]
- 14.Jensen, A. T., J. Curtis, J. Montgomery, E. Handman, and T. G. Theander. 2001. Molecular and immunological characterisation of the glucose regulated protein 78 of Leishmania donovani. Biochim. Biophys. Acta 1549:73-87. [DOI] [PubMed] [Google Scholar]
- 15.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707. [DOI] [PubMed] [Google Scholar]
- 16.Kyes, S. A., Z. Christodoulou, A. Raza, P. Horrocks, R. Pinches, J. A. Rowe, and C. I. Newbold. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Mol. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 17.Lanzer, M., S. P. Wertheimer, D. De Bruin, and J. V. Ravetch. 1993. Plasmodium: control of gene expression in malaria parasites. Exp. Parasitol. 77:121-128. [DOI] [PubMed] [Google Scholar]
- 18.Oguariri, R. M., S. Borrmann, M. Q. Klinkert, P. G. Kremsner, and J. F. Kun. 2001. High prevalence of human antibodies to recombinant Duffy binding-like α domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect. Immun. 69:7603-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeder, J. C., A. F. Cowman, K. M. Davern, J. G. Beeson, J. K. Thompson, S. J. Rogerson, and G. V. Brown. 1999. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl. Acad. Sci. USA 96:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson, S. J., R. Tembenu, C. Dobaño, S. Plitt, T. E. Taylor, and M. E. Molyneux. 1999. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am. J. Trop. Med. Hyg. 61:467-472. [DOI] [PubMed] [Google Scholar]
- 22.Rowe, J. A., S. A. Kyes, S. J. Rogerson, H. A. Babiker, and A. Raza. 2002. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J. Infect. Dis. 185:1207-1211. [DOI] [PubMed] [Google Scholar]
- 23.Salanti, A., A. T. R. Jensen, H. D. Zornig, T. Staalsoe, L. Joergensen, M. A. Nielsen, A. Khattab, D. E. Arnot, M. Q. Klinkert, L. Hviid, and T. G. Theander. 2002. A sub-family of common and highly conserved var genes expressed by CSA-adhering Plasmodium falciparum. Mol. Biochem. Parasitol. 122:111-115. [DOI] [PubMed] [Google Scholar]
- 23a.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. R. Jensen, M. P. K. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. Selective up-regulation of a single distinctly structured var gene in CSA-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol., in press. [DOI] [PubMed]
- 24.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 25.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 26.Staalsoe, T., A. T. R. Jensen, T. G. Theander, and L. Hviid. 2002. Novel Plasmodium falciparum malaria vaccines: evidence-based searching for variant surface antigens as candidates for vaccination against pregnancy-associated malaria. Immunol. Lett. 84:133-136. [DOI] [PubMed] [Google Scholar]
- 27.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]