Abstract
Deterioration of lung function in patients with cystic fibrosis (CF) is closely associated with chronic pulmonary infection with mucoid Pseudomonas aeruginosa. The mucoid exopolysaccharide (MEP) from P. aeruginosa has been shown to induce opsonic antibodies in mice that are protective against this chronic infection. MEP-specific opsonic antibodies are also commonly found in the sera of older CF patients lacking detectable P. aeruginosa infection. When used in a human vaccine trial, however, MEP only minimally induced opsonic antibodies. To evaluate whether conjugation of MEP to a carrier protein could improve its immunogenicity, we bound thiolated MEP to keyhole limpet hemocyanin (KLH) by using succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) as a linker. In contrast to the native MEP polymer, the MEP-KLH conjugate vaccine induced high titers of MEP-specific immunoglobulin G (IgG) in C3H-HeN mice and in a rabbit. Sera from mice immunized with MEP-KLH conjugate, but not from animals immunized with comparable doses of native MEP, demonstrated opsonic killing activity. Vaccination with MEP-KLH conjugate induced opsonic antibodies broadly cross-reactive to heterologous mucoid strains of P. aeruginosa. Preexisting nonopsonic antibodies to MEP are found in normal human sera, including young CF patients, and their presence impedes the induction of opsonic antibodies. Induction of nonopsonic antibodies by either intraperitoneal injection of MEP or injection or feeding of the cross-reactive antigen, seaweed alginate, reduced the level of overall IgG elicited by follow-up immunization with the MEP-KLH conjugate. However, the opsonic activity was lower only in the sera of MEP-KLH conjugate-immunized mice with preexisting antibodies induced by MEP but not with antibodies induced by seaweed alginate. Immunization with MEP-KLH elicited a significant proportion of antibodies specific to epitopes involving O-acetate residues, and this subpopulation of antibodies mediated opsonic killing of mucoid P. aeruginosa in vitro. These results indicate that conjugation of MEP to KLH significantly enhances its immunogenicity and the elicitation of opsonic antibodies in mice and rabbits, that the conjugate induces opsonic antibodies in the presence of preexisting nonopsonic antibodies, and that opsonic antibodies to MEP are directed at epitopes that include acetate residues on the uronic acid polymer.
Chronic pulmonary infection in patients with cystic fibrosis (CF) is predominantly due to infection by mucoid strains of Pseudomonas aeruginosa. A phenotypic characteristic of these strains is elaboration of large quantities of mucoid exopolysaccharide (MEP), also referred to as alginate. MEP is a random polymer of d-mannuronic acid (M) and l-guluronic acid (G) residues linked β1-4 (36). M residues are variably O acetylated on C-2 and C-3 (36). The emergence of the mucoid phenotype of P. aeruginosa during the clinical course of CFs correlates with the onset of significant deterioration in lung function (5, 21). Although patients with CF generate a vigorous antibody response to MEP, these antibodies generally lack opsonic activity and are evidently ineffective at eliminating the bacteria (31). However, among a small subset of older, uninfected CF patients (ca. 5% of all patients) naturally acquired MEP-specific opsonic antibodies can be found (32) and have been associated with resistance to infection. In a rodent model of chronic endobronchial infection with mucoid P. aeruginosa, opsonic but not nonopsonic antibodies to MEP mediated protection (33). Opsonic antibodies differ functionally from nonopsonic antibodies by their ability to deposit complement factor C3 on the outer bacterial surface (30). MEP-based active and passive immunization of naive animals elicits opsonic antibodies and leads to clearance of mucoid P. aeruginosa from the respiratory tract in mice and rats (33). In addition, immunization with a single MEP antigen gives rise to antibodies that have opsonic activity against a broad variety of mucoid strains (28, 31). MEP is not only expressed in mucoid strains but its synthesis is also increased in nonmucoid strains of P. aeruginosa exposed to a hypoxic milieu like that found in the mucus plugs in the airway lumen of CF lungs (41). In addition, a recent study demonstrates that MEP expression is critical for the chronic colonization of the upper respiratory tract of cystic fibrosis transmembrane conductance regulator-deficient mice by both mucoid and nonmucoid strains (3).
As a result of the numerous studies underlining the protective potential of a MEP-vaccine, human trials were initiated (28). When tested at doses between 10 and 300 μg, the MEP was shown to be safe and generally well tolerated (28). However, with only 2 of 23 vaccinees producing long-lived titers of opsonic antibodies, the results of this initial trial were disappointing (28). Reevaluation of the vaccine in animal studies indicated that only the highest-molecular-size polymers of MEP are able to induce opsonic antibodies in mice with preexisting levels of nonopsonic antibodies (10). Nonopsonic antibodies frequently are seen in healthy individuals as well as in most patients with CF even before the onset of detectable infection (10). As a consequence of these findings, a second vaccine lot composed of high-molecular-weight MEP was evaluated in humans (28). Compared to the initial lot, more volunteers immunized with high-molecular-weight MEP generated opsonic antibodies, but the overall outcome (only 35% of a total of 188 vaccinated volunteers produced measurable opsonic antibodies) was nevertheless unsatisfactory (28).
An immensely successful approach to enhance the immunogenicity of bacterial polysaccharides has been their conjugation to carrier proteins (19, 20). In fact, a conjugate vaccine of MEP with exotoxin A of P. aeruginosa as a carrier protein has been synthesized and evaluated previously (4). This conjugate improved the immunogenicity of MEP considerably but utilized a significantly depolymerized and de-O-acetylated polysaccharide. Results concerning cross-reactivity of MEP-specific immunoglobulin G (IgG) induced by this vaccine conflict; data from rat immunization studies indicate that only low levels of cross-reactive antibodies were induced by the MEP-exotoxin A conjugate (4, 15, 16). With only one heterologous strain tested, the cross-reactivity of opsonic antibodies induced by this exotoxin A conjugate vaccine was poorly defined (4). Furthermore, protection by the MEP-exotoxin A conjugate could not be demonstrated, since bead-infected rats at the end of the observation period of 28 days had cleared the bacteria regardless of whether they had been immunized with the vaccine or the adjuvant alone (15).
Overall, our knowledge of the immune response to MEP conjugate vaccines remains incomplete. In addition, evidence from the literature suggests that both high-molecular-weight and native O-acetate content are critical to the induction of opsonic antibodies and should thus be conserved during vaccine synthesis (10, 27) In the present study, we describe the synthesis and characterization of a conjugate vaccine of MEP by using the native, nondepolymerized, and nondeacetylated polymer, demonstrating its ability to induce broadly cross-reactive opsonic antibodies in naive mice, in mice with preexisting antibodies to MEP, and in a rabbit.
MATERIALS AND METHODS
Bacterial strains.
Mucoid P. aeruginosa strains 2192 (10), FRD1 (25), 258 (33), 027, 8050, 5681, and 287 are all clinical isolates obtained from CF patients.
Animals.
C3H-HeN mice were obtained from Harlan-Sprague-Dawley Farms, Chicago, Ill. A New Zealand White rabbit was from Millbrock Breeding Labs, Amherst, Mass.
Reagents.
Protanal LF 120 M and Scogin XXL seaweed alginates derived from Laminaria hyperborea were obtained from FMC Biopolymers, Philadelphia, Pa.Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)-deri-vatized keyhole limpet hemocyanin (KLH) was from Pierce Endogen, Rockford, Ill. 1-Ethyl-3(3-dimethylaminopropyl)carbodiimide (EDC), dithiothreitol (DTT), dithionitrobenzoic acid (Ellman reagent), cystamine, and cysteine were from Sigma-Aldrich, St. Louis, Mo. meta-Hydroxybiphenyl was purchased from Eastman Kodak, Rochester, N.Y. The Limulus amebocyte lysate assay was from Cape Cod Associates, Woods Hole, Mass. Sephacryl S-500 and S-1000, Sephadex G-50, and PD-10 columns were from Amersham Biotech, Piscataway, N.J. Polyethersulfone ultrafiltration membranes with a molecular-weight cutoff of 10,000 were obtained from Millipore, Bedford, Mass. Immunolon 4HBX microtiter plates were from Thermo Labsystems, Franklin, Mass. DNase I, RNase A, and proteinase K were from Worthington Biochemical Corp., Lakewood, N.Y. Rabbit anti-KLH antibody was from Bethyl Laboratories, Montgomery, Tex. Alkaline phosphatase conjugates of goat anti-mouse IgG (Fc specific), goat anti-mouse IgM (μ-chain specific), and goat anti-rabbit IgG (whole molecule) were from Sigma. Baby rabbit complement was from Cedarlane Laboratories, Hornby, Ontario, Canada.
Purification of MEP.
MEP-alginate was purified from P. aeruginosa strain 2192 (10). Bacteria were grown at 37°C for 72 h in 4 liters of modified Mian’s medium. Bacteria were removed from the culture by repeated centrifugation, and crude MEP was precipitated from the supernatant by the addition of ethanol (80% final concentration). The precipitate was collected by centrifugation, washed three times in ethanol, dialyzed against deionized water (dH2O), and lyophilized. Freeze-dried crude MEP was redissolved at 2 mg/ml in phosphate-buffered saline (PBS) supplemented with 5 mM MgCl2 and 1 mM CaCl2, and then DNase I and RNase A were added (each at 100 μg/ml). After overnight incubation at 37°C, proteinase K was added (100 μg/ml) for 4 h with incubation at 56°C. The mixture was then extracted with an equal volume of 90% phenol (65°C, 15 min). After being cooled to 4°C, the aqueous phase was recovered and dialyzed extensively against dH2O and lyophilized. For size exclusion chromatography, MEP was dissolved at 2 mg/ml in 0.4 M ammonium carbonate buffer and applied to a 5-by-90-cm Sephacryl S-500 column. Eluted fractions were assayed for uronic acid content, and positive fractions eluting near the void volume of the column were pooled and precipitated with ethanol. Finally, the precipitate was collected by centrifugation, dialyzed against dH2O, and lyophilized. Contaminating lipopolysaccharide (LPS) was removed by ultracentrifugation of MEP (1.5 mg/ml in dH2O) at 100,000 × g for 18 h.
Acetylation and deacetylation of MEP-alginate.
For acetylation of seaweed alginate, the polysaccharide (Protanal LF 120 M; FMC Biopolymers) was uniformly suspended in pyridine (2 mg/ml), and an equal volume of acetic anhydride was added. The mixture was incubated with stirring at 20°C for 72 h and then chilled to 4°C, and the acetic anhydride was inactivated by the slow addition of an equal volume of dH2O. The acetylated seaweed alginate was dialyzed extensively against dH2O and freeze-dried. A high degree of O acetylation was obtained by subjecting seaweed alginate to this procedure repeatedly. For deacetylation, MEP was suspended (2.5 mg/ml) in 0.1 N ammonium hydroxide and then incubated at 95°C for 5 h. After it cooled to room temperature, the reaction mixture was dialyzed against dH2O and lyophilized. The success of the acetylation and deacetylation was confirmed by 1H nuclear magnetic resonance (NMR) analysis.
Chemical analysis of alginates.
The purified antigen was analyzed for uronic acid content by the meta-hydroxybiphenyl assay with sodium alginate as the standard (Protanal LF 120 M; FMC Biopolymers) (9), for nucleic acid by absorption at 254 nm with salmon sperm DNA as the standard (Sigma) (10), for protein by the Bradford assay with bovine serum albumin (BSA) as the standard (Sigma) (2), for LPS by the Limulus amebocyte lysate assay with E. coli O113:H10 endotoxin as the standard (Cape Cod Associates). The degree of O acetylation, and the ratio of mannuronic to guluronic acid was determined by 1H NMR analysis according to the method of Skjak-Braek et al. (36).
Preparation of conjugates.
MEP (5 mg/ml) and cystamine (0.1 M) were dissolved in 0.05 M MES (2-[N-morpholino]ethanesulfonic acid) buffer, and the pH was adjusted to 5.0. EDC was added (2 mM final concentration), and the reaction mixture was stirred at room temperature for 4 h while the pH was maintained between 4.9 and 5.1. The reaction mixture was dialyzed exhaustively against dH2O at 4°C and lyophilized. The extent of thiolation was measured on an aliquot of polysaccharide reduced with 0.05 M DTT for 2 h and then passed through a PD-10 column equilibrated with the conjugation buffer (50 mM PBS, 0.9 M NaCl, 5 mM EDTA; pH 7.4) for removal of low-molecular-weight material. Void volume fractions were assayed for sulfhydryl and uronic acid content, and the degree of derivatization was expressed as the molar percentage of cystamine per monosaccharide unit (6). For conjugation to SMCC-derivatized KLH, 25 mg of MEP-cystamine was dissolved in conjugation buffer (1 mg/ml) and reduced with 0.05 M DTT for 2 h. The mixture was then desalted with a 2.6-by-24-cm Sephadex G-50 column equilibrated with conjugation buffer, and volume fractions that assayed positive for free sulfhydryl groups and uronic acid were pooled and concentrated under nitrogen by ultrafiltration (molecular-weight cutoff, 10,000) to a final concentration of 5 mg/ml. A total of 12 mg of SMCC-derivatized KLH (456 M SMCC/M KLH according to the manufacturer) was added to the reduced cystamine derivative of MEP, and the mixture was stirred at room temperature for 1 h and at 4°C overnight. Nonderivatized SMCC remaining after overnight incubation was quenched by the addition of 10 mM cysteine. Precipitated material was removed by centrifugation, and the supernatant was passed through a 1.6-by-95-cm Sephacryl S-1000 column with PBS used as running buffer. Void-volume fractions that assayed positive for both protein and uronic acid were designated polysaccharide-protein conjugate and were pooled, dialyzed against PBS, and lyophilized in aliquots. The amounts of protein and MEP present in the conjugate were quantified by assaying for protein and uronic acid as described above.
Immunization of mice and rabbits.
Female C3H/HeN mice, 6 to 8 weeks old, were injected subcutaneously (s.c.) in groups of five on days 0, 7, and 14 with either a mixture of MEP and KLH or the MEP-KLH conjugate suspended in PBS. Blood was obtained from the tail-vein on days 7, 14, 21, 28, and 35. Some animals were bled every 7 to 14 days for up to 84 days. All immunizations were done without adjuvant. A female New Zealand White rabbit was immunized s.c. with 10 μg of conjugate vaccine suspended in complete Freund adjuvant (Sigma), followed by the same dose s.c. suspended in incomplete Freund adjuvant (Sigma) on day 7. The rabbit was boosted intravenously with four 10-μg doses the following week. Further booster doses of 10 μg were given intravenously at intervals of 14 days. For studies in mice preimmunized with seaweed alginate or native MEP, 10 C3H-HeN mice, 6 to 8 weeks old, were immunized intraperitoneally (i.p.) with three 50-μg doses of seaweed alginate (Scogin XXL; FMC Biopolymers) or of native MEP administered at 1-week intervals. A third group of mice received seaweed alginate with their drinking water (1 mg/ml) for 21 days. MEP-specific IgG and IgM were determined by enzyme-linked immunosorbent assay (ELISA) on day 28 and again at day 183. Starting on day 190, five mice from each group received three 1-μg doses, at 1-week intervals, of the polysaccharide as a mixture of native MEP and KLH or as a conjugate. Serum was obtained 35 days after the first dose of the specific immunization with the mixed or conjugated vaccines.
ELISA.
ELISAs were performed by standard methods as described previously (29). In brief, microtiter plates were coated with MEP or deacetylated MEP derived from P. aeruginosa strain 2192 (10 μg/ml in 0.04 M phosphate buffer [pH 7.0]) and kept overnight at 4°C. Cross-reactivity of rabbit and mouse antisera was assessed on plates coated with MEP from heterologous strains of mucoid P. aeruginosa obtained by ethanol precipitation of heat-denatured culture supernatants. The precipitates were washed three times in ethanol, dialyzed against dH2O, lyophilized, and used for coating as described for highly purified MEP preparations. For quantification of antibodies binding to KLH, the plates were coated with KLH (5 μg/ml) in carbonate buffer (pH 9.6). Between incubation steps, plates were washed three times with PBS containing 0.05% Tween 20 (PBS-Tw). Blocking was performed with 3% skim milk in PBS-0.02% sodium azide for 2 h at 37°C. Individual mouse sera were assayed as duplicates of threefold serial dilutions. Rabbit sera were measured in twofold serial dilutions. An alkaline phosphatase conjugate diluted 1:1,000 was used as secondary antibody, and p-nitrophenyl phosphate was used as a substrate (Sigma; 1 mg/ml in diethanolamine buffer, 0.5 mM MgCl2 [pH 9.8]). After 60 min of incubation at 37°C, the absorbance was measured at 405 nm. For competition inhibition ELISAs, 10-fold dilutions of inhibiting polysaccharide (native and de-O-acetylated MEP) ranging from 100 to 10−6 μg/ml (final concentration) were mixed with a single serum dilution of rabbit antiserum previously shown by ELISA to be near the middle of the linear portion of the binding curve.
A heterologous sandwich ELISA was used to confirm covalent coupling of MEP to KLH (40). In brief, microtiter plates were sensitized with anti-KLH rabbit IgG capture antibody (5 μg/ml), washed, and blocked with 5% BSA-PBS. The plates were then incubated with either the MEP-KLH conjugate (diluted in 5% BSA-0.05% Tween 20) or a weight-adjusted mixture of MEP and KLH at concentrations ranging from 0.02 to 50 μg/ml. Mouse serum cross-reactive to MEP (raised against acetylated seaweed alginate conjugated to BSA) was added for detection of bound MEP. An alkaline phosphatase conjugate antibody to mouse IgG served as a secondary antibody.
Opsonophagocytic assay.
An opsonophagocytic assay was used as previously described (10). A modification of this protocol was the use of unabsorbed infant rabbit serum as a complement source instead of absorbed human serum. The opsonic activity of immune sera was compared to that of sera obtained before vaccination. Serum from a rabbit hyperimmunized with native MEP served as a positive control. Negative controls included tubes from which polymorphonuclear leukocytes, complement, or serum was omitted. For all assays involving mouse sera, pooled serum from members of the respective immunization groups was used. The opsonic activity of the serum was calculated as follows: [1 − (CFU immune serum at 90 min/CFU of preimmune serum at 90 min)] × 100. For inhibition studies, rabbit antiserum diluted to a concentration resulting in >90% bacterial killing was incubated for 90 min at 4°C with 0.01 to 100 μg/ml with either native MEP, de-O-acetylated MEP, native algal alginate (Protanal LF 120 M) or chemically O-acetylated algal alginate. Subsequently, the antiserum was centrifuged, and the supernatant used in the opsonophagocytic assay as described above.
Statistical analysis.
ELISA titers were calculated by linear regression analysis of duplicate measurements versus the log10 of the serum dilution. The serum titers were defined as the serum dilution, which equals an optical density of 0.4 (∼3 to 4 times above the plate background level). Differences in ELISA and opsonic titers were compared after log transformation by analysis of variance (ANOVA) by using the Bonferroni test for comparisons of pairs of means or the Dunnet test for comparisons to values obtained with control sera. Statistical analysis was done by using Prism GraphPad software (San Diego, Calif.).
RESULTS
Physiochemical characterization of alginates.
Only the large-size polymer fraction of MEP eluting near the void volume (Kd = 0.18) of a Sephacryl S-500 size-exclusion column was used for synthesis of MEP-KLH conjugates. The purified antigen contained 96% (wt/wt) uronic acid, <1% protein, and <0.0025% LPS. MEP from strain 2192 was composed of 74% mannuronic acid and 26% guluronic acid, and 49% of its monosaccharide units were O acetylated as determined by 1H NMR analysis. Seaweed alginate derived from L. hyperborea contained 52% mannuronic acid and 48% guluronic acid and was 37% O acetylated after treatment with acetic anhydride. On a Sephacryl S-500 column, seaweed alginate eluted at a higher volume than MEP (Kd = 0.42), indicating a somewhat smaller molecular size.
Synthesis and composition of conjugates.
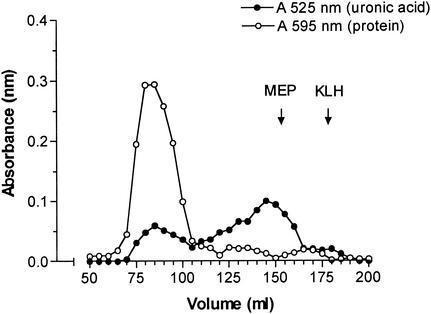
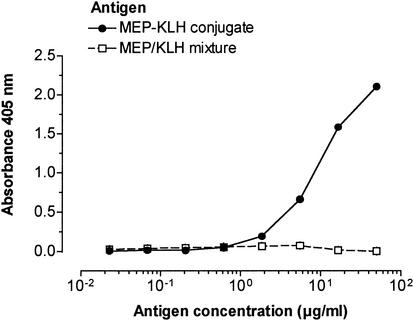
Initial attempts to conjugate MEP in an EDC-mediated reaction directly to KLH were not successful. EDC preferentially self-polymerized the carrier protein or produced highly cross-linked, water-insoluble polysaccharide-protein complexes. In contrast, the derivatization of MEP with cystamine was well controlled by varying the amount of EDC added to the reaction. For conjugation to KLH, 2.3% of the carboxylate groups of MEP (which represents 1 per 43 monosaccharide units) were coupled to cystamine. An inhibition ELISA showed no significant difference between native MEP and its cystamine derivative in the ability to inhibit binding of MEP-specific IgG to the native antigen (data not shown). MEP and MEP-cystamine also did not differ in the ability to inhibit antibody-mediated killing of mucoid P. aeruginosa in the opsonophagocytic killing assay by using antisera raised to native MEP (data not shown). Thus, no difference in epitope specificity was found between native MEP and cystamine-derivatized MEP. Gel filtration of the MEP-KLH conjugate on a Sephacryl S-1000 column yielded a large peak at void volume consisting both of protein and polysaccharide, two minor protein peaks at higher elution volumes, and a broad second peak positive for uronic acid with a maximum at Kd of 0.63 (Fig. 1). Since native MEP elutes on this resin at a broad range around its Kd of 0.68, only fractions eluting at void volume were presumed to be free of nonconjugated polysaccharide and denoted MEP-KLH conjugate. The MEP-KLH conjugate contained 76% (wt/wt) protein and 24% (wt/wt) uronate. Attempts to increase the polysaccharide content of the conjugate by using MEP with a higher degree of derivatization resulted in insoluble conjugates. The covalent bond between KLH and MEP was confirmed by capture ELISA (Fig. 2), wherein a capture rabbit antibody specific to KLH and a detection mouse antibody to MEP showed a positive result.
FIG. 1.
Sephacryl S-1000 gel filtration profile of MEP conjugated to KLH. Fractions were assayed for protein by the Bradford assay (595 nm) and for uronic acid by the meta-hydroxybiphenyl assay (525 nm).
FIG. 2.
Sandwich ELISA with rabbit anti-KLH IgG for antigen capture and mouse anti-MEP serum for detection of the binding of the polysaccharide component of the MEP-KLH conjugate. A mixture of MEP and KLH did not bind anti-MEP IgG. In contrast, wells incubated with the MEP-KLH conjugate reacted with the anti-MEP antibody.
Serum antibody response to native and conjugated MEP.
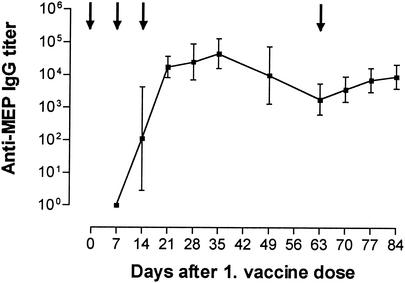
A mixture of native MEP and KLH elicited a significant rise in MEP-specific IgM and KLH-specific IgG but failed to induce IgG antibodies to MEP (Table 1). A higher dose of native MEP (10 μg per mouse) likewise did not elicit MEP-specific IgG (data not shown). In contrast, immunization with MEP-KLH conjugate elicited not only MEP-specific IgM but also IgG (Table 1). A rise in MEP-specific IgG was observed 7 days after the second vaccine dose, and a third immunization boosted antibody levels significantly (Table 1). The immunization with the MEP-KLH conjugate also induced high titers of IgG against the carrier protein (Table 1). In dose-response studies with vaccine doses ranging from 0.1 to 10 μg per dose per mouse, a 1-μg injection of MEP-KLH conjugate induced optimal levels of MEP-specific IgG (data not shown). Peak levels of anti-MEP IgG in mice immunized with MEP-KLH conjugate were reached 35 days after a series of three weekly vaccinations (Fig. 3). Titers slowly declined in the following weeks, but a vaccine booster given on day 63 increased IgG levels significantly (Fig. 3). Over this follow-up period, mice immunized with native MEP never developed MEP-specific IgG (data not shown). A hyperimmunized New Zealand White rabbit also generated high titers of MEP-specific IgG (titers of 1:50,000 to 1:80,000 after day 21 [data not shown]). Serum IgG induced by MEP-KLH was broadly cross-reactive to MEP derived from heterologous mucoid strains of P. aeruginosa (see Fig. 5).
TABLE 1.
Antibody levels of female C3H-HeN 6- to 8-week-old mice injected s.c. with 1 μg of either native MEP mixed with KLH or an MEP-KLH conjugate
| Immunogen | Target antigena | Isotype | Geometric mean (n = 5) serum titer (range) on:
|
||
|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |||
| MEP-KLH conjugate | MEP | IgG | <50b | 110c (<50-1,680) | 17,263c (6,980-30,050) |
| MEP | IgM | 600d (340-2,500) | 530 (380-1,530) | 940 (640-1,730) | |
| KLH | IgG | 60e (0-1,550) | 17,180e (5,790-45,470) | 13,020e (2,930-25,570) | |
| MEP plus KLH mixture | MEP | IgG | <50b | <50b | <50b |
| MEP | IgM | 430d (280-670) | 370 (310-600) | 290 (230-340) | |
| KLH | IgG | 70e (30-300) | 29,340e (11,830-45,500) | 97,540e (66,540-149,390) | |
MEP, microtiter plates coated with MEP derived from strain 2192.
Serum dilutions started at 1:50.
MEP-KLH conjugate elicited a significant rise in anti-MEP IgG after the second injection (P < 0.001 [as determined by repeated-measures ANOVA and the Bonferroni test for pairwise comparison]) and after a third injection (P < 0.01).
IgM titers after immunization with either MEP-KLH conjugate or a mixture of MEP and KLH rose significantly after the first injection (P < 0.001) but not after the booster injection.
KLH elicited a significant increase in anti-KLH IgG after the first injection (P < 0.001) and a booster response after the second injection (P < 0.001) in mice immunized with either MEP-KLH conjugate or with a mixture of MEP and KLH.
FIG. 3.
Titers of anti-MEP IgG in C3H-HeN mice over a period of 84 days after the beginning of immunization. Lines represent the geometric mean of ELISA titers, and error bars indicate the range. Arrows indicate the time points of immunization. For numerical values of titers for days 0 to 21, see Table 1. Titers of anti-MEP antibodies peaked at day 35 and declined gradually until day 63 (P < 0.001 [as determined by repeated measures ANOVA and Bonferroni test for pairwise comparisons of the time points]). A booster dose given on day 63 elicited a significant rise in titers by day 84 (P < 0.05).
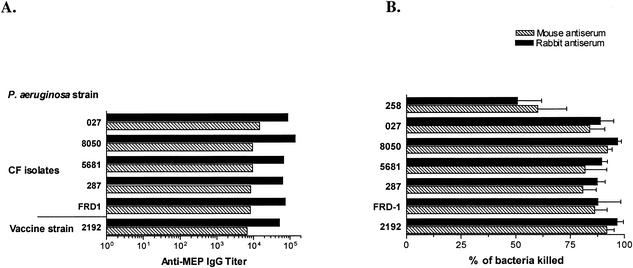
FIG. 5.
Cross-reactive binding and opsonic killing activity of rabbit and mouse antisera raised to MEP-KLH conjugate vaccine. (A) IgG titers against the vaccine strain (strain 2192) and multiple mucoid, LPS rough P. aeruginosa isolates from CF patients. (B) Opsonic activity of a serum dilution of 1:10 of the rabbit and mouse sera after immunization with MEP-KLH conjugate against the same target strains. No significant difference was found in binding to MEP isolated from the vaccine strain or heterologous strains of mouse and rabbit antisera. Also, the opsonic activity of the antisera against heterologous mucoid strains of P. aeruginosa did not differ significantly from their activity against strain 2192, except for strain 258, for which the killing activity was significantly lower than that against the vaccine strain (P < 0.001 [as determined by ANOVA with the Dunnet test for comparison with strain 2192]). The sera tested were obtained from a hyperimmunized rabbit and from five C3H-HeN mice 35 days after the first immunization. The mouse sera were pooled for analysis. MEP from strain 258 was not tested by ELISA for cross-reactive IgG, since this strain reverts to the nonmucoid phenotype when grown in the Mian’s medium used to produce MEP. The bars represent the mean of titers of two replicates determined by linear regression (A) and the means of four replicates (B); error bars indicate the standard error of the mean in panel B.
Opsonic activity of mouse and rabbit sera against mucoid P. aeruginosa.
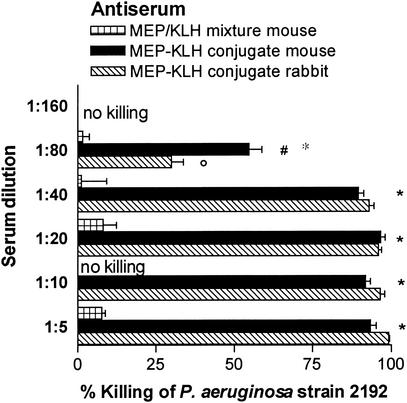
High levels of opsonic antibodies to MEP correlate with clearance of mucoid P. aeruginosa from the lung in rodent models of chronic infection (33). We therefore compared the potential of native and conjugated MEP to induce opsonic antibodies specific for MEP. In the opsonophagocytic killing assay, antisera from mice and rabbits immunized with the MEP-KLH conjugate mediated phagocytic killing ≥50% up to serum dilutions of 1:40 (rabbit) and 1:80 (mouse) against the vaccine strain in contrast to mice immunized with native MEP, which did not generate detectable opsonic antibodies (Fig. 4). Mouse and rabbit sera were also highly active in mediating killing of a panel of six mucoid, LPS rough clinical CF isolates (Fig. 5). With the exception of strain 258, the opsonic activity at a serum dilution of 1:10 against these heterologous strains did not differ significantly from the activity against the P. aeruginosa strain 2192 used to produce the vaccine (Fig. 5).
FIG. 4.
Opsonophagocytic killing of P. aeruginosa strain 2192 (vaccine strain) by serum from C3H-HeN mice after immunization with either MEP-KLH conjugate or native MEP mixed with KLH and by serum from a rabbit hyperimmunized with MEP-KLH. Bars represent the mean percent killing of four replicates relative to the respective preimmune serum, and error bars represent the standard error of the mean. At serum dilutions ranging from 1:5 to 1:80, sera of mice immunized with MEP-KLH generated significantly higher killing activity than did the sera of mice immunized with the nonconjugated polysaccharide (✽, P < 0.001; ○, P < 0.01 [as determined by ANOVA with the Bonferroni test for pairwise comparison]). At a serum dilution of 1:80, mouse serum maintained a significantly higher opsonic activity than rabbit serum (#, P < 0.01).
Antibody response in mice with preexisting antibodies to MEP.
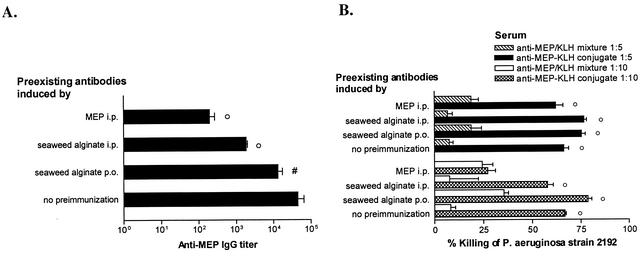
Nonopsonic antibodies to MEP have been shown to interfere with the development of opsonic antibodies when the former are present prior to immunization. Nonopsonic antibodies could potentially be induced not only by environmental exposure to Pseudomonas spp., many of which produce MEP-alginate but also by exposure to algal alginate, which is a widely used agent for stabilizing, gelling, and thickening of food products (26; C. Theilacker, Y. Wang, M. Grout, and G. B. Pier, Abstr. 101th Gen. Meet. Am. Soc. Microbiol., abstr. E-33, 2001), or even by other potential cross-reactive antigens. To determine the ability of the MEP conjugate vaccine to overcome suppression of antibody production in the presence of preexisting nonopsonic antibodies, we tested the immunogenicity of the MEP-KLH conjugate in mice with preexisting nonopsonic antibodies to MEP. Vaccination i.p. with both MEP and seaweed alginate elicited IgM antibodies binding to MEP, but no IgG antibodies were induced (data not shown). It is interesting that IgM cross-reactive to MEP also was found after a 21-day exposure to seaweed alginate in drinking water (data not shown). After 190 days, a period likely to more accurately reflect the interval between environmental exposure to antigen and deliberate immunization, we vaccinated the preimmunized mice with either MEP-KLH or the native MEP mixed with KLH. As observed for naive mice, native MEP did not elicit IgG antibodies in any of the groups of the preimmunized mice (data not shown). The mice immunized with MEP-KLH conjugate did generate MEP-specific IgG, but titers were significantly lower in these preimmunized mice compared to mice lacking any preexisting antibody (Fig. 6). Preimmunized mice boosted with the MEP-KLH conjugate also generated opsonic antibodies to MEP but, depending on the type of the preimmunization, these antibodies were also of a lower titer compared with those in mice lacking any preexisting antibody (Fig. 6). As observed in naive mice, native MEP did not elicit opsonic antibodies in the presence of nonopsonic antibodies (Fig. 6).
FIG. 6.
(A) MEP-specific IgG in mice preimmunized with 50 μg of native MEP given i.p., 50 μg of seaweed alginate given i.p., or seaweed alginate given in the drinking water (1 mg/ml) compared to that in naive mice. Weekly starting at 190 days after preimmunization, the mice were immunized with three 1-μg doses of MEP-KLH, and serum was collected on day 225. Mice with preexisting antibodies to MEP produced significantly lower titers than those in naive mice (○, P < 0.01; #, P < 0.05 [as determined by ANOVA with the Dunnet test for comparison to naive mice]). Bars represent the geometric mean of individual titers of five mice, and error bars represent the standard error of the mean. (B) Opsonic activity of sera pooled from mice with preexisting antibodies to MEP after vaccination with three 1-μg doses of MEP-KLH conjugate or a mixture of MEP and KLH. At a serum dilution of 1:5, the opsonic activity in sera from mice immunized with the conjugate vaccine was significantly higher than in sera from mice that had received nonconjugated polysaccharide, regardless of the presence of preexisting antibodies (○, P < 0.001 [as determined by ANOVA and Bonferroni test for pairwise comparison]). There was no significant difference in the opsonic activity at this serum dilution among any of the groups given the MEP-KLH conjugate regardless of the preimmunization regimen. At a serum dilution of 1:10, the sera of mice initially immunized i.p. with MEP showed no difference in opsonic killing activity regardless of whether the animals were immunized with MEP-KLH conjugate or the mixture. The sera of mice preimmunized with seaweed alginate and boosted with the MEP-KLH conjugate vaccine had a significantly higher killing activity at a 1:10 serum dilution than did sera from mice immunized with the mixture of MEP and KLH (○, P < 0.001). The opsonic killing activity of sera of naive mice immunized with the MEP-KLH conjugate was significantly higher than that of sera from animals vaccinated with MEP-KLH conjugate that were preimmunized with MEP (P < 0.001 [as determined by ANOVA by using the Dunnet test for comparison with naive mice]). Preimmunization with alginic acid either i.p. or perorally did not reduce the opsonic killing activity of sera compared to that in sera from nonpreimmunized mice given the conjugate vaccine. Bars represent mean percent killing of four replicates compared to preimmune serum, and the error bars represent the standard error of the mean.
Epitope specificity of the opsonic antibodies elicited by MEP-KLH.
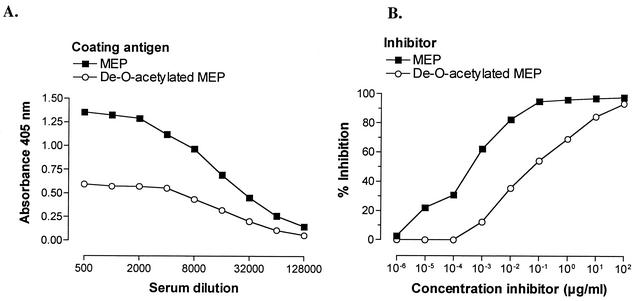
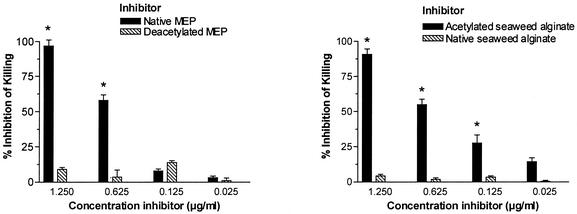
Previous studies of MEP-specific monoclonal and polyclonal opsonic antibodies have suggested that the epitope recognized by these antibodies contains O-acetyl groups (27). We therefore wanted to determine whether O-acetate-specific antibodies are induced by vaccination with the MEP-KLH conjugate and whether these antibodies mediate opsonic activity. IgG elicited by the MEP-KLH conjugate bound significantly less to de-O-acetylated MEP than to the native polysaccharide (Fig. 7). In a competitive inhibition ELISA, a 316-fold-higher concentration of de-O-acetylated MEP than of native MEP was necessary to inhibit 50% of the binding of MEP-specific IgG, indicating that the immunization with MEP-KLH raised a significant population of O-acetate-specific antibodies (Fig. 7). When deacetylated MEP was used as the solid-phase antigen, native and deacetylated MEP did not differ in potency to inhibit antibody binding (data not shown). The opsonic activity of antiserum after immunization with MEP-KLH was abrogated completely after preincubation of serum with native MEP, confirming that opsonic activity was MEP-specific (Fig. 8). After de-O acetylation, MEP lost its ability to inhibit opsonic killing (Fig. 8). Similar to MEP-alginate from P. aeruginosa seaweed alginate is a linear random polymer of l-guluronic and d-mannuronic acid but, unlike the P. aeruginosa antigen, algal alginate is not O acetylated. If opsonic antibodies bind to O acetate, one could speculate that these antibodies should recognize O-acetate residues on chemically O-acetylated seaweed alginate. Indeed, opsonic activity of sera after immunization with MEP-KLH was inhibited equally by chemically O-acetylated seaweed alginate and MEP but not by native nonacetylated seaweed alginate (Fig. 8).
FIG. 7.
Effect of acetate substituents on the binding of conjugate-induced antisera to MEP. (A) Binding of rabbit IgG after immunization with the MEP-KLH conjugate vaccine to MEP and de-O-acetylated MEP as the coating antigen in an ELISA. (B) Competitive inhibition ELISA with native MEP and de-O-acetylated MEP to inhibit binding of conjugate-induced antibody to native MEP as the solid-phase antigen. The percent inhibition was determined by comparison of the absorbance at 405 nm in the presence or absence of inhibitor. Native and de-O-acetylated MEP used for coating and inhibition were derived from P. aeruginosa strain 2192.
FIG. 8.
Inhibition of opsonic killing of rabbit serum raised against MEP-KLH by various inhibitors. All inhibitors were tested in concentrations up to 100 μg/ml, without any increase in inhibition at concentrations higher than 1.25 μg/ml (data not shown). (A) Comparative inhibition with native or deacetylated MEP. In concentrations of ≥0.625 μg/ml, native MEP inhibited opsonic killing activity significantly better than did de-O-acetylated MEP (✽, P < 0.001). (B) Comparative inhibition with native, non-O-acetylated, or chemically O-acetylated seaweed alginate. O-acetylated seaweed alginate inhibited opsonophagocytic killing activity significantly better than did the native polysaccharide (✽, P < 0.001 for inhibitor concentrations ≥0.125 μg/ml). The target strain in the opsonophagocytic killing assay was 2192. Native and de-O-acetylated MEP used for inhibition were also derived from strain 2192. Bars represent the mean of four replicates, and the error bars represent the standard error of the mean. Inhibition of opsonophagocytic killing was compared by ANOVA and Bonferroni test for pairwise comparison of O-acetylated and non-O-acetylated polysaccharide.
DISCUSSION
Probably because of the very large molecular weight of MEP, conjugating it to carrier proteins to produce immunogenic vaccines has proven difficult. For a previous conjugate vaccine of MEP constructed by Cryz et al. (4), the antigen had to be depolymerized by acid hydrolysis to facilitate its conjugation to exotoxin A. This approach, however, has some potential disadvantages. Conformational epitopes of bacterial polysaccharides often are stabilized by polymer length, which can be destroyed by depolymerization (18). Accordingly, it has been shown in mice that opsonic antibodies to MEP are preferentially induced by the large-molecular-weight fraction of the polysaccharide (10). Conditions necessary to depolymerize MEP by acid hydrolysis also result in its partial de-O acetylation. For the MEP conjugate produced by Cryz et al., acid hydrolysis resulted in a threefold reduction of O acetylation (4). The data presented in the present study and previous publications show, however, that O-acetyl groups contribute to the binding epitope of MEP-specific opsonic antibodies (27).
We therefore sought a conjugation scheme that would not involve denaturing the MEP polymer. Initially we tried to conjugate native, nondepolymerized MEP in an EDC-mediated reaction directly to KLH. We found that, similar to the observations of Cryz et al. (4), the reaction was difficult to control chemically and resulted in insoluble, highly cross-linked complexes and in excessive self-polymerization of the carrier protein. A modified reaction scheme utilizing a thiol-derivative of MEP to conjugate it to a malemide-activated carrier protein was more successful (39). The introduction of a defined amount of cystamine onto the MEP polymer avoided problems with overderivatization of the polymer. In fact, inhibition studies comparing native and cystamine-derivatized MEP showed that no antigenic epitopes are lost due to the incorporation of cystamine. Using this technology, we were able to construct a water-soluble conjugate of native, large-molecular-weight MEP under conditions that preserve O-acetate groups. A potential disadvantage of the chosen carrier protein and conjugation chemistry is that neither KLH nor the cross-linker used in the present study, SMCC, have yet been used in bacterial polysaccharide conjugate vaccines injected into humans. Conjugates of KLH made by using analogous maleimide-containing cross-linkers, however, have been constructed and tested in humans for a variety of carbohydrate and polypeptide conjugate vaccines directed primarily at tumor antigens (11, 24, 34, 37). In these trials, side effects of the vaccination were restricted to transient local skin reactions and mild flu-like symptoms. For our initial studies of an MEP conjugate vaccine, we wanted to provide evidence that conjugation in fact improves immunogenicity of this polysaccharide. Accordingly, we chose a carrier protein with superior immunogenicity (13). SMCC was chosen because its forms a chemically robust thio-ether bond and avoids problems associated with the more labile disulfide bond formed by other heterobifunctional cross-linkers such as N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP) (7, 14, 17). Further studies concerning the safety of this construct will be necessary if a use in humans is considered.
In the present study, native MEP did not elicit a measurable IgG response to vaccine doses up to 10 μg, thus behaving like a typical T-cell-independent antigen. Conjugation to KLH successfully induced high titers of IgG and a booster response upon repeated immunization, indicating a T-cell dependence of the immune response to the conjugate. High levels of MEP-specific IgG antibodies were maintained in mice until day 35 and declined slowly thereafter. A further booster dose increased IgG levels and stabilized them for the remaining observation period. Whereas IgG titers determined by ELISA are useful in screening sera for serologic responses, the levels of opsonic antibodies are the best predictor of protective efficacy in animal models (33) and are associated with resistance of patients with CF to chronic mucoid P. aeruginosa infection (31). In the opsonophagocytic killing assay, MEP conjugated to KLH was clearly superior to the native polysaccharide in its ability to induce opsonic antibodies in mice. Likewise, the MEP-KLH conjugate raised substantial opsonic titers in a hyperimmunized rabbit. In contrast to the result found here, Garner et al. reported the induction of opsonic antibodies in mice after their immunization with native MEP (10). A possible explanation for these conflicting results may be the different compositions of the MEP preparations used in the two studies: the MEP antigens differed in Kd, the ratio of mannuronric acid to guluronic acid, and acetate content (10). Also, small variations in impurities such as LPS may account for the differing immunogenicities, since such contaminants can bolster immune responses significantly. Despite its ability to induce opsonic antibodies in mice, however, the vaccine lot used by Garner et al. was poorly immunogenic in an immunization trial in human volunteers (28). In this vaccine study, only 35% of vaccinated individuals receiving an optimal dose of nonconjugated MEP had a rise at all in their titers of opsonic antibody, and most increases were quite modest (28).
The difficulties in inducing high titers of opsonic MEP-specific antibodies in humans have been suggested to be due to the presence of preexisting nonopsonic antibodies to MEP, which can be found in most humans and a large majority of CF patients regardless of whether they are colonized by P. aeruginosa (31). To test the immune response to the MEP-KLH conjugate in the setting of preexisting nonopsonic antibodies, we mimicked this state in mice by prior immunization with either MEP or seaweed alginate (10). It is interesting that oral administration of seaweed alginate also elicited IgM antibodies cross-reactive to MEP. As seen for naive mice, nonconjugated MEP did not elicit IgG antibodies in the context of preexisting nonopsonic antibodies. However, mice with preexisting nonopsonic antibodies that then had received the MEP-KLH conjugate vaccine generated MEP-specific IgG titers, although their ELISA titers were lower compared to those in mice that had not been preimmunized. Thus, not only MEP itself but also seaweed alginates, which are probably encountered frequently in the environment and food, have the potential to induce antibodies that interfere with subsequent MEP-based vaccination. Our results indicate that conjugation of MEP can partly overcome the inhibitory effects of preexisting nonopsonic antibodies. Further work will need to address the immunogenicity of MEP-conjugates in order to obtain higher levels of MEP-specific antibodies in individuals preexposed to this antigen. In addition, this finding could have important implications for the vaccination of CF patients. According to our data, an optimal immune response to an MEP conjugate vaccine is obtained before environmental exposure to bacterial or algal alginates gives rise to nonopsonic antibodies to MEP. With screening programs for detecting CF at birth becoming more common, early childhood vaccination of CF patients may be a feasible approach (42).
A key feature of any vaccine is its ability to protect against infection with strains of the organism heterologous to the one from which the vaccine was derived. For MEP, with its considerable variation in the ratio of mannuronic to guluronic acid and degree of O acetylation, it is crucial to establish that vaccination with a single preparation induces antibodies reactive against heterologous mucoid P. aeruginosa strains (35). Our data suggest that IgG induced by MEP-KLH is cross-reactive to a wide variety of heterologous mucoid strains and that opsonic activity of immune sera also has broad specificity for multiple strains. In contrast to our results, cross-reactive IgG was virtually absent after immunization of rats with a MEP-exotoxin A conjugate (15, 16). These data suggest that extensive depolymerization and/or de-O acetylation may lead to the loss of epitopes shared between heterologous strains.
O-acetyl groups on capsular polysaccharides have been shown to be important for antigenicity and elicitation of opsonic antibodies against a variety of encapsulated bacterial pathogens. For example, O-acetyl groups are critical for the induction of opsonic antibodies to serogroup A polysaccharide from Neisseria meningitidis (1). Opsonic antibodies to serogroup C polysaccharide from N. meningitidis, on the other hand, are preferentially induced by the de-O-acetylated polysaccharide (23). Finally, epitopes for opsonic antibodies are found both on the backbone structure and associated with the O-acetyl groups of Staphylococcus aureus type 5 and type 8 capsular polysaccharide (8). According to our data, the immunization with MEP-KLH induced two distinct populations of antibodies binding either to O-acetated epitopes or to epitopes localized on the portion of the polysaccharide lacking O acetates. In an inhibition opsonophagocytic killing assay, however, only the native, O-acetylated polysaccharide inhibited killing of the target strain, indicating that MEP-specific opsonic antibodies recognize O-acetyl substituents. The finding that nonopsonic antibodies to MEP can normally kill mutant strains of mucoid P. aeruginosa that produce nonacetylated MEP supports these results (27). In contrast, only opsonic antibodies recognizing O-acetylated epitopes can kill wild-type strains (27). Previous work (22, 30) indicates that nonopsonic antibodies to MEP activate but fail to deposit the key complement opsonin, iC3b, onto the bacterial surface. However, we can only speculate as to why backbone-specific nonopsonic antibodies are immunodominant. The data here implicate environmental or food exposure to nonacetylated seaweed alginate, or a similar antigen, in inducing a state predisposing to the immunodominance of the nonopsonic antibodies.
Seaweed alginate is similar to MEP, since both are β1-4-linked linear random polymers of d-mannuronic and l-guluronic acid but, unlike MEP, seaweed alginate is nonacetylated (12). In theory, chemical O acetylation could be sufficient to establish epitopes on seaweed alginate recognized by opsonic MEP-specific antibodies. To test this hypothesis, we generated O-acetylated seaweed alginate by treating it with acetic anhydride (38). When used as an inhibitor in the opsonophagocytic killing assay, O-acetylated algal alginate had the same inhibitory potency as native MEP. Encouraged by this finding, we wanted to test whether MEP-specific opsonic antibodies can also be raised by immunization with O-acetylated algal alginate (C. Theilacker, Y. Wang, M. Grout, and G. B. Pier, Abstr. 101th Gen. Meet. Am. Soc. Microbiol., abstr. E-33, 2001). We synthesized two conjugate vaccines of O-acetylated algal alginates that differed slightly in their mannuronic acid to guluronic acid ratio and O-acetate content. Rabbit serum after immunization with these vaccines elicited high titers of IgG antibodies cross-reactive to MEP. Opsonic activity of the sera raised against the acetylated seaweed alginate conjugates, however, was disappointingly low. We had to conclude that, despite great antigenic similarity between the two polysaccharides, opsonic epitopes are not immunogenic when expressed on acetylated algal alginate. The lack of induction of mucoid P. aeruginosa-specific opsonic antibodies could be due to the subtle chemical differences between these bacterial and seaweed alginates: unlike MEP, algal alginate contains blocks of neighboring guluronic acid residues (12). Also, chemical O acetylation will acetylate G and M residues on the seaweed alginate indiscriminately, whereas only M residues are O acetylated on bacterially produced MEP (36).
In summary, conjugation of MEP to KLH utilizing thiolation of the polysaccharide and conjugation to a malemide-activated carrier yielded a conjugate vaccine that elicited high titers of MEP-specific IgG. Antisera raised against the conjugate had high opsonic activity not only against the vaccine strain but also against a variety of mucoid strains of P. aeruginosa. The MEP conjugate also was able to induce antibodies in mice that had preexisting nonopsonic antibodies against MEP, although the titer was lower than that achieved in naive mice. Finally, opsonic antibodies elicited by MEP-KLH were specific for epitopes containing O-acetyl groups. These data indicate that an MEP-based vaccine has significant potential to protect against chronic infection with mucoid P. aeruginosa in the CF host.
Acknowledgments
We thank G. Skjak-Braek for the 1H NMR analysis of the alginate samples.
This work was supported by NIH grant R01 AI 48917 and by the Eli Lilly International Foundation (C.T.).
Editor: V. J. DiRita
REFERENCES
- 1.Berry, D. S., F. Lynn, C. H. Lee, C. E. Frasch, and M. C. Bash. 2002. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 70:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Coleman, F. T., S. Mueschenborn, G. Meluleni, C. Ray, V. J. Carey, S. O. Vargas, C. L. Cannon, F. M. Ausubel, and G. B. Pier. 2003. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc. Natl. Acad. Sci. USA 100:1949-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryz, S. J., Jr., E. Furer, and J. U. Que. 1991. Synthesis and characterization of a Pseudomonas aeruginosa alginate-toxin A conjugate vaccine. Infect. Immun. 59:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demko, C. A., P. J. Byard, and P. B. Davis. 1995. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J. Clin. Epidemiol. 48:1041-1049. [DOI] [PubMed] [Google Scholar]
- 6.Ellman, G. L. 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82:70-77. [DOI] [PubMed] [Google Scholar]
- 7.Fattom, A., J. Shiloach, D. Bryla, D. Fitzgerald, I. Pastan, W. W. Karakawa, J. B. Robbins, and R. Schneerson. 1992. Comparative immunogenicity of conjugates composed of the Staphylococcus aureus type 8 capsular polysaccharide bound to carrier proteins by adipic acid dihydrazide or N-succinimidyl-3-(2-pyridyldithio)propionate. Infect. Immun. 60:584-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattom, A. I., J. Sarwar, L. Basham, S. Ennifar, and R. Naso. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filisetti-Cozzi, T. M., and N. C. Carpita. 1991. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 197:157-162. [DOI] [PubMed] [Google Scholar]
- 10.Garner, C. V., D. DesJardins, and G. B. Pier. 1990. Immunogenic properties of Pseudomonas aeruginosa mucoid exopolysaccharide. Infect. Immun. 58:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilewski, T., S. Adluri, G. Ragupathi, S. Zhang, T. J. Yao, K. Panageas, M. Moynahan, A. Houghton, L. Norton, and P. O. Livingston. 2000. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin. Cancer Res. 6:1693-1701. [PubMed]
- 12.Grasdalen, H. 1983. High-field, 1H-NMR spectroscopy of alginate: sequential structure and linkage conformations. Carbohydr. Res. 118:255-260. [Google Scholar]
- 13.Helling, F., A. Shang, M. Calves, S. Zhang, S. Ren, R. K. Yu, H. F. Oettgen, and P. O. Livingston. 1994. GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 54:197-203. [PubMed] [Google Scholar]
- 14.Hermanson-GT. 1996. Bioconjugate techniques. Academic Press, Inc., San Diego, Calif.
- 15.Johansen, H. K., F. Espersen, S. J. Cryz, Jr., H. P. Hougen, A. Fomsgaard, J. Rygaard, and N. Hoiby. 1994. Immunization with Pseudomonas aeruginosa vaccines and adjuvant can modulate the type of inflammatory response subsequent to infection. Infect. Immun. 62:3146-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen, H. K., H. P. Hougen, S. J. Cryz, Jr., J. Rygaard, and N. Hoiby. 1995. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am. J. Respir. Crit. Care Med. 152:1337-1346. [DOI] [PubMed] [Google Scholar]
- 17.Kossaczka, Z., F. Y. Lin, V. A. Ho, N. T. Thuy, P. Van Bay, T. C. Thanh, H. B. Khiem, D. D. Trach, A. Karpas, S. Hunt, D. A. Bryla, R. Schneerson, J. B. Robbins, and S. C. Szu. 1999. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 67:5806-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laferriere, C. A., R. K. Sood, J. M. de Muys, F. Michon, and H. J. Jennings. 1998. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect. Immun. 66:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, F. Y., V. A. Ho, H. B. Khiem, D. D. Trach, P. V. Bay, T. C. Thanh, Z. Kossaczka, D. A. Bryla, J. Shiloach, J. B. Robbins, R. Schneerson, S. C. Szu, M. N. Lanh, S. Hunt, L. Trinh, and J. B. Kaufman. 2001. The efficacy of a Salmonella typhi Vi conjugate vaccine in two- to-five-year-old children. N. Engl. J. Med. 344:1263-1269. [DOI] [PubMed] [Google Scholar]
- 20.Lindberg, A. A. 1999. Glycoprotein conjugate vaccines. Vaccine 17(Suppl. 2):S28-S36. [DOI] [PubMed] [Google Scholar]
- 21.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meluleni, G. J., M. Grout, D. J. Evans, and G. B. Pier. 1995. Mucoid Pseudomonas aeruginosa growing in a biofilm in vitro are killed by opsonic antibodies to the mucoid exopolysaccharide capsule but not by antibodies produced during chronic lung infection in cystic fibrosis patients. J. Immunol. 155:2029-2038. [PubMed] [Google Scholar]
- 23.Michon, F., C. H. Huang, E. K. Farley, L. Hronowski, J. Di, and P. C. Fusco. 2000. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev. Biol. 103:151-160. [PubMed] [Google Scholar]
- 24.Musselli, C., P. O. Livingston, and G. Ragupathi. 2001. Keyhole limpet hemocyanin conjugate vaccines against cancer: the Memorial Sloan Kettering experience. J. Cancer Res. Clin. Oncol. 127(Suppl. 2):R20-R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohman, D. E., and A. M. Chakrabarty. 1981. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect. Immun. 33:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onsoyen, E. 1996. Commercial applications of alginates. Carbohydr. Eur. 14:26-31. [Google Scholar]
- 27.Pier, G. B., F. Coleman, M. Grout, M. Franklin, and D. E. Ohman. 2001. Role of alginate O-acetylation in the resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect. Immun. 69:1895-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pier, G. B., D. DesJardin, M. Grout, C. Garner, S. E. Bennett, G. Pekoe, S. A. Fuller, M. O. Thornton, W. S. Harkonen, and H. C. Miller. 1994. Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect. Immun. 62:3972-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pier, G. B., D. Desjardins, T. Aguilar, M. Barnard, and D. P. Speert. 1986. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J. Clin. Microbiol. 24:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pier, G. B., M. Grout, and D. Desjardins. 1991. Complement deposition by antibodies to Pseudomonas aeruginosa mucoid exopolysaccharide (MEP) and by non-MEP specific opsonins. J. Immunol. 147:1869-1876. [PubMed] [Google Scholar]
- 31.Pier, G. B., W. J. Matthews, Jr., and D. D. Eardley. 1983. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J. Infect. Dis. 147:494-503. [DOI] [PubMed] [Google Scholar]
- 32.Pier, G. B., J. M. Saunders, P. Ames, M. S. Edwards, H. Auerbach, J. Goldfarb, D. P. Speert, and S. Hurwitch. 1987. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N. Engl. J. Med. 317:793-798. [DOI] [PubMed] [Google Scholar]
- 33.Pier, G. B., G. J. Small, and H. B. Warren. 1990. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science 249:537-540. [DOI] [PubMed] [Google Scholar]
- 34.Ragupathi, G., R. R. Koganty, D. Qiu, K. O. Lloyd, and P. O. Livingston. 1998. A novel and efficient method for synthetic carbohydrate conjugate vaccine preparation: synthesis of sialyl Tn-KLH conjugate using a 4-(4-N-maleimidomethyl)cyclohexane-1-carboxyl hydrazide (MMCCH) linker arm. Glycoconj. J. 15:217-221. [DOI] [PubMed] [Google Scholar]
- 35.Sherbrock-Cox, V., N. J. Russell, and P. Gacesa. 1984. The purification and chemical characterization of the alginate present in extracellular material produced by mucoid strains of Pseudomonas aeruginosa. Carbohydr. Res. 135:147-154. [DOI] [PubMed] [Google Scholar]
- 36.Skjak-Braek, G., H. Grasdalen, and B. Larsen. 1986. Monomer sequence and acetylation pattern in some bacterial alginates. Carbohydr. Res. 154:239-250. [DOI] [PubMed] [Google Scholar]
- 37.Slovin, S. F., G. Ragupathi, S. Adluri, G. Ungers, K. Terry, S. Kim, M. Spassova, W. G. Bornmann, M. Fazzari, L. Dantis, K. Olkiewicz, K. O. Lloyd, P. O. Livingston, S. J. Danishefsky, and H. I. Scher. 1999. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl. Acad. Sci. USA 96:5710-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szu, S. C., S. Bystricky, M. Hinojosa-Ahumada, W. Egan, and J. B. Robbins. 1994. Synthesis and some immunologic properties of an O-acetyl pectin [poly(1→4)-alpha-d-GalpA]-protein conjugate as a vaccine for typhoid fever. Infect. Immun. 62:5545-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szu, S. C., A. L. Stone, J. D. Robbins, R. Schneerson, and J. B. Robbins. 1987. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J. Exp. Med. 166:1510-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Wiel, P. A., M. H. Witvliet, D. Evenberg, H. J. Derks, and E. C. Beuvery. 1987. O-polysaccharide-protein conjugates induce high levels of specific antibodies to Pseudomonas aeruginosa immunotype 3 lipopolysaccharide. Vaccine 5:33-38. [DOI] [PubMed] [Google Scholar]
- 41.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, S. S., M. Kharrazi, M. Pearl, and G. Cunningham. 2001. Cystic fibrosis screening in newborns: results from existing programs. Curr. Opin. Pulmon. Med. 7:427-433. [DOI] [PubMed] [Google Scholar]