Abstract
Chronic enterocolitis is the leading cause of morbidity in colonies of captive rhesus macaques (Macaca mulatta). This study's aim was to identify the common enteric pathogens frequently associated with chronic enterocolitis in normal, immunocompetent rhesus monkeys and to elucidate the influence of this clinical syndrome on the host immune system. We analyzed the fecal specimens from 100 rhesus macaques with or without clinical symptoms of chronic diarrhea. Retrospective analysis revealed an increased incidence of Campylobacter spp. (Campylobacter coli and Campylobacter jejuni), Shigella flexneri, Yersinia enterocolitica, adenovirus, and Strongyloides fulleborni in samples collected from animals with chronic diarrhea (P < 0.05). The presence of additional enteric pathogens, such as Escherichia coli, carrying the eaeA intimin or Stx2c Shiga toxin virulence genes, Balantidium coli, Giardia lamblia, Enterocytozoon bieneusi, and Trichuris trichiura was found in all animals regardless of whether diarrhea was present. In addition, the upregulation of interleukin-1α (IL-1α), IL-3, and tumor necrosis factor alpha cytokine genes, accompanied by an increased presence of activated (CD4+ CD69+) T lymphocytes was found in gut-associated lymphoid tissues collected from animals with chronic enterocolitis and diarrhea in comparison with clinically healthy controls (P < 0.05). These data indicate that chronic enterocolitis and diarrhea are associated, in part, with a variety of enteric pathogens and highlight the importance of defining the microbiological status of nonhuman primates used for infectious disease studies. The data also suggest that chronic colitis in rhesus macaques may have potential as a model of inflammatory bowel disease in humans.
A high incidence of chronic enterocolitis associated with the presence of opportunistic and/or obligate pathogens known to cause disease of the gastrointestinal tract has been recorded from colonies with captive rhesus monkeys (13, 25, 28). In colonies of nonhuman primates, recurring diarrhea is the leading cause of animal morbidity requiring veterinary care (13, 25). The success of the rhesus macaque model of AIDS and the identification of the intestinal immune system as a primary target of human immunodeficiency virus and simian immunodeficiency virus (SIV) has resulted in increased demands for microbiologically and genetically defined animals. The need for pathogen defined rhesus monkeys has reached critical proportions (9, 10, 27). At present, most rhesus monkey specific-pathogen-free colonies are defined by negative testing for four viral agents (SIV, type D simian retrovirus, simian T lymphotropic virus type 1, and simian herpes B virus). However, AIDS research focusing on opportunistic infections, novel vaccine strategies, immune modulating pharmaceutical agents and gene therapy approaches may require that rhesus monkeys be free of additional viruses, protozoa, and bacteria. Developing strategies to prevent or treat enteric infections and elucidate their role, or lack thereof, in AIDS enteropathy and disease progression is one objective of research at the Tulane National Primate Research Center (TNPRC) (32, 33). Thus, the aim of the present study was to determine the incidence of infectious microorganisms commonly associated with symptoms of chronic enterocolitis in captive rhesus macaques, thereby improving the research value of this animal model. In addition, we evaluated the level of general immune response indicators in regard to systemic and mucosal lymphoid tissues from these animals by measuring the expression of CD69 and CD45RA by CD4+ and CD8+ T lymphocytes and RNA expression of 23 cytokine genes. We hypothesized that chronic inflammation of the colon in rhesus monkeys might not only clinically but also immunologically resemble the inflammatory bowel disease (IBD) of a human patient.
MATERIALS AND METHODS
Rhesus macaques: categories and fecal sample collection.
One hundred rhesus macaques (Macaca mulatta) of either sex were examined as they were admitted to veterinary clinic at TNPRC in between January and June 2002. The ages of studied animals ranged from 6.5 months to 24.5 years. All animals originated from the TNPRC and were housed in biosecurity level 2 (BL2) in accordance with standards of the Guide for the Care and Use of Laboratory Animals and the Association for Assessment and Accreditation of Laboratory Animal Care. Only simian retrovirus (SIV, simian retrovirus, and simian T lymphotropic virus)- and tuberculosis-negative animals were used. Investigators adhered to the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council. Fifty animals had no clinical symptoms of diarrhea (surgery-related admissions), and the other fifty animals showed symptoms of chronic diarrhea at the time of sample collection (Table 1). Chronic diarrhea was defined by the manifestation of multiple outbreaks of clinical diarrhea over a period of at least 1 month. In general, every animal with or without a history of diarrhea that was presented to the veterinary clinic at TNPRC was clinically monitored on a daily basis. To obtain stools, cage pans were cleaned in the morning and stools were collected in the afternoon. Collected samples were made into 50% suspensions with phosphate-buffered saline (PBS; pH 7.4) and divided into aliquots for virus, bacterium, and parasite screening. Samples to detect bacteria and parasites were processed on the day of sample collection. Samples for viral analysis were stored frozen at −80°C until tested.
TABLE 1.
Clinical summary of 100 rhesus macaques from which samples were obtained
| Group (n) | Mean age (yr) ± SD | Male/ female ratio | Clinical diarrhea (%) | Mean stool consistencya ± SD | History of diarrhea (%) |
|---|---|---|---|---|---|
| Clinically healthy (50) | 6.2 ± 6.2 | 0.32 | 0 | 1 ± 0 | 32 |
| Chronic diarrhea (50) | 6.0 ± 5.8 | 0.42 | 100 | 3.8 ± 0.4 | 100 |
Stool consistency was assigned based on a scale from 1 to 5: 1, normal; 2, pasty; 3, semiliquid; 4, watery; 5, watery with the presence of blood.
Histopathological evaluation and tissue sample collection.
Tissues from six rhesus macaques were collected. Three animals with clinical symptoms of chronic diarrhea (J079, CJ41, and CJ51) and three age-matched control animals without clinical symptoms of diarrhea (T080, DE22, and V176) were randomly selected. All six animals received a complete necropsy and histopathologic examination. Samples of systemic tissues (spleen), gut-associated lymphoid tissues (mesenteric lymph nodes), and the gastrointestinal tract had been collected immediately after euthanasia with an intravenous overdose of pentobarbital. The small and large intestines were removed and separated into the duodenum; jejunum; ileocecal junction; proximal, middle, and distal colon; and rectum. Tissues were fixed in 10% neutral buffered formalin, routinely processed for histopathologic examination, sectioned at 6 μm, and stained with hematoxylin and eosin (H&E). In addition, the spleens and mesenteric lymph nodes were used for (i) isolation of mononuclear cells and flow cytometry as previously described (31, 38) and (ii) frozen at −80°C for later extraction of total RNA.
Detection of gastrointestinal pathogens.
The presence of a variety of viral, bacterial, and parasitic agents known to infect nonhuman primates was examined.
Viral agents.
A commercially available immunoassay (Dako, Cambridgeshire, United Kingdom) that is capable of detecting the presence of any of the 49 known adenovirus serotypes (product code K6021) was used according to the manufacturer's instructions to examine the presence of adenoviruses in 100 of the fecal specimens. In addition, all samples were tested by enzyme-linked immunosorbent assay for the presence of rotavirus antigens at the Cincinnati Children's Hospital as described previously (23).
Bacterial agents.
In order to detect the presence of common bacterial pathogens, the following method was used. Stool samples were plated on (i) xylose-lysine-deoxycholate agar (BBL, Cockeysville, Md.), (ii) Hektoen enteric agar (BBL), (iii) eosin methylene blue agar (BBL), (iv) Campylobacter selective agar (BBL), (v) Yersinia selective agar (Oxoid, Ltd., Basingstoke, England), and (vi) Yersinia enrichment broth (Oxoid). Plates 1, 2, and 3 were examined after 18 to 24 h of incubation at 37°C. Colonies indicative of Salmonella sp. or Shigella sp. were subcultured to triple sugar iron agar (BBL), incubated for 18 to 24 h at 37°C, and typed with the appropriate antisera. Colonies suggestive of Escherichia coli were identified and tested for the presence of enteropathogenic Escherichia coli (EPEC) and enterotoxigenic Escherichia coli strains at the Veterinary Research Institute, Brno, Czech Republic, and Tulane University Health Sciences Center, respectively, as described previously (6, 7). Multiplex PCR was used to test for the presence of genes responsible for eaeA intimin and Stx1, Stx2, and Stx2c Shiga toxin as described previously (24). Positive strains were tested for the production of Vero toxins (6). Plate 4 was incubated for 48 h at 42°C in an atmosphere of 10% carbon dioxide and 5% oxygen, with a balance of nitrogen. Confirmation of Campylobacter identification was done by Gram stain and determining susceptibility to nalidixic acid and cephalothin. The hippurate test was used to differentiate Campylobacter coli and Campylobacter jejuni (14). Plate 5 was incubated at room temperature for 48 h. Colonies indicative of Yersinia were subcultured to TSIA slants, urea broth, phenylalanine slants, and analytical profile index (bioMérieux, Marcy l'Étoile, France) identification panels. The Yersinia cold broth (plate 6) remained in the refrigerator (4°C) for 5 to 7 days. Subsequently, it was subcultured onto a Yersinia selective agar (Oxoid) plate, and the procedure for plate 5 was repeated.
Parasitic agents.
For the detection of parasitic agents, a direct fecal smear, followed by a concentration technique, was used (1). Briefly, by the use of an applicator stick, 2 mg of stool was added to a drop of PBS (pH 7.4) on a slide. After thorough mixing, a 22-by-22-mm coverslip was added. The motility of the organisms was observed under a light microscope (magnification, ×40 to ×100). In addition to the direct smear, a flotation method was performed as described previously (1), and the preparations were examined by light microscopy. For detection and characterization of microsporidia (Enterocytozoon bieneusi), calcofluor staining to screen the samples and nested PCR to confirm the results with Enterocytozoon bieneusi primers were used as previously described (16, 33).
Cytokine gene expression.
Total RNA was isolated from 3 g of mesenteric lymph node or spleen tissues and tested for the expression of 23 common cytokine genes (interleukin-1α [IL-1α], IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12α, IL-12β, IL-13, IL-14, IL-15, IL-16, IL-17, IL-18, gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], and TNF-β) and internal standard control genes (β-actin, GAPDH [glyceraldehyde-3-phosphate dehydrogenase], and pUC18) by using a Nonrad-GEArray kit (SuperArray, Inc., Bethesda, Md.). Briefly, biotinylated cytokine DNA probes were constructed from the total RNA templates by reverse transcription of mRNA by using a cytokine-specific primer mix supplied by the manufacturer, biotin-16-dUTP (Promega, Madison, Wis.) and Moloney murine leukemia virus reverse transcriptase (Promega). The labeling reaction was stopped after 4 h, and the probes were denatured and added to a hybridization solution. During the labeling reaction, a gene expression array cytokine membrane (GEArray membrane) was treated by prehybridization with salmon sperm DNA (Invitrogen, Inc., Carlsbad, Calif.) to prevent nonspecific binding. The GEArray membrane was then hybridized overnight with the probe solution in a sealed plastic bag. After hybridization, the probe solution was washed off and the GEArray membrane was incubated with a 1:4,000 alkaline phosphatase-streptavidin solution (supplied with GEArray) and subsequently with CDP-Star color substrate solution for the visualization of the hybridization (spot) reaction. A sheet of X-ray film (Kodak, Rochester, N.Y.) was used to develop an image of the membrane. Relative mRNA expression was calculated from the films via local background corrected spot densiometry measurements with ScanAlyze v2.44 software (Stanford University, Stanford, Calif.) and comparison with the β-actin (positive) and pUC18 (negative) controls built into the array.
Flow cytometry.
Spleen and mesenteric lymph nodes were used for isolation of mononuclear cells as previously described (31). Gating was set based on the CD3+ “bright” T-lymphocyte population as determined by single-color staining. Proportions of CD69+ (activated T lymphocytes) and CD45RA (naive T lymphocytes) in CD4+ and CD8+ (T helper and cytotoxic/suppressor lymphocytes) populations were assessed by four-color flow cytometry. The combination of CD69, CD45RA, CD4, and CD8 was used for the mononuclear cells isolated from mesenteric lymph nodes and spleens as described previously (31, 33). Briefly, the extracted, purified, and stained mononuclear cells were fixed in PBS (pH 7.4) containing 1% paraformaldehyde (Sigma, St. Louis, Mo.). A FACSCalibur flow cytometer with CellQuest software (Becton-Dickinson, Franklin Lakes, N.J.) was used to perform the quantitation of naive and activated CD4+- and CD8+-T-lymphocyte populations.
Statistical analysis.
The prevalence of each infectious agent in the chronic diarrhea group was compared to its prevalence in the clinically healthy group by using the chi-square test. The effect of multiple bacterial, viral, and parasite infections, specifically adenovirus, Campylobacter spp., Shigella flexneri, Yersinia enterocolitica, and Strongyloides fulleborni, on the health status (clinically healthy versus chronic diarrhea) was statistically analyzed by using the chi-square distribution test. The cytokine gene expression and T-lymphocyte populations were compared between the two groups described above by a paired-sample Student t test with two-tailed distribution.
RESULTS
Histopathology findings.
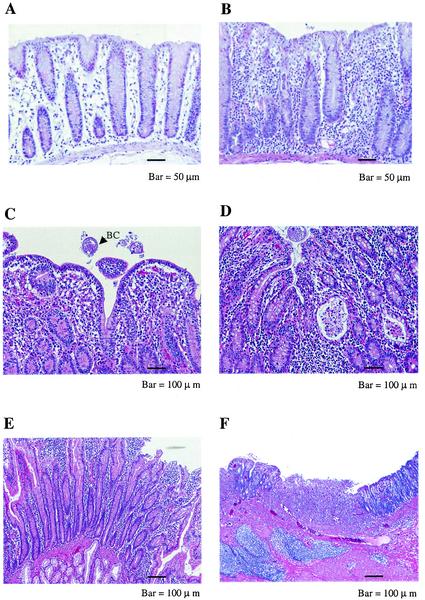
A range of histopathological changes were observed in rhesus macaques with clinical symptoms of chronic diarrhea that were euthanized (Fig. 1). The most typical and prominent lesions included the infiltration of colon lamina propria with inflammatory cells, such as lymphocytes, neutrophils, and plasma cells; the loss of goblet cells; crypt dilation; amyloidosis; and the accumulation of necrotic debris in the lumen of the crypts. The colon and terminal ileum were the most affected parts of chronically inflamed intestines. Other parts of the intestinal tract, such as the duodenum, ileum, and cecum, were also affected with the accumulation of inflammatory cells, mostly in the lamina propria (Fig. 1).
FIG. 1.
H&E-stained tissue sections of small and large intestine tissues from selected animals with symptoms of chronic diarrhea (J079, CJ41, and CJ51) and a clinically normal control (T080). (A) Normal colon (T080) with straight crypts, the presence of goblet cells, and lamina propria with a few mononuclear cells. (B) Chronic colitis (J079). The lamina propria is densely infiltrated by mononuclear cells. (C) Chronic colitis (CJ51). The loss of goblet cells from mucosal epithelium and the presence of Balantidium coli (BC) is shown. (d) Active chronic colitis (CJ51). Moderately dense infiltration of lamina propria by mononuclear cells, crypt dilatation, and crypt abscess with accumulation of granulocytes and necrotic debris in the lumen of the gland can be seen. (E) Duodenum, chronic enteritis (CJ51). Inflammatory cells are present in the lamina propria with inflammation confined to the mucosa. (F) Ileocecal junction, severe chronic ulcerative ileocolitis (CJ41). Both acute and chronic inflammatory cells are present. Numerous neutrophils are present in the ulcer. Lymphocytes, neutrophils, plasma cells and fibroblasts are present in the lamina propria.
Gastrointestinal agents identified.
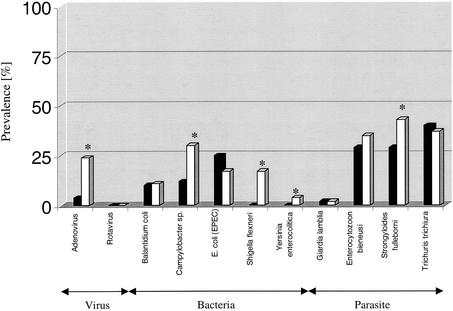
A variety of infectious agents were identified in both clinically healthy animals and animals with chronic diarrhea. Microorganisms and/or enteric pathogens that were detected more frequently (P < 0.05) in animals with chronic diarrhea than in clinically healthy animals included adenovirus; enteric bacteria including Campylobacter spp. (Campylobacter coli and Campylobacter jejuni species), Shigella flexneri, and Yersinia enterocolitica; and the parasitic nematode Strongyloides fulleborni (Fig. 2). In contrast, enteric pathogens detected in all animals without being significantly more prevalent in macaques with chronic diarrhea included EPEC carrying the eaeA intimin or Stx2c Shiga toxin virulence genes; the protozoan parasites Balantidium coli and Giardia lamblia; the helminth Trichuris trichiura; and the microsporidium Enterocytozoon bieneusi (Fig. 2). The effect of multiple bacterial, viral, and parasite infections, specifically adenovirus, Campylobacter spp., Shigella flexneri, Yersinia enterocolitica, and Strongyloides fulleborni, on the health status (i.e., clinically healthy animals versus animals with chronic diarrhea) was statistically examined. The cumulative effect of these infectious agents was found to be significant at a P value of <0.002 (df = 4). The production of verotoxin was detected by cytotoxicity assay in two of seven of Stx2c Escherichia coli strains. No rotavirus was detected in any of the animals tested. In addition to the enteric pathogens described above, we identified up to a 90% incidence of ameboid protozoa (Entamoeba coli and Iodoamoeba spp.) and a 26% incidence of protozoan flagellates (Trichomonas and and Chilomastix spp.) regardless of whether the animals did or did not have chronic diarrhea.
FIG. 2.
Percentages of infectious agents detected from stools of 100 rhesus macaques either with (□, n = 50) or without (▪, n = 50) symptoms of chronic diarrhea. A statistically significant difference (P < 0.05) between the two groups is indicated by an asterisk.
T-lymphocyte populations.
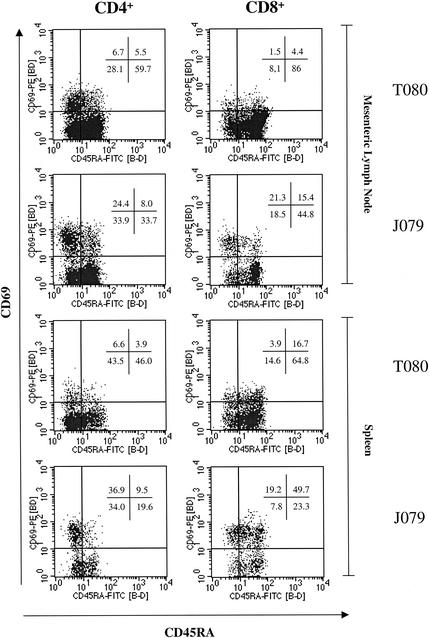
Four-color flow cytometry was used to determine the distribution of activated and/or naive CD4+ and CD8+ T lymphocytes in gut-associated lymphoid tissues (mesenteric lymph nodes) and spleens. In clinically healthy animals both gut-associated lymphoid tissues and spleen CD4+ and CD8+ T lymphocyte populations contained a higher proportion of naive (CD45RA+) cells (48.5% ± 8.2% and 77.7% ± 8.9%, respectively) and a lower proportion of activated (CD69+) cells (12.3% ± 4.0% and 4.7% ± 2.6%) compared to animals that exhibited clinical symptoms of chronic diarrhea (Table 2 and Fig. 3). Statistically significant elevations (P < 0.05), however, were only found in CD4+ CD69+ T lymphocytes in the mesenteric lymph nodes of animals with chronic diarrhea (23.3% ± 4.0%, Table 2). However, the trend (P > 0.05) for an increased % of activated lymphocyte populations in animals with chronic diarrhea was observed in both the mesenteric lymph node and the spleen. When the CD4+ and CD8+ populations were compared, a larger proportion of activated cells (CD69+) and a lower proportion of naive cells (CD45RA+) were observed in the CD4+ T-cell population than in the CD8+ T-cell population in both groups of animals (Table 2).
TABLE 2.
Activated-T-cell phenotypesa
| Antigen expression | Mean % activated T cells ± SD in:
|
|||
|---|---|---|---|---|
| Mesenteric lymph nodes
|
Spleens
|
|||
| Clinically healthy animals | Animals with chronic diarrhea | Clinically healthy animals | Animals with chronic diarrhea | |
| CD4+ | ||||
| CD45RA+ CD69− | 48.5 ± 8.2 | 24.1 ± 7.1 | 42.3 ± 2.8 | 24.7 ± 7.3 |
| CD45RA− CD69+ | 12.3 ± 4.0 | 23.3 ± 4.0b | 12.6 ± 4.3 | 24.0 ± 9.8 |
| CD45RA+ CD69+ | 5.1 ± 0.7 | 5.2 ± 2.4 | 5.9 ± 1.7 | 8.9 ± 3.7 |
| CD8+ | ||||
| CD45RA+ CD69− | 77.7 ± 8.9 | 50.7 ± 4.4 | 52.6 ± 9.3 | 33.5 ± 9.9 |
| CD45RA− CD69+ | 4.7 ± 2.6 | 13.9 ± 5.3 | 11.3 ± 5.2 | 10.8 ± 5.9 |
| CD45RA+ CD69+ | 5.6 ± 1.0 | 9.8 ± 4.2 | 19.3 ± 2.8 | 43.5 ± 10.9 |
Values represent subsets of animals with chronic diarrhea (n = 3) and clinically healthy controls (n = 3).
A statistically significant difference (P < 0.05) between the two groups is indicated.
FIG. 3.
Flow cytometry histograms of mononuclear cells isolated from lymphoid tissues of an animal with chronic diarrhea (J079) and a clinically healthy control (T080). Gating was performed on population of bright CD3+ T lymphocytes as determined by a single-color-stained sample. Major differences between the two animals can be seen between the percentages of activated (CD69) and naive (CD45RA+) CD4+ and CD8+ lymphocyte subsets in both mesenteric lymph nodes and spleens.
Cytokine gene expression.
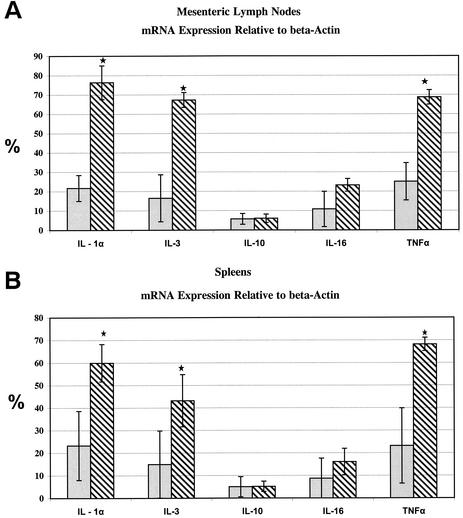
Statistically significant upregulation (P < 0.05) of cytokine genes specific for IL-1α, IL-3, and TNF-α in both the mesenteric lymph nodes and the spleens of animals with chronic diarrhea versus clinically healthy animals (Fig. 4) was detected. Each of these cytokine genes showed at least a 2.5-fold increase in animals with chronic diarrhea compared to those without diarrhea. The level of expression of these cytokine genes in normal animals was 20 to 30% of the internal standard control (β-actin), whereas in animals with chronic diarrhea these cytokine genes were expressed at 60 to 70% of the internal standard control. IL-10 and IL-16 gene expression in both the mesenteric lymph nodes and the spleen was not significantly different between the two groups (Fig. 4). The remainder of the 18 tested cytokine genes were expressed at very low or undetectable levels.
FIG. 4.
The relative abundance of mRNA transcripts specific for IL-1α, IL-3, IL-10, IL-16, and TNF-α cytokine genes is shown and compared between the animals with chronic diarrhea (▧, n = 3) and clinically healthy controls ( , n = 3) in mesenteric lymph nodes (A) and spleens (B). The means ± the standard deviations are shown for cytokine genes that were found to be significantly different (a P value of <0.05 is indicated by an asterisk for IL-1α, IL-3, and TNF-α), along with the two genes (IL-10 and IL-16), for which no significant difference was observed. The IL-1α, IL-3, and TNF-α gene expression in mesenteric lymph nodes was significantly higher in animals with chronic diarrhea (P < 0.02, 0.03, and 0.02, respectively). Similarly, IL-1α, IL-3, and TNF-α gene expression in the spleen was significantly higher in animals with chronic diarrhea at P < 0.05, 0.03, and 0.05, respectively.
DISCUSSION
The gastrointestinal immune system has recently been implicated as important in the pathogenesis of numerous diseases and syndromes, including AIDS (39, 40), and of IBD disorders such as ulcerative colitis and Crohn's disease (34). A healthy gastrointestinal tract is critical for research studies involving pathogenesis, as well as novel mucosal vaccine and gene therapy approaches utilizing genetically modified viruses, bacteria, or synthetic delivery systems. Intestinal malabsorption, chronic diarrhea, and wasting are frequent manifestations of human immunodeficiency virus infection and, in many cases, the presenting illness (17, 21, 30). Similar findings have been observed in the SIV/macaque model of AIDS (18, 35). In SIV-infected macaques, large numbers of infected lymphocytes are found throughout the intestinal lamina propria within days of intravenous virus inoculation (18, 29, 38). Many of the pathological changes, such as the rapid loss of intestinal CD4+ T lymphocytes, are clearly due to SIV infection. However, other alterations, such as the morphological changes and persistent diarrhea, may be associated with opportunistic infections or alteration in intestinal flora. Developing strategies to prevent or treat these enteric infections and elucidate their role, or lack thereof, in AIDS enteropathy and disease progression is the focus of numerous studies (a search of the CRISP database for the terms opportunistic infection and AIDS returned 469 hits for the year 2001). Many of these studies are hampered by the fact that most captive macaques in existing colonies have been exposed to these agents. Thus, our study sought to identify the enteric pathogens most frequently associated with chronic diarrhea in normal, immunocompetent rhesus macaques and to elucidate their impact on the occurrence of chronic enterocolitis and the host immune system in general.
We identified the presence of gastrointestinal pathogens such as Campylobacter spp., Shigella flexneri, Yersinia enterocolitica, adenovirus, and Strongyloides fulleborni in rhesus macaques with chronic diarrhea. These organisms are known to be associated with chronic enterocolitis not only in nonhuman primates but also in humans (5, 8, 20, 22, 36, 37). A wide range of histopathological changes in intestinal tissues collected from animals with chronic diarrhea reflected the presence of a number of enteric pathogens and their cumulative effect. It is likely that additional agents beyond those identified in the present study were also contributing to the chronic enterocolitis. However, the detection of microorganisms such as Mycobacterium spp. (Mycobacterium avium and Mycobacterium simiae), the Campylobacter-like bacterium Helicobacter cinaedi, Brachyspira sp., or cytomegalovirus would require more focused and specialized examination (15, 19).
Although the opportunistic microsporidium Enterocytozoon bieneusi was more prevalent in animals with chronic diarrhea in the present study than in clinically healthy animals, it was not significantly associated with the onset of diarrhea. This is consistent with our earlier findings. We previously reported that there is a significant increase in Enterocytozoon bieneusi shedding only in individuals with very low levels of peripheral CD4+ T lymphocytes (33).
Rotaviruses were not detected in the present study. Compared to human infants, maximum shedding of rotavirus antigens in feces of rhesus monkeys is expected at the age of approximately 4 months, a time when passive, maternally acquired immunity subsides. In our study, the age of monkeys was 6.5 months and older, and these animals were showing symptoms of chronic diarrhea; therefore, we would not have expected to detect rotavirus in association with their disease. However, exposure to rotavirus will occur in most conventionally reared rhesus macaques at very young ages when it is likely to result in infection that produces lower levels of shedding and mild disease. In the majority of adult rhesus macaques that possess actively acquired rotavirus immunity, reinfections occur only subclinically or the disease is very mild, with little virus shedding in the feces (unpublished results).
Detection of adenoviruses in our study was based on the use of a commercial polyclonal reagent that is capable of detecting the presence of any of the 49 known adenovirus serotypes (Dako), including those reported to cause gastroenteritis in humans (serotypes 40 and 41) and monkeys (serotypes 17, 20, and 32) (36, 37). Adenoviruses were associated in our study with chronic diarrhea.
EPEC strains were previously recognized as opportunistic pathogens in SIV-infected rhesus macaques (22). Among rhesus macaques with AIDS, EPEC was identified in 28% of animals (22). Moreover, in 7.3% of animals dying with AIDS, EPEC was the sole pathogen detected in the gastrointestinal tract (22). In our study, the Escherichia coli strains that were identified as carriers of enteric virulence genes were found in up to 25% of animals regardless of clinical symptoms of diarrhea. It is important to note, however, that among our immunocompetent animals, of the seven Stx2c-virulence gene-carrying Escherichia coli isolates only two actively produced Vero cell toxins. The fact that 32% of clinically healthy animals had some past history of clinical diarrhea might explain the detection of several gastrointestinal pathogens from this “clinically healthy” category.
We attempted to elucidate what differences, if any, would be seen with respect to activated and/or naive CD4+ and CD8+ lymphocytes in gut-associated inductive lymphoid tissues and systemic inductive lymphoid tissues from animals with or without chronic enterocolitis. Immunological involvement of both major T-lymphocyte populations (CD4+ and CD8+) in animals with chronic diarrhea was indicated by the increased presence of activated (CD69+) cells, although a significant increase was measured only in the CD4+ T lymphocytes isolated from mesenteric lymph nodes. This increased presence of activated T lymphocytes with T helper cell characteristics most likely reflected the presence of multiple enteric pathogens over a long period of time, possibly associated with ligand reactivities to multiple antigens. In patients with IBD, typically with chronic inflammation of the terminal ileum and colon, CD4+ T lymphocytes, cross-reactive with different species of aerobic and anaerobic bacteria, have been detected (12). Although the precise etiology of IBD is not clear, it is speculated that hyperresponsive T lymphocytes in IBD patients react with commensal bacterial microflora of the gut and cause chronic inflammation (12). In the present study the presence of multiple enteric pathogens in rhesus macaques was associated with chronic inflammation of the colon and an increased percentage of activated (namely, CD4+) T lymphocytes in gut-associated and systemic lymphoid tissues.
Consistent with the presence of inflammation and the increased percentage of activated cells, we observed upregulation of IL-1-α, IL-3, and TNF-α cytokine genes. This finding is in accord with studies conducted with murine and rhesus models of human chronic colitis wherein increased expression of IL-1 and TNF-α was related to the development of intestinal pathology (4, 26, 41). Chronic transmural colitis in STAT4 transgenic mice was characterized by the infiltration of CD4+ T lymphocytes secreting TNF and IFN-γ (41). In vitro-cultured explants of a rhesus colon with conditions of colitis resulted in increased production of IL-1 and TNF-α in contrast to cultures derived from a normal colon (26). It was suggested that inhibition of inducible nitric oxide synthase (iNOS) might abrogate the severity of chronic colitis (26). However, the complexity of factors that may affect IBD, such as cytokine network interactions with intestinal vascular endothelium and/or neurons that are known to constitutively express iNOS, still needs to be fully elucidated. The recent focus of research on the development of novel strategies for treating human patients with IBD has shifted to immune therapies. Trials with Crohn's disease patients treated with anti-TNF-α antibodies demonstrated dramatic improvement, as scored by colonoscopy findings (2, 3, 11).
The increased immunological reactivities (i.e., the presence of CD69+ T lymphocytes and the upregulation of IL-1α, IL-3, and TNF-α cytokine genes) were detected in the present study not only in gut-associated lymphoid tissues but also in systemic lymphoid tissues represented by the spleen. This suggests that the prevention of chronic enterocolitis in nonhuman primates is critical in order to increase the usefulness of this animal model for studies involving vaccine or gene therapy research. On the other hand, the rhesus colitis model could also be explored as an in vivo model in trials with new drugs and immune regimens that are aimed to reduce or abrogate IBD.
Acknowledgments
This study was supported by Public Health Service grant DK50550. Partial support was provided by TNPRC base grant RR00164.
The technical assistance of Ayanna Jefferson, Ann Bennett, Lisa Bowers, Maurice Duplantis, and Kevin Callahan is greatly appreciated. We thank Gary B. Baskin and John D. Clements for careful review and suggestions regarding the manuscript.
Editor: A. D. O'Brien
REFERENCES
- 1.Ash, L. R., and T. C. Orihel. 1987. Parasites: a guide to laboratory procedures and identification, p. 18-33. In L. R. Ash and T. C. Orihel (ed.), Parasites: a guide to laboratory procedures and identification. ASCP Press, Chicago, Ill.
- 2.Baert, F. J., and P. R. Rutgeerts. 1999. Anti-TNF strategies in Crohn's disease: mechanisms, clinical effects, indications. Int. J. Colorectal Dis. 14:47-51. [DOI] [PubMed] [Google Scholar]
- 3.Bell, S., and M. A. Kamm. 2000. Antibodies to TNF alpha as treatment for Crohn's disease. Lancet 355:858-860. [DOI] [PubMed] [Google Scholar]
- 4.Bhan, A. K., E. Mizoguchi, R. N. Smith, and A. Mizoguchi. 2000. Spontaneous chronic colitis in TCR alpha-mutant mice; an experimental model of human ulcerative colitis. Int. Rev. Immunol. 19:123-138. [DOI] [PubMed] [Google Scholar]
- 5.Butler, T., M. Islam, A. K. Azad, M. R. Islam, and P. Speelman. 1987. Causes of death in diarrhoeal diseases after rehydration therapy: an autopsy study of 140 patients in Bangladesh. Bull. W. H. O. 65:312-323. [PMC free article] [PubMed] [Google Scholar]
- 6.Cizek, A., P. Alexa, I. Literak, J. Hamrik, P. Novak, and J. Smola. 1999. Shiga toxin-producing Escherichia coli O157 in feedlot cattle and Norwegian rats from a large-scale farm. Lett. Appl. Microbiol. 28:435-439. [DOI] [PubMed] [Google Scholar]
- 7.Clements, J. D., K. L. Lowe, L. Bonham, and S. el-Morshidy. 1985. Intracellular distribution of heat-labile enterotoxin in a clinical isolate of Escherichia coli. Infect. Immunol. 50:317-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerinx, J., J. Bogaerts, H. Taelman, J. B. Habyarimana, A. Nyirabareja, P. Ngendahayo, and P. Van De Perre. 1995. Chronic diarrhea among adults in Kigali, Rwanda: association with bacterial enteropathogens, rectocolonic inflammation, and HIV infection. Clin. Infect. Dis. 21:1282-1284. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, J. 2000. Vaccine studies stymied by shortage of animals. Science 287:959-960. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers, R. C. 1997. The value of specific pathogen-free rhesus monkey breeding colonies for AIDS research. AIDS Res. Hum. Retrovir. 13:5-6. [DOI] [PubMed] [Google Scholar]
- 11.Dotan, I., D. Yeshurn, A. Hallak, N. Horowitz, E. Tiomny, S. Reif, Z. Halpern, and D. Rachmilewitz. 2001. Treatment of Crohn's disease with anti TNF alpha antibodies-the experience in the Tel Aviv Medical Center. Harefuah 140:289-293. [PubMed] [Google Scholar]
- 12.Duchmann, R., E. May, M. Heike, P. Knolle, M. Neurath, K.-H. Meyer zum Buschenfelde. 1999. T-cell specificity and cross reactivity towards enterobacteria, Bacteroides, Bifidobacterium, and antigens from resident intestinal flora in humans. Gut 44:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmore, D. B., J. H. Anderson, D. W. Hird, K. D. Sanders, and N. W. Lerche. 1992. Diarrhea rates and risk factors for developing chronic diarrhea in infant and juvenile rhesus monkeys. Lab. Anim. Sci. 42:356-359. [PubMed] [Google Scholar]
- 14.Forbes, B. A., D. F. Sahm, and A. S. Weissfield. 1998. Campylobacter, Arcobacter, and Helicobacter, p. 570-572. In B. A. Forbes, D. F. Sahm, and A. S. Weissfield (ed.), Diagnostic microbiology, 10th ed. Mosby, New York, N.Y.
- 15.Fox, J. G., L. Handt, B. J. Sheppard, S. Xu, F. E. Dewhirst, S. Motzel, and H. Klein. 2001. Isolation of Helicobacter cineadi from colon, liver, and mesenteric lymph node of a rhesus monkey with chronic colitis and hepatitis. J. Clin. Microbiol. 39:1580-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, L. C., P. J. LeBlanc, and E. S. Didier. 2000. Discrimination between viable and dead Encephalitozoon cuniculi (Microsporidian) spores by dual staining with sytox green and calcofluor white M2R. J. Clin. Microbiol. 10:3811-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise, C., S. Dandekar, P. Kumar, R. Duplantier, R. M. Donovan, and C. H. Halsted. 1991. Human immunodeficiency virus infection of enterocytes and mononuclear cells in human jejunal mucosa. Gastroenterology 100:1521-1527. [DOI] [PubMed] [Google Scholar]
- 18.Heise, C., C. J. Miller, A. Lackner, and S. Dandekar. 1994. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169:1116-1120. [DOI] [PubMed] [Google Scholar]
- 19.Kaup, F., K. Matz-Rensing, H. Kuhn, P. Hunerbein, C. Stahl-Hennig, and G. Hunsmann. 1998. Gastrointestinal pathology in rhesus monkeys with experimental SIV infection. Pathobiology 66:159-164. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy, F. M., J. Astbury, J. R. Needham, and T. Cheasty. 1993. Shigellosis due to occupational contact with non-human primates. Epidemiol. Infect. 110:247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotler, D. P., H. Gaetz, M. Lange, E. B. Klein, and P. R. Holt. 1984. Enteropathy associated with the acquired immune deficiency syndrome. Ann. Intern. Med. 101:421-428. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield, K. G., K. C. Lin, J. Newman, D. Schauer, J. MacKey, A. A. Lackner, and A. Carville. 2001. Identification of enteropathogenic Escherichia coli in SIV-infected infant and adult rhesus macaques. J. Clin. Microbiol. 39:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeal, M. M., J. L. VanCott, A. H. C. Choi, M. Basu, J. A. Flint, C. S. Stone, J. D. Clements, and R. L. Ward. 2002. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G). J. Virol. 76:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng, J., S. Zhao, M. P. Doyle, S. E. Mitchell, and S. Kresovich. 1997. A multiplex PCR for identifying Shiga-like toxin-producing Escherichia coli O157:H7. Lett. Appl. Microbiol. 24:172-176. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Zanzi, C. A., M. C. Thurmond, D. W. Hird, and N. W. Lerche. 1999. Effect of weaning time and associated management practices on postweaning chronic diarrhea in captive rhesus monkeys (Macaca mulatta). Lab. Anim. Sci. 49:617-621. [PubMed] [Google Scholar]
- 26.Ribbons, K. A., M. G. Currie, J. R. Connor, P. T. Manning, P. C. Allen, P. Didier, M. S. Ratterree, D. A. Clark, and M. J. S. Miller. 1997. The effect of inhibitors of inducible nitric oxide synthase on chronic colitis in the rhesus monkey. J. Pharmacol. Exp. Ther. 280:1008-1015. [PubMed] [Google Scholar]
- 27.Roberts, J. A., D. G. Smith, and A. Hendickx. 2000. Managing the rhesus supply. Science 287:1591. [DOI] [PubMed] [Google Scholar]
- 28.Russell, R. G., S. L. Rosenkranz, L. A. Lee, H. Howard, R. F. DiGiacomo, M. A. Bronsdon, G. A. Blakley, C. C. Tsai, and W. R. Morton. 1987. Epidemiology and etiology of diarrhea in colony-born Macaca nemestrina. Lab. Anim. Sci. 37:309-316. [PubMed] [Google Scholar]
- 29.Sasseville, V. G., Z. Du, L. V. Chalifoux, D. R. Pauley, H. L. Young, P. K. Sehgal, R. C. Desrosiers, and A. A. Lackner. 1996. Induction of lymphocyte proliferation and severe gastrointestinal disease in macaques by a nef gene variant of SIVmac239. Am. J. Pathol. 149:163-176. [PMC free article] [PubMed] [Google Scholar]
- 30.Serwadda, D., N. K. Sewankambo, J. W. Carswell, A. C. Bayley, R. S. Tedder, R. A. Weiss, R. D. Mugerwa, A. Lwegaba, G. B. Kirya, R. G. Downing, S. A. Clayden, and A. G. Dalgleish. 1985. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet 11:849-852. [DOI] [PubMed] [Google Scholar]
- 31.Sestak, K., J. H. Lee, J. Brisben, M. Beauchemin, and S. Tzipori. 2000. Tacrolimus induced immunosuppression in gnotobiotic piglets. Vet. Immunol. Immunopathol. 77:289-300. [DOI] [PubMed] [Google Scholar]
- 32.Sestak, K., L. A. Ward, A. Sheoran, X. Feng, D. Akiyoshi, H. D. Ward, and S. Tzipori. 2002. Variability among Cryptosporidium parvum genotype 1 and 2 immunodominant surface glycoproteins. Parasite Immunol. 24:213-219. [DOI] [PubMed] [Google Scholar]
- 33.Sestak, K., P. P. Aye, M. Buckholt, K. G. Mansfield, A. A. Lackner, and S. Tzipori. 2003. Quantitative evaluation of Enterocytozoon bieneusi infection in SIV-infected rhesus monkeys. J. Med. Primatol. 32:74-81. [DOI] [PubMed] [Google Scholar]
- 34.Singh, B., F. Powrie, and N. Mortensen. 2001. Immune therapy in inflammatory bowel disease and models of colitis. Br. J. Surg. 88:1558-1569. [DOI] [PubMed] [Google Scholar]
- 35.Stone, J. D., C. C. Heise, C. J. Miller, C. H. Halsted, and S. Dandekar. 1994. Development of malabsorption and nutritional complications in simian immunodeficiency virus-infected rhesus macaques. AIDS 8:1245-1256. [DOI] [PubMed] [Google Scholar]
- 36.Stuker, G., L. S. Oshiro, N. J. Schmidt, C. A. Holmberg, J. H. Anderson, C. A. Glaser, and R. V. Hendrickson. 1979. Virus detection in monkeys with diarrhea: the association of adenoviruses with diarrhea and the possible role of rotaviruses. Lab. Anim. Sci. 29:610-616. [PubMed] [Google Scholar]
- 37.Trevino, M., E. Prieto, D. Penalver, A. Aguilera, A. Garcia-Zabarte, C. Garcia-Riestra, and B. J. Regueiro. 2001. Diarrhea caused by adenovirus and astrovirus in hospitalized immunodeficient patients. Enferm. Infecc. Microbiol. Clin. 19:7-10. [DOI] [PubMed] [Google Scholar]
- 38.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 39.Veazey, R. S., and A. A. Lackner. 1998. The gastrointestinal tract and the pathogenesis of AIDS. AIDS 12(Suppl. A):S35-S42. [PubMed] [Google Scholar]
- 40.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626-633. [DOI] [PubMed] [Google Scholar]
- 41.Wirtz, S., S. Finotto, S. Kanzler, A. W. Lohse, M. Blessing, H. A. Lehr, P. R. Galle, and M. F. Neurath. 1999. Cutting edge: chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF- plus IFN-γ-producing CD4+ T cells that respond to bacterial antigens. J. Immunol. 162:1884-1888. [PubMed] [Google Scholar]