Abstract
Macrophages secrete transforming growth factor α (TGF-α) to trigger proliferation of cancer cells. Here, we report a new role for TGF-α in modulating the direct cellular proliferation of a parasitic protozoan, Trypanosoma cruzi. Amastigotes present two classes of receptors for TGF-α with different binding affinities. 125I-TGF-α binding was competed by an excess of cold epidermal growth factor and TGF-α but not by an irrelevant molecule. Upon binding of TGF-α to amastigotes, the ligand is internalized, inducing trypanosome tyrosine phosphorylation of 90- and 87-kDa proteins and increasing DNA synthesis and proliferation of amastigotes. Furthermore, exposure of macrophages to TGF-α induced increased amastigote proliferation. These results describe a novel mechanism used by amastigotes to regulate their proliferation mediated by a TGF-α-dependent signal transduction pathway.
Transforming growth factor α (TGF-α) is a potent mitogen for a wide range of cells and regulates metastasis, transformation of normal cells, chemotaxis, and acute-phase response (20). TGF-α displays 33% homology to epidermal growth factor (EGF), binds to EGF receptor (EGFR) on mammalian cells, activates tyrosine phosphorylation of the receptor, and stimulates cell proliferation (20). TGF-α is produced by macrophages (6, 18, 19, 23), cells that play a pivotal role in the immunomodulation of Trypanosoma cruzi infection (15). However, the role of TGF-α in microbial infections is unknown, as is the potential direct role that TGF-α may have on the proliferation of parasitic protozoan parasites.
Chagas' disease, or American trypanosomiasis, is a chronic and debilitating heart and digestive tract disease that affects millions of people, causing significant morbidity and mortality (10, 11). The invasive trypomastigotes invade mammalian cells and transform into amastigotes, the multiplicative form of the parasite, which disseminate the disease (1). Despite significant advances in the understanding of the pathogenesis of T. cruzi infection, very little is known about the host factors that regulate cellular proliferation of the mammalian multiplicative forms of the parasite.
Encouraged by recent studies from our group indicating that T. cruzi amastigotes present receptors for human EGF (5), we hypothesized that TGF-α binds to T. cruzi amastigotes to signal these cells, inducing cell proliferation. Testing this hypothesis may be important, since macrophages, the main cells infected by T. cruzi, produce this growth factor, which may be involved in the development of the parasite. In the present study we demonstrate that TGF-α binds to a receptor(s) on amastigotes and is internalized, that this binding can be competed by an excess of cold TGF-α and EGF, and that TGF-α enhances tyrosine phosphorylation events, leading to DNA synthesis and amastigote proliferation.
125I-labeled TGF-α binds to receptors on T. cruzi amastigotes and is internalized, and binding is competed by excess TGF-α and EGF.
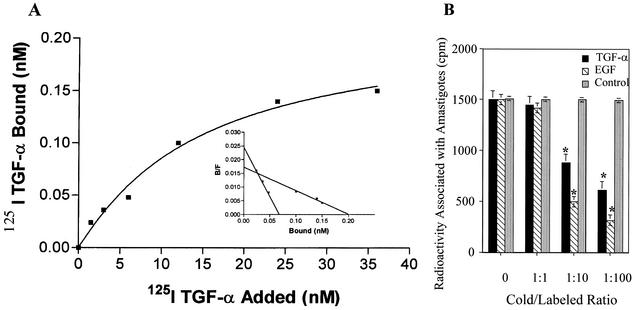
To evaluate the specificity of 125I-TGF-α binding to amastigotes, ligand-binding analysis was conducted with labeled TGF-α as described previously (14). Briefly, for all experiments described in this paper, T. cruzi amastigotes released from Vero cells (American Type Culture Collection; grown in minimum essential medium supplemented with 10% fetal bovine serum in the presence of 100 U of penicillin/ml and 100 μg of streptomycin/ml) were purified by differential centrifugation on a metrizamide gradient as previously described (21). The pure axenic amastigotes were grown in cell-free medium supplemented with fetal bovine serum at 37°C in 5% CO2 (21), extensively washed with Hanks balanced salt solution (pH 7.2) (HBSS), and starved before exposure to TGF-α. Binding of TGF-α to amastigotes was assessed by adding to amastigotes (5 × 106/ml) increasing concentrations of labeled 125I-TGF-α (NEN) in HBSS-bovine serum albumin (BSA), HBSS-BSA alone, or HBSS-BSA containing 100-fold excess unlabeled TGF-α to determine nonspecific binding. Each point was done in triplicate. The tubes were incubated at 4°C for 1 h with constant shaking. Unbound radiolabeled TGF-α was removed by washing with HBSS, and the radioactivity associated with the cellular pellet was determined in a gamma counter. Specific binding was determined by subtracting nonspecific binding (determined in the presence of excess unlabeled TGF-α from the total amount of counts). Scatchard analysis was performed with Prism (San Diego, Calif.) software. TGF-α binds to amastigotes in a concentration-dependent manner (Fig. 1A). Binding was saturable, indicating that amastigotes have specific receptors for this growth factor (Fig. 1A, inset). By using the Prism software, a specialized software for binding and Scatchard analysis, we found that amastigotes present two classes of receptors for TGF-α with high and low affinities. Amastigotes presented 2.88 × 104 receptors per cell that bound TGF-α with a low-affinity Kd of 1.25 μM and 6.72 × 103 receptors per cell that bound TGF-α with a high-affinity Kd of 0.18 μM. Preincubation of amastigotes with different concentrations of excess (0- to 100-fold) cold EGF and TGF-α resulted in significant inhibition of subsequent 125I-TGF-α binding (Fig. 1B). Since mammalian TGF-α and EGF bind to the same mammalian receptor, the fact that amastigotes express approximately twice the number of receptors for TGF-α might be dependent on the state of development of the amastigotes used for binding assays. Alternatively, amastigotes may express other homologue receptors that may interact with TGF-α with similar affinity. Furthermore, it has been documented that TGF-α binds to the human EGFR receptor with less affinity than EGF (20). EGF was better able to compete with TGF-α binding to amastigotes than TGF-α itself. There was no significant reduction in binding in the presence of the same concentrations of bovine pituitary extract, a control peptide (Fig. 1B). To differentiate cell surface-bound from internalized ligand, we examined the release of 125I-TGF-α from amastigotes by acid treatment, using previously described methods (14). As shown in Table 1, when 125I-TGF-α bound to amastigotes under conditions in which ligand-receptor complex internalization does not occur (4°C for 1 h), it was readily dissociated from the parasite surface. However, 125I-TGF-α bound to amastigotes at 37°C for 1 h was not dissociated by acid treatment, indicating that TGF-α had attained an intracellular location and was no longer susceptible to dissociation from its receptor (Table 1). These data indicate that amastigotes possess specific receptors for TGF-α and that the growth factor attains an intracellular location subsequent to binding to surface receptors. All published work to date describes common receptors for TGF-α and EGF (4). No unique receptor has been identified for TGF-α. TGF-α binds to amastigotes with a much lower affinity (Kd of 0.18 μM) than that with which EGF binds to the amastigote EGFR (Kd of 0.8 nM) (5). This is consistent with other reports, which demonstrate that mammalian EGFR binds TGF-α with a lower affinity than EGF (20) except for chicken EGFR, which binds TGF-α and EGF with equal affinity (12). The fact that both EGF and TGF-α compete with radiolabeled TGF-α for binding to its receptor on amastigotes also indicates that they bind to a common receptor and that EGF is a better competitor for binding to the amastigote EGFR than TGF-α itself (Fig. 1B). This is the first report showing that TGF-α binds to receptors on trypanosomes or on any other parasite. Furthermore, TGF-α binds to multiplicative amastigotes but not to nonmultiplicative but invasive trypomastigotes (results not shown).
FIG. 1.
125I-TGF-α binds to amastigotes, and this binding is inhibited by cold excess TGF-α and EGF. (A) TGF-α binds to amastigotes. (Inset) Scatchard analysis of the binding data. B/F, bound/free (unbound) TGF-α. (B) Competition of 125I-TGF-α binding by TGF-α, EGF, and bovine pituitary extract. The same concentration of amastigotes (5 × 106 parasites ml−1) were preincubated with different excess amounts (0- to 100-fold) of cold EGF and TGF-α followed by exposure to 125I-TGF-α. Bars show means for triplicate samples of one representative experiment (± 1 standard deviation) selected from three independent experiments with similar results. *, significant difference compared to values for 0 and 1:1 cold/labeled ratios or to bovine pituitary extract values for 0 to 1:100 cold/labeled ratios (P < 0.05). The results are representative of three independent experiments.
TABLE 1.
125I-labeled TGF-α is internalized by amastigotes of T. cruzia
| Incubation temp (°C) | Radioactivity (cpm) in amastigotes washed in:
|
|
|---|---|---|
| HBSS | Acid | |
| 4 | 1,907 ± 179 | 468 ± 35 |
| 37 | 2,006 ± 90 | 2,197 ± 153 |
Amastigote samples in triplicate (2 × 107/ml in HBSS) received 10 ng of 125I-labeled TGF-α or HBSS alone for 1 h at 4 or 37°C. Unbound 125I-labeled TGF-α was removed by centrifugation at 4°C. One half of the samples were tested for dissociation of bound TGF-α by treatment with acidified NaCI solution. Each point represents the mean of triplicate determinations ± 1 standard deviation. Differences between values for cells with and without acid treatment at 4°C were statistically significant (P < 0.05). Data are from a set of representative experiments of three performed with similar results.
TGF-α induces tyrosine phosphorylation of T. cruzi amastigote proteins.
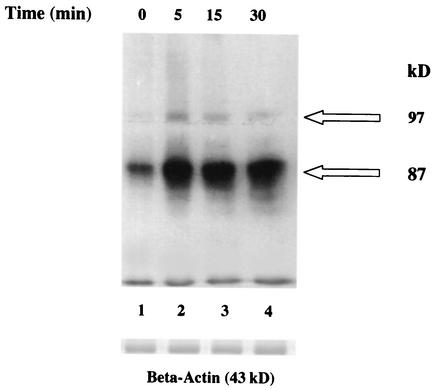
Exposure of T. cruzi amastigotes to 2 ng of TGF-α per ml for 5, 15, and 30 min induced significant tyrosine phosphorylation, in a time-dependent manner, of two amastigote proteins of 87 and 97 kDa (Fig. 2). The enhancement of tyrosine phosphorylation of the 87-kDa protein of amastigotes can be seen as early as 5 min and continues at 15 and 30 min, whereas the enhancement of tyrosine phosphorylation of the 97-kDa protein of amastigotes peaks at 5 min and then decreases over the 30-min exposure. The 87- and 97-kDa proteins are surface proteins. This was shown by exposing live biotinylated amastigotes to EGF and TGF-α. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted on nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. The blots were then stripped and probed with avidin-conjugated horseradish peroxidase and developed by enhanced chemiluminescence. These results indicated that EGF and TGF-α induced tyrosine phosphorylation of surface 87- and 97-kDa proteins (data not shown). The concentration of 2 ng/ml was chosen because treatment of amastigotes with different concentrations of TGF-α indicated that the tyrosine phosphorylation effect induced by this growth factor is concentration dependent and 2 ng of TGF-α per ml induced optimal (highest) phosphorylation (data not shown). Growth factor binding to the EGFR in mammalian cells induces tyrosine phosphorylation (22), including autophosphorylation. Stripping the blots and probing with anti-β-actin indicated equal loading amounts of amastigote proteins in these blots (Fig. 2). The fact that TGF-α induced tyrosine phosphorylation that was time and concentration dependent is consistent with published reports which identify 2 ng/ml as the physiological concentration of TGF-α in mammalian systems (7) and 15 to 30 min as the amount of time the ligand-bound EGFR remains on the cell surface before undergoing receptor-mediated endocytosis (2). These proteins could be any of a number of proteins in the EGFR signal transduction pathway, including a putative parasite EGFR.
FIG. 2.
TGF-α induces tyrosine phosphorylation of amastigote proteins. Amastigotes (5 × 106 organisms) were incubated with TGF-α (2 ng/ml) for 5, 15, and 30 min at 4°C. Lysate samples (10 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto nitrocellulose, probed with antiphosphotyrosine monoclonal antibody PY20, and developed by chemiluminescence. Control parasites did not receive any growth factor. Blots were stripped, probed with anti-β-actin antibodies, and developed by chemiluminescence as a loading control. Results are from a representative experiment of three performed independently with similar results. Arrows point to enhanced protein tyrosine phosphorylation induced by TGF-α with respect to controls in the absence of growth factor treatment.
TGF-α increases DNA synthesis and amastigote growth in cell-free medium and within macrophages.
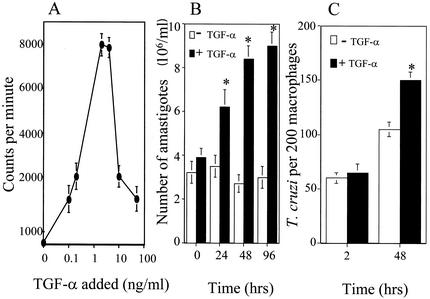
Addition of different concentrations of TGF-α to pure amastigotes grown in ML-15 axenic medium in the absence of host cells (20) induced increases in the incorporation of [3H]thymidine into DNA (Fig. 3A), indicating that amastigote DNA synthesis was increased by exposure to the growth factor. The increase in DNA synthesis was optimal at 2 ng/ml and was accompanied by a TGF-α-induced increase in the growth of amastigotes over time, as shown in Fig. 3B. Controls were MCF-7 breast cancer cells (American Type Culture Collection) treated with 2 ng of TGF-α per ml or mock treated for 24 and 48 h; TGF-α is mitogenic for MCF-7 cells, as observed by significant increases in [3H]thymidine incorporation (results not shown). These results are in agreement with previous findings obtained with MCF-7 cells exposed to TGF-α (9). Pretreatment of starved amastigotes with AG178 (10 μM), a specific EGFR inhibitor (3), in the presence of 2 ng of TGF-α per ml in ML-15 medium at 24, 48, and 96 h significantly inhibited amastigote multiplication with respect to control values up to 79% ± 2.1% (24 h), 87.2% ± 3.1% (48 h), and 98.3% ± 3.5% (96 h). The growth inhibition seen is time dependent. The concentration of AG178 used was not toxic for amastigotes, since it did not affect amastigote morphology and typical in situ vibrational motion of amastigotes (21) with respect to mock-treated amastigotes. This indicates that the proliferation induced by TGF-α in amastigotes is TGF-α specific and is mediated by the receptor. Furthermore, as shown in Fig. 3C, exposure of unelicited CBA/J mouse peritoneal macrophages (13) to 2 ng of TGF-α per ml and T. cruzi trypomastigotes as described previously (13) induced a significant increase in amastigote replication at 48 h compared to mock-treated macrophages. The major downstream effect of TGF-α/EGF activation of the EGFR is proliferation of mammalian cells (22). Taken together, these results show that TGF-α induces increases in the growth of amastigotes in cell-free media and in macrophages (Fig. 3), indicating that this host growth factor is mitogenic for amastigotes.
FIG. 3.
TGF-α stimulates DNA synthesis and proliferation of T. cruzi amastigotes in cell-free medium and within macrophages. (A) Incorporation of [3H]thymidine into amastigote DNA. Overnight-starved amastigotes (3 × 106 organisms/ml) in RPMI with 1% BSA were incubated at 37°C with different concentrations of TGF-α and 1 μCi of [3H]thymidine. After 24 h, the organisms were harvested and the incorporation of [3H]thymidine was determined by measuring acid-insoluble radioactivity. (B) Amastigote proliferation in cell-free medium. Overnight-starved amastigotes (3 × 106 organisms/ml) were incubated at 37°C in ML-15 medium alone (open bars) or in ML-15 medium containing 2 ng of TGF-α per ml (closed bars). The number of organisms was determined at 24-h intervals. (C) Amastigote proliferation within macrophages. Unelicited CBA/J mouse peritoneal macrophages were cultured with 2 ng of TGF-α per ml and trypomastigotes at a ratio of five parasites per macrophage. Parasite uptake was measured at 2 h and parasite multiplication was measured at 48 h as previously described (13). Bars represent the mean of triplicate samples of one representative experiment (± 1 standard deviation) selected from three independent experiments with similar results. *, significant difference compared to values at 0 and 2 h (P < 0.05).
The fact that TGF-α is directly involved in signaling and growth of amastigotes suggests that this growth factor modulates the pathogenesis of T. cruzi in mammalian cells. Amastigotes could come into contact with TGF-α after it is endocytosed by macrophages, since TGF-α is not immediately degraded by lysosomes (16). It may therefore be available for binding to intracellular amastigotes. This is being investigated by our group. T. cruzi amastigotes have been shown to use host-specific proteins for interacting with cells (8, 17) and surviving in mammalian cells (5, 13, 14). This work contributes to the list of host proteins that amastigotes bind to via specific receptors, such as fibronectin (17), lactoferrin (13), transferrin (14), mannose binding protein (8), and EGF (5). Since TGF-α is produced by macrophages, which are the main cells involved in host defense against the parasite in the mammalian host (14), we suggest that this may be an effective mechanism to subvert host defenses against T. cruzi for parasite survival in the mammalian host. In summary, we describe a novel mechanism used by T. cruzi amastigotes to regulate their proliferation mediated by a TGF-α-dependent signal transduction pathway.
Acknowledgments
This work was supported partially by NIH grants HL 03156 and RR 03032 and a grant-in-aid from the American Heart Association to M.F.L. Doctoral studies of A.D.A. were supported partially by NIH grant F31GM018918 and by predoctoral fellowships from the Department of Education and the Sloan Foundation.
Editor: J. M. Mansfield
REFERENCES
- 1.Brenner, Z. 1973. Biology of Trypanosoma cruzi. Annu. Rev. Microbiol. 27:347-382. [DOI] [PubMed] [Google Scholar]
- 2.Earp, H. S., T. L. Dawson, X. Li, and H. Yu. 1995. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res. Treatment 35:115-132. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi, S., K. Numaguchi, H. Iwasaki, T. Matsumoto, T. Yamakawa, E. D. Utsunomiya, H. Kawakatsu, K. M. Owada, Y. Hirta, F. Marumo, and T. Inagami. 1998. Calcium-dependent epidermal growth factor receptor transactivator II-induced mitogen-activated in vascular smooth muscle. J. Biol. Chem. 273:8890-8896. [DOI] [PubMed] [Google Scholar]
- 4.Garrett, T. P., N. M. McKern, M. Lou, T. C. Elleman, T. E. Adams, G. O. Lovrecz, H.-J. Zhu, F. Walker, M. J. Frenkel, P. A. Hoyne, R. N. Jorissen, E. C. Nice, A. W. Burgess, and C. W. Ward. 2002. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor-α. Cell 110:763-773. [DOI] [PubMed] [Google Scholar]
- 5.Ghansah, T. J., E. Ager, P. Freeman-Junior, F. Villalta, and M. F. Lima. 2002. Epidermal growth factor binds to a receptor on Trypanosoma cruzi amastigotes inducing signal transduction events and cell proliferation. J. Eukaryot. Microbiol. 49:383-390. [DOI] [PubMed] [Google Scholar]
- 6.Hallbeck, A. L., T. M. Walz, and A. Wasteson. 2001. Interleukin-6 enhances transforming growth factor alpha mRNA expression in macrophage-like human monocytoid (U-937-1) cells. Biosci. Rep. 21:325-339. [DOI] [PubMed] [Google Scholar]
- 7.Harigava, T., S. Tsunoda, M. Mizuno, and H. Nagasawa. 1994. Different gene expression of mouse transforming growth factor-alpha between pregnant mammary glands and mammary tumors in C3H/He mice. Zool. Sci. 11:625-627. [PubMed] [Google Scholar]
- 8.Kahn, S. J., M. Wleklinski, R. A. Ezekowitz, D. Coder, A. Aruffo, and A. Farr. 1996. The major surface glycoproteins of T. cruzi amastigotes are ligands of the human serum mannose-binding protein. Infect. Immun. 64:2649-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karey, K. P., and D. A. Sibarsku. 1988. Differential responsiveness of human breast cancer cell lines MCF-7 and TD47D to growth factors and 17b-estradiol. Cancer Res. 48:4083-4092. [PubMed] [Google Scholar]
- 10.Kierszenbaum, F. 1996. Can a killer be arrested? Nat. Med. 2:10. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhoff, L. V. 1993. Chagas' disease. American trypanosomiasis. Infect. Dis. Clin. N. Am. 7:487-502. [PubMed] [Google Scholar]
- 12.Lax, I., A. Johnson, R. Howk, J. Sap, F. Bellot, M. Winkler, A. Ullrich, B. Vennstrom, J. Schlessinger, and D. Givol. 1988. Chicken epidermal growth factor receptor: cDNA cloning, expression in mouse cells, and differential binding of EGF and transforming growth factor-alpha. Mol. Cell. Biol. 8:1970-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima, M. F., and F. Kierszenbaum. 1985. Lactoferrin effects on phagocytic cell function. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J. Immunol. 134:4179-4183. [PubMed] [Google Scholar]
- 14.Lima, M. F., and F. Villalta. 1990. Trypanosoma cruzi amastigote receptors for human transferrin and their role. Mol. Biochem. Parasitol. 38:245-252. [DOI] [PubMed] [Google Scholar]
- 15.Lopes, M. F., C. G. Freire-de-Lima, and G. A. DosReis. 2000. The macrophage haunted by cell ghosts: a pathogen grows. Immunol. Today 21:489-494. [DOI] [PubMed] [Google Scholar]
- 16.Maeda, K., Y. Kato, and Y. Sugiyama. 2002. pH dependent receptor/ligand dissociation as a determining factor for intracellular sorting of ligands for epidermal growth factor receptors in rat hepatocytes. J. Control Release 82:71-82. [DOI] [PubMed] [Google Scholar]
- 17.Noisin, E. L., and F. Villalta. 1989. Fibronectin increases Trypanosoma cruzi binding to and uptake by murine macrophages and human monocytes. Infect. Immun. 57:1030-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeifer, R. W., and L. A. Adams. 1994. Transforming growth-factor alpha expression in peritoneal macrophages elicited from SENCAR and B6C3F31 mice: responses to lipopolysaccharide and 12-O-tetradecaonoylphorpbol-13-acetate. Mol. Carcinog. 10:142-150. [DOI] [PubMed] [Google Scholar]
- 19.Russell, S. W., and S. Gordon. 1992. Macrophage biology and activation. Curr. Top. Microbiol. Immun. 181:103-105. [Google Scholar]
- 20.Sporn, M. B., and A. B. Roberts. 1991. Peptide growth factors and their receptors. Springer-Verlag, New York, N.Y.
- 21.Villalta, F., and and F. Kierszenbaum. 1982. Growth of isolated amastigotes of Trypanosoma cruzi in cell-free medium. J. Protozool. 29:570-576. [DOI] [PubMed] [Google Scholar]
- 22.Wiley, L. M., E. D. Adamson, and E. C. Tsark. 1995. Epidermal growth factor receptor function in early mammalian development. BioEssays 17:839-846. [DOI] [PubMed] [Google Scholar]
- 23.Zhu, J. Q., J. Wu, D. X. Zhu, A. Scharfman, G. Lamblin, and K. K. Han. 1991. Recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF) induces human macrophage production of transforming growth factor alpha. Cell. Mol. Biol. 37:413-419. [PubMed] [Google Scholar]