Abstract
Leishmania amazonensis is one of the major etiologic agents of a broad spectrum of clinical forms of leishmaniasis and has a wide geographical distribution in the Americas, which overlaps with the areas of transmission of many other Leishmania species. The LACK and A2 antigens are shared by various Leishmania species. A2 was previously shown to induce a potent Th1 immune response and protection against L. donovani infection in BALB/c mice. LACK is effective against L. major infection, but no significant protection against L. donovani infection was observed, in spite of the induction of a potent Th1 immune response. In an attempt to select candidate antigens for an American leishmaniasis vaccine, we investigated the protective effect of these recombinant antigens (rLACK and rA2) and recombinant interleukin-12 (rIL-12) against L. amazonensis infection in BALB/c mice. As expected, immunization with either rA2-rIL-12 or rLACK-rIL-12 induced a robust Th1 response prior to infection. However, only the BALB/c mice immunized with rA2-rIL-12 were protected against infection. Sustained gamma interferon (IFN-γ) production, high levels of anti-A2 antibodies, and low levels of parasite-specific antibodies were detected in these mice after infection. In contrast, mice immunized with rLACK-rIL-12 displayed decreased levels of IFN-γ and high levels of both anti-LACK and parasite-specific antibodies. Curiously, the association between rA2 and rLACK antigens in the same vaccine completely inhibited the rA2-specific IFN-γ and humoral responses and, consequently, the protective effect of the rA2 antigen against L. amazonensis infection. We concluded that A2, but not LACK, fits the requirements for a safe vaccine against American leishmaniasis.
Leishmaniasis is one of the six major tropical diseases of developing countries, according to the World Health Organization (16). Control of leishmaniasis in Central and South America is a difficult task because of the zoonotic features of transmission and the sylvatic nature of reservoirs and vectors. In this context, the development of a prophylactic vaccine is strongly desirable. One of the main reasons that an efficient vaccine has not yet emerged is the fact that, in the Americas, the disease is caused by at least eight different species of the genus Leishmania, each one with its own natural history, determinants of virulence, and pathogenesis, but many of these species have common areas of transmission (2, 15, 16, 29). In this context, one might anticipate that to be effective against American leishmaniasis, the vaccine's components should be immunogenic and shared by different species and should present few polymorphisms.
The LACK (Leishmania homologue of receptors for activated C kinase) antigen is a 36-kDa protein expressed in promastigote and amastigote forms of different Leishmania species, including Leishmania amazonensis (7, 33). The LACK amino acid sequence, including an immunodominant epitope located between amino acids 158 and 173, is highly conserved (31, 33). Immunization of BALB/c mice with a truncated (24-kDa) version of LACK, delivered either as protein or DNA or as part of a multicomponent vaccine, confers protection against L. major infection (17, 18, 33). However, no significant protection against L. donovani infection was observed (31).
A2 antigens were identified as an amastigote stage-specific protein family in L. donovani (6). The A2 proteins are composed predominantly of multiple copies of a 10-amino-acid repeat, and depending on the number of repeats within each protein, the protein size may range from 45 to 100 kDa (38). Karyotype analysis, performed with a panel of samples representative of different Leishmania species, revealed that A2 genes are conserved in species of the L. donovani complex and the L. mexicana complex (10). Recently, it was demonstrated that immunization with A2, as protein or DNA, protects against L. donovani infection (13, 14).
In this work, we have investigated the protective effect of vaccination with the recombinant LACK (rLACK) and recombinant A2 (rA2) proteins against L. amazonensis infection. Our findings demonstrated significant protection in mice immunized with rA2 but not in those immunized with rLACK. Protection was associated with the preferential and sustained induction of a Th1 immune response. Curiously, the association between rA2 and rLACK antigens in the same vaccine inhibited the protective effect of the rA2 antigen against L. amazonensis infection. Thus, we present evidence that A2 is a candidate antigen for a vaccine against American leishmaniasis.
MATERIALS AND METHODS
Parasites.
L. amazonensis (IFLA/BR/67/PH-8) and L. major (MHOM/IL/80/Friedlin) were kindly provided by Maria Norma Melo (Department of Parasitology, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte, Brazil) and Leda Quércia Vieira (Department of Biochemistry and Immunology, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte, Brazil), respectively. The parasites were grown at 23°C in Schneider's medium (Sigma, St. Louis, Mo.) supplemented with 20% heat-inactivated fetal bovine serum (Sigma), 20 mM l-glutamine, 200 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 μg of gentamicin/ml at pH 7.4.
Expression and purification of recombinant proteins.
The rLACK protein was obtained by cloning of a PCR product amplified from genomic DNA from L. chagasi promastigotes. Genomic DNA was isolated by a phenol-chloroform extraction, as previously described (11). The amplification product was isolated by low-melting-point agarose (FMC BioProducts, Rockland, Maine) gel electrophoresis and cloned into the pMAL-c2 vector (New England Biolabs, Inc., Beverly, Mass.). The insert was sequenced by using a Thermo Sequenase Fluorescent labeled primer cycle sequencing kit with 7-deaza-dGTP (Amersham Life Science, Piscataway, N.J.) and an automated, fluorescent DNA sequencer (Pharmacia Biotech, Piscataway, N.J.). rLACK was expressed as a fusion protein with maltose-binding protein (MBP). Recombinant protein expression was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside; Promega, Montreal, Canada) to Escherichia coli DH5α cells. After 3 h, cells were lysed by five cycles of freezing and thawing followed by mild ultrasonic treatment (five cycles of 30 s each with an ultrasonic processor, model GEX 600) and then centrifuged at 13,000 × g for 20 min at 4°C. The rLACK protein and recombinant MBP were purified by affinity chromatography in an amylase column (New England Biolabs). Cloning, expression, and purification of the L. donovani rA2 protein containing a tag of six residues of histidine were carried out as previously described (5).
Antigen preparation.
Soluble Leishmania antigen (SLA) was prepared from late-log-phase promastigotes of L. amazonensis after a few passages in liquid culture, as previously described (35). Briefly, 2 × 108 promastigotes/ml were washed three times in 5 ml of cold sterile phosphate-buffered saline (PBS). After five cycles of freezing and thawing, the suspension was centrifuged at 8,000 × g for 20 min at 4°C and supernatant containing SLA was collected and stored at −70°C. The protein concentrations were estimated by the Bradford method (4).
Immunization.
Groups of eight 4- to 6-week-old female BALB/c mice (Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte, Brazil) were immunized subcutaneously in their left hind footpads with 50 μg of one of the recombinant proteins (rA2 or rLACK), with a combination of the proteins, or with SLA from L. amazonensis promastigotes. Additional groups were immunized with MBP-recombinant interleukin-12 (MBP-rIL-12) or MBP-rA2-rIL-12. Two vaccine doses were administered at 2-week intervals. rIL-12 (1 μg; Genetics Institute) adsorbed to alum (1.5 μg of antigen to 1 μg of alum [Rehydragel low-viscosity gel; Reheis, Inc., Berkeley Heights, N.J.]) or not adsorbed was used as an adjuvant. Control mice received only 20 μl of sterile PBS or the recombinant proteins in the absence of adjuvants.
Challenge infection.
Three weeks after the last vaccine dose, 106 late-log-phase L. amazonensis promastigotes were injected into each mouse's right hind footpad. The course of the disease was monitored at weekly intervals by measuring footpad thickness with a metric caliper, and results were expressed as the increase in the thickness of the infected hind foot compared to the thickness of the uninfected left foot. All groups of mice were evaluated for lesion development for up to 8 or 9 weeks. Then, animals were sacrificed and spleens, serum samples, and tissue fragments were harvested for parasite quantification and immunological analysis.
Parasite quantitation.
The number of viable parasites at the site of infection was determined by a limiting dilution assay, as described elsewhere (1). Briefly, skin fragments were excised and homogenized in Schneider's modified medium supplemented with 20% fetal bovine serum. Each tissue homogenate was serially diluted in a 96-well Maxisorb plate (Nunc, Roskilde, Denmark). Samples, in duplicate, were incubated at 23°C. The wells containing motile promastigotes were identified with a microscope (Axiovert 25; Zeiss), and the number of viable parasites per milligram of tissue was determined from the highest dilution at which promastigotes had grown after 7 days of incubation.
Cytokine production assays.
Splenocyte cultures and cytokine assays were performed as described previously (10). Briefly, single-cell preparations from spleen tissue were plated in duplicate in 24-well plates (Nunc) at 2 × 106 cells/ml. Cells were incubated in Dulbecco modified Eagle medium alone (background control) or separately stimulated with SLA from L. amazonensis (50 μg), concanavalin A (positive control; 5 μg), or recombinant proteins (10 μg) at 37°C in 5% CO2 for 48 h. Levels of gamma interferon (IFN-γ) and IL-4 in supernatants were assessed by sandwich enzyme-linked immunosorbent assay (ELISA) by using Inter Test mouse IFN-γ and IL-4 (Pharmingen, San Diego, Calif.). IL-10 levels were measured by using the DuoSet ELISA development system (R&D Systems, Minneapolis, Minn.).
ELISA for parasite-specific IgG1 and IgG2a isotypes.
Levels of parasite-specific immunoglobulin G1 (IgG1) and IgG2a were measured by ELISA as described elsewhere (8). A titration curve was plotted to determine the best protein concentration and antibody dilution. In brief, 96-well plates were sensitized with rA2 or rLACK (250 ng/100 μl/well) overnight at 4°C. Plates were blocked with PBS-10% bovine serum albumin at 37°C for 1 h and treated successively with 1:100 dilutions of mouse serum samples for 2 h at 37°C. Peroxidase-conjugated anti-mouse IgG1 or IgG2a isotype (Sigma) was diluted at 1:5,000 and added for 2 h at 37°C. Reactions were developed by incubation with H2O2 and o-phenylenediamine. Optical densities were read at 492 nm with an optical density reader (model 2550; Bio-Rad, Richmond, Calif.).
Western blot analysis.
Purified recombinant proteins (5 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (0.2-μm pore size; Sigma) by Western blotting by using standard protocols (37). Membranes were blocked with PBS-2% bovine serum albumin and incubated for 18 h at 37°C before the first incubation with a pool of sera collected from immunized mice after challenge infection and diluted 1:100 in PBS. Peroxidase-conjugated anti-mouse IgG (1:10,000; Sigma) or anti-rabbit IgG (1:20,000; Sigma) was used as a second antibody. Reactions were revealed by the addition of chloronaphthol, diaminobenzidine, and H2O2. The anti-A2 monoclonal antibody C9 (38) diluted 1:100 or a rabbit anti-rLACK polyclonal serum diluted 1:100 was used as a positive control.
Statistical analysis.
All data comparisons were tested for significance by using unpaired Student's t test; P values of <0.05 were considered statistically significant.
RESULTS
Immunization with rA2, but not rLACK, confers protection against L. amazonensis infection in BALB/c mice.
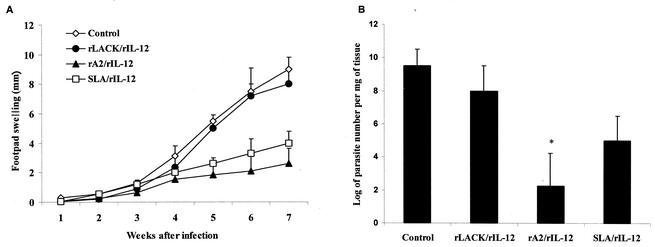
The protective effect of immunization with the proteins rLACK and rA2 against L. amazonensis infection in BALB/c mice was evaluated by measuring lesion development (Fig. 1A) and parasite loads (Fig. 1B) in the infected footpads. No significant protection or reduction in parasite loads was observed in the animals immunized with rLACK-rIL-12, in spite of the fact that the same immunization protocol induced partial protection and a significant reduction in parasite loads (105-fold) in BALB/c mice challenged with L. major. Similar results were observed with mice immunized with a LACK DNA vaccine (data not shown). In contrast, mice immunized with rA2-rIL-12 or SLA-rIL-12 showed a delay in both the onset and course of L. amazonensis infection, resulting in a significant decrease in edema levels in the infected footpads compared with those in the footpads of the control group. The reduction in lesion development observed in rA2-rIL-12-treated mice correlated with a 106-fold decrease in parasite loads in the infected footpads compared to those in the footpads of the control group (Fig. 1B). No significant differences were observed in lesion development rates among the groups immunized with rA2-rIL-12 or SLA-rIL-12 adsorbed or not adsorbed to alum or among groups given different infective doses (106 or 105 L. amazonensis promastigotes). Immunization with the rA2 protein in the absence of rIL-12 did not induce protection against L. amazonensis infection (data not shown).
FIG. 1.
Results of L. amazonensis protection assays. Groups of eight BALB/c mice were immunized with two subcutaneous injections at 15-day intervals with 1 μg of rIL-12 as an adjuvant and 50 μg of rLACK, rA2, or L. amazonensis SLA in the left hind footpads. Each mouse was challenged with 106 late-log-phase L. amazonensis promastigotes in the right hind footpads, as described in Material and Methods. Control mice received PBS. (A) Lesion development (footpad swelling) in immunized groups was monitored weekly with a caliper. Each point represents the average, and error bars represent standard deviations. (B) Parasite loads detected in infected footpads of each immunized group 9 weeks postchallenge. Numbers of viable parasites were determined by a limiting dilution assay as described in Materials and Methods. Each bar represents the average, and error bars represent standard deviations (n = 4). The asterisk indicates that differences are statistically significant (P < 0.05).
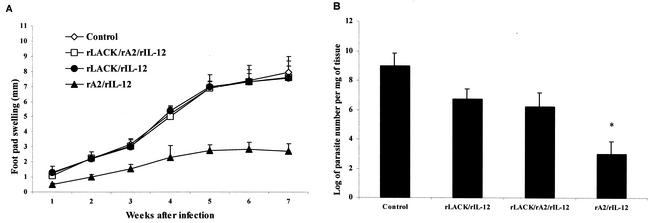
Simultaneous immunization with rLACK completely inhibits the protection induced by immunization with rA2 in L. amazonensis-infected mice.
We next examined the effect of immunization with a combined rLACK-rA2-rIL-12 vaccine (Fig. 2). In this experiment and as previously observed, immunization with rA2-rIL-12, but not with rLACK-rIL-12, induced significant protection, measured by either lesion development or parasite loads. Interestingly, in the animals immunized with the combined vaccine, the protection induced by rA2 was completely abolished (Fig. 2A). No significant differences in lesion development rates or parasite loads (Fig. 2B) were observed among the control group (mice receiving PBS) and those groups receiving rLACK-rIL-12 or rLACK-rA2-rIL-12. Since LACK was produced as a fusion protein with MBP, we also investigated protection levels in MBP-rIL-12- and MBP-rA2-rIL-12-immunized mice. No significant protection was observed in animals immunized with MBP-rIL-12. In contrast, protection induced by rA2 was not inhibited in animals immunized with MBP-rA2-rIL-12 (data not shown).
FIG. 2.
Results of L. amazonensis protection assays for mice immunized with rLACK-rA2-rIL-12. Groups of eight BALB/c mice were immunized with two subcutaneous injections at 15-day intervals with 50 μg of rLACK, rA2, or a combination of the two recombinant proteins in the left hind footpads and challenged with 106 late-log-phase L. amazonensis promastigotes in the right hind footpads, as described in Materials and Methods. rIL-12 (1 μg) was used as an adjuvant. Control mice received PBS. (A) Lesion development (footpad swelling) was monitored weekly with a caliper. Each point represents the average, and error bars represent standard deviations. (B) Parasite loads detected in infected footpads of each immunized group 9 weeks postchallenge. Numbers of viable parasites were determined by a limiting dilution assay as described in Materials and Methods. Each bar represents the average, and error bars represent standard deviations (n = 4). The asterisk indicates that differences are statistically significant (P < 0.05).
Characterization of the humoral and cytokine (IFN-γ, ΙL-4, and IL-10) responses in immunized animals.
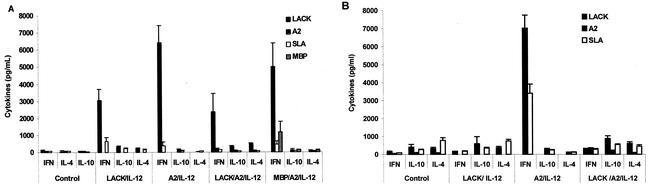
Since activation of a Th1 immune response and sustained IFN-γ production are important requirements for protection against L. amazonensis infection, we analyzed the production of IFN-γ, IL-4, and IL-10 by spleen cells of immunized animals prior to (15 days after the second vaccine dose) and after challenge infection.
Prior to challenge infection, spleen cells taken from animals immunized with either rLACK-rIL-12 or rA2-rIL-12 produced, in response to each recombinant protein, high levels of IFN-γ and very low levels of IL-4 and IL-10 (Fig. 3A). Cells from rA2-rIL-12-immunized animals produced significantly higher levels of IFN-γ than did splenocytes of rLACK-rIL-12-immunized animals. Thus, immunization with either rA2-rIL-12 or rLACK-rIL-12 primed the animals for an antigen-specific Th1 immune response. In contrast, in the animals immunized simultaneously with the two recombinant proteins, the strong IFN-γ production induced by immunization with rA2-rIL-12 was almost completely inhibited. No significant differences were observed between the levels of IFN-γ, IL-4, and IL-10 produced by rLACK-stimulated cells from these animals and those produced by cells from rLACK-rIL-12-immunized animals. The high levels of IFN-γ production observed after stimulation of spleen cells with rA2 were not suppressed in animals immunized with MBP-rA2-rIL-12 (Fig. 3A). Also, no significant differences were detected in the levels of IL-4 and IL-10 produced by spleen cells of rA2-rIL-12-, rLACK-rA2-rIL-12-, or MBP-rA2-rIL-12-immunized animals when the cells were stimulated with rA2.
FIG. 3.
Levels of IFN-γ, IL-4, and IL-10 in BALB/c mice from control and immunized groups prior to (A) and 3 months after (B) challenge infection with L. amazonensis. Single-cell suspensions were obtained from spleens and stimulated for 48 h with rLACK, rA2, or L. amazonensis SLA. IFN-γ, IL-4, and IL-10 were measured in culture supernatants by capture ELISAs. Each bar represents the average, with error bars representing standard deviations, of cytokine levels determined for eight mice per group.
In spite of the high IFN-γ levels detected in cultures of splenocytes from rLACK-immunized animals prior to challenge (Fig. 3A), a decrease (approximately 10-fold) in the IFN-γ production was observed after infection with L. amazonensis. Production of IFN-γ from splenocyte cultures stimulated with either SLA or rLACK was significantly lower than that in rLACK-immunized animals before challenge and was comparable to that observed in nonimmunized animals. No significant differences were observed in IL-4 or IL-10 levels in response to rLACK or SLA in rLACK-rIL-12-immunized animals (Fig. 3B). Consistent with this cytokine profile observed after the infection of mice immunized with rLACK-rIL-12, reduced levels of IFN-γ were also observed in culture supernatants of rLACK-stimulated spleen cells from mice immunized with rLACK-rA2-rIL-12. Furthermore, the rA2-specific IFN-γ production remained suppressed in these mice after challenge infection. This is in sharp contrast to the sustained IFN-γ production in response to rLACK observed in rLACK-immunized animals after L. major (data not show) or L. donovani (31) challenge infection. SLA- or rLACK-stimulated splenocytes from mice immunized with SLA-rIL-12 produced significantly higher levels of IFN-γ (data not shown) than splenocytes from rLACK-rIL-12-immunized animals. No significant differences were observed in IL-4 or IL-10 levels. In contrast, mice immunized with rA2 showed sustained and enhanced IFN-γ production after L. amazonensis infection. Moreover, spleen cells from these mice produced high levels of IFN-γ in response to either rA2 or SLA but undetectable levels of IL-4 or IL-10 in response to both antigens.
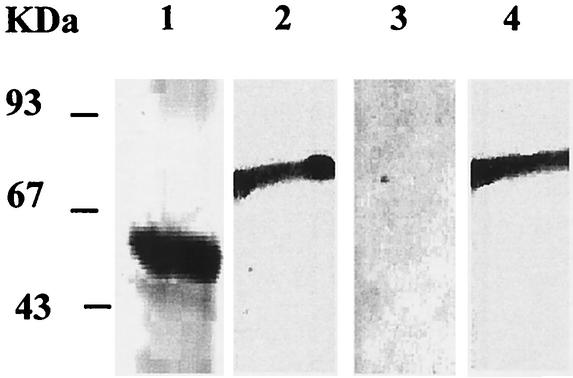
After challenge infection, a Western blot analysis (Fig. 4) revealed that sera from either rLACK-rIL-12- or rLACK-rA2-rIL-12-immunized mice recognized the rLACK (78 kDa). In contrast, rA2 (53 kDa) was recognized only by sera of rA2-rIL-12-immunized animals and not by sera of rLACK-rA2-rIL-12-immunized animals. Thus, besides T-cell responses, immunization with rLACK also impaired the antibody response to rA2.
FIG. 4.
Detection of anti-rA2- or anti-rLACK-specific antibodies in sera of immunized BALB/c mice after challenge infection. rA2 (lanes 1 and 3) and rLACK (lanes 2 and 4) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and incubated with sera of mice immunized with rA2-rIL-12 (lane 1), rLACK-rIL-12 (lane 2), or rLACK-rA2-rIL-12 (lanes 3 and 4). The anti-A2 monoclonal antibody C9 and a rabbit anti-rLACK polyclonal serum were used as positive controls (data not shown).
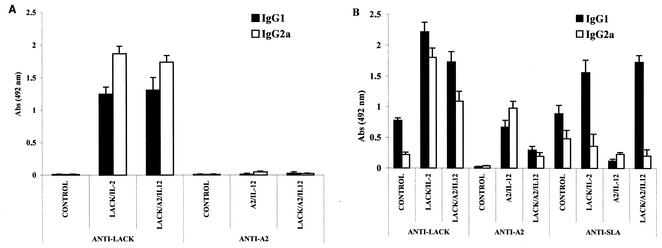
Levels of IgG (isotypes 1 and 2a) were measured in sera of control and immunized animals before and after challenge infection (Fig. 5). This analysis revealed another striking difference between the two antigens studied. While immunization with rLACK-rIL-12 induced a strong humoral response (Fig. 5A) characterized by significantly higher levels of anti-rLACK IgG2a antibodies, immunization with rA2-rIL-12 induced very low levels of both IgG1 and IgG2a antibodies. This same profile was observed in mice immunized with rLACK-rA2-rIL-12.
FIG. 5.
Levels of parasite-specific IgG isotypes in sera of immunized BALB/c mice prior to (A) and 2 months after (B) challenge infection with L. amazonensis. IgG1 and IgG2a levels were assessed by ELISA in sera, diluted 1:100, from control and immunized mice as described in Materials and Methods. Bars represent the averages, with error bars representing standard deviations, of levels determined for eight mice per group. Abs, absorbance.
After infection, however, these profiles were significantly altered (Fig. 5B). In rLACK-rIL-12- or rLACK-rA2-rIL-12-immunized mice, higher anti-rLACK IgG1 levels supplanted, significantly, the IgG2a levels observed before challenge. Levels of anti-rA2 or anti-SLA antibodies were significantly lower in LACK-rA2-rIL-12-immunized animals. Infected control mice showed significantly higher levels of anti-rLACK- or anti-SLA-specific IgG1 antibodies than of IgG2a antibodies. Increased levels of anti-rA2 IgG2a antibodies were detected in rA2-rIL-12-immunized animals compared to the levels detected prior to infection. In addition, in these mice, levels of anti-rA2- or anti-SLA-specific IgG2a antibodies were significantly higher than those of IgG1 antibodies. An antibody titration experiment, performed after infection, indicated that anti-rA2 antibody titers were 1:2,000 and 1:200 in rA2-rIL-12- and rLACK-rA2-rIL-12-immunized animals, respectively. In contrast, anti-rLACK antibody titers were 1:12,800 in rLACK-immunized animals. Consistent with the IgG data, the IgM levels were found to be undetectable for A2 and present for LACK in the first month after immunization of animals with rLACK-rA2-rIL-12 (data not shown).
DISCUSSION
L. amazonensis is one of the major etiologic agents of American leishmaniasis and is associated with a broad spectrum of diseases in humans, ranging from asymptomatic infection to the disfiguring forms of leishmaniasis or the potentially fatal visceral form of the disease (2). In addition, L. amazonensis has a broad distribution in the Americas which overlaps with the areas of occurrence of many other Leishmania species (15). In an effort to identify candidate antigens for a defined vaccine applicable to such a complex epidemiological scenario, we have investigated the protective effect against L. amazonensis infection of two antigens, rLACK and rA2, which are expressed in L. amazonensis amastigotes (5, 33) as well as in those of other species (12, 31).
Although immunization with either rA2 or rLACK induced a robust Th1 response prior to infection, BALB/c mice vaccinated with rA2-rIL-12, but not those vaccinated with rLACK-rIL-12, were protected against L. amazonensis infection. The significant protection induced by the L. donovani rA2 protein against L. amazonensis infection was associated with high IFN-γ levels and null IL-4 or IL-10 production. This finding is in agreement with those of previous reports, which showed that immunization with rA2 protein or DNA induces a specific IFN-γ response and is protective against L. donovani infection (14, 15). This is also consistent with the fact that production of high IFN-γ levels, a surrogate marker of Th1 response, is critical for protection against these two species (1, 9, 21, 25, 28, 34).
Immunization, or tolerization, of BALB/c mice with LACK induced protection against L. major infection. In fact, during L. major infection, depletion of LACK-reactive T cells in LACK-tolerized mice diminishes the early IL-4 response, allowing the development of a protective Th1 response mediated by IL-12-dependent IFN-γ production (23). However, these mice were not protected against L. mexicana infection (36). These findings led to the conclusion that T-cell responses to LACK do not have an important role in L. mexicana infection. In our experiments, infection with L. amazonensis resulted in a significant alteration of the rLACK-specific immune response induced by immunization. We have observed that, in rLACK-rIL-12-immunized mice, IFN-γ levels were significantly decreased after L. amazonensis challenge infection. Curiously, in another study, immunization with LACK did not protect BALB/c mice against L. donovani infection, in spite of a sustained IFN-γ antigen-specific recall response after challenge infection (31). No significant differences were observed in IL-4 or IL-10 levels between rLACK-immunized and control infected animals or between animals before and after challenge. Thus, it seems that the down-regulation of the IFN-γ response in LACK-immunized mice cannot be attributed to increased levels of IL-4 or IL-10. This is in agreement with the fact that LACK-tolerized mice were not protected against L. mexicana infection, despite the fact that their splenocytes secreted significantly less IL-4 than control, infected wild-type BALB/c mice (36). Second, it has been previously shown that IL-4−/− and IL-10−/− mice remain susceptible to L. amazonensis infection and that, apparently, these cytokines do not contribute to the IL-12 unresponsiveness and susceptibility to L. amazonensis infection (21, 22). Suppression of the immune response to LACK, after infection with L. amazonensis, seems to be a more general phenomenon of parasite antigens. Thus, we also observed suppression of SLA- and A2-specific immune responses in unprotected mice with high loads of parasitism (those immunized with rLACK-rIL-12 and rLACK-rA2-rIL-12). These findings are in contrast with the immune response of protected mice that showed a strong IFN-γ response to both rA2 and SLA.
Together, these data support previous observations indicating that the mechanisms underlying susceptibility to different Leishmania species are, in fact, widely distinct. Susceptibility to L. major infection is linked to a polarized Th2 response characterized by early IL-4 production and differential expression of the IL-12 receptor (19, 24, 27). In contrast, IL-4 or IL-10 does not play a critical role in susceptibility to L. amazonensis infection (21, 22). Susceptibility to L. amazonensis as well as to L. chagasi and L. donovani seems to be due to a failure to mount a Th1 response rather than to the development of a Th2 response (1, 20, 28, 31, 35). Moreover, it has been shown that, in L. amazonensis infection, susceptibility is determined by an IL-4-independent inhibition of IL-12 responsiveness (21). In fact, administration of anti-IL-4 antibody to susceptible mice has no effect on L. amazonensis disease progression (1). Recently, it was reported that no significant bias toward any T-cell receptor-bearing CD4+ cells is observed following L. amazonensis infection (20). In addition, at different stages of infection, CD4+ cells of draining lymph nodes contained comparable frequencies of Th1 and Th2 cells, which became, however, highly polarized to a Th2 phenotype after in vitro stimulation. These cells preferentially expressed Vα2, Vβ4, or Vβ8.1/Vβ8.2 and displayed weak proliferative responses to the L. major LACK antigen or its dominant epitope.
Another striking difference between rLACK- and rA2-immunized animals was the humoral responses. Levels of anti-rA2 IgG1 or IgG2a antibody in the sera of rA2-immunized animals were lower than those of the rLACK-specific antibodies present in the sera of rLACK-immunized animals. The presence of anti-rA2 antibodies in rA2-immunized animals may have an important role in preventing amastigote internalization during L. amazonensis infection, as has been demonstrated in the case of L. donovani-challenged animals (14). On the other hand, it is possible that the high antiparasite antibody titers observed in rLACK-immunized animals may have also contributed to disease progression in these animals. Antibodies may in fact have a deleterious role in L. amazonensis infection, since infection was impaired in the absence of circulating antibodies or in mice lacking the Fc receptors' common γ chain (26). Furthermore, H-2q syngeneic (Biozzi) mice with high and low antibody responses were shown to be susceptible and resistant, respectively, to L. amazonensis infection (30).
An interesting finding of our study was the fact that simultaneous immunization with rLACK and rA2 inhibited the development of the rA2-specific cellular and humoral response. This is the first description, to our knowledge, of defined antigen interference in the development of a response to a vaccine against a parasite infection. One plausible explanation for this finding is the presence of an immunodominant epitope between the amino acid positions 158 and 173 of the rLACK antigen. This epitope is identical in all Leishmania species. It has been shown that, in L. major infection, this immunodominant epitope is recognized by IL-4-secreting, disease-promoting T cells that express a Vβ4+/Vα8+ T-cell receptor and that, after antigen presentation, undergo rapid clonal expansion (24, 27). Besides IL-4, T cells recognizing LACK were shown to produce IL-10 in patients with American cutaneous leishmaniasis (3). After immunization with rLACK protein and rIL-12, CD4+ cells are the main T-cell subset responsible for IFN-γ production in L. major- and L. donovani-infected BALB/c mice. Protocols combining different Leishmania antigens, including LACK, have been tested against L. major infection, with favorable results (32). It has been suggested by others (31) that, in cases in which immunization with LACK induces sustained IFN-γ production, LACK could potentially contribute to the protective response generated by multicomponent vaccines. However, our data suggest that, through its immunodominant effect, LACK may considerably restrict the repertoire of T-cell effectors recognizing A2 or other nondominant antigens when these antigens are present in the same vaccine with LACK. This is an important aspect that should be considered in the development of a vaccine against Leishmania infection.
Acknowledgments
This work was supported in part by CNPq, CNPq/PADCT SBIO (62.0106/95-6), CNPq/Pronex, and FAPEMIG. A.P.F., C.A.P.T., and R.T.G. are CNPq research fellows. G.M. acknowledges the support from CIHR. E.A.F.C. is a graduate student with a scholarship from CAPES. K.F.C. is an undergraduate student with a scholarship from FAPEMIG.
Editor: J. M. Mansfield
REFERENCES
- 1.Afonso, L. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barral, A., D. Pedral-Sampaio, H. Momen, D. McMahon-Pratt, A. R. De Jesus, R. Almeida, R. Badaró, M. Barral-Neto, E. M. Carvalho, W. D. Johnson, and G. Grimaldi, Jr. 1991. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am. J. Trop. Med. Hyg. 44:536-546. [DOI] [PubMed] [Google Scholar]
- 3.Bottrel, R. L. A., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho, F. A. A., H. Charest, C. A. P. Tavares, G. Matlashewski, E. P. Valente, A. Rabello, R. T. Gazzinelli, and A. P. Fernandes. 2002. Diagnosis of American visceral leishmaniasis in humans and dogs using the recombinant Leishmania donovani A2 antigen. Diagn. Microbiol. Infect. Dis. 43:289-295. [DOI] [PubMed] [Google Scholar]
- 6.Charest, H., and G. Matlashewski. 1994. Developmental gene expression in Leishmania donovani: differential cloning and analysis of an amastigote-stage-specific gene. Mol. Cell. Biol. 14:2975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courret, N., E. Prina, E. Mougneau, E. M. Saraiva, D. L. Sacks, N. Glaichenhaus, and J. C. Antonie. 1999. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 29:762-773. [DOI] [PubMed] [Google Scholar]
- 8.Faria, A. M., S. M. Ficker, E. Speziali, J. S. Menezes, B. Stransky, B. A. Verdolin, W. M. Lahmann, V. S. Rodrigues, and N. M. Vaz. 1998. Aging and immunoglobulin isotype patterns in oral tolerance. Braz. J. Med. Biol. Res. 31:35-48. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes, A. P., E. C. Herrera, W. Mayrink, R. T. Gazzinelli, W. Y. Liu, C. A. Costa, C. A. P. Tavares, M. N. Melo, M. S. M. Michalick, R. Gentz, and E. Nascimento. 1997. Immune responses induced by a Leishmania (Leishmania) amazonensis recombinant antigen in mice and lymphocytes from vaccinated subjects. Rev. Inst. Med. Trop. Sao Paulo 39:70-78. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes, A. P., F. A. A. Carvalho, C. A. P. Tavares, H. C. Santiago, G. A. Castro, W. L. Tafuri, L. A. M. Ferreira, and R. T. Gazzinelli. 2001. Combined interleukin-12 and topical chemotherapy for established leishmaniasis drastically reduces tissue parasitism and relapses in susceptible mice. J. Infect. Dis. 183:1646-1652. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira-Pinto, K. C., A. L. Miranda-Vilela, C. Anacleto, A. P. S. M. Fernandes, M. C. B. Abdo, M. L. Petrillo-Peixoto, and E. S. A. Moreira. 1996. Leishmania (V.) guyanensis: isolation and characterization of glucantime-resistant cell lines. Can. J. Microbiol. 42:944-949. [DOI] [PubMed] [Google Scholar]
- 12.Ghedin, E., H. Charest, and G. Matlashewski. 1998. A2 rel: a constitutively expressed Leishmania gene linked to an amastigote-stage-specific gene. Mol. Biochem. Parasitol. 93:23-29. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, A., S. Labrecque, and G. Matlashewski. 2001. Protection against Leishmania donovani infection by DNA vaccination: increased DNA vaccination efficiency through inhibiting the cellular p53 response. Vaccine 19:3169-3178. [DOI] [PubMed]
- 14.Ghosh, A., W. W. Zhang, and G. Matlashewski. 2002. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine 20:59-66. [DOI] [PubMed] [Google Scholar]
- 15.Grimaldi, G., Jr., R. B. Tesh, and D. McMahon-Pratt. 1989. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am. J. Trop. Med. Hyg. 41:687-725. [DOI] [PubMed] [Google Scholar]
- 16.Grimaldi, G., Jr., and R. B. Tesh. 1993. Leishmaniasis of the New World: current concepts and implications for future research. Clin. Microbiol. Rev. 6:230-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 19.Himmelrich, H., C. Parra-Lopez, F. Tacchni-Cottier, J. A. Louis, and P. Launois. 1998. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 161:6156-6163. [PubMed] [Google Scholar]
- 20.Ji, J., J. Sun, H. Qi, and L. Soong. 2002. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am. J. Trop. Med. Hyg. 66:338-345. [DOI] [PubMed] [Google Scholar]
- 21.Jones, D. E., L. U. Buxbaum, and P. Scott. 2000. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J. Immunol. 165:364-372. [DOI] [PubMed] [Google Scholar]
- 22.Jones, D. E., M. R. Ackermann, U. Wille, C. A. Hunter, and P. Scott. 2002. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect. Immun. 70:2151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julia, V., M. Rassoulzadegan, and N. Glaichenhaus. 1996. Resistance to Leishmania major induced by tolerance to a single antigen. Science 274:421-423. [DOI] [PubMed] [Google Scholar]
- 24.Julia, V., and N. Glaichenhaus. 1999. CD4+ T cells which react to the Leishmania major LACK antigen rapidly secrete interleukin-4 and are detrimental to the host in resistant B10.D2 mice. Infect. Immun. 67:3641-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaye, P. M., A. J. Curry, and J. M. Balckwell. 1991. Differential production of Th1- and Th2-derived cytokines does not determine genetically controlled vaccine-induced rate of cure in murine visceral leishmaniasis. J. Immunol. 146:2763-2770. [PubMed] [Google Scholar]
- 26.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, M. J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 191:1063-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xénarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. McDonald, and J. A. Louis. 1997. IL-4 rapidly produced by Vβ4+ Vα8+ CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann, J., K. H. Enssle, I. Lehmann, A. Emmendorff, and M. L. Lohman-Matthes. 2000. The capacity to produce IFN-gamma rather than the presence of IL-4 determines resistance and the degree of susceptibility to Leishmania donovani infection in mice. J. Interferon Cytokine Res. 20:63-77. [DOI] [PubMed] [Google Scholar]
- 29.Leon, L. L., G. M. C. Machado, L. E. Carvalho Paes, and G. Grimaldi, Jr. 1990. Antigenic differences of Leishmania amazonensis isolates causing diffuse cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 84:678-680. [DOI] [PubMed] [Google Scholar]
- 30.Lima, G. M. C., A. Puel, C. Decreusefond, J. C. Bouthillier, I. A. Abrahamsohn, and D. Mouton. 1998. Susceptibility and resistance to Leishmania amazonensis in H-2q syngeneic high and low antibody responder mice (Biozzi mice). Scand. J. Immunol. 48:144-151. [DOI] [PubMed] [Google Scholar]
- 31.Melby, P. C., J. Yang, W. Zhao, L. E. Perez, and J. Cheng. 2001. Leishmania donovani p36 (LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 69:4719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendez, S., Y. Belkaid, R. A. Seder, and D. Sacks. 2002. Optimization of DNA vaccination against cutaneous leishmaniasis. Vaccine 20:3702-3708. [DOI] [PubMed] [Google Scholar]
- 33.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z. E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 34.Soong, L., S. M. Duboise, P. Kima, and D. McMahon-Pratt. 1995. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 63:3559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soong, L., C. H. Chang, J. Sun, Jr., B. D. Longley, N. H. Ruddle, R. A. Flavell, and D. McMahon-Pratt. 1997. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 158:5374-5383. [PubMed] [Google Scholar]
- 36.Torrentera, F. A., N. Glaichenhaus, J. D. Lanan, and Y. Carlier. 2001. T-cell responses to immunodominant LACK antigen do not play a critical role in determining susceptibility of BALB/c mice to Leishmania mexicana. Infect. Immun. 69:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, W. W., H. Charest, E. Ghedin, and G. Matlashewski. 1996. Identification and overexpression of the A2 amastigote-specific protein in Leishmania donovani. Mol. Biochem. Parasitol. 78:79-90. [DOI] [PubMed] [Google Scholar]