Abstract
Cholera toxin (CT) and related Escherichia coli enterotoxins LTI and LTIIb have a conserved hydrophobic region at the AB5 interface postulated to be important for toxin assembly. Hydrophobic residue F223 in the A subunit of CT (CTA) as well as residues 174, L77, and T78 in the B subunit of CT (CTB) were replaced individually with aspartic acid, and the resulting CTA and CTB variants were analyzed for their ability to assemble into holotoxin in vivo. CTA-F223D holotoxin exhibited decreased stability and toxicity and increased susceptibility to proteolysis by trypsin. CTB-L77D was unable to form functional pentamers. CTB-I74D and CTB-T78D formed pentamers that bound to GM1 and d-galactose but failed to assemble with CTA to form holotoxin. In contrast, CTB-T78D and CTA-F223H interacted with each other to form a significant amount of holotoxin in vivo. Our findings support the importance of hydrophobic interactions between CTA and CTB in holotoxin assembly. We also developed an efficient method for assembly of CT in vitro, and we showed that CT assembled in vitro was comparable to wild-type CT in toxicity and antigenicity. CTB-I74D and CTB-T78D did not form pentamers or holotoxin in vitro, and CTA-F223D did not form holotoxin in vitro. The efficient system for in vitro assembly of CT described here should be useful for future studies on the development of drugs to inhibit CT assembly as well as the development of chimeric CT-like molecules as potential vaccine candidates.
Cholera toxin (CT) is a heterohexameric AB5 toxin that is produced by Vibrio cholerae and is responsible for the profuse and life-threatening diarrheal disease resulting from infection. The pentameric B subunit of CT (CTB) binds to ganglioside GM1 receptors and triggers uptake of CT holotoxin into intestinal epithelial cells by endocytosis. Subsequent reduction of the proteolytically nicked A subunit (CTA) within the endoplasmic reticulum releases the active toxin component CTA1 (18). The CTA1 fragment is translocated to the cytoplasm, where it catalyzes the NAD-dependent ADP-ribosylation of Gsα, leading to the constitutive activation of adenylate cyclase. The subsequent increase in cyclic AMP concentration induces the secretion of fluids and electrolytes into the lumen of the small intestine (4). The resulting diarrhea may exceed 6 liters per hour, and there are estimated to be over 185,000 cases of V. cholerae infection worldwide each year (2, 34).
CT is a member of the V. cholerae-Escherichia coli family of AB5 heat-labile enterotoxins, which includes the highly homologous E. coli heat-labile type I enterotoxin (LTI) and the structurally related but less homologous type II enterotoxins (LTIIa and LTIIb) (8, 9). The LTIIa and LTIIb variants share the AB5 structure and enzymatic activity of CT and LTI, but they differ significantly in the amino acid sequences of their A2 domains and B subunits (33). The A2 peptides of these enterotoxins penetrate the central pore of the corresponding B pentamers. The interactions between the A2 domain and the B pentamer that occur within the upper end of the pentamer pore are exclusively hydrophobic, while the interactions that occur in the lower regions of the pentamer pore are largely hydrophilic (33). The presence of a large hydrophobic surface located within the upper pore of CT and other AB5 toxins is highly conserved, but the specific amino acids that participate in these hydrophobic interactions are not conserved (26, 33, 35). In the absence of the A subunit, this hydrophobic surface in the pore of the B pentamer is solvent accessible, but it is largely buried in the holotoxin structure as a consequence of hydrophobic interactions between the A and B subunits. For CT, this 549-Å2 hydrophobic surface is composed largely of residues I74, L77, and T78 of the CTB subunit that interact with residues of the CTA2 domain, including F223 and S228 (33).
Although CTA and CTB interact tightly at the AB5 interface of holotoxin, the B pentamer alone is highly stable and is able to bind to ganglioside GM1 in the presence or absence of CTA. In addition, CTA will only associate with CTB while CTB subunits are in the process of forming a pentamer (5). Studies have determined that the rate of holotoxin assembly is faster than that of B-pentamer assembly alone (5, 27). The conserved hydrophobic ring in the upper part of the pore of these AB5 toxins is postulated to be important in promoting CTA-CTB interactions and increasing the rate of holotoxin assembly over that of B pentamer (33).
In this study, we tested the hypothesis that interactions between CTA and CTB within the conserved hydrophobic region at the upper end of the pore of the CTB pentamer are important for holotoxin assembly. Four hydrophobic residues, including F223 in CTA and I74, L77, and T78 in CTB, were changed individually by site-directed mutagenesis of the corresponding structural gene to a charged aspartic acid residue. Variant toxins were expressed in E. coli, and in vivo assembly was characterized. The results presented here indicate that residues I74 and T78 of CTB are essential for holotoxin assembly but not for the formation of CTB pentamers, while L77 is essential for pentamer formation. In addition, holotoxin stability is dependent upon the hydrophobic F223 of CTA, and a CTA-F223H variant can partially complement the phenotypic defect of the CTB-T78D variant for holotoxin assembly.
To further characterize holotoxin assembly, we also developed an efficient method of in vitro assembly of CT. Earlier studies indicated that in vitro assembly of holotoxin could be accomplished by denaturing CT in acidic urea and allowing reassembly of the CTA and CTB polypeptides to occur after neutralization and removal of urea by dialysis (3, 30). These studies analyzed the holotoxin produced in vitro by using biological activity assays and gel electrophoresis. More recently, we and others have noted difficulty in obtaining reproducible assembly of CT holotoxin in vitro under the conditions described (5, 6). As we report here, we obtained efficient and reproducible assembly of wild-type CT in vitro when CTB pentamers were dissociated at low pH, neutralized, and allowed to interact at neutral pH with CTA in urea during sequential dialysis. The purified variant toxin subunits did not reassemble in vitro by this method. Nonetheless, in vitro assembly may aid in the future characterization of wild-type toxin assembly as well as the evaluation of CT assembly inhibitors for use as potential therapeutic compounds (1, 7, 11). In addition, this in vitro assembly method has been used successfully to construct CT-like chimeric molecules for testing as potential vaccines.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli TE1 is a ΔendA derivative of TX1 [F′::Tn10 proA+B+ lacIq Δ(lacZ)M15, glnV44 Δ(hsdM-mcrB)5 Δ(lac-proAB)thi] (16). This strain was used for cloning of recombinant plasmids as well as expression of variant toxins. Cultures were maintained on Luria-Bertani (24) agar plates supplemented with the appropriate antibiotics. For protein expression, cultures were grown in Terrific Broth (31) at 25°C, 30°C, or 37°C. Antibiotics were added at 100 or 50 μg/ml to select for ampicillin resistance or 25 μg/ml to select for chloramphenicol resistance.
Vector construction and mutagenesis.
pARCT5 is an arainose-inducible clone derived from pAR3 (21) expressing an operon containing the ctxA and ctxB genes with signal sequences derived from the LTIIb B gene and using the T7g10 Shine-Dalgarno sequence (15, 16). pARCT5 is a derivative of pARCT4 containing a unique, translationally silent PstI site within ctxA1 (13, 14). Oligonucleotide-derived site-directed mutagenesis was performed directly on pARCT5 with the Quick-change mutatgenesis method (Stratagene) as described. Whenever possible, a unique restriction site was introduced into the primer to allow rapid screening of mutations. pJKT4 was constructed to introduce a Phe to Asp substitution at position 223 within mature CTA (forward primer: GTTAAAAGACAAATCGATTCAGGCTAT; base changes in bold and ClaI restriction site underlined). pJKT5, pJKT6, and pJKT7 were constructed to introduce Ile, Leu, and Thr to Asp replacements at positions 74, 77, and 78, respectively, within mature CTB. The forward primers were pJKT5 (GATACCCTGAGGGATGCATATCTTACT, with NsiI site underlined), pJKT6 (GAGGATTGCATATGATACTGAAGCTAAAG, with NdeI site underlined), and pJKT7 (GGATTGCATATCTAGATGAAGCTAAAGTC, with XbaI site underlined). The double variants pJKT16 and pJKT17 were constructed by site-directed mutagenesis of pJKT5 directly to introduce a Ser to Ala and a Ser to His replacement, respectively, of residue 228 within mature CTA with forward primers pJKT16 (CAGGCTATCAAGCTGATATCGATACAC, with EcoRV site underlined) and pJKT17 (CAGGCTATCAACATGATATCGATACAC, with EcoRV site underlined) Similarly, pJKT18 and pJKT19 were constructed by mutagenesis of pJKT7 to introduce a Phe to Ala and Phe to His replacement, respectively, of residue 223 within mature CTA with forward primers pJKT18 (GACAAATAGCTTCCGGATATCAATCTG, with BspEI site underlined) and pJKT19 (GACAAATACATTCCGGATATCAATCTG, with BspEI site underlined).
To determine the effect of the F223A and F223H CTA substitutions independently from the substitutions within CTB, ctxA was cloned from pJKT18 and pJKT19 as an NdeI fragment and ligated into NdeI-digested pARCT5 to generate pJKT24 and pJKT27, respectively. The S228A and S228H CTA substitutions were also cloned independently into vectors expressing wild-type CTB in a similar manner. To isolate CTA containing the F223D mutation for purification and in vitro reassembly, plasmid pJKT26 was constructed by digesting pJKT4 with NdeI, followed by blunt ending and ligating the isolated ctxA variant into the arabinose-inducible vector pAR3 (21). All plasmids were sequenced through the ctxA and ctxB genes to confirm the predicted nucleotide substitutions.
Toxin and toxin subunit purification.
For the purification of wild-type and variant forms of holotoxin, small-scale cultures (25 ml) were grown in Terrific Broth to an optical density at 600 nm (OD600) of approximately 1.0 before induction with 0.1% l-arabinose. The cultures were then incubated for an additional 3 h before the cells were collected by centrifugation. Cells were then resuspended at 25 × concentration relative to the original culture in 20 mM Tris-buffered saline (TBS, pH 7.5) with 1 mg of polymyxin B per ml and shaken at 37°C for 15 min. Insoluble debris was removed from these periplasmic extracts by centrifugation at 12,000 × g for 15 min. Wild-type and variant holotoxins were then further purified from the periplasm over a 0.2-ml bed volume of d-galactose-agarose resin (Pierce) (32). The columns were washed with 3 to 5 column volumes of 50 mM Tris-HCl (pH 8.0), and bound holotoxin was eluted with 4 × 0.5 ml of 50 mM Tris-HCl (pH 8.0)-1 M d-galactose. The resulting eluates were analyzed either directly by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot or pooled, dialyzed in TBS, and stored at 4°C for further characterization.
For in vitro assembly of wild-type CT and the F223D variant, wild-type CTB was purified from the supernatant of V. cholerae 569BΔA, which contains a chromosomal deletion of ctxA and harbors the multicopy plasmid pLDR5 expressing CTB from a pLac promoter with an LTIIb leader sequence (16). One liter of this strain was grown overnight at 37°C in modified syncase medium to an OD600 of 2.5 (17). The next day, the cells were harvested, resuspended in fresh medium, and allowed to grow another 24 h. CTB was purified from the supernatant by sodium hexametaphosphate precipitation essentially as described by Lebens et al. (17). I74D and T78D variants of CTB for in vitro assembly were purified on d-galactose-agarose from the periplasm of E. coli TE1 strains harboring pJKT5 and pJKT7 as described above.
Wild-type CTA was purified from E. coli BL21(DE3)/pLysS (Novagen) harboring plasmid pT7CTA, which expresses the ctxA gene from the pT7φ 10 promoter in the absence of the periplasmic signal sequence (29). After growth of the culture in LB with ampicillin (100 μg/ml) and chloramphenicol (25 μg/ml) to an OD600 of 1.0 and induction with 0.5 mM IPTG (isopropylthiogalactopyranoside), the culture was allowed to grow overnight. Cells were then pelleted and resuspended in TBS plus 0.1 % Triton X-100 and lysed by repeated freezing and thawing. The lysed cells were incubated in DNase I (10 μg/ml) at 25°C for 10 min and pelleted. The resulting pellet of CTA inclusion bodies was repeatedly washed with 1% Triton X-100 and solubilized in 8 M urea-50 mM Tris (pH 8.0). To purify CTA containing the F223D mutation for in vitro reassembly, E. coli TE1 containing plasmid pJKT26 was grown and induced with 0.1% l-arabinose. The variant CTA subunit was isolated in soluble form from the periplasm after preparing extracts with polymyxin B and removing insoluble debris by centrifugation, as described above.
Assays for holotoxin assembly, toxicity, and stability.
Toxin samples purified from d-galactose resin were mixed with 1/2 volume of 4 × Laemmli sample buffer and boiled for 3 min in the presence of 3% β-mercaptoethanol or 75 mM dithiothreitol before loading onto a sodium dodecyl sulfate-12% polyacrylamide gel for analysis of assembly (24). SDS-PAGE analysis was reproducible and performed at least three times for each variant. For Western blotting, proteins were transferred to nitrocellulose by semidry electroblotting as described by the manufacturer (Bio-Rad) and detected with either a rabbit anti-CTA polyclonal antibody or a rabbit anti-CTB polyclonal antibody. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin secondary antibody was then detected by chemiluminescence as described by the manufacturer (Dupont/NEN). CTA and CTB ratios were determined by GM1 enzyme-linked immunosorbent assay (ELISA), with the above CT-specific antibodies, as described previously (10). Holotoxin stability was assessed by a variation of the GM1 ELISA in which the bound toxin was exposed to phosphate-buffered saline (PBS, pH 7.5), PBS (pH 7.5) plus 0.05% SDS, or McIlvaine buffer pH 5.5 plus 0.05% SDS for 30 min at room temperature before binding of the primary antibody, as described by Rodighiero et al. (22). The GM1 ELISAs presented are representative and were performed at least two times for each experiment.
Susceptibility to limited trypsin treatment was determined by treating 1.5 μg of purified toxin in 50 μl for various times at 37°C with 2% (wt/vol) bovine trypsin and stopping the reactions with 4% (wt/vol) soybean trypsin inhibitor. Proteolytic cleavage of CTA into the 22-kDa CTA1 fragment was assessed by SDS-PAGE and Western blotting. Relative toxicity of variant holotoxins was determined by exposing mouse Y1 adrenal cells to various concentrations of toxin and assessing cell rounding after overnight incubation, as described previously (20).
In vitro reassembly of cholera toxin.
For in vitro reassembly experiments, purified wild-type CTB was dissociated into monomeric subunits with acid treatment in a manner similar to that described by Ruddock et al. (23). We diluted 500 μl of purified CTB at a concentration of 3 mg/ml into 500 μl of 0.1 M KCl-HCl (pH 1.0) for 30 s. The acid was quickly neutralized with 500 μl of 50 mM Tris-HCl (pH 8.0), and the sample was mixed with 1 ml of purified wild-type CTA at 4 mg/ml to give a 1:4 molar ratio of pentameric CTB to CTA. In early experiments, the neutralization step was omitted and resulted in decreased efficiency of holotoxin reassembly. The mixture, at a final volume of 2.5 ml, was dialyzed at 4°C against 500 ml of 50 mM Tris-HCl (pH 8.0)-8 M urea, with changes of buffer containing sequential twofold dilutions of urea every 4 h to overnight, to a final concentration of 0.5 M urea. The resulting sample was purified by absorption to and subsequent elution from a 500-μl bed of d-galactose resin as described above. Holotoxin assembly was analyzed by SDS-PAGE, Western blot, and GM1 ELISA as described above. Protein concentrations were determined spectrophotometrically by a Bradford Coomassie dye-based protein assay (Pierce). In vitro assembly experiments with CTB and CTA variants were performed essentially identically except that each variant toxin subunit was purified separately and mixed with a complementary wild-type or variant subunit, as noted in the text.
RESULTS
Construction and characterization of an F223D variant of CTA.
By using oligonucleotide-directed site-specific mutagenesis, phenylalanine 223 within the hydrophobic interface of the CTA2 domain was substituted with the negatively charged residue aspartic acid. The rationale for selecting F223 for modification was based upon detailed analysis of the crystal structure for the closely related E. coli LTI toxin. F223 in the A polypeptide has five hydrophobic contacts with T78 in the B polypeptide (26), and both CTA-F223 and CTB-T78 are conserved in CT and LTI. Figure 1 illustrates the hydrophobic interface between CTA and CTB at the upper end of the toxin pore and shows the residues modified in this study, with F223 of CTA in red. The plasmid coexpressing the CTA-F223D variant and wild-type CTB was transformed into E. coli, and the toxin assembled in the periplasm was isolated and affinity purified on a d-galactose-agarose column. SDS-PAGE analysis of holotoxin containing the F223D variant of CTA as well as the purified wild-type toxin is shown in Fig. 2A and B. Based on the relative concentrations of the bands corresponding to CTA and CTB, it appeared that the F223D variant of CTA assembled less efficiently into holotoxin than wild-type CTA. A Western blot of induced whole-cell lysates indicated that CTA-F223D was not degraded more rapidly in the cell than wild-type CTA (Fig. 2C).
FIG. 1.
Cartoon representation of crystal structure of CT and residues mutated in this study. (A) Holotoxin with only two B subunits is shown for clarity. Red residue is CTA-F223, purple is CTA-S228, yellow is CTB-I74, blue is CTB-L77, and green is CTB-T78. (B) Close-up view of the upper hydrophobic region of the pore.
FIG. 2.
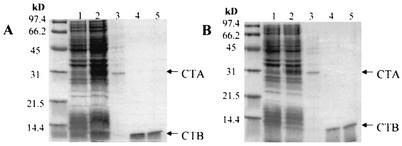
Purification of (A) wild-type CT and (B) the CTA-F223D variant holotoxin from the E. coli periplasm on d-galactose resin. In panels A and B, the numbered samples are as follows: 1, whole-cell lysate before induction; 2, whole-cell lysate 3 h postinduction with 0.1% l-arabinose; 3, d-galactose flowthrough; 4, d-galactose eluate 1; 5, d-galactose eluate 2. (C) Anti-CTA Western blot of twofold dilutions of wild-type (WT) to (OD600 0.143) and the CTA-F223D (OD600 0.15) variant whole-cell lysates. Samples 1 to 4 were uninduced; 5 and 6 were induced with 0.1% l-arabinose for 3 h.
The stability of the assembled wild-type and mutant forms of holotoxin was determined by an ELISA in which a polyclonal antibody to CTA was allowed to interact with bound toxin after it had been incubated at 25°C with PBS, PBS plus 0.5% SDS, or McIlvaine buffer at pH 5.5 plus 0.5% SDS. The results indicated that holotoxin containing the F223D variant of CTA was less stable than wild-type toxin (Fig. 3A). The unnicked F223D variant was found to be comparable in toxicity to unnicked wild-type holotoxin, but unlike wild-type holotoxin, it was not activated by treatment with trypsin (Fig. 3B). In addition, the F223D holotoxin variant exhibited increased susceptibility of the CTA1 domain to proteolysis by trypsin, as shown in Fig. 4A and B.
FIG. 3.
(A) GM1 stability ELISA with anti-CTA polyclonal antibodies. WT, wild-type holotoxin. F223D is holotoxin containing the CTA-F223D variant. The assay is representative of three independent experiments. (B) Y1 toxicity assays. Toxins were nicked with trypsin at 37°C for 10 min. Percentages are the averages of three independent experiments.
FIG. 4.
(A) Susceptibility to trypsin. Wild-type (wt) holotoxin and holotoxin containing CTA-F223D were treated with 2% (wt/vol) trypsin at 37°C for 30 min and analyzed by SDS-PAGE. (B) Western blot with anti-CTA polyclonal antibodies against wild-type toxin and the F223D, F223H, and F223A variants. Lanes 1 to 4 show samples 0 min, 15 min, 30 min, and 60 min after the addition of trypsin and incubation at 37°C, respectively, (C) Assembly of holotoxin during growth at 37°C and 25°C. Lanes 1 and 2, wild-type d-galactose flowthrough and d-galactose eluate, respectively; lanes 3 and 4, F223D variant d-galactose flowthrough and d-galactose eluate, respectively.
For later complementation studies, F223 of CTA was also replaced with a small, uncharged alanine residue or a positively charged histidine residue. Both of these variants appeared to assemble normally with native CTB to form holotoxin, as determined by purification on d-galactose resin (data not shown), but the variant containing CTA-F223H exhibited dramatically increased susceptibility to trypsin (Fig. 4B). Furthermore, the in vivo assembly of the CTA-F223D variant into holotoxin was temperature sensitive, and significantly less holotoxin containing CTA-F223D was produced in cultures grown at 37°C than in cultures grown at 25°C (Fig. 4C).
Construction and characterization of CTB variants L77D, I74D, and T78D.
Site-specific mutagenesis was also used to construct single-substitution variants of CTB with aspartic acid. Substitutions were made in each of the hydrophobic residues (Fig. 1; I74 yellow, L77 blue, and T78 green) that are conserved in CT and LTI, surface exposed, located at the upper end of the central pore of the B pentamer, and believed to interact directly with the CTA2 domain (33). We characterized the ability of each of these CTB variants to assemble into holotoxin with wild-type CTA by affinity purification on d-galactose resin of periplasmic extracts from E. coli grown at 30°C. SDS-PAGE and Western blot analyses of the L77D variant were performed (data not shown). We were unable to detect either the CTA or CTB subunit in the d-galactose eluate. Western blots with anti-CTA and anti-CTB antibodies of whole-cell lysates revealed that CTB-L77D was expressed in significant amounts from the plasmid, although it could not be eluted from d-galactose, indicating that this CTB variant could not form stable pentamers or holotoxin.
SDS-PAGE analysis of the CTB-I74D and CTB-T78D variants is shown in Fig. 5. Both of these variants were able to form functional pentamers that bound to d-galactose, but neither was able to assemble with CTA to form holotoxin. Analysis of these purified mutants by Western blot confirmed that no wild-type CTA was associated with the purified pentamers of CTB-I74D and CTB-T78D, and GM1 ELISA assays showed that these B-pentamer variants were able to bind GM1 as efficiently as wild-type CTB pentamers (data not shown). In addition, growth at 37°C or 25°C did not significantly affect the stability of these CTB pentamer variants or allow holotoxin formation at the 25°C temperature previously shown to be permissive for formation of holotoxin containing the F223D variant of CTA (Fig. 4C).
FIG. 5.
Analysis by purification on d-galactose resin and subsequent SDS-PAGE of the ability of CTB variants (A) I74D and (B) T78D to assemble into B pentamer and holotoxin in the periplasm of E. coli. Lanes: 1, whole-cell lysate before induction; 2, whole-cell lysate 3 h postinduction with 0.1% l-arabinose; 3, d-galactose resin flowthrough; 4, d-galactose eluate 1; and 5, d-galactose eluate 2.
Construction of complementing amino acid substitutions in CTA.
The I74D and T78D variants of CTB were of special interest, as they appeared to be competent for formation of pentameric B subunits but defective in holotoxin assembly. We constructed variants with specific substitutions within the CTA2 domain that we predicted might complement the assembly defects of these CTB variants. Using the Ras-Mol molecular modeling program (25) and the known structure of CT (35), we identified residues in CTA that were most likely to interact with I74 and T78 of CTB. S228 of CTA was found to be within 3.6 Å of I74 and F223 of CTA within 3.6 Å of T78. Residues F223 and S228 in CTA were substituted with small, uncharged alanine residues or larger, positively charged histidine residues. These substitutions were designed to test the hypothesis that a positively charged histidine at either position in CTA might interact electrostatically with the corresponding negatively charged aspartic acid at either position 74 or 78 in CTB to permit toxin assembly or that the small, uncharged alanine at either position in CTA might permit assembly by decreasing steric hindrance with residue 74 or 78 of CTB within the pore. Figure 1 shows the locations of the wild-type residues F223 (red) and S228 (purple) of CTA within the pore.
The I74D variant of CTB did not assemble with either the S228H or S228A variant of CTA (data not shown). In contrast, however, the T78D variant was able to assemble with CTA-F223H but not with CTA-F223A to form holotoxin with low but detectable efficiency. Figure 6 shows the SDS-PAGE, Western blot, and GM1 ELISA analyses of the CTB-T78D variant with wild-type CTA compared to the CTB-T78D CTA-F223H double variant. From the results of the GM1 ELISA assay with anti-CTA antibodies, we estimated that assembly of this double variant occurred with an efficiency of 50% of that of wild-type CTA (Fig. 6C). The results of these structure-based complementation experiments support the conclusion that F223 of CTA interacts directly with T78 of CTB at the subunit interface and that the interaction of these residues is involved in toxin assembly (26).
FIG. 6.
Failure of CTB-T78D variant to assemble with wild-type CTA (T78D/WT) and partial complementation of the assembly defect of CTB-T78D by the CTA-F223H variant (T78D/F223H). (A) SDS-PAGE of d-galactose-purified toxins. (B) Western blot analysis of d-galactose-purified toxin with anti-CTA. Lanes: 1, T78D/wild-type d-galactose flowthrough; 2, T78D/wild-type d-galactose eluate 1; 3, T78D/F223H double variant d-galactose flowthrough; and 4, T78D/F223H double variant d-galactose eluate 1. (C) Anti-CTA GM1 ELISA of wild-type holotoxin (WT), the T78D/wild-type variant, and the T78D/F223H double variant. The assay is representative of two independent experiments.
As indicated above, the CTA-F223H variant was also analyzed independently for its effect on toxin assembly and found to assemble with wild-type CTB in a manner similar to wild-type CTA. In addition, the F223A, S228A, and S228H CTA substitutions were found to have no significant effect on holotoxin assembly with wild-type CTB (data not shown). These results indicate that CTB-T78D is unable to interact with wild-type CTA to form holotoxin, but the F223H substitution partially restores the ability of CTA to assemble with CTB-T78D to form holotoxin.
In vitro reassembly of wild-type CT.
To further characterize cholera toxin assembly, we developed a method of in vitro reassembly of wild-type CT based upon earlier studies in which individual subunits were purified and allowed to renature (3, 23, 30). Pentameric CTB purified from V. cholerae overexpressing CTB was acid denatured for 30 s in 0.1 M HCl-KCl (pH 1.0) and rapidly neutralized with 50 mM Tris-HCl (pH 7.0). CTB monomer was immediately mixed with purified CTA in 8 M urea (pH 8.0) in a 1:4 (1 CTB pentamer:4 CTA) molar ratio, and the CTB-CTA mixture was dialyzed sequentially against 50 mM Tris (pH 8.0) buffer containing serial twofold dilutions of urea from 8 M to a final concentration of 0.5 M. Reassembly of holotoxin was analyzed by SDS-PAGE after purification of the dialyzed toxin over a d-galactose-agarose column.
Figure 7A shows the fractions from the d-galactose column after in vitro reassembly of wild-type toxin. Western blot analyses of the separately purified subunits and reconstituted nicked and unnicked holotoxin are shown in Fig. 7B. The reassembled toxin was analyzed by GM1 ELISA and found to contain a ratio of immunoreactive CTA to CTB comparable to that of the in vivo-assembled wild-type CT control (Fig. 8A). Y1 toxicity assays also showed that the reassembled toxin was similar to the in vivo-prepared control, with a significantly higher level of toxicity after nicking of the toxin with trypsin (Fig. 8B). These results demonstrate that the in vitro reassembly procedure described above is an efficient method of reconstituting functional wild-type holotoxin.
FIG. 7.
(A) SDS-PAGE of in vitro-reassembled holotoxin after purification on immobilized d-galactose. Lanes: 1, flowthrough; 2, eluate 1; 3, eluate 2; 4, eluate 3. (B) SDS-PAGE and Western blot with anti-CTA and anti-CTB antibodies. Lanes: 1, CTA starting material; 2, CTB starting material; 3, reassembled holotoxin; 4, reassembled holotoxin nicked with trypsin.
FIG. 8.
(A) GM1 ELISA of in vivo-produced standard CT compared to in vitro-reassembled toxin with anti-CTA and anti-CTB antibodies. The assay is representative of three independent experiments. (B) Y1 toxicity assays comparing the activity of the nicked standard to that of the reassembled nicked and unnicked toxin. Percentages are the averages of three independent experiments.
Behavior of CTA-F223D, CTB-I74D, and CTB-T78D variants under in vitro assembly conditions.
To further characterize the assembly proficiency of the CTA-F223D, CTB-I74D, and CTB-T78D variants, we analyzed the ability of each purified variant subunit to reassemble in vitro with the complementary wild-type subunit. The results are shown in Fig. 9. The CTA-F223D variant was cloned independently of CTB, as described in Materials and Methods, and purified from periplasmic extracts. This variant polypeptide was soluble, unlike the wild-type CTA subunit, and thus was not initially present in a solution of 8 M urea. After mixing the CTA-F223D variant with acid-denatured wild-type CTB and sequential dialysis, reassembly of the variant holotoxin in vitro was not observed (Fig. 9A). In addition, unlike wild-type CTB, acid denaturation, neutralization, and dialysis of the variant CTB pentamers I74D and T78D with wild-type CTA produced neither holotoxin nor B pentamers, indicating that the monomeric CTB variants were incompetent for reassembly into holotoxin or B pentamers in vitro (Fig. 9B).
FIG. 9.
Effects of specific amino acid substitutions in CTA and CTB on assembly of CT holotoxin in vitro. (A) Assembly of wild-type CTA (1 to 3) or CTA-F223D (4 to 6) with wild-type CTB. d-galactose resin flowthrough (1 and 4); eluate 1 (2 and 5), and eluate 2 (3 and 6). (B) Assembly of wild-type CTA with wild-type (1 to 3), I74D (4 to 6), or T78D (7 to 9) CTB. d-Galactose resin flowthrough (1, 4, and 7), eluate 1 (2, 5, and 8), and eluate 2 (3, 6, and 9).
To determine if denaturation of these CTB variants under less extreme acidic conditions would facilitate their subsequent reassembly, we generated monomeric CTB-T74D and CTB-T78D with citric acid at pH 2.3, as described by Hatic et al. (6). Although wild-type CTB monomers produced under these milder conditions were assembly competent, the variant CTB-I74D and CTB-T78D polypeptides remained unable to reassemble into pentamers (data not shown). Thus, although the conditions that we developed promote efficient assembly of wild-type CTA and CTB into holotoxin in vitro, they are significantly less optimal for assembly than the conditions that exist in the periplasm of V. cholerae. Furthermore, variant polypeptides that retained the ability to assemble into holotoxin (CTA-F223D) or B pentamers (CTB-I74D and CTB-T78D) in vivo were not capable of reassembling into the same multimeric forms in vitro.
DISCUSSION
In 1993, Sixma et al. determined by crystal structure that the surface of the upper narrow end of the central pore of the B pentamer of E. coli LTI is dominated by hydrophobic residues I74 and L77 of the B subunit as well as the hydrophobic methyl group of T78 of the B subunit (26). These residues tightly contact the A2 helix and exclude water molecules within this region. In addition, this study revealed that F223 of the LTA2 domain participates in five hydrophobic contacts with the T78 residues of the B pentamer. Later, X-ray crystallography of CT confirmed its structural homology to E. coli LTI and demonstrated the conservation of these hydrophobic stabilizing interactions between subunits A and B within the central pore (35). Van den Akker et al. (33) crystalized the related E. coli LTIIb toxin and identified a conserved ring of hydrophobic residues in the upper pore that is common to all AB5 toxins. These studies suggested that the hydrophobic residues within the pore are crucial in the assembly of the family of AB5 heterohexameric toxins that includes CT, LTI, and LTII as well as Shiga toxin and pertussis toxin. The studies described here were designed to analyze the role of specific hydrophobic residues of CTA and CTB located within this conserved hydrophobic motif in the assembly of CTB pentamers and CT holotoxin.
In these studies, we used oligonucleotide-directed site-specific mutagenesis to replace hydrophobic residues I74, L77, and T78 of CTB as well as F223 of CTA with a charged aspartic acid residue. Characterization of the holotoxin variant containing CTA-F223D revealed that residue 223 of CTA is a significant determinant of holotoxin assembly and stability. The CTA-F223D variant exhibited temperature-sensitive assembly of holotoxin, increased susceptibility of the CTA polypeptide to trypsin, and reduced stability of holotoxin in the presence of SDS and/or low pH in a GM1 ELISA. Some of these properties were specific for the F223D substitution, as the CTA-F223A and CTA-F223H variants appeared to be able to assemble into holotoxin after d-galactose purification and analysis by SDS-PAGE. However, the CTA-F223H polypeptide in holotoxin exhibited a markedly increased susceptibility to degradation by trypsin, indicative of structural instability. The CTB-L77D variant was unable to form functional pentamers or assemble into holotoxin. In contrast, the CTB-I74D and CTB-T78D variant formed pentamers that retained the ability to bind to GM1 but were unable to assemble into holotoxin. Taken together, these findings support the structure-based hypothesis that the interactions of these hydrophobic residues of wild-type CTA and CTB are important for toxin assembly. Further support for this hypothesis is provided by the ability of the F223H substitution variant of CTA to partially complement the inability of the CTB-T78D variant to assemble into holotoxin in vivo. This finding indicates that the hydrophobic interactions that normally occur between residues F223 in CTA and T78 in CTB during assembly of wild-type holotoxin can be replaced to some extent by hydrophilic or electrostatic interactions between H223 in CTA and D78 in CTB.
In the absence of the CTA subunit, CTB is able to form stable pentamers. Mature B pentamers however, cannot subsequently interact with CTA to form holotoxin. During biogenesis of CT in the periplasm of V. cholerae, CTA interacts with immature oligomeric forms of CTB that are not yet pentameric. In addition, the assembly of CTB into holotoxin in vivo when the A subunit is present is threefold faster that the assembly of CTB into pentamers in the absence of CTA (5, 27). The observation that AB3 or AB4 assembly intermediates act to attract additional monomeric B subunits to the exposed hydrophobic A2 helix provides a plausible explanation for the stimulating effect of CTA on assembly (5, 19). The phenotypes reported here for CTA-F223D, CTB-I74D, and CTB-T78D provide direct experimental evidence that hydrophobic interactions between CTA and CTB at the upper end of the pore of the B pentamer are important for holotoxin assembly and stability. A better understanding of the role of the AB intermediates and the hydrophobic residues involved in AB5 toxin assembly may aid in the development of compounds that inhibit toxin assembly and have potential value as therapeutic agents for treatment of cholera. One such compound is 3-methylthio-1,4-diphenyl-1H-1,3,4-triazolium (MDT), whichwas shown to interact with the hydrophobic region of the CT pore by crystal structure analysis (11).
In 1974 Finkelstein et al. reported the first successful separation and reassociation of the noncovalently associated CTA and CTB subunits in vitro (3). After CTA and CTB were separated by gel filtration in urea under acidic conditions (pH 3.2), the subunits were mixed and dialyzed against sequential changes of buffer at pH 7.5 containing decreasing concentrations of urea. The reconstituted toxin was subsequently isolated in its native form. In 1981, the in vitro formation of hybrid toxins between E. coli LTI and CT was reported by Takeda et al. (30). Fractions from a Sephadex column were eluted in urea at pH 4.0, and toxin was reconstituted by dialyzing the pooled fractions against buffer for 16 h. In 1996, Ruddock et al. analyzed the assembly of B subunit pentamers of both CT and LTI (23). This study revealed that assembly-competent monomers of CTB were formed with high efficiency by exposing CTB pentamers to a pH of less than 2.0 for short periods, followed by rapid neutralization to pH 7.0.
In an effort to further characterize assembly-deficient variants of holotoxin, we developed a reproducible method of in vitro reassembly of wild-type CT based on these earlier findings. This method has consistently produced a high efficiency of reassembly of wild-type toxin. In 2001, Hatic et al. reported the assembly of recombinant green fluorescent protein (GFP)-CTA2 polypeptides with CTB in vitro to form GFP-CT chimeras with approximately 20% efficiency based on the concentration of resulting protein (6). From our GM1 ELISA analysis of in vitro-assembled CT compared to a CT holotoxin standard produced in vivo (Fig. 8), we estimate that the preparation of CT produced in vitro consists of at least 90% holotoxin, with few or no free CTB pentamers. These data indicate that assembly-competent CTB polypeptides associate with CTA in vitro with very high efficiency when CTA is present in a fourfold molar excess over pentameric CTB.
There are many potential applications for consistent and reproducible methods of in vitro reassembly of cholera toxin and related enterotoxins. Compounds such as MDT, described above, can be tested in vitro for their inhibitory effects on assembly. In addition, the kinetics of toxin assembly can be studied in vitro under biochemical conditions that are more precisely defined than the conditions that exist in the periplasm of toxin-producing bacteria. In vitro assembly is also advantageous for promoting the proper folding of chimeras that are insoluble when expressed in vivo in E. coli. We have begun to investigate the use of in vitro assembly of CTB with fusion proteins containing a carboxyl-terminal CTA2 domain for use as potential vaccines (12), with preliminary success. One such chimera that we were able to produce in vitro but not in vivo contains a fragment of the toxin-coregulated pilus (Tcp) antigen of V. cholerae that is of interest as a candidate vaccine against cholera (28).
Despite our initial success with in vitro reassembly of wild-type and chimeric cholera toxins, the conditions that we have developed for in vitro assembly are significantly less optimal than the conditions for toxin assembly that exist within the periplasm of V. cholerae. Both a peptidyl proline isomerase and the thiol-disulfide oxidoreductase, DsbA, appear to be essential for B pentamer formation within the periplasm (23). In addition, protein concentration in the periplasm is an important factor for efficient assembly (19). The CTA and CTB variants reported here that were found to assemble with holotoxin in vivo but not in vitro may be useful as tools in future studies both to optimize in vitro assembly conditions further and to identify critical differences between in vitro and in vivo assembly that explain their different efficiencies.
In summary, we used genetic and biochemical methods to characterize the role of specific residues within conserved hydrophobic regions of CTA and CTB in the assembly and stability of cholera holotoxin both in vivo and in vitro. Understanding the factors that determine holotoxin assembly and stability at the molecular level will facilitate future studies on the development of therapeutic agents to interfere with toxin assembly or action and on the use of cholera toxin and related enterotoxins for the development of novel vaccines and improved immunomodulatory agents.
Acknowledgments
This work was supported in part by an NIH postdoctoral National Research Service Award (T32AI07537) to J.K.T. and an NIH research grant (RO1AI-31940) to R.K.H.
We thank Michael G. Jobling for expert opinions and assistance during the course of this work and Claire J. O'Neal for critical insight during the development of the manuscript.
Editor: J. D. Clements
REFERENCES
- 1.Fan, E., E. A. Merritt, C. L. M. J. Verlinde, and W. G. J. Hol. 2000. AB5 toxins: structures and inhibitor design. Curr. Opin. Struct. Biol. 10:680-686. [DOI] [PubMed] [Google Scholar]
- 2.Faruque, S. M., J. M. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein, R. A., M. Boesman, S. H. Neoh, M. K. LaRue, and R. Delaney. 1974. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J. Immunol. 113:145-150. [PubMed] [Google Scholar]
- 4.Gill, D. M., and R. Meren. 1978. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc. Natl. Acad. Sci. USA 75:3050-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy, S. J. S., J. Holmgren, S. Johansson, J. Sanchez, and T. R. Hirst. 1988. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc. Natl. Acad. Sci. USA 85:7109-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatic II, S. O., J. A. McCann, and W. D. Picking. 2001. In vitro assembly of novel cholera toxin-like complexes. Anal. Biochem. 292:171-177. [DOI] [PubMed] [Google Scholar]
- 7.Hirst, T. R. 2001. A toxin with emerging therapeutic potential. Lancet 358:s7. [DOI] [PubMed]
- 8.Holmes, R. K. 1997. Heat-labile enterotoxins (Escherichia coli), p. 30-33. In C. Montecucco and R. Rappuoli (ed.), Guidebook to protein toxins and their use in cell biology. Oxford University Press, Oxford, England.
- 9.Holmes, R. K., M. G. Jobling, and T. D. Connell. 1995. Cholera toxin and related enterotoxins of gram-negative bacteria, p. 225-256. In J. Moss, B. Iglewski, M. Vaughan, and A. Tu (ed.), Handbook of natural toxins: bacterial toxins and virulence factors in disease, vol. 8. Marcel Dekker, Inc., New York, N.Y.
- 10.Holmes, R. K., and E. M. Twiddy. 1983. Characterization of monoclonal antibodies that react with unique and cross-reacting determinants of cholera enterotoxin and its subunits. Infect. Immun. 42:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovey, B. T., C. L. M. J. Verlinde, E. Merritt, and W. G. J. Hol. 1999. Structure-based discovery of a pore-binding ligand: towards assembly inhibitors for cholera and related AB5 toxins. J. Mol. Biol. 285:1169-1178. [DOI] [PubMed] [Google Scholar]
- 12.Jobling, M. G., and R. K. Holmes. 1992. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect. Immun. 60:4915-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jobling, M. G., and R. K. Holmes. 2001. Biological and biochemical characterization of variant A subunits of cholera toxin constructed by site-directed mutagenesis. J. Bacteriol. 183:4024-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobling, M. G., and R. K. Holmes. 2000. Identification of motifs in cholera toxin A1 polypeptide that are required for its interaction with human ADP-ribosylation factor 6 in a bacterial two-hybrid system. Proc. Natl. Acad. Sci. USA 97:14662-14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jobling, M. G., and R. K. Holmes. 2002. Mutational analysis of ganglioside GMI-binding ability, pentamer formation, and epitopes of cholera toxin B (CTB) subunits and CTB/heat-labile enterotoxin B subunit chimeras. Infect. Immun. 70:1260-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobling, M. G., L. M. Palmer, J. L. Erbe, and R. K. Holmes. 1997. Construction and characterization of versatile cloning vectors for efficient delivery of native foreign proteins to the periplasm of E. coli. Plasmid 38:158-173. [DOI] [PubMed] [Google Scholar]
- 17.Lebens, M., S. Johansson, J. Osek, M. Lindblad, and J. Holmgren. 1993. Large-scale production of Vibrio cholerae Toxin B subunit for use in oral vaccines. Bio/Technology 11:1574-1578. [DOI] [PubMed] [Google Scholar]
- 18.Lencer, W. I., T. R. Hirst, and R. K. Holmes. 1999. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450:177-190. [DOI] [PubMed] [Google Scholar]
- 19.Lesieur, C., M. J. Cliff, R. Carter, R. F. James, A. R. Clarke, and T. R. Hirst. 2002. A kinetic model of intermediate formation during assembly of cholera toxin B-subunit pentamers. J. Biol. Chem. 277:16697-704. [DOI] [PubMed] [Google Scholar]
- 20.Maneval, D. R., R. R. Colwell, S. W. J. Grays, and S. T. Donta. 1981. A tissue culture method for the selection of bacterial enterotoxins. J. Tissue Cult. Methods 6:85-90. [Google Scholar]
- 21.Perez-Perez, J., and J. Gutierrez. 1995. An arabinose-inducible expression vector, pAR3, compatible with ColE1-derived plasmids. Gene 158:141-142. [DOI] [PubMed] [Google Scholar]
- 22.Rodighiero, C., A. T. Aman, M. J. Kenny, J. Moss, W. I. Lencer, and T. R. Hirst. 1999. Structural basis for the differential toxicity of cholera toxin and Escherichia coli heat-labile enterotoxin. J. Biol. Chem. 274:3962-3969. [DOI] [PubMed] [Google Scholar]
- 23.Ruddock, L. W., J. J. Coen, C. Cheesman, R. B. Freedman, and T. R. Hirst. 1996. Assembly of the B subunit pentamer of Escherichia coli heat-labile enterotoxin. J. Biol. Chem. 271:19118-19123. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Sayle, R. A., and E. J. Milner-White. 1995. RasMol: biomolecular graphics for all. Trends Biochem. Sci. 20:374. [DOI] [PubMed] [Google Scholar]
- 26.Sixma, T. K., K. H. Kalk, B. M. van Zanten, Z. Dauter, J. Kingma, B. Witholt, and W. G. J. Hol. 1993. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J. Mol. Biol. 230:890-918. [DOI] [PubMed] [Google Scholar]
- 27.Streatfield, S. J., M. Sandkvist, T. K. Sixma, M. Bagdasarian, W. G. J. Hol, and T. R. Hirst. 1992. Intermolecular interactions between the A and B subunits of heat-labile enterotoxins from Escherichia coli promote holotoxin assembly and stability in vivo. Proc. Natl. Acad. Sci. USA 89:12140-12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun, D., J. J. Mekalanos, and R. K. Taylor. 1990. Antibodies directed against the toxin-coregulated pilus isolated from Vibro cholerae provide protection in the infant mouse experimental cholera model. J. Infect. Dis. 161:1231-1236. [DOI] [PubMed] [Google Scholar]
- 29.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for the controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda, Y., T. Honda, S. Taga, and T. Miwatani. 1981. In vitro formation of hybrid toxins between subunits of Escherichia coli heat-labile enterotoxin and those of cholera enterotoxin. Infect. Immun. 34:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tartof, K. D., and C. A. Hobbs. 1987. Improved media for growing plasmid and cosmid clones. Focus 9:12.
- 32.Uesaka, Y., Y. Otsuka, Z. Lin, S. Yamasaki, J. Yamaoka, H. Kurazono, and Y. Takeda. 1994. Simple method of purification of Escherichia coli heat-labile enterotoxin and cholera toxin using immobilized galactose. Microb. Pathog. 16:71-76. [DOI] [PubMed] [Google Scholar]
- 33.van den Akker, F., S. Sarfaty, E. M. Twiddy, T. D. Connell, R. K. Holmes, and W. G. Hol. 1996. Crystal structure of a new heat-labile enterotoxin, LTIIb. Structure 4:665-678. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2002. Cholera in 2001. Wkly. Epidemiol. Rec. 77:257-268.12189690 [Google Scholar]
- 35.Zhang, R.-G., D. L. Scott, M. L. Westbrook, S. Nance, B. D. Spangler, G. G. Shipley, and E. M. Westbrook. 1995. The three dimensional crystal structure of cholera toxin. J. Mol. Biol. 251:563-573. [DOI] [PubMed] [Google Scholar]