Abstract
Mycobacterium tuberculosis produces a variety of molecules capable of activating Toll-like receptors, a family of pattern recognition receptors expressed by macrophages and a variety of other cells. To determine whether Toll-like receptor 4 (TLR4) was critical in resistance to M. tuberculosis infection, we compared the morbidity and mortality of TLR4-defective C3H/HeJ mice to those of TLR4-sufficient C3H mouse substrains. TLR4-defective C3H/HeJ mice and TLR4-sufficient C3H/HeSnJ, C3HeB/FeJ, and C3H/HeOuJ mice were infected by the aerosol route with M. tuberculosis. TLR4-defective C3H/HeJ mice had levels of cytokines in their bronchoalveolar lavage fluids and in vitro mycobacterial antigen-specific recall responses similar to those of other C3H mouse substrains. In addition, bacterial replication and long-term survival of mice following infection appeared to be independent of TLR4. Interestingly, C3HeB/FeJ mice were significantly more susceptible to M. tuberculosis infection, indicating that genetic heterogeneity among inbred C3H mouse substrains modifies resistance to infection. Therefore, cautious interpretation is required when the C3H/HeJ strain is used as a model of a TLR4-defective mouse strain, as there are significant allelic differences between C3H/HeJ and other C3H mouse substrains in response to M. tuberculosis infection. With this caveat, our data indicate that TLR4 may not be required for optimal immunity of mice to M. tuberculosis.
Recognition of microbial pathogens by the innate immune system leads to the early activation of both humoral and cellular immune responses. Bacterial products directly activate leukocytes, induce the synthesis and release of proinflammatory cytokines, and up-regulate adhesion molecules, all of which amplify the host immune response to infection (41). During the last decade, it has been recognized that the initial activating effect of bacterial products is mediated by a diverse set of cell surface receptors that recognize pathogen-associated molecular patterns (PAMPs) present on microbes. One well-characterized family of mammalian PAMP receptors is the Toll-like receptors (TLRs) (18).
The Toll protein was originally identified in Drosophila melanogaster mutants exhibiting profound susceptibility to fungal infections. To date, 10 related genes have been identified in humans (tlr1 to tlr10) (5, 9, 12, 26, 28). Several agonists have been identified, and each TLR appears to have a distinct nonoverlapping specificity (2). For example, TLR2 agonists include microbial cell wall products, such as lipoproteins, peptidoglycan, lipoarabinomannan, and glycosylphosphatidylinositol (19, 26). In contrast, TLR4 is activated by gram-negative bacterial lipopolysaccharide (LPS), and TLR9 is activated by double-stranded RNA (14, 17, 31, 32). The capacity to recognize diverse PAMPs makes the TLR family well suited to functioning as an early warning system for detection of the presence of microorganisms by the host immune system. Activation of the TLR signal transduction pathway leads to the induction of numerous genes that function in host defense, including those for inflammatory cytokines, chemokines, antigen-presenting molecules, and costimulatory molecules (39). Thus, TLRs have a central role in the initiation of innate cellular immune responses and the subsequent adaptive immune responses to microbial pathogens.
Tuberculosis is caused by the bacterium Mycobacterium tuberculosis, which is transmitted by aerosols and infects alveolar macrophages. Several macrophage cell surface receptors mediate the binding and internalization of M. tuberculosis, including complement receptor 3, complement receptor 4, and the macrophage mannose receptor (34, 35). Although these receptors can signal, they are not major mediators of M. tuberculosis-induced cytokine production (13). In contrast, TLR proteins recognize mycobacterial products and activate signaling pathways, some of which are shared with the interleukin 1 (IL-1) receptor family, leading to NF-κB signal transduction and production of proinflammatory cytokines (16, 22). TLR4-mediated signaling requires other accessory molecules, including CD14, a high-affinity LPS receptor that can be secreted into the serum or found on the surface of macrophages. It also requires MD-2, a small protein that lacks a transmembrane region and is expressed on the cell surface in association with TLR4 (28, 36, 42). Because TLR2 and TLR4 are expressed by macrophages and dendritic cells, they are considered candidate pattern recognition receptors (PRRs) for mycobacterial PAMPs. Means et al. demonstrated that both TLR2 and TLR4 mediate M. tuberculosis-induced cellular activation and that this activity is CD14 independent (24). Recently, Abel et al. found that phosphatidyl-myo-inositol tetra- and hexamannosides, which are similar to lipids that activate TLR2, are agonists for TLR4 (1). The presence of TLR4 ligands on M. tuberculosis suggests that this molecule may play a role in the immune response to mycobacterial infection. Therefore, it is of great interest to determine whether TLR4 plays a role in host resistance to mycobacterial disease.
The C3H/HeJ strain of mice was characterized as LPS hyporesponsive about 30 years ago (15, 38). The phenotype of LPS resistance is controlled by a single locus (Lps), and homozygosity for a codominant allele (Lpsd) is responsible for endotoxin unresponsiveness. In contrast, other C3H substrains, such as C3HeB/FeJ, C3H/HeOuJ, and C3H/HeSnJ, which originated from the same stock as C3H/HeJ mice, carry the Lpsn allele (32). In 1998, it was shown that the tlr4 gene was identical to the Lps locus: a single point mutation in the tlr4 gene resulted in a defective TLR4 protein that caused LPS unresponsiveness in C3H/HeJ mice. This finding was subsequently confirmed with TLR4 knockout mice (17, 31). TLR4-defective C3H/HeJ mice are more susceptible to certain infections, including Escherichia coli-induced polynephritis as well as infections caused by Salmonella enterica serovar Typhimurium and Leishmania donovani, demonstrating the utility of these mice in determining the role of TLR4 in microbial immunity (29, 30).
In the present study, we sought to determine whether TLR4 participates in the immune response to M. tuberculosis infection. If TLR4 plays a role in innate resistance to M. tuberculosis and participates in the initiation of the adaptive immune response, then TLR4-defective C3H/HeJ mice may be more susceptible to M. tuberculosis infection than wild-type mice. To test this hypothesis, we infected mice by the aerosol route with virulent M. tuberculosis and studied the immune responses during the course of infection in TLR4-defective C3H/HeJ mice and TLR4-sufficient C3H/HeSnJ, C3H/HeOuJ, and C3HeB/FeJ mice.
MATERIALS AND METHODS
Mice.
Age-matched female TLR4-defective C3H/HeJ or TLR4 wild-type C3H/HeSnJ, C3H/HeOuJ, and C3HeB/FeJ mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were housed in a biosafety level 3 facility under specific-pathogen-free conditions at the Animal Biohazard Containment Suite (Dana Farber Cancer Institute, Boston, Mass.) and were used in a protocol approved by the institution.
Bacteria and aerosol infection.
Virulent M. tuberculosis (Erdman strain) was passaged through mice and grown in Middlebrook 7H9 medium supplemented with oleic acid-albumin-dextrose complex (Difco, Detroit, Mich.) as described previously (8). Mice were infected by the aerosol route by using a nose-only exposure unit (Intox Products, Albuquerque, N.Mex.) (7). By plating serial dilutions of homogenized lung tissues (see below) obtained 16 h following inoculation by the aerosol route, we determined that the average inoculum (mean and standard deviation) was 350 ± 200 CFU per mouse. Five experiments were done: one for comparing C3H/HeJ and C3H/HeOuJ mice; two for comparing C3H/HeJ and C3H/SnJ mice; one for comparing C3H/HeJ, C3H/SnJ, and C3HeB/FeJ mice; and one for comparing all four substrains.
BAL.
After the mice were euthanatized, the trachea was exposed and cannulated by using a 19-gauge blunt-ended needle. Lavage was performed by introducing 1 ml of sterile 0.005 M EDTA in phosphate-buffered saline into the lungs, followed by aspiration, reinjection, and reaspiration two additional times. The bronchoalveolar lavage (BAL) fluid was stored at −80°C prior to its use in cytokine enzyme-linked immunosorbent assays (ELISAs).
In vitro restimulation assays.
Splenocyte or lung mononuclear cell (MNC) in vitro restimulation assays were done as previously described (7). Briefly, single cell suspensions were prepared from lungs and spleens of infected mice by mechanical dissociation and by a combination of collagenase digestion and mechanical dissocation, respectively. The red blood cells were lysed with lysis buffer (0.15 M NaCl, 1 mM KHCO3, 0.1 mM sodium EDTA [pH 7.3]). All cells were washed and then resuspended in complete medium (RPMI 1640, 10% fetal calf serum, 2% HEPES, 10% l-glutamine, 10% penicillin-streptomycin, 0.1% β-mercaptoethanol). Lung MNCs (3 × 106 cells/ml) in a volume of 2 ml were incubated with complete medium containing 1 μg of concanavalin A/ml, with M. tuberculosis H37Ra sonicate (3) (diluted 1:1,000, 1:5,000, or 1:25,000), with culture filtrate protein (CFP; obtained from John Belisle, Colorado State University) at 3.3 or 10 μg/ml, or with medium alone for 48 h at 37°C. Culture supernatants were assayed for cytokines by ELISAs with antibody pairs and cytokines from Pharmingen (San Diego, Calif.) (4). To confirm that the antigen-dependent gamma interferon (IFN-γ) production observed was attributable to T cells, blocking studies were done with monoclonal antibodies specific for class II major histocompatibility complex (MHC), class I MHC, or CD4 or a nonbinding isotype control (clones M5/114, M1/42, GK1.5, and Y13-238, respectively, from the American Type Culture Collection). Five million splenocytes were cultured in 2 ml of complete medium with or without 5 μg of CFP/ml in the presence or absence of antibodies at a concentration of 20 μg/ml. To confirm that C3H/HeJ mice were hyporesponsive to LPS compared to TLR-sufficient C3H mouse substrains, splenocytes from all four C3H substrains were stimulated in vitro with 8, 80, or 800 ng of LPS/ml for 48 h. Cellular proliferation was determined by measuring 3H-thymidine incorporation with standard techniques.
CFU determination.
After the mice were euthanatized by CO2 inhalation, the inferior vena cava was severed and blood was purged from the lungs by perfusion with RPMI 1640 through the right ventricle of the heart. The left lung and half of the spleen were aseptically removed and individually homogenized in 0.9% NaCl- 0.02% Tween 80 with a Mini-Bead Beater-8 (Biospec Products, Bartlesville, Okla.). CFUs were quantified by plating 10-fold serial dilutions of organ homogenates on 7H11 Mitchinson agar plates (Remel, Lenexa, Kans.). Colonies were counted after 3 weeks of incubation at 37°C.
Histological analysis.
Lung tissues were preserved in 10% buffered formalin and then embedded in paraffin. Sections 5 μm thick were stained with hematoxylin and eosin or stained for acid-fast bacilli to confirm the bacterial load in the lungs (3). Low-power images were obtained by using a SprintScan 4000 digital film scanner (Polaroid Corporation, Cambridge, Mass.) in combination with a PathScan Enabler (Meyer Instruments, Houston, Tex.).
Statistics.
One-way analysis of variance with Bonferroni posttests was used to compare the bacterial burdens in the organs of the C3H substrains of mice after log transformation of the individual CFUs. All analyses were carried out with the Prism software program (Graphpad, San Diego, Calif.). The log rank test with the Kaplan-Meier method was used to compare differences in the rates of survival of mouse strains.
RESULTS
TLR4 is not necessary for the production of inflammatory cytokines in BAL fluids of M. tuberculosis-infected mice.
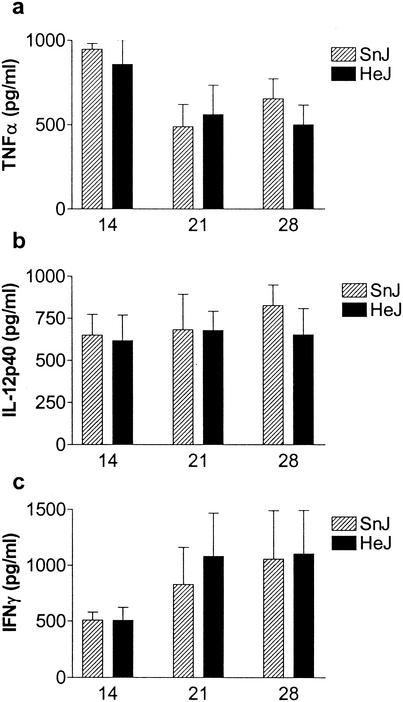
The presence of cytokines in BAL fluid is an early sign of pulmonary inflammation that can be detected in both tuberculosis patients and infected mice (40). As TLR4 agonists stimulate the cellular production of inflammatory cytokines, we examined whether differences existed in the amounts of tumor necrosis factor alpha (TNF-α) and IL-12p40 found in the BAL fluids of TLR4-defective C3H/HeJ mice or other C3H mouse substrains following aerosol infection with M. tuberculosis. Although TNF-α and IL-12p40 were both detected in the BAL fluids of infected mice, no significant differences were observed between TLR4-defective C3H/HeJ and TLR4-sufficient C3H/HeSnJ mice at the times studied (Fig. 1a and b). IFN-γ was also measured to determine whether TLR4 signaling resulted in a subsequent alteration in T-cell-mediated immunity; however, there were no significant differences between C3H/HeJ mice and other C3H mouse substrains (Fig. 1c).
FIG. 1.
Cytokines in BAL fluids from M. tuberculosis-infected C3H mice. Levels of TNF-α, IL-12p40, and IFN-γ in BAL fluids from C3H/HeJ (HeJ) and C3H/HeSnJ (SnJ) mice at 14, 21, or 28 days after aerosol inoculation with M. tuberculosis were determined by ELISAs. Each group contained six mice; data represent means and standard deviations.
TLR4 does not modulate in vitro recall responses to mycobacterial antigens.
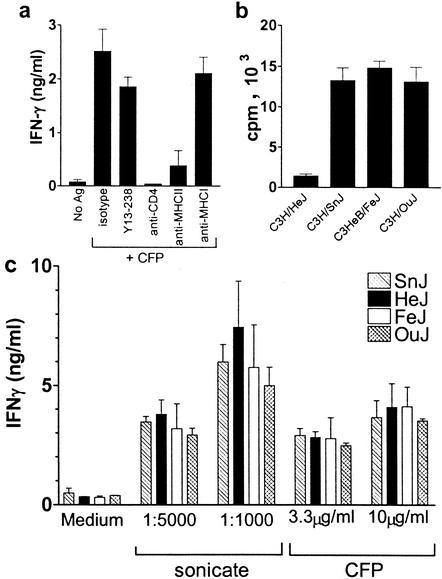
A potent cell-mediated immune response is generated following infection of mice with M. tuberculosis by the aerosol route. This response can be studied in vitro by stimulating splenocytes or lung MNCs with mycobacterial antigens and monitoring the production of IFN-γ (7). The in vitro response is mediated primarily by class II MHC-restricted CD4+ T cells and, in C3H mice, peaks at 3 to 4 weeks following infection (Fig. 2a). We first confirmed that our C3H/HeJ mice were impaired in their responses to LPS. Splenocytes from C3H/HeJ, C3H/HeOuJ, C3H/HeSnJ, and C3HeB/FeJ mice were stimulated in vitro with LPS for 48 h, and the proliferative responses were determined. Compared to splenocytes from TLR4-sufficient C3H substrains, TLR4-defective C3H/HeJ splenocytes failed to proliferate (Fig. 2b), as observed by others (10). To determine whether TLR4 signaling during M. tuberculosis infection modulated the CD4+ T-cell response, we examined whether TLR4-defective C3H/HeJ mice had a diminished pulmonary recall response to mycobacterial antigen compared to TLR4-sufficient C3H mouse substrains. At 4 weeks following aerosol infection, lung MNCs from infected mice were stimulated in vitro with whole M. tuberculosis sonicate or CFP. All C3H substrains made similar levels of IFN-γ. In general, more IFN-γ was made in response to M. tuberculosis sonicate than to CFP. Significant differences in antigen-stimulated IFN-γ production by lung MNCs were not detected between TLR4-defective and TLR4-sufficient mice (Fig. 2c).
FIG. 2.
TLR4 does not affect in vitro T-cell recall responses to mycobacterial antigens. (a) Splenocytes from infected C3H/SnJ-H2b mice were cultured without further manipulation (no antigen [No Ag]) or in the presence of M. tuberculosis CFP (5 μg/ml). To some wells, isotype control or blocking antibody to CD4, class II MHC, or class I MHC was added. An ELISA was used to measure the amounts of IFN-γ produced 48 h after stimulation. (b) The cellular proliferation of splenocytes from the four C3H substrains was determined 48 h after stimulation with 80 ng of LPS/ml. Proliferation levels in the absence of LPS were 1,314 cpm (C3H/HeJ), 1,959 cpm (C3H/SnJ), 2,987 cpm (C3H/HeOuJ), and 2,108 cpm (C3HeB/FeJ). (c) Lungs were removed from C3H/HeSnJ (SnJ), C3H/HeJ (HeJ), C3H/HeOuJ (OuJ), and C3HeB/FeJ (FeJ) mice 28 days after aerosol inoculation with M. tuberculosis. One half of the lungs was used to determine the CFUs (see Fig. 4), and lung MNCs were prepared from the other half to determine in vitro recall responses to M. tuberculosis antigens. The dilutions of M. tuberculosis sonicate or concentrations of M. tuberculosis CFP used are indicated. Each group contained six mice; data represent means and standard deviations.
M. tuberculosis burden does not correlate with TLR4 genotype.
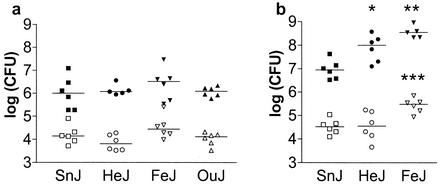
The bacterial burdens in the lungs and spleens of C3H/HeJ mice and several control C3H mouse substrains were determined at different times following infection to determine whether a defective tlr4 gene product impaired the ability of mice to control bacterial replication. In three independent experiments, no significant differences in lung M. tuberculosis burdens were observed between TLR4-sufficient C3H/HeSnJ and TLR4-defective C3H/HeJ mice (Fig. 3a). In a fourth experiment, C3HeB/FeJ and C3H/HeJ mice had significantly higher mycobacterial loads in their lungs than C3H/HeSnJ mice at 4 weeks following infection (Fig. 3b). In the same experiment, bacterial burdens in the spleens of C3HeB/FeJ mice were significantly higher than those in either C3H/HeSnJ or C3H/HeJ mice. These data support the hypothesis that the control of mycobacterial replication is not entirely dependent on TLR4 signaling, since C3H/HeJ mice were no more susceptible than other TLR4-sufficient C3H mouse substrains. However, the variations in lung bacterial loads between different C3H substrains suggest the existence of genetic heterogeneity that affects resistance to M. tuberculosis infection.
FIG. 3.
Bacterial burden is independent of TLR4. Two representative experiments (a and b) are shown for C3H/HeSnJ (SnJ), C3H/HeJ (HeJ), C3H/HeOuJ (OuJ), and C3HeB/FeJ (FeJ) mice. CFUs in the lungs (closed symbols) and spleens (open symbols) were determined 28 days after aerosol inoculation with M. tuberculosis. The lung inocula were 144 CFU (a) and 472 CFU (b). Horizontal lines indicate median CFUs. Significance levels determined by one-way analysis of variance with Bonferroni multiple-comparison tests were as follows: *, P < 0.05 (lungs, SnJ versus HeJ); **, P < 0.001 (lungs, SnJ versus FeJ); and ***, P < 0.05 (spleens, HeJ versus FeJ) and P < 0.01 (spleens, SnJ versus FeJ).
Survival of C3H mouse substrains following inoculation with M. tuberculosis does not depend on TLR4.
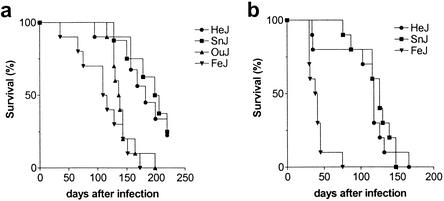
To determine whether the resistance of C3H/HeJ mice to M. tuberculosis infection was impaired by their TLR4 defect, the rates of survival of TLR4-defective and TLR4-sufficient mice were compared following inoculation with virulent M. tuberculosis by the aerosol route. All of the C3H mouse substrains succumbed to infection earlier than B6 mice (data not shown), reemphasizing the fact that susceptibility to tuberculosis is a general feature of all C3H mice. Interestingly, the most susceptible C3H substrain was C3HeB/FeJ, which consistently died more rapidly following infection than any of the other substrains (Fig. 4). In contrast, the survival of TLR4-defective C3H/HeJ mice was similar to that of TLR4-sufficient C3H/HeSnJ mice. For example, following the administration of a lung inoculum of 472 CFU, C3HeB/FeJ mice had a median survival time (MST) of 39.5 days (Fig. 4b). In contrast, C3H/HeJ mice had an MST of 118 days, and C3H/HeSnJ mice had an MST of 126 days (P < 0.0006 and P < 0.0001, respectively, compared to C3HeB/FeJ mice) (Fig. 4b). Similar results were obtained when a slightly smaller inoculum of 144 CFU was delivered to the lungs (Fig. 4a). These data parallel the bacterial burdens in the lungs of these mice (Fig. 3) and demonstrate that there exists among C3H substrains genetic heterogeneity that affects resistance to tuberculosis.
FIG. 4.
Survival of C3H substrains following aerosol inoculation with M. tuberculosis. Groups of 10 mice per strain were inoculated by the aerosol route, and their survival was monitored. The lung inocula were144 CFU (a) and 472 CFU (b).
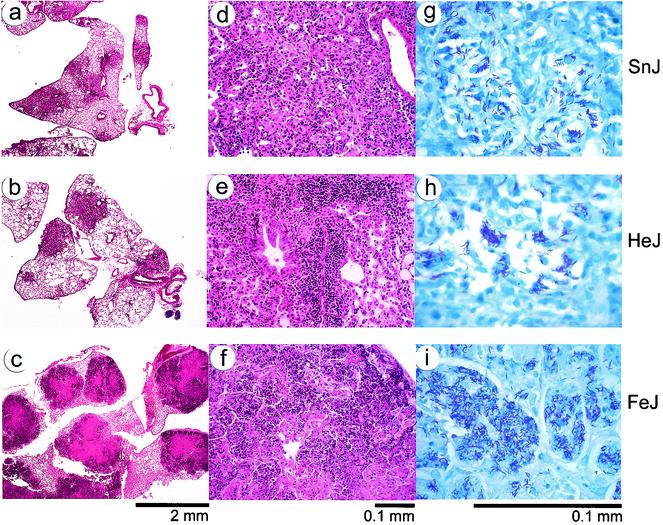
FIG. 5.
Lung pathology in M. tuberculosis-infected mice. Lung tissues were obtained from C3H/HeSnJ (a, d, and g), C3H/HeJ (b, e, and h), and C3HeB/FeJ (c, f, and i) mice 28 days after aerosol infection with M. tuberculosis. Shown are representative sections from formalin-fixed, paraffin-embedded lung tissues stained with hematoxylin and eosin at low power (a to c) and high power (d to f) or stained for acid-fast bacilli (g to i).
Pathological changes in lungs.
Extensive granuloma formation was observed in the lungs of C3H mice at 4 weeks following aerosol infection with M. tuberculosis (Fig. 5). The histological appearances of the granulomas in TLR4-defective C3H/HeJ and wild-type C3H/HeSnJ mice were similar. The medium-sized inflammatory nodules were dominated by macrophages accompanied by abundant neutrophils and scattered lymphoid cells (Fig. 5a, b, d, and e). Little necrosis was observed at this time. In contrast, massive tissue destruction was seen in the lungs of C3HeB/FeJ mice (Fig. 5c and f). Prominent necrotic areas of degenerating cells and dense infiltrates of neutrophils dominated the granulomas. Furthermore, while lung tissues from C3H/HeJ and C3H/HeSnJ mice had abundant mycobacteria in clumps that appeared to be intracellular, the granulomas in C3HeB/FeJ mice had large confluent areas of bacteria, especially in necrotic areas, which may have arisen from extracellular bacterial replication (Fig. 5g to i). These observations confirm that C3HeB/FeJ mice have altered responses and impaired resistance following infection with M. tuberculosis.
DISCUSSION
The complexity of the innate immune system has evolved from the necessity to protect the host from a vast and divergent microbial world. The recognition of PAMPs present on the surface of pathogenic microbes by the PRRs of the innate immune system is critical in host defense against infection (18, 27). The members of the TLR family of PRRs have an important role in mammalian immunity, as their primary functions include opsonization, phagocytosis, activation of complement and coagulation cascades, activation of proinflammatory signaling pathways, and induction of apoptosis (19). Consequently, there has been considerable interest in determining whether TLRs play a role in immunity to mycobacterial diseases and whether defects in the TLR signaling pathway could account for the susceptibility of certain individuals to infectious diseases.
We found that TLR4-defective C3H/HeJ mice were no more susceptible than TLR4-sufficient mice, suggesting that TLR4 does not play a significant role in host immunity to M. tuberculosis. Neither the production of cytokines in BAL fluid nor the antigen-specific recall response was diminished in TLR4-defective C3H/HeJ mice compared to other C3H mouse substrains. More importantly, the tlr4 genotype did not affect the outcome of infection, as the long-term survival of TLR4-defective C3H/HeJ mice was not diminished compared to that of TLR4-sufficient C3H mouse substrains following inoculation with M. tuberculosis by the aerosol route. These results are similar to the previous finding that the susceptibility of C3H/HeJ mice following intravenous infection with M. tuberculosis was independent of TLR4 (8). A similar conclusion was reached by Reiling et al., who observed no differences in the mortalities of C3H/HeJ and C3H/HeN mice following either low- or high-dose aerosol challenge with M. tuberculosis (33). In contrast, Means et al. showed that TLR4-deficient C3H/HeJ mice were significantly more susceptible than normal C3H/HeOuJ mice to intraperitoneal M. bovis bacillus Calmette-Guérin (BCG) infection (23). This finding supported their earlier work showing that mycobacteria contain TLR4 agonists (24). Recently, using virulent M. tuberculosis in a low-dose aerosol model, Abel and colleagues showed that C3H/HeJ mice had a diminished proinflammatory response, had poor macrophage recruitment and activation, and succumbed to chronic infection earlier than C3H/HeN mice, suggesting that TLR4 is required for optimum protection in mice (1). The Erdman strain of M. tuberculosis was used in our studies; and while bacterial strain differences could be important, especially if their expression of TLR4 ligands differs, we do not believe this to be the case. Differences in the choice of bacterial strain cannot explain the opposite conclusions reached by the studies Abel et al. and Reiling et al., since both groups used H37Rv. This information suggests that some other experimental variable has an important effect. The differences in the results of the studies may arise from the choice of the control mouse strain. We observed significant heterogeneity in morbidity and mortality for different TLR4-sufficient C3H mouse substrains following aerosol infection with virulent M. tuberculosis. For example, C3HeB/FeJ mice tended to have higher bacterial burdens early during infection, had more lung necrosis, and succumbed earlier than other C3H mouse substrains. The sst1 locus accounts for much of the susceptibility of C3HeB/FeJ mice compared to C57BL/6 mice (20). However, the status of this locus in other C3H substrains remains to be defined. Consequently, the relative resistance of C3H/HeJ mice to tuberculosis infection will depend in part on the choice of the control strain. If C3HeB/FeJ had been used as the only control strain, then the erroneous conclusion that TLR4 is a susceptibility factor might have been reached.
How to choose the best control strain for C3H/HeJ mice is not obvious. Strong originally developed the C3H strain in 1920 from crosses of Bagg albino mice with DBA mice. In 1941, Heston obtained strain C3H/He, which was the parental stock for the C3H/HeJ, C3H/HeSnJ, C3H/HeOuJ, and C3HeB/FeJ substrains. C3H/He mice were passed to Jackson Laboratories (C3H/HeJ) in 1947 (22a). It is estimated that the mutation in the tlr4 gene became fixed in the C3H/HeJ substrain between 1960 and 1968 (10). Fekete developed C3HeB/FeJ in 1948 by transferring fertilized C3H/HeJ ova to C57BL/6 foster mothers. This transfer clearly occurred before the tlr4 mutation, as C3HeB/FeJ mice are LPS responsive and lack the mutation present in C3H/HeJ mice (32).
The basis of the observed heterogeneity is likely genetic, as we have tried to control for as many environmental variables as possible by obtaining all of the mice from the same vendor, housing the mice together in the same facility, infecting and analyzing the mice at the same time, and handling the mice in the same way. Differences in the C3H substrains with respect to biochemical markers, skin grafting, and skeletal characteristics have been noted; in addition, it appears that C3H substrains maintained in different closed colonies around the world have diverged (11, 21, 37). These variations may have originated from residual genetic heterozygosity or as a consequence of mutation, as documented for the LPS response. Consequently, it is not possible to definitively conclude whether TLR4 has a role in host immunity, as the control strains have potential allelic differences that alter their susceptibility to tuberculosis. This caveat applies to all studies that compare the susceptibilities of C3H/HeJ mice and TLR4-sufficient C3H mouse substrains as controls (1, 23, 33). In addition, this issue will not be easily circumvented through the use of knockout mice, because the commonly used embryonic stem cells are derived from 129/SvJ mice, which are susceptible to M. tuberculosis (25). Consequently, exhaustive backcrossing will be required to ensure that the disrupted tlr4 gene is segregated away from other potential susceptibility genes within the 129/SvJ genome.
Although TLR4 is a critical molecule in innate immunity, our data suggest that it is not a critical determinant of susceptibility to acute or chronic infection by M. tuberculosis. In contrast, TLR2 may play a more important role. Mycobacteria produce well-defined ligands, such as arabinose-capped lipoarabinomannan and the 19-kDa lipoprotein, that activate TLR2 (6, 24). Furthermore, it was recently shown that TLR2 knockout mice have reduced survival compared to control mice following aerosol infection with virulent M. tuberculosis (33). Interestingly, in that study, this phenotype was apparent only with larger inocula, suggesting that even TLR2 plays a minor or redundant role in innate immunity to mycobacterial infection. As discussed above, a heterogeneous mixture of susceptibility and resistance genes derived from parental C57BL/6 and 129/Sv mouse strains may obscure a more prominent role for TLR2. Alternatively, PRRs other than TLR family members may play a more critical role in immunity to tuberculosis. However, despite the evolution of the mammalian innate immune system to recognize microbes, successful pathogens may have evolved multiple mechanisms to evade such defenses.
Acknowledgments
This work was supported by National Institutes of Health grant HL64540. Materials were provided by Colorado State University through NIH/NIAID contract NO1-AI-75320 (Tuberculosis Research Materials and Vaccine Testing).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abel, B., N. Thieblemont, V. J. Quesniaux, N. Brown, J. Mpagi, K. Miyake, F. Bihl, and B. Ryffel. 2002. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J. Immunol. 169:3155-3162. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Behar, S. M., C. C. Dascher, M. J. Grusby, C. R. Wang, and M. B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behar, S. M., T. A. Podrebarac, C. J. Roy, C. R. Wang, and M. B. Brenner. 1999. Diverse TCRs recognize murine CD1. J. Immunol. 162:161-167. [PubMed] [Google Scholar]
- 5.Belvin, M. P., and K. V. Anderson. 1996. A conserved signaling pathway: the Drosophila Toll-dorsal pathway. Annu. Rev. Cell Dev. Biol. 12:393-416. [DOI] [PubMed] [Google Scholar]
- 6.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 7.Chackerian, A. A., J. M. Alt, T. V. Perera, C. C. Dascher, and S. M. Behar. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chackerian, A. A., T. V. Perera, and S. M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang, T., and R. J. Ulevitch. 2001. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim. Biophys. Acta 1518:157-161. [DOI] [PubMed] [Google Scholar]
- 10.Glode, L. M., and D. L. Rosenstreich. 1976. Genetic control of B cell activation by bacterial lipopolysaccharide is mediated by multiple distinct genes or alleles. J. Immunol. 117:2061-2066. [PubMed] [Google Scholar]
- 11.Green, E. L. 1953. A skeletal difference between sublines of the C3H strain on mice. Science 117:81-82. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto, C., K. L. Hudson, and K. V. Anderson. 1988. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269-279. [DOI] [PubMed] [Google Scholar]
- 13.Heldwein, K. A., and M. J. Fenton. 2002. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 4:937-944. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 15.Heppner, G., and D. W. Weiss. 1965. High susceptibility of strain A mice to endotoxin and endotoxin-red blood cell mixtures. J. Bacteriol. 90:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 18.Janeway, C. A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp. Quant. Biol. 54:1-13. [DOI] [PubMed] [Google Scholar]
- 19.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 20.Kramnik, I., W. F. Dietrich, P. Demant, and B. R. Bloom. 2000. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:8560-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krog, H. H., and R. Moutier. 1978. Identification of inbred strains of mice. II. Characterization of different substrains of the C3H strain. J. Hered. 69:66-70. [DOI] [PubMed] [Google Scholar]
- 22.Lemaitre, B., J. M. Reichhart, and J. A. Hoffmann. 1997. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 94:14614-14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Lyon, M. F., S. Rastan, and S. D. M. Brown (ed.). 1996. Genetic variants and strains of the laboratory mouse, 3rd ed. Oxford University Press, Oxford, England.
- 23.Means, T. K., B. W. Jones, A. B. Schromm, B. A. Shurtleff, J. A. Smith, J. Keane, D. T. Golenbock, S. N. Vogel, and M. J. Fenton. 2001. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J. Immunol. 166:4074-4082. [DOI] [PubMed] [Google Scholar]
- 24.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 25.Medina, E., and R. J. North. 1998. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 93:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 29.Nowicki, B., J. Singhal, L. Fang, S. Nowicki, and C. Yallampalli. 1999. Inverse relationship between severity of experimental pyelonephritis and nitric oxide production in C3H/HeJ mice. Infect. Immun. 67:2421-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien, A. D., D. L. Rosenstreich, and B. A. Taylor. 1980. Control of natural resistance to Salmonella typhimurium and Leishmania donovani in mice by closely linked but distinct genetic loci. Nature 287:440-442. [DOI] [PubMed] [Google Scholar]
- 31.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 34.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 35.Schlesinger, L. S., T. M. Kaufman, S. Iyer, S. R. Hull, and L. K. Marchiando. 1996. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J. Immunol. 157:4568-4575. [PubMed] [Google Scholar]
- 36.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvia, O. J., and N. Urosevic. 1999. Variations in LPS responsiveness among different mouse substrains of C3H lineage and their congenic derivative sublines. Immunogenetics 50:354-357. [DOI] [PubMed] [Google Scholar]
- 38.Sultzer, B. M. 1968. Endotoxin-induced resistance to a staphylococcal infection: cellular and humoral responses compared in two mouse strains. J. Infect. Dis. 118:340-348. [DOI] [PubMed] [Google Scholar]
- 39.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 40.Tsao, T. C., J. Hong, C. Huang, P. Yang, S. K. Liao, and K. S. Chang. 1999. Increased TNF-alpha, IL-1 beta and IL-6 levels in the bronchoalveolar lavage fluid with the upregulation of their mRNA in macrophages lavaged from patients with active pulmonary tuberculosis. Tuber. Lung Dis. 79:279-285. [DOI] [PubMed] [Google Scholar]
- 41.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]