Abstract
Thioredoxins are a family of small redox proteins that undergo NADPH-dependent reduction by thioredoxin reductase. This results in a supply of reducing equivalents that cells use in a wide variety of biological reactions, which include maintaining reduced forms of the enzymes important for protection against damage from high-energy oxygen radicals, the regulation of transcription factor activity, and the inhibition of apoptosis. Here we report on a new member of the thioredoxin family of proteins from the filarial nematode Brugia malayi, Bm-TRX-1, which defines a new subclass of 16-kDa thioredoxins that occur widely in nematodes, including Caenorhabditis elegans. In addition to being larger than the thioredoxins found in mammalian and bacterial species, the putative active site sequence of Bm-TRX-1, WCPPC, does not conform to the highly conserved WCGPC reported for thioredoxins from mammals to bacteria. Interestingly, an allelic form of Bm-TRX-1 was identified with an active site sequence WCPQC, which appears to be unique to the thioredoxins from filarial species. Bm-TRX-1 was between 98% and 35% identical to thioredoxins from other nematodes and ≈20% identical to the thioredoxins from mammals and Escherichia coli. Bm-TRX-1 was constitutively transcribed throughout the B. malayi life cycle, and Bm-TRX protein was detectable in somatic extracts and excretory-secretory products from adults and microfilariae. Recombinant Bm-TRX-1 had thiodisulfide reductase activity, as measured by the reduction of insulin, and protected DNA from the nicking activity of oxygen radicals. Overexpression of Bm-TRX-1 in a human monocyte cell line negatively regulated tumor necrosis factor alpha-induced p38 mitogen-activated protein kinase activity, suggesting a possible role of the 16-kDa Bm-TRX-1 in immunomodulation.
Human lymphatic filariasis is a seriously debilitating vector-borne parasitic disease that affects nearly 120 million people worldwide (23). The etiological agents of lymphatic filariasis in humans are the nematode species Wuchereria bancrofti, Brugia malayi, and Brugia timori. The World Health Organization ranks the complex of clinical conditions caused by lymphatic filariae as second on the list of infectious disease that lead to permanent and long-term disability (24). In areas where it is endemic, humans become infected early in life and are persistently infected for decades with adult parasites residing within the lymphatic system. The ability to endure long term in the hostile immunological environment of the mammalian host suggests that the parasite has evolved measures to counter a variety of potentially harmful effector molecules.
Indeed, there are a number of reports that indicate that filarial parasites have evolved measures to counter the host's innate and adaptive immune responses (14, 41). In this regard, one of the areas of intense study is the capacity of the parasite to neutralize host-derived reactive oxygen species such as H2O2, superoxide radicals, hydroxyl ions, and nitric oxide. Filarial parasites produce a number of stress-inducible secretory proteins that include thioredoxin peroxidases, selenium-independent glutathione peroxidases, and superoxide dismutase (reviewed in reference 16). Several of these antioxidant enzymes are known to require the thioredoxin system as a source of reducing equivalents to remain active. As part of an effort to better understand the regulation of the antioxidant system in filarial parasites and the control of homeostasis in general, we cloned and characterized thioredoxin from the model filarial parasite Brugia malayi.
The thioredoxins that have been characterized to date are nearly uniformly small (12-kDa) ubiquitous proteins that are critical for redox regulation of protein function and signaling. Thioredoxins function in a variety of cellular processes that can be generalized into two major roles. First, they act as electron carriers necessary for the catalytic cycles of biosynthetic and antioxidant enzymes such as ribonucleotide reductases, methionine sulfoxide reductases, and the peroxidoxins. Second, thioredoxin functions to protect cytosolic proteins from aggregation or inactivation via oxidant-mediated formation of intra- or intermolecular disulfides (2). In addition, there is a growing body of evidence that thioredoxins have important regulatory effects on immune responses through their ability to potentate cytokine release (36), enhance cytotoxicity (37), block the action of chemokines (4), and control the DNA binding activity of immunologically active transcription factors, including NF-κB and AP-1 (38).
Here we describe the cloning and partial characterization of a thioredoxin from the filarial nematode B. malayi, Bm-TRX-1, as a representative of a new subclass of 16-kDa thioredoxins that are found in a spectrum of nematode species. Although the putative active site sequence WCPPC did not conform to the highly conserved WCGPC reported for the 12-kDa class of thioredoxins, Bm-TRX-1 exhibited oxidoreductase activity and antioxidant activity comparable to that of 12-kDa thioredoxins. In addition to is putative role as a reducing equivalent, that Bm-TRX-1 is secreted by the parasite and its ability to inhibit tumor necrosis factor alpha (TNF-α)-induced activation of p38 mitogen-activated protein (MAP) kinase raises the possibility that Bm-TRX-1 may function as a component of the parasite's immune evasion mechanism.
MATERIALS AND METHODS
Isolation and sequencing of B. malayi thioredoxin.
The B. malayi cDNA clones selected for characterization in these studies were initially identified as part of the gene discovery initiative of the Filarial Genome Project (6). The cDNA clone AS3ISB007T3 was selected as a representative member of cluster BMC00153 (NEMBASE; http://nema.cap.ed.ac.uk/nematodeESTs/nembase.html). All cDNAs were sequenced completely in both directions by automated sequencing (Applied Biosystems, Foster City, Calif.) and determined to be partial 3′ clones. Additional 5′ sequence was obtained by sequencing PCR products generated with primers W5308 (see below) and SL-1 (GGTTTAATTACCCAAGTTTGAG).
The DNA and deduced amino acid sequences of all the clones were compared to the protein, nucleotide, and expressed sequence tag (EST) databases by Blast (1). Motif searches and other analyses were carried out with Internet resources for the parasite genome projects (5). The B. malayi thioredoxin cDNA was designated Bm-trx-1 (accession number AY117545) according to the Filarial Uniform Nomenclature Kommission (FUNK) nomenclature convention (7).
Isolation and sequencing of genomic sequence of B. malayi thioredoxin.
The sequence corresponding to the Bm-trx-1 open reading frame was labeled and used as a probe to screen a HindIII B. malayi bacterial artificial chromosome (BAC) library (10). BAC clone 41G1 was isolated and used as a template to amplify the genomic sequence of Bm-trx-1 by PCR with primers W5317 and W5308 (see below). The resulting PCR product was purified (QIAquick, Qiagen, Chatsworth, Calif.) and sequenced as outlined above.
Expression and purification of recombinant proteins.
The sequence corresponding to the Bm-trx-1 open reading frame was isolated by PCR from mRNA or cDNA. The 5′ primer W5317 (5′-CGCGGATCCGATGGCTGATTTACTTGCTAATATC-3′) contained a recognition site for BamHI and 24 bp of Bm-trx-1 open reading frame that included the codon for the initiating methionine (underlined). The 3′ primer W5308 (5′-CCCAAGCTTCTACGCTGCTGCTAACCAGC-3′) contained a recognition site for HindIII, a stop codon (underlined), and the last 17 bp of the Bm-trx-1 open reading frame. After 30 cycles of amplification, the PCR products were subcloned in frame into pET11b (Novagen, Madison, Wis.) as a T7 fusion protein. Recombinant plasmids were used to transform Escherichia coli BL21(DE3), and recombinant Bm-TRX-1 (rBm-TRX-1) protein synthesis was induced with 0.5 mM isopropylthiogalactopyranoside (IPTG) for 2 h at 37°C. The cells were subjected to sonication, and the soluble proteins were fractionated by mass to produce a preparation that was highly enriched for rBm-TRX-1.
Antiserum production.
Antibodies against the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-purified rBm-TRX-1 were produced in mice. Initial immunizations were carried out with Freund's complete adjuvant and subsequent boosters with Freund's incomplete adjuvant. The antisera used in these studies were obtained after the third immunization.
Insulin reduction assay.
Thioredoxin-mediated catalysis of insulin reduction was measured spectrophotometrically at 650 nm and 25°C as an increase in turbidity resulting from precipitation of the free insulin β-chain (18). The assay mixture contained 100 mM potassium phosphate, 2 mM EDTA (pH 7.0), 0.13 mM insulin (0.75 mg/ml), and various concentrations (25, 50, and 100 mg per reaction) of Spirulina thioredoxin (Sigma) or rBm-TRX-1. The reaction was initiated by the addition of 0.33 mM dithiothreitol (DTT). Anti-Bm-TRX-1 and control sera were used in the assay at a dilution of 1:500.
DNA nicking assay.
pTracer EF/VS-His (Invitrogen, Carlsbad, Calif.) plasmid DNA was used as a substrate for detecting DNA damage mediated by the mixed function oxidase (MFO) system (13). The MFO system consisted of 66 mM FeCl3, 10 mM DTT, and 2 mM EDTA in a 25 mM HEPES buffer, pH 7. Plasmid DNA (100 ng) was incubated in the MFO system at 37°C with or without rBm-TRX-1. The extent of MFO-mediated nicking was evaluated on ethidium bromide-stained agarose gels. Recombinant B. malayi SXP (Bm-SXP, accession number M98813 [32]) expressed in pRSET as a 26-kDa fusion protein with a 6-His tag was used as a negative control, and Spirulina thioredoxin (Sigma) was used as a positive control.
Prothrombin assay.
Thromboplastin (200 ml; Diagnostic Grifols, S.A.) was suspended in distilled water and combined with rBm-TRX-1 (50, 100, or 200 μg/ml), bovine serum albumin (10 mg/ml), and crude E. coli extract (100 μg/ml) or Bm-SXP (200 μg/ml) at 37°C. After 30 s, 100 μl of oxalated plasma was quickly added from a pipette, and the tube was gently inverted at 37°C. The time required to form a gel was monitored and taken as the endpoint of the assay. All tests were performed in duplicate.
p38 activation assay.
The human myeloblastic leukemic cell line ML-1 (21) was maintained at 37°C in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Gibco-BRL). ML-1 cells were grown to a density of 2 × 105 cells/ml, and differentiation was induced by treatment with 0.3 ng of tetradecanoyl phorbol acetate (TPA) per ml in dimethyl sulfoxide (final concentration, 0.03%) for 3 days. The TPA-containing medium was then removed and replaced with fresh medium lacking TPA, and the cells were incubated for an additional 3 days. Cell viability was determined by trypan blue dye exclusion.
In preliminary experiments, TPA-differentiated ML-1 cells were stimulated with TNF-α (100 IU/well) for 5, 10, and 30 min, and the phosphorylation status of p38 MAP kinase was analyzed on immunostained Western blots of cell extracts with phosphoPlus p38 MAP kinase antibody kit (Cell Signaling Technology, Beverly, Mass.). Under the conditions used in these studies, maximal activation of p38 MAP kinase was seen 5 min after addition of TNF-α (data not shown), and thus this time point was used for the assays reported here.
The open reading frame of Bm-trx-1 was cloned in-frame as a KpnI-XbaI fragment into the eukaryotic expression vector pTracer EF/VS-His. The predicted organization of the plasmid was confirmed by DNA sequencing. Differentiated ML-1 cells were seeded in six-well plates at 2 × 106 cells per well in 12 ml of serum-free growth medium containing 4 μg of Bm-trx-1 plasmid DNA (or empty vector) and Lipofectamine (Gibco-BRL). After a 6-h incubation, the transfection medium was removed and replaced with 2 ml of RPMI 1640 supplemented with 10% fetal bovine serum. At 48 h posttransfection, P38 phosphorylation was induced by treating the ML-1 cells with TNF-α at 100 IU per well. After 5 min of incubation, the transfected and nontransfected cells were suspended in lysis buffer and processed for SDS-PAGE and immunoblot analysis of p38 MAP kinase phosphorylation.
Parasites.
Aedes aegypti (Liverpool, Blackeye strain) were fed on B. malayi-infected gerbils (Meriones unguiculatus) and dissected on days 10 to 12 postinfection to obtain vector-derived third-stage (L3) parasites (28). To obtain fourth-stage (L4) larvae, ≈2,000 vector-stage L3s were introduced into the peritoneal cavity of a naïve male gerbil (6 to 8 weeks of age) and allowed to develop for 14 to 16 days. The L4 parasites were collected by peritoneal lavage, washed, and snap frozen in liquid nitrogen. Adult female parasites and first-stage larvae (L1 or microfilariae) were obtained by lavage from the peritoneal cavity of infected gerbils. Excretory/secretory (ES) products from microfilariae and adult B. malayi were collected as described previously (28) and concentrated with an Ultrafree MC concentrator (Millipore) with a molecular mass cutoff of 5 kDa.
RESULTS
Cloning and sequencing the thioredoxin gene from Brugia malayi.
The cDNA encoding B. malayi thioredoxin-1 (Bm-trx-1; accession number AY117545; NEMBASE cluster BMC00153; TIGR Gene Index TC1868) was 698 bp. The mature Bm-trx-1 mRNA was trans-spliced with the conserved 21-nucleotide spliced leader (SL-1) positioned 55 nucleotides upstream from the initiating ATG.
Genomic organization of B. malayi thioredoxin.
It was determined that B. malayi BAC clone 41G1 contained Bm-trx-1 by probe hybridization. A 1,662-bp genomic fragment was amplified by PCR from the 41G1 template and sequenced (accession number AY161166) to reveal that Bm-trx-1 contained four introns (bp 175, 563, 205, and 284) and five exons (bp 75, 75, 140, 100, and 48). The splice acceptor and splice donor sites conformed to the consensus splice junctions for most nematodes and other eukaryotic organisms (8). The nucleotide sequence encoding the putative active site resided within the second exon. The positions of the intron-exon junctions as they relate to the protein sequence are indicated in Fig. 2.
FIG. 2.
Alignment of protein sequence of B. malayi thioredoxin with other 16-kDa thioredoxins derived from nematodes. Bm-TRX-1, B. malayi, GenBank accession AY117545; Wb-TRX-1, Wuchereria bancrofti, accession AY142707; Ov-TRX-1, Onchocerca volvulus, accession AY142708; Ov-TRX-2, O. volvulus, accession AY142706; Oc-TRX, O. cervacalis, accession AI363557; Ls-TRX-5, Litomosoides carinii, accession AW152732; As-TRX-1, Ascaris suum, accession BM516048; Ac-TRX, Ancylostoma canimun, accession BM077365; Ts-TRX-1, Trichinella spiralis, accession BG322259; Ce-TRX-5, Caenorhabditis elegans, accession NP_495275; Ce-TRX-6, C. elegans, accession NP_503440; Ce-TRX-8, C. elegans, accession NP_500478; Ss-TRX-1, Strongyloides stercoralis, accession BG226973; Gr-TRX-I, Globodera rostochiensis, accession BM354327; Hg-TRX-1, Heterodera glycines, accession BF013813; Mi-TRX-1, Meloidogyne incognita, accession AW783656; Pp-TRX-1, Pristionchus pacificus, accession AW097135. Residues highlighted in black are an exact match to the Bm-TRX-1 sequence. Residues highlighted in red are invariant for all of the sequences tested. The arrows indicate the positions of the intron-exon junctions found in the Bm-trx-1 genomic sequence (accession AY161166).
Transcription of B. malayi thioredoxin.
The results of reverse transcription-PCR experiments carried out on all of the major developmental stages of B. malayi indicated that transcription of Bm-trx-1 could be detected throughout the life cycle (data not shown). However, an examination of the NEMBASE and the TIGR Brugia malayi Gene Index cluster databases showed that a majority of the ESTs (28 of 41) were derived from the L3 or L4 library. This L3/L4 bias in expression of Bm-trx-1 is supported by the EST expression patterns of Onchocerca volvulus trx-1 (Ov-trx-1) and Ov-trx-2 (see below). Of the 42 Ov-trx-1 plus Ov-trx-2 ESTs, 32 were derived from the libraries produced from third-stage larvae.
Comparison of protein sequence of B. malayi thioredoxin with other thioredoxins.
The deduced sequence of Bm-trx-1 encoded a protein of 145 residues with a predicted molecular mass of 16.1 kDa and predicted pI of 5.4. Overall, Bm-TRX-1 was approximately 20% identical to the 12-kDa class of thioredoxins from mammalian, avian, insect, protozoan, and bacterial species (data not shown). A majority of this identity was clustered in the region surrounding the active site that extended from a conserved Val eight residues upstream from the active site to a highly conserved Asp residue positioned 29 to 36 residues downstream from the active site (Fig. 1). While a number of residues in and around the invariant WCGPCK active site of the 12-kDa thioredoxins were also found in the Bm-trx-1 sequence, the putative active site of Bm-trx-1 contained the sequence WCPPCR. Interestingly, two non-12-kDa thioredoxins, Trypanosoma brucei TRX-2 (16 kDa, accession number CAA07003) and human nucleoredoxin (27 kDa, accession number XM_091674), also contain putative WCPPCR active sites and were 50% and 36% identical to Bm-trx-1, respectively (Fig. 1).
FIG. 1.
Alignment of region surrounding the active site of the 16-kDa thioredoxin from B. malayi with comparable regions from 12-kDa class thioredoxins. The thioredoxins tested were Bm-TRX-1, B. malayi, GenBank accession AAM51563; Hs-TRX, Homo sapiens, accession XM_091674 PP27; Tb-TRX-2, Trypanosoma brucei, accession CAA07003; Hs-TRX-1, Homo sapiens, accession AAF86466; Mm-TRX-1, Mus musculus, accession NP_035790; Rn-TRX, Rattus norvegicus, accession NP_446252; Ec-TRX, Equus caballus, accession BAA37154; Ss-TRX, Sus scrofa, accession AAK60272; Sc-TRX, Saccharomyces cerevisiae, accession TXBY1; Oa-TRX, Ovis aries, accession CAA81083; Gg-TRX, Gallus gallus, accession AAA49092; Oh-TRX, Ophiophagus hannah (king cobra), accession AAK09384; Eg-TRX, Echinococcus granulosus, accession AAC14584; Pf-TRX, Plasmodium falciparum, accession CAB90828; Lm-TRX, Leishmania major, accession NP_047052; Tb-TRX-1, Trypanosoma brucei, accession CAB81781; Ecoli-TRX, Escherichia coli, accession P00274. The numbering begins with the initiating methionine for each sequence. Residues highlighted in black are an exact match to the Bm-TRX-1 sequence. Residues highlighted in red are invariant for all of the sequences tested.
An examination of a database of genes from Onchocerca volvulus, a closely related filarial nematode, identified two genes encoding members of the thioredoxin family of proteins, which were designated Ov-trx-1 (614 bp; accession number AY142708; NEMBASE cluster OVC03096; TIGR Gene Index TC1915) and Ov-trx-2 (539 bp; accession number AY142706; NEMBASE cluster OVC00287; TIGR Gene Index TC2322). Both O. volvulus genes were trans-spliced with SL-1 and encoded 16.3-kDa thioredoxins. The nucleotide sequences of Ov-trx-1 and Ov-trx-2 were 78% identical to each other, and they were both 80% identical to Bm-trx-1. At the protein level, Ov-TRX-1 and Ov-TRX-2 were 76% identical to each other and 86% and 79% identical to Bm-TRX-1, respectively.
A search of the major protein databases revealed a number of putative thioredoxin sequences from a spectrum of nematode species. A vast majority of the nematode-derived proteins were in the 16-kDa class of thioredoxins. An alignment of the 16-kDa nematode thioredoxins (Fig. 2) revealed that they were between 97% and 35% identical. None of the nematode thioredoxins contained an obvious signal sequence. The identity between the nematode-derived thioredoxins included 21 invariant residues and 32 highly conserved residues (positions where the residue was identical in at lease 13 of the 17 aligned sequences) (Fig. 2).
A majority (12 of 17) of the 16-kDa nematode-derived thioredoxins contained a putative WCPPCR active-site sequence. Alternative active-site sequences for the 16-kDa thioredoxins included WCPQCR, WCPSCR, and WCGSCR. Another notable difference between the primary sequences of the 12-kDa and 16-kDa classes of thioredoxins was that the 12-kDa thioredoxins contained three conserved cysteine residues at positions 62, 69, and 73 (residue positions in human TRX-1, accession no. AAF86466) that are absent from the 16-kDa thioredoxins. These cysteine residues are thought to impart important biological properties to the 12-kDa thioredoxins (12).
A careful examination of the ESTs within the Bm-trx-1 cluster revealed that the sequences could be organized into two groups based on the sequence of codon 41 of the Bm-trx-1 open reading frame. In one group (23 of 40), codon 41 was CCG and encoded the second Pro in the active site of Bm-TRX-1. In the second group (17 of 40), codon 41 was CAG, resulting in an active site sequence WCPQCR. This difference in codon 41 was the only difference in the nucleotide sequence within the cluster. Sequencing a number of clones from each group to completion in both directions confirmed that the single nucleotide polymorphism was not a sequencing artifact. It was concluded that this was an allelic form of trx in B. malayi. This is the first report of a PQ in a thioredoxin active site from any species. Interestingly, thioredoxins from two closely related organisms from the genus Onchocerca, Ov-TRX-2 and Oc-TRX-2 (accession number AI363557), also contain WCPQCR active sites (Fig. 2).
Native Bm-TRX-1.
When used to immunostain Western blots of somatic extracts from adult parasites and microfilariae, the anti-Bm-TRX antiserum specifically recognized a protein with a molecular mass of ≈16 kDa (Fig. 3). Interestingly, Bm-TRX was also detected as a 16-kDa molecule in the excretory-secretory products released from cultured microfilariae and adults.
FIG. 3.
Bm-TRX-1 is a secreted as a 16-kDa protein by adult and larval stages. First-stage larvae (microfilariae, M) and adult (A) parasites were placed in culture for 18 h. The culture medium was concentrated, and the secreted proteins were separated on a 12% polyacrylamide gel under reducing conditions. Following Western blotting, the nitrocellulose membrane was immunostained with anti-Bm-TRX-1 antibodies. A/ES and M/ES designate lanes containing the excretory-secretory products from adult parasites and microfilariae, respectively. The mass of the recombinant Bm-TRX-1 (lane r) was 17 kDa due to the addition of a 1-kDa T7 tag peptide derived from the expression vector.
B. malayi thioredoxin catalyzes the reduction of insulin.
The interchain disulfides of insulin are substrates of thioredoxin. Reduction of the disulfide linkage releases the A and B chains of insulin, the latter of which precipitates. The level of turbidity caused by precipitation of the B chain is directly proportional to the number of bonds reduced. A dithiothreitol-mediated insulin reduction assay was carried out at pH 7.0 in the presence and absence of Brugia or Spirulina thioredoxin (Fig. 4). In reactions containing only DTT, DTT plus bovine serum albumin, or DTT plus recombinant B. malayi SXP, no measurable precipitation was observed through 60 min. With the addition of rBm-TRX-1, precipitate was detected within 2 min. The kinetics of disulfide reduction observed for the Brugia-derived thioredoxin was nearly identical to that of the positive-control Spirulina thioredoxin. Insulin reduction was significantly inhibited by addition of the anti-Bm-TRX antibody (Fig. 4), whereas it was not affected by incubation with preimmune serum (data not shown). The maximal rate of precipitation, 1.5 ΔA650/min/mg of Bm-TRX, was similar to that reported for E. coli and human thioredoxins (40) but about half the rate reported for Trypanosoma brucei brucei and E. coli thioredoxins run under slightly different conditions (33).
FIG. 4.
Two allelic forms of B. malayi thioredoxin catalyze reduction of insulin. The increase in turbidity measured at 650 nm was plotted against reaction time. The conditions under which the catalytic activity of the WCPPCR- and WCPQCR-encoding alleles of Bm-trx-1 were measured in the insulin reduction assay are outlined in the text. Commercially available thioredoxin from Spirulina (Spi-TRX) was used as a positive control. B. malayi-derived SXP (Bm-SXP), bovine serum albumin (BSA), and the proteins extracted from induced, pET11b-containing E. coli (pET11b) were used as negative controls. In addition, the WCPPCR form of Bm-TRX-1 was incubated with anti-Bm-TRX-1 antibodies for 1 h before being added to the reaction mixture (Bm-TRX-1 plus Ab).
Also of note were the results of the insulin reduction assays with the allelic form of Bm-TRX-1 containing the novel active site WCPQCR. Although the maximum rate of precipitate formation was significantly lower than that observed for the WCPPCR-containing rBm-TRX-1 or Spirulina thioredoxin at the same concentration (1.0 ΔA650/min/mg), the WCPQCR-containing Bm-TRX was also capable of catalyzing the DTT-mediated reduction of insulin (Fig. 4).
B. malayi thioredoxin functions as an antioxidant.
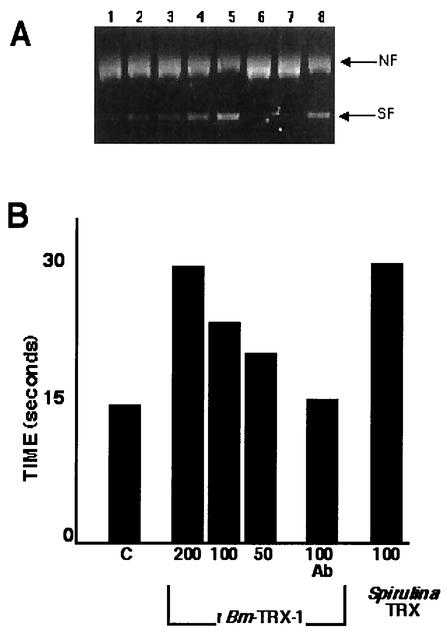
Thioredoxin is detectable in human plasma up to concentrations of 6 nM, and it has been suggested that it plays a direct role as an antioxidant (26). A DNA nicking assay was performed to determine whether rBm-TRX-1 was capable of functioning as an antioxidant protein. The assay employed the mixed function oxidation (MFO) system that generates hydroxyl (OH−) and thiol (RS*) radicals capable of damaging a DNA template (13). The extent of DNA damage was evaluated by assessing the shift in gel mobility of a plasmid as it was converted from the supercoiled to the nicked form. When the plasmid DNA was exposed to the products of the MFO system, essentially all of the plasmid DNA was converted to the nicked form in 60 min (Fig. 5, lane 1). Bm-TRX-1 inhibited the nicking reaction in a dose-dependent fashion between 1.2 μg and 10 μg (Fig. 5, lanes 1 to 5). When anti-Bm-TRX-1 was added to the reaction, the protection was abolished (Fig. 5, lane 7). The plasmid DNA was nicked to completion when rBm-SXP was added to the reaction (Fig. 5, lane 5), demonstrating that this protection was specific to Bm-TRX-1 and not simply due to the presence of protein in the reaction mix.
FIG. 5.
(A) Bm-TRX-1 functions as an antioxidant to protect DNA from nicking. Plasmid DNA was used as a substrate for detecting DNA damage mediated by the mixed-function oxidase (MFO) system in the presence of 0, 12, 25, 50, or 100 μg of the WCPPCR form of Bm-TRX-1 (lanes 1 through 5, respectively). The extent of MFO-mediated nicking was evaluated on ethidium bromide-stained agarose gels. Recombinant B. malayi SXP (lane 6) and Spirulina thioredoxin (lane 8) served as negative and positive controls, respectively. Anti-Bm-TRX-1 was used to block the antioxidant activity of Bm-TRX-1 (lane 7). NF, nicked form; SF, supercoiled form. (B) Bm-TRX alters clotting time. Thromboplastin was suspended in distilled water and combined with rBm-TRX-1 alone (at 50, 100, or 200 μg/ml), rBm-TRX-1 (100 μg/ml) plus anti-rBm-TRX-1, or Bm-SXP (200 μg/ml) at 37°C. After 30 s, oxalated plasma was added from a pipette, and the tube was gently inverted at 37°C. The time required to form a gel was monitored and taken as the endpoint of the assay. The data are representative of triplicate experiments.
B. malayi thioredoxin influences the rate of clotting.
The most abundant form of the parasite in the human host, the first-stage larvae or microfilariae, resides in the peripheral circulation in steady-state numbers that can exceed 2,000 parasites/ml of whole blood. Despite their numbers and relatively large size (≈250 μm), the larvae do not induce thrombosis. The finding that Bm-TRX-1 was secreted by larvae (Fig. 3) raises the possibility that Bm-TRX-1 may function to inhibit the intrinsic coagulation pathway during infection. To test this possibility, recombinant versions of the two allelic forms of Bm-TRX-1 were tested for their ability to delay coagulation. A dose-dependent delay in coagulation was observed in the presence of both the WCPPCR (Fig. 5) and WCPQCR (data not shown) forms of rBm-TRX-1. The addition of anti-Bm-TRX-1 antibody inhibited the activity of the Bm-TRX-1 in the assay.
B. malayi thioredoxin blocks activation of p38 MAP kinase.
One of the mechanisms used by the parasite to establish long-term chronic infections is to modulate the activity of the host's immune response. Molecules released by the parasite are thought to be key to this modification of immune reactivity. One of the possible mechanisms through which parasites can affect modifications of immunity is by altering the normal signaling of host immune cells. A number of key signaling activities of antigen-presenting cells are mediated by phosphorylation. Activation of p38 mitogen-activated protein kinase is mediated by dual phosphorylation of the threonine and tyrosine residues of p38 MAP kinase. The levels of threonine and tyrosine phosphorylation reflect the activation state of p38 MAP kinase (31). Therefore, we examined the threonine and tyrosine phosphorylation of p38 MAP kinase in TNF-α-stimulated ML-1 cells transfected with either form of Bm-TRX-1. The TNF-α-mediated activation of p38 MAP kinase through phosphorylation was significantly inhibited in the presence of the WCPPC and WCPQC forms of rBm-TRX-1 (Fig. 6). These results suggest that both allelic forms of Bm-TRX have the potential to mediate altered cell signaling, resulting in immunomodulation.
FIG. 6.
Bm-TRX-1 inhibits activation of p38 MAP kinase. The WCPPCR and WCPQCR alleles of Bm-trx-1 were cloned separately into a eukaryotic expression plasmid vector, and the plasmids were introduced into TPA-differentiated ML-1 cells. The transfected ML-1 cells were stimulated with TNF-α (100 IU/well) for 5 min, and the phosphorylation status of p38 MAP kinase was analyzed on immunostained Western blots of cell extracts. Controls included cells maintained in medium (medium control), cells exposed to the transfection regent (transfection control), and cells transfected with a plasmid containing no insert (plasmid control).
DISCUSSION
A majority of the thioredoxins that have been reported to date from eukaryotic and prokaryotic organisms are initially translated as a 12-kDa protein. Here we report on a class of 16-kDa thioredoxins from nematodes that, despite notable differences in mass and the sequence of the catalytic site, appear to be functionally similar to the 12-kDa thioredoxins.
The members of the thioredoxin family of proteins contain a conserved catalytic site that undergoes reversible oxidation to the cysteine disulfide through the transfer of reducing equivalents from the cysteines in the catalytic site to, typically, a disulfide substrate. The oxidized form of thioredoxin is reduced by an NADPH-dependent, thioredoxin reductase-mediated reaction. For nearly all of the mammalian, avian, insect, yeast, and bacterial 12-kDa thioredoxins, the sequence of the catalytic site is WCGPCK. In contrast, the putative active-site sequences from the nematode-derived 16-kDa thioredoxins is variable, with the dominant sequence being WCPPCR (Fig. 2). That the WCPPCR allele of Bm-TRX-1 was shown to catalyze reducing and antioxidant activities similar to that reported for the eukaryotic and prokaryotic thioredoxins (Fig. 4 and 5) indicates that this active site is functional.
Although the sequence of the catalytic site from the 12-kDa class of thioredoxins is highly conserved, members of the thioredoxin protein family that are larger than 12 kDa have variable residues associated with the active-site motif. For example, a 27-kDa nucleus-associated thioredoxin from the mouse (20) and a 16-kDa thioredoxin from the protozoan parasite T. brucei (22) have functional catalytic sites with a sequence identical to the WCPPCR-encoding allele of Bm-TRX-1. A novel catalytic site sequence, WCPQCR, was found for a subset of filarial nematode-derived thioredoxin proteins, and the allelic form of Bm-TRX-1 containing this sequence was shown to be catalytically active (Fig. 4 and 6). Thus, it appears that there is a considerable degree of flexibility in the two residues positioned between the invariant cysteines of the active site, so that the consensus motif is WCXXCK/R.
A search of the Caenorhabditis elegans genome identified over 30 genes that contain the thioredoxin active site consensus motif (data not shown). Thirteen of these genes, ranging from 12 to 18 kDa, are putative thioredoxins, including five distinct sequences that are predicted to be members of the 16-kDa subclass of thioredoxin proteins (Ce-TRX-5, Ce-TRX-6, and Ce-TRX-8 are presented in Fig. 2). The active-site sequences of the five 16-kDa C. elegans thioredoxins include WCPPCR, WCGPCR, and WCGSCR. The functional roles of these multiple thioredoxins in C. elegans are not known. With the exception of the filarial parasite O. volvulus, in which two distinct thioredoxin sequences have been reported (Fig. 2), only one thioredoxin has been reported in the databases for each parasitic nematode species. It is possible that when further genomic and expression data are available for parasitic nematode species, genes that encode additional thioredoxin proteins will be identified.
The remaining thioredoxin motif-containing proteins in C. elegans are predicted to be between 24 kDa and 80 kDa, with limited sequence and structural identity to members of the thioredoxin family. These include 10 members of the protein disulfide isomerase protein family and the dpy-11 gene, which is required for body and sensory organ morphogenesis in C. elegans (19).
In addition to the two conserved cysteines in the active site, mammalian thioredoxins have three conserved cysteine residues at positions 62, 69, and 73 (numbering based on human thioredoxin). The C-terminal Cys73 is important in dimer formation, which in turn dictates several unique biological properties of mammalian thioredoxins (17). As reported for the bacterial thioredoxins, all three of these conserved cysteines are absent from the 16-kDa thioredoxin proteins.
Role as secreted protein.
Despite the lack of a signal sequence, Bm-TRX is secreted by the adult and microfilarial stages of B. malayi. Thioredoxin is also secreted by a variety of mammalian cell types, including fibroblasts, airway epithelial cells, and activated T cells and B cells (reviewed in reference 30) after exposure to stresses such as hypoxia (3), lipopolysaccharide (LPS) (9), and viral infection (4, 35). Similar to the 16-kDa thioredoxins from nematodes, the mammalian 12-kDa thioredoxins have no signal sequence. The mechanism of secretion of mammalian thioredoxins is unknown, but it does appear to share features with the secretion pathway of interleukin-1β (34).
Secreted human TRX-1 acts as a growth factor for lymphocytes (39), fibroblasts (27), and tumor cells (25). This action is not receptor mediated and appears to be due to the ability of thioredoxin to sensitize cells to the presence of growth factors (11, 29). In addition, secreted thioredoxin increases expression of the cytokines interleukins-1, -2, -6, and -8 and TNF-α (36, 42) and acts as a chemotactic factor for monocytes, neutrophils, and T lymphocytes (4). Where studied, the growth factor and proinflammatory activity of thioredoxin is dependent on the conserved Cys32 and Cys35 residues of the catalytic site. The activity, if any, of Bm-TRX in the areas of cell growth and inflammation is not known.
It is interesting that while secreted thioredoxin is proinflammatory, high-level intracellular production of TRX-1 downregulates the expression of cytokines through a mechanism that results in inhibition of the activity of p38 MAP kinase (15). When expressed intracellularly in mammalian cells, Bm-TRX-1 also inhibits the activation of p38 MAP kinase (Fig. 6). It is interesting to speculate that, in the mammalian host, the secreted form of filarial nematode-derived thioredoxin may accumulate locally to sufficient levels to influence host immune cell activity. This is particularly relevant for the adult stage, which takes up residence in the lumen of lymphatic vessels just downstream from major lymph node clusters, the sites where adaptive immune responses are initiated and regulated.
Reducing equivalent for antioxidant proteins.
Filarial parasites constitutively secrete a number of antioxidant proteins that include thioredoxin peroxidases, selenium-independent glutathione peroxidases, and superoxide dismutase (reviewed in reference 16). In both prokaryotic and eukaryotic systems, thioredoxin has been shown to be an efficient electron donor for glutathione peroxide and members of the peroxiredoxin superfamily of proteins (reviewed in reference 30). It is possible that the secreted form of Bm-TRX-1 is an important source of reducing equivalents required by these antioxidant enzymes to maintain their activity and protect the parasite from radicals produced by cells of the immune response. Active-site mutants are being developed to aid in defining the physiological role of Bm-TRX-1 in filarial parasites.
Acknowledgments
We thank John Parkinson (University of Edinburgh) for help in the cluster analysis, S. K. Pandey and M. Bernier (Diabetes Section, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health) for assistance in the analysis of p38 MAP kinase, and Geetha Muthukumar (CBT, Anna University) and Aimee Marson (Johns Hopkins University). The ML-1 cell line was kindly provided by M. Trush (Johns Hopkins University).
This work was supported by a grant from the World Health Organization/UNDP/World Bank Special Programme for Research and Training in Tropical Diseases.
Editor: J. M. Mansfield
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Arner, E. S., and A. Holmgren. 2000. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267:6102-6109. [DOI] [PubMed] [Google Scholar]
- 3.Berggren, M., A. Gallegos, J. R. Gasdaska, P. Y. Gasdaska, J. Warneke, and G. Powis. 1996. Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 16:3459-3466. [PubMed] [Google Scholar]
- 4.Bertini, R., O. M. Howard, H. F. Dong, J. J. Oppenheim, C. Bizzarri, R. Sergi, G. Caselli, S. Pagliei, B. Romines, J. A. Wilshire, M. Mengozzi, H. Nakamura, J. Yodoi, K. Pekkari, R. Gurunath, A. Holmgren, L. A. Herzenberg, and P. Ghezzi. 1999. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 189:1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaxter, M., and M. Aslett. 1997. Internet resources for the parasite genome projects. Trends Genet. 13:40-41. [DOI] [PubMed] [Google Scholar]
- 6.Blaxter, M., M. Aslett, D. Guiliano, and J. Daub. 1999. Parasitic helminth genomics. Filarial Genome Project. Parasitology 118:S39-S51. [DOI] [PubMed] [Google Scholar]
- 7.Blaxter, M. L., D. B. Guilano, A. L. Scott, and S. A. Williams. 1997. A unified nomenclature for filarial genes. Parasitol. Today 13:416-417. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal, T., and K. Steward. 1997. RNA processing and gene structure, p. 117-145. In D. Riddle, T. Bluementhal, B. Meyer, and J. Priess (ed.), Caenorhabditis elegans II. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 9.Ejima, K., T. Koji, H. Nanri, M. Kashimura, and M. Ikeda. 1999. Expression of thioredoxin and thioredoxin reductase in placentae of pregnant mice exposed to lipopolysaccharide. Placenta 20:561-566. [DOI] [PubMed] [Google Scholar]
- 10.Foster, J. M., I. H. Kamal, J. Daub, M. C. Swan, J. R. Ingram, M. Ganatra, J. Ware, D. Guiliano, A. Aboobaker, L. Moran, M. Blaxter, and B. E. Slatko. 2001. Hybridization to high-density filter arrays of a Brugia malayi BAC library with biotinylated oligonucleotides and PCR products. BioTechniques 30:1216-1218, 1220, 1222 passim. [DOI] [PubMed]
- 11.Gasdaska, J. R., M. Berggren, and G. Powis. 1995. Cell growth stimulation by the redox protein thioredoxin occurs by a novel helper mechanism. Cell Growth Differ. 6:1643-1650. [PubMed] [Google Scholar]
- 12.Gasdaska, J. R., D. L. Kirkpatrick, W. Montfort, M. Kuperus, S. R. Hill, M. Berggren, and G. Powis. 1996. Oxidative inactivation of thioredoxin as a cellular growth factor and protection by a Cys73→Ser mutation. Biochem. Pharmacol. 52:1741-1747. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, I., S. W. Eisinger, N. Raghavan, and A. L. Scott. 1998. Thioredoxin peroxidases from Brugia malayi. Mol. Biochem. Parasitol 91:207-220. [DOI] [PubMed] [Google Scholar]
- 14.Harnett, W., M. R. Deehan, K. M. Houston, and M. M. Harnett. 1999. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 21:601-608. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto, S., K. Matsumoto, Y. Gon, S. Furuichi, S. Maruoka, I. Takeshita, K. Hirota, J. Yodoi, and T. Horie. 1999. Thioredoxin negatively regulates p38 MAP kinase activation and IL-6 production by tumor necrosis factor-alpha. Biochem. Biophys. Res. Commun. 258:443-447. [DOI] [PubMed] [Google Scholar]
- 16.Henkle-Duhrsen, K., and A. Kampkotter. 2001. Antioxidant enzyme families in parasitic nematodes. Mol. Biochem. Parasitol. 114:129-142. [DOI] [PubMed] [Google Scholar]
- 17.Holmgren, A. 1985. Thioredoxin. Annu. Rev. Biochem. 54:237-271. [DOI] [PubMed] [Google Scholar]
- 18.Holmgren, A. 1979. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 254:9627-9632. [PubMed] [Google Scholar]
- 19.Ko, F. C., and K. L. Chow. 2002. A novel thioredoxin-like protein encoded by the C. elegans dpy-11 gene is required for body and sensory organ morphogenesis. Development 129:1185-1194. [DOI] [PubMed] [Google Scholar]
- 20.Kurooka, H., K. Kato, S. Minoguchi, Y. Takahashi, J. Ikeda, S. Habu, N. Osawa, A. M. Buchberg, K. Moriwaki, H. Shisa, and T. Honjo. 1997. Cloning and characterization of the nucleoredoxin gene that encodes a novel nuclear protein related to thioredoxin. Genomics 39:331-339. [DOI] [PubMed] [Google Scholar]
- 21.Li, Y., A. Lafuente, and M. A. Trush. 1994. Characterization of quinone reductase, glutathione and glutathione S-transferase in human myeloid cell lines: induction by 1,2-dithiole-3-thione and effects on hydroquinone-induced cytotoxicity. Life Sci. 54:901-916. [DOI] [PubMed] [Google Scholar]
- 22.Ludemann, H., M. Dormeyer, C. Sticherling, D. Stallmann, H. Follmann, and R. L. Krauth-Siegel. 1998. Trypanosoma brucei tryparedoxin, a thioredoxin-like protein in African trypanosomes. FEBS Lett. 431:381-385. [DOI] [PubMed] [Google Scholar]
- 23.Michael, E. 2000. The population dynamics and epidemiology of lymphatic filariasis, p. 41-81. In T. B. Nutman (ed.), Lymphatic filariasis. Imperial College Press, London, England.
- 24.Molyneux, D. H., and M. J. Taylor. 2001. Current status and future prospects of the Global Lymphatic Filariasis Programme. Curr. Opin. Infect. Dis. 14:155-159. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura, H., H. Masutani, Y. Tagaya, A. Yamauchi, T. Inamoto, Y. Nanbu, S. Fujii, K. Ozawa, and J. Yodoi. 1992. Expression and growth-promoting effect of adult T-cell leukemia-derived factor, a human thioredoxin homologue in hepatocellular carcinoma. Cancer 69:2091-2097. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, H., K. Nakamura, and J. Yodoi. 1997. Redox regulation of cellular activation. Annu. Rev. Immunol. 15:351-369. [DOI] [PubMed] [Google Scholar]
- 27.Oblong, J. E., M. Berggren, P. Y. Gasdaska, and G. Powis. 1994. Site-directed mutagenesis of active site cysteines in human thioredoxin produces competitive inhibitors of human thioredoxin reductase and elimination of mitogenic properties of thioredoxin. J. Biol. Chem. 269:11714-11720. [PubMed] [Google Scholar]
- 28.Pastrana, D. V., N. Raghavan, P. FitzGerald, S. W. Eisinger, C. Metz, R. Bucala, R. P. Schleimer, C. Bickel, and A. L. Scott. 1998. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect. Immun. 66:5955-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powis, G., J. R. Gasdaska, P. Y. Gasdaska, M. Berggren, D. L. Kirkpatrick, L. Engman, I. A. Cotgreave, M. Angulo, and A. Baker. 1997. Selenium and the thioredoxin redox system: effects on cell growth and death. Oncol. Res. 9:303-312. [PubMed] [Google Scholar]
- 30.Powis, G., and W. R. Montfort. 2001. Properties and biological activities of thioredoxins. Annu. Rev. Biophys. Biomol. Struct. 30:421-455. [DOI] [PubMed] [Google Scholar]
- 31.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 32.Rao, K. V., M. Eswaran, V. Ravi, B. Gnanasekhar, R. B. Narayanan, P. Kaliraj, K. Jayaraman, A. Marson, N. Raghavan, and A. L. Scott. 2000. The Wuchereria bancrofti orthologue of Brugia malayi SXP1 and the diagnosis of bancroftian filariasis. Mol. Biochem. Parasitol. 107:71-80. [DOI] [PubMed] [Google Scholar]
- 33.Reckenfelderbaumer, N., H. Ludemann, H. Schmidt, D. Steverding, and R. L. Krauth-Siegel. 2000. Identification and functional characterization of thioredoxin from Trypanosoma brucei brucei. J. Biol. Chem. 275:7547-7552. [DOI] [PubMed] [Google Scholar]
- 34.Rubartelli, A., A. Bajetto, G. Allavena, E. Wollman, and R. Sitia. 1992. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 267:24161-24164. [PubMed] [Google Scholar]
- 35.Sasada, T., H. Nakamura, H. Masutani, S. Ueda, H. Sono, A. Takabayashi, and J. Yodoi. 2002. Thioredoxin-mediated redox control of human T cell lymphotropic virus type I (HTLV-I) gene expression. Mol. Immunol. 38:723-732. [DOI] [PubMed] [Google Scholar]
- 36.Schenk, H., M. Vogt, W. Droge, and K. Schulze-Osthoff. 1996. Thioredoxin as a potent costimulus of cytokine expression. J. Immunol. 156:765-771. [PubMed] [Google Scholar]
- 37.Silberstein, D. S., S. McDonough, M. S. Minkoff, and M. K. Balcewicz-Sablinska. 1993. Human eosinophil cytotoxicity-enhancing factor. Eosinophil-stimulating and dithiol reductase activities of biosynthetic (recombinant) species with COOH-terminal deletions. J. Biol. Chem. 268:9138-9142. [PubMed] [Google Scholar]
- 38.Tanaka, T., H. Nakamura, A. Nishiyama, F. Hosoi, H. Masutani, H. Wada, and J. Yodoi. 2001. Redox regulation by thioredoxin superfamily: protection against oxidative stress and aging. Free Radic. Res. 33:851-855. [DOI] [PubMed] [Google Scholar]
- 39.Wakasugi, N., Y. Tagaya, H. Wakasugi, A. Mitsui, M. Maeda, J. Yodoi, and T. Tursz. 1990. Adult T-cell leukemia-derived factor/thioredoxin, produced by both human T-lymphotropic virus type I- and Epstein-Barr virus-transformed lymphocytes, acts as an autocrine growth factor and synergizes with interleukin 1 and interleukin 2. Proc. Natl. Acad. Sci. USA 87:8282-8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollman, E. E., L. d'Auriol, L. Rimsky, A. Shaw, J. P. Jacquot, P. Wingfield, P. Graber, F. Dessarps, P. Robin, F. Galibert, et al. 1988. Cloning and expression of a cDNA for human thioredoxin. J. Biol. Chem. 263:15506-15512. [PubMed] [Google Scholar]
- 41.Yamaoka, K. A., J. P. Kolb, N. Miyasaka, G. Inuo, and K. Fujita. 1994. Purified excretory-secretory component of filarial parasite enhances Fc epsilon RII/CD23 expression on human splenic B and T cells and IgE synthesis while potentiating T-helper type 2-related cytokine generation from T cells. Immunology 81:507-512. [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida, S., T. Katoh, T. Tetsuka, K. Uno, N. Matsui, and T. Okamoto. 1999. Involvement of thioredoxin in rheumatoid arthritis: its costimulatory roles in the TNF-alpha-induced production of IL-6 and IL-8 from cultured synovial fibroblasts. J. Immunol. 163:351-358. [PubMed] [Google Scholar]