Abstract
To assess the role of glutamine synthetase (GS), an enzyme of central importance in nitrogen metabolism, in the pathogenicity of Mycobacterium tuberculosis, we constructed a glnA1 mutant via allelic exchange. The mutant had no detectable GS protein or GS activity and was auxotrophic for l-glutamine. In addition, the mutant was attenuated for intracellular growth in human THP-1 macrophages and avirulent in the highly susceptible guinea pig model of pulmonary tuberculosis. Based on growth rates of the mutant in the presence of various concentrations of l-glutamine, the effective concentration of l-glutamine in the M. tuberculosis phagosome of THP-1 cells was ∼10% of the level assayed in the cytoplasm of these cells (4.5 mM), indicating that the M. tuberculosis phagosome is impermeable to even very small molecules in the macrophage cytoplasm. When complemented by the M. tuberculosis glnA1 gene, the mutant exhibited a wild-type phenotype in broth culture and in human macrophages, and it was virulent in guinea pigs. When complemented by the Salmonella enterica serovar Typhimurium glnA gene, the mutant had only 1% of the GS activity of the M. tuberculosis wild-type strain because of poor expression of the S. enterica serovar Typhimurium GS in the heterologous M. tuberculosis host. Nevertheless, the strain complemented with S. enterica serovar Typhimurium GS grew as well as the wild-type strain in broth culture and in human macrophages. This strain was virulent in guinea pigs, although somewhat less so than the wild-type. These studies demonstrate that glnA1 is essential for M. tuberculosis virulence.
Glutamine and glutamate are central molecules in nitrogen metabolism. Glutamine is used as the nitrogen donor for many nitrogen-containing molecules in the cell and is synthesized from l-glutamate, ammonia, and ATP by the enzyme glutamine synthetase (GS) (33). The internal l-glutamine pool has been shown to be a sensor of external nitrogen limitation for Salmonella enterica serovar Typhimurium (21). GS is the only known biosynthetic pathway for the synthesis of glutamine and along with glutamate synthetase is responsible for ammonia assimilation under nitrogen-limiting growth conditions. In enteric bacteria, glutamate dehydrogenase can assimilate ammonia directly into glutamate at high concentrations of ammonia. However, for bacteria such as Mycobacterium tuberculosis which lack glutamate dehydrogenase, GS and glutamate synthetase are the sole means of ammonia assimilation. Due to its central role in nitrogen metabolism, GS is subject to varied and complex forms of transcriptional and posttranslational regulation as well as feedback inhibition by several products of glutamine metabolism (7, 33).
There are at least four major forms of GS (25). In enteric bacteria, a single glnA gene encodes a GS type I (GSI) enzyme, and glnA null mutants are glutamine auxotrophs. Other bacteria have been shown to possess two or three different types of GS. In the case of Sinorhizobium meliloti (formerly Rhizobium meliloti), all three GS genes must be inactivated to generate a strain that is auxotrophic for l-glutamine (35). M. tuberculosis has a glnA1 gene that encodes a GSI enzyme that is transcriptionally and posttranslationally regulated in a manner similar to that of the Escherichia coli GS as well as three other glnA genes (glnA2, glnA3, and glnA4) that are predicted to encode GSI type enzymes (3, 10). However, in our previous biochemical characterization of the M. tuberculosis GS, we found that GlnA1 seemed to account for the vast majority of GS activity (reference 10 and our unpublished observations).
Our interest in M. tuberculosis GS arose from our identification of GS as a major component of M. tuberculosis culture filtrates as well as the finding that M. tuberculosis is quite sensitive to the GS inhibitor l-methionine-SR-sulfoximine (MSO), particularly in comparison to the nonpathogenic Mycobacterium smegmatis (10, 13, 14, 39). In this study, we have constructed and characterized an M. tuberculosis glnA1 mutant. Like enteric bacteria with glnA null mutations and an M. smegmatis glnA1 mutant, the M. tuberculosis glnA1 mutant is also a glutamine auxotroph (33, 39). The mutant requires a relatively high level of exogenous l-glutamine for robust growth in vitro and possesses no detectable GS activity. The mutant is attenuated for intracellular growth in differentiated THP-1 cells and is avirulent in guinea pigs infected by the aerosol route, indicating that the M. tuberculosis phagosome is limited in l-glutamine and that glnA1 is essential for M. tuberculosis virulence.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. M. tuberculosis strains were grown on Middlebrook 7H10 or 7H11 agar (Difco) containing 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC) (Becton Dickinson) and 0.5% (vol/vol) glycerol or as unshaken cultures in 7H9 broth (Difco) supplemented with 10% (vol/vol) OADC, 0.05% (wt/vol) Tween 80, and 0.2% (vol/vol) glycerol (7H9-OADC-TW) at 37°C in an atmosphere of 5% CO2-95% air. Hygromycin (50 μg ml−1) and/or kanamycin (20 or 50 μg ml−1) were included as appropriate. l-Glutamine was sterilized by filtration and added aseptically to broth and agar after autoclaving when required.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | Gibco BRL | |
| E. coli XL10-Gold | Stratagene | |
| M. tuberculosisa | Wild-type Erdman strain | ATCC 35801 |
| M. tuberculosis pNBV1 | 39 | |
| M. tuberculosis glnA1 | Insertionally inactivated glnA1 locus; Kmr | This study |
| M. tuberculosis glnA1 pNBV1 | This study | |
| M. tuberculosis glnA1 pNBV1-MtbGS | This study | |
| M. tuberculosis glnA1 pNBV1-StGS | This study | |
| Plasmids | ||
| pUC19 | E. coli cloning vector | 42 |
| pPR27 | Mycobacterial allelic exchange vector; ori(ts) sacB Gmr | 31 |
| pEX1 | Mycobacterial allelic exchange vector derived from pPR27 and pGFPuv; ori(ts) sacB Hygr GFP | This study |
| pNBV1 | E. coli mycobacterial shuttle vector; Hygr | 20 |
| pNBV1-MtbGS | M. tuberculosis glnA1 expression vector | 39 |
| pNBV1-StGS | S. enterica serovar Typhimurium glnA with M. tuberculosis glnA1 promoter | 39 |
| pGFPuv | UV-optimized Aequorea victoria GFP gene | Clontech |
All M. tuberculosis strains are derived from the Erdman wild-type strain.
E. coli strains DH5α and XL10-Gold were used for cloning purposes and were grown on Luria-Bertani agar or Terrific Broth II (QBiogene) at 37°C. Ampicillin (100 μg ml−1), hygromycin (250 μg ml−1), and kanamycin (50 μg ml−1) were included as appropriate.
Recombinant DNA methods.
Plasmid DNA was isolated using Quantum Prep (Bio-Rad) miniprep kits. Genomic DNA was isolated from M. tuberculosis by phenol extraction and ethanol precipitation as previously described (39).
Southern hybridizations.
Restriction fragments of genomic DNA were electrophoresed in agarose gels, transferred to positively charged nylon membranes (Hybond-N+; Amersham Pharmacia Biotech) in 0.4 M NaOH, and hybridized to a biotinylated M. tuberculosis glnA1 probe. The probe was biotinylated by random priming, and hybridization and detection were performed using the North2South complete biotin random prime labeling and detection kit (Pierce) according to the manufacturer's instructions.
Construction of the M. tuberculosis glnA1 mutant.
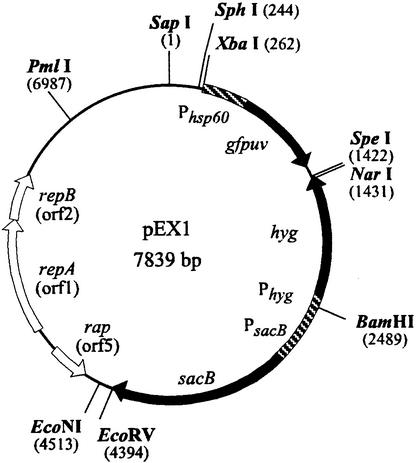
An allelic exchange vector based on the temperature-sensitive sacB vector pPR27 (31) was created that replaced the gentamicin resistance gene with a hygromycin resistance gene and incorporated GFPuv (UV-optimized green fluorescent protein) as a screenable marker as follows. (i) pGFPuv, a pUC19-derived plasmid, was digested with BspHI and SpeI, and a 1.9-kb fragment containing the pUC ori and gfpuv was ligated to a hygromycin resistance gene (amplified from pNBV1). (ii) The sacB gene (amplified from pPR27) was inserted into the PstI and EcoRV sites upstream of the hygromycin resistance gene. (iii) A 2.6-kb EcoRV-HpaI fragment from pPR27 containing a temperature-sensitive mycobacterial origin of replication was inserted into the unique EcoRV site downstream of sacB. (iv) Finally, the gfpuv gene was replaced with an XbaI-SpeI fragment from pNBV1-GFPuv containing the gfpuv gene downstream of the Mycobacterium bovis BCG hsp60 promoter to drive its expression in mycobacteria. The intermediate and final constructs were confirmed by restriction analysis, and a map of the final vector, designated pEX1, is shown in Fig. 1.
FIG. 1.
Allelic exchange vector, pEX1, used in the construction of the glnA1 mutant. Unique restriction sites are shown.
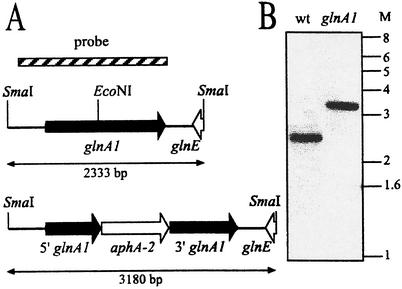
The 1.8-kb M. tuberculosis glnA1 genomic locus previously cloned in pSMT3 was transferred as a BamHI→HindIII fragment (5′ to 3′) into the multiple cloning site of pUC19 (13). The resulting plasmid was digested with EcoNI (linearizing the plasmid at a unique site in the coding region of glnA1), and the 5′ overhangs were filled with T4 DNA polymerase. A nonpolar, promoterless Kmr cassette (39) was ligated into the vector, and a clone was identified by restriction analysis that had the Kmr cassette in the same orientation as glnA1, allowing for expression of the kanamycin resistance gene from the glnA1 promoter (Fig. 2A). Immediately downstream of the aphA-2 stop codon, the Kmr cassette provides a ribosomal binding site and ATG start codon which is in frame with the 3′ portion of glnA1 to allow for translation of the 3′ portion of the disrupted glnA1. The disrupted gene was released from pUC19 by digestion with BamHI and HindIII. The HindIII site was modified by ligation to a HindIII-BamHI adapter, the fragment was redigested with BamHI and cloned into pEX1 linearized with BglII, and the product was designated pEX1-Mtb-glnA1::Kmr. (There are two BglII sites in pEX1, and the fragment was determined to be in the site located between the gfpuv and hyg genes. The fragment was not cloned into the BamHI site because at the time it was believed that it was not unique.)
FIG. 2.
Construction of the M. tuberculosis glnA1 mutant. (A) Maps of the wild-type glnA1 locus and the disrupted allele, which contains a Kmr cassette (aphA-2) inserted into the unique EcoNI site in the middle of the glnA1 coding region. Only a small portion of the 3′ end of glnE is present on the SmaI fragment. (B) Genomic DNA from the M. tuberculosis wild-type strain and the glnA1 mutant was digested to completion with SmaI and probed with a 1.8-kb fragment containing glnA1 (hatched bar in panel A). M, molecular mass markers in kilobases; wt, wild-type.
The allelic exchange construct (pEX1-Mtb-glnA1::Kmr) was electroporated into M. tuberculosis, and transformants were selected on 7H11 containing hygromycin and kanamycin at 32°C. Attempts to obtain a glnA1 mutant through a double crossover in a single selection were unsuccessful, so a two-step protocol was used (28). Initial transformants obtained at 32°C were green fluorescent when viewed under long-wavelength UV light due to expression of GFPuv. The transformants were replated on 7H11 with hygromycin and kanamycin at 39°C to select for those cells that integrated the plasmid. Pooled colonies were inoculated into 7H9 containing 20 mM l-glutamine and kanamycin, and after 3 weeks of growth at 39°C, the culture was plated on 7H11 containing 2% (wt/vol) sucrose, 20 mM l-glutamine, and kanamycin to select for clones that had undergone a second homologous recombination event. Colonies were examined under long-wavelength UV light to identify nonfluorescent clones that had lost the plasmid. Three of six nonfluorescent clones were confirmed to have the glnA1 mutation by immunoblotting and Southern analysis. A single clone was selected for all further characterization.
Complementation of the mutant was achieved by electroporation of plasmids pNBV1-MtbGS and pNBV1-StGS (Table 1), with the parent plasmid pNBV1 serving as a control. Preparation of electrocompetent cells and electroporation were performed as previously described (39).
Biochemical analysis of M. tuberculosis strains.
Triplicate 25-ml 7H9-OADC-TW (± 20 mM l-glutamine) cultures of each strain were inoculated to an initial A550 of ∼0.003 and grown for 10 days. Twenty-milliliter aliquots from each culture were centrifuged, and the cell pellets were washed by resuspending in 10 ml of phosphate-buffered saline (PBS)-0.05% Tween 80, followed by recentrifugation. The cell pellets were stored frozen at −80°C until analysis. After resuspension in 5 ml of PBS, the cells were lysed by sonicating once for 3 min on ice with a Heat Systems Ultrasonics W-375 sonicator (50% pulse, maximum setting with a microtip). Cellular debris was removed by centrifugation, and the cleared lysate was sterilized by filtration (pore size, 0.8 and 0.2 μm). The filtered lysates were assayed for total protein and GS activity and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
GS was assayed by the γ-glutamyl transferase reaction (41) as previously described (39). Reactions were linear with time and with enzyme concentration. Assays were performed in triplicate for each lysate. A unit is defined as the amount of enzyme that catalyzes the formation of 1 μmol of γ-glutamic acid hydroxamate per minute under the assay conditions.
Total protein in cell lysates was assayed with the bicinchoninic acid reagent (Pierce) using bovine serum albumin as a standard. Assays were performed in triplicate for each lysate.
Aliquots of cell lysates containing ∼18 μg of total protein were analyzed on 12.5% SDS-polyacrylamide gels. The gels were stained with colloidal Coomassie brilliant blue G-250 (26) or the proteins were transferred to a nitrocellulose membrane and probed with a mixture of rabbit polyclonal antibodies specific for the M. tuberculosis GlnA1 (diluted 1:10,000) (10) and the M. tuberculosis SodA (diluted 1:10,000) (12). The membranes were subsequently incubated with horse radish peroxidase-conjugated goat anti-rabbit antibodies (Bio-Rad; diluted 1:250,000), a chemiluminescent substrate (SuperSignal West Pico, Pierce) was added, and the proteins were visualized by exposure to X-ray film.
l-Glutamine requirement of M. tuberculosis glnA1 in broth culture.
Duplicate or triplicate 30-ml 7H9-OADC-TW cultures containing 0, 0.2, 0.5, 1, 1.5, 2, 5, or 20 mM l-glutamine were inoculated with bacteria from log-phase cultures (A550, 0.3 to 0.6), which were diluted to obtain an initial calculated A550 of 0.0001, and grown for 14 days. The maximum carryover of l-glutamine from the inoculum was 7 μM. The culture flasks were shaken once a day to resuspend settled bacteria before removal of aliquots for absorbance measurements and plating for CFU. The aliquots were serially diluted, and 20-μl drops of dilutions were spotted in triplicate on 7H11 plates containing 20 mM l-glutamine. Plates were incubated 10 to 16 days at 37°C, at which point the colonies were large enough to be counted readily but not so large as to coalesce with neighboring colonies. Longer incubations did not result in increased CFU.
Intracellular growth in human THP-1 macrophage monolayers.
THP-1 cells, a human monocytic cell line, were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 20 mM HEPES, and 2 mM l-glutamine at 37°C in an atmosphere of 5% CO2-95% air. Cells were seeded at 2 × 105 cells per well in 2-cm2 24-well tissue culture plates and differentiated with 100 nM phorbol 12-myristate 13-acetate (PMA) for 3 days. The bacterial inocula were prepared by dilution of log-phase cultures grown in 7H9-OADC-TW (plus 20 mM l-glutamine for the glnA1 mutant) into tissue culture medium (containing 2 mM l-glutamine) which included 10% human serum type AB (Irvine Scientific) in place of the heat-inactivated fetal bovine serum. The monolayers were infected with M. tuberculosis strains at a multiplicity of infection of 0.2 to 1 bacterium per THP-1 cell for 2 h at 37°C in triplicate wells, after which the medium was removed and the monolayers were washed twice with medium. One ml of medium containing 0.2, 0.5, 1, 2, or 10 mM l-glutamine was added to the monolayers, and the plates were incubated at 37°C for 0 to 6 days. The medium was replaced with fresh medium at 3 days for those wells to be harvested after day 3. CFU were enumerated at various times (Fig. 5 and 6) as follows. The culture medium (1 ml) was removed and added to 8 ml of dilution medium (7H9-OADC-TW with 5 mM l-glutamine). The monolayer was then lysed with 1 ml of 0.1% SDS in sterile distilled water, and the lysate was immediately added to the dilution tube. CFU were determined as described above for broth cultures. The low level of l-glutamine (0.2 mM) did not appear to have a detrimental effect on the differentiated THP-1 cells, as monolayers incubated with 0.2 mM l-glutamine were indistinguishable from monolayers incubated with 2 mM l-glutamine.
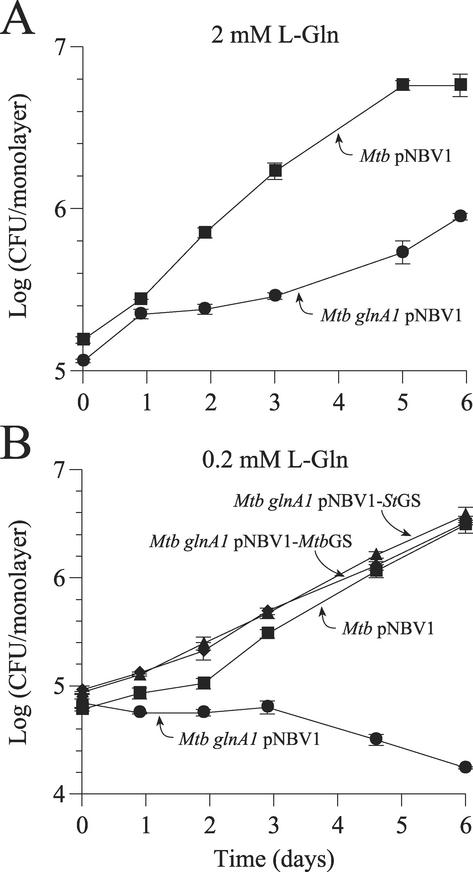
FIG. 5.
Intracellular growth of M. tuberculosis wild-type, glnA1, and complemented strains in human THP-1 macrophages. The tissue culture medium included the standard amount of l-glutamine (2 mM) (A) or 0.2 mM l-glutamine (B). When THP-1 cells were cultured in the presence of 2 mM l-glutamine, the glnA1 mutant multiplied intracellularly but at a reduced rate compared with the wild-type strain. When THP-1 cells were cultured in the presence of only 0.2 mM l-glutamine, the strain did not multiply and slowly died (∼0.5 log reduction in 6 days). The wild-type strain and both complemented strains grew normally in the presence of 0.2 mM l-glutamine. Data are the means ± standard errors for three wells per time point. In many instances, the error bars are smaller than the symbols. For all measurements, the standard error was <2% of the mean. The experiment was repeated once with similar results. Mtb, M. tuberculosis.
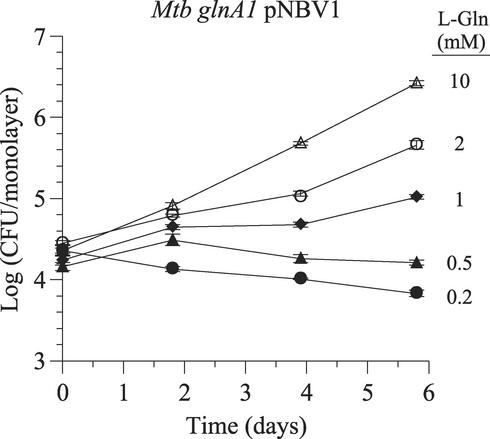
FIG. 6.
Glutamine requirement of the M. tuberculosis glnA1 mutant during intracellular growth in human THP-1 macrophages. The concentration of l-glutamine in the tissue culture medium was varied from 0.2 to 10 mM. At the highest concentration, the mutant grew at a rate similar to that of the wild-type strain. Data are the means ± standard errors for three wells per time point. In many instances, the error bars are smaller than the symbols. For all measurements, the standard error was <2% of the mean. The experiment was repeated once with similar results. Mtb, M. tuberculosis.
Determination of the intracellular amino acid pool in THP-1 macrophage monolayers.
THP-1 cells were seeded at ∼107 cells (in 10 ml of RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 20 mM HEPES, and 2 mM l-glutamine) per 75-cm2 tissue culture flask and differentiated with 100 nM PMA for 3 days. After 3 days of differentiation, the medium was replaced with 10 ml of fresh medium containing 0.2 or 2 mM l-glutamine, and the cells were incubated for 1 day (20 to 24 h). The medium was removed, and the monolayer was washed twice quickly (∼30 s each wash) with 10 ml of cold (4°C) PBS. For each l-glutamine concentration, three flasks were subjected to amino acid analysis and three were assayed for intracellular water content. For amino acid analysis, the cells were extracted with 5 ml of cold (4°C) 70% ethanol for ∼10 min with occasional shaking, and the extract was taken to dryness on a centrifugal vacuum concentrator. Amino acid analysis of the extracts was performed by the molecular structure facility at UC Davis using a Beckman 6300 (Li-citrate-based) amino acid analyzer. Amino acid analysis of tissue culture medium was performed similarly after removal of protein by precipitation with ethanol (70% final concentration). When monolayers were spiked with l-glutamine (50 to 200 nmol) before ethanol extraction of amino acids, recovery of l-glutamine was very high (84 to 105%, n = 4).
To determine the total cellular water content of the monolayer, 10 ml of PBS was added to the washed monolayer, and the cells were detached with a cell scraper. The cells were counted and centrifuged into a preweighed tube, and the wet weight of the pellet was determined. The cell pellet was dried at 60°C under vacuum, and the water weight was calculated by subtracting the dry weight from the wet weight of the cell pellet. Extracellular water in the packed cell pellet was assumed to be negligible (32). No significant difference in the water weight per cell was found between cells grown with 0.2 or 2 mM l-glutamine, so the results were combined. Differentiated THP-1 cells were found to have a water content of 2.8 ± 0.6 pl/cell (mean ± standard error of two experiments with six individual flasks per experiment). For comparative purposes, suspension-grown THP-1 cells (harvested at ∼106 cells/ml) were also analyzed for their water content, which was determined to be 1.00 ± 0.06 pl/cell (mean ± standard error of three separate experiments). This value is in good agreement with a volume measurement for THP-1 cells of 0.97 pl/cell determined with a Coulter counter (8). The intracellular amino acid concentration was calculated for each amino acid by dividing the total nanomoles of amino acid in the extract by the volume of intracellular water.
Virulence in guinea pigs.
Pathogen-free outbred male Hartley strain guinea pigs (650 to 750 g) were administered an aerosol dose of M. tuberculosis generated from a 10-ml suspension of bacteria containing a total of 5 × 104 CFU of the M. tuberculosis wild-type strain, M. tuberculosis glnA1, M. tuberculosis glnA1 pNBV1-MtbGS, or M. tuberculosis glnA1 pNBV1-StGS. The aerosol delivered ∼20 live organisms to the lungs of each animal (19). In addition, the M. tuberculosis glnA1 strain was administered by aerosol at 10× and 100× concentrations (5 × 105 and 5 × 106 total CFU). The wild-type strain was prepared from a recent guinea pig passage, as previously described (18). The other strains were prepared from log-phase broth cultures. The guinea pigs were killed at 10 weeks, and the right lung and spleen of each animal were cultured for CFU of M. tuberculosis on 7H11 plates containing 20 mM l-glutamine. Colonies were scored after 3 weeks of incubation at 37°C. The mean log CFU in the lung and spleen of each challenge group was compared with that of the wild-type strain by analysis of variance. Animal research was conducted in compliance with all relevant federal guidelines and University of California—Los Angeles policies.
M. smegmatis mc2 155 genome sequence.
Preliminary sequence data was obtained from The Institute for Genomic Research website at http://www.tigr.org.
RESULTS
Construction and characterization of an M. tuberculosis glnA1 mutant.
The M. tuberculosis glnA1 gene was disrupted by insertion of a Kmr cassette in a unique EcoNI site located near the middle of the glnA1 coding region, and the disrupted allele was cloned into pEX1 for allelic exchange. The pEX1 vector (Fig. 1), based on the temperature-sensitive sacB vector pPR27 (31), includes gfpuv and allows for the screening of M. tuberculosis colonies after counterselection (colonies maintaining the plasmid and expressing GFPuv are green fluorescent under long-wavelength UV light). Although we had previously applied a one-step allelic exchange protocol (31) in generating an M. smegmatis glnA1 mutant (39), we were unsuccessful in creating an M. tuberculosis glnA1 mutant in this manner. Therefore, we generated the mutant with a two-step protocol (28). First, we selected for integration of the plasmid into the chromosome by growth at the restrictive temperature in the presence of hygromycin and kanamycin, followed by sucrose counterselection to isolate clones that had undergone a second homologous recombination event. Construction of the mutant was confirmed by Southern blot analysis (Fig. 2).
To assess the glutamine requirements of the strain, bacteria were plated at a low density (∼102 CFU per plate) on 7H10 plates containing 0, 1, 2, 5, 10, or 20 mM l-glutamine and incubated for 3 weeks. Absolutely no growth was observed at 0 mM l-glutamine. In fact, no growth was observed in the absence of l-glutamine even when heavy inocula (107 to 108 CFU) were spread on 7H10 plates. Colonies were just barely visible at 1 mM l-glutamine, and colony size increased with increasing l-glutamine concentration until, at 10 and 20 mM l-glutamine, the colonies were comparable in size to those of the wild-type strain (data not shown).
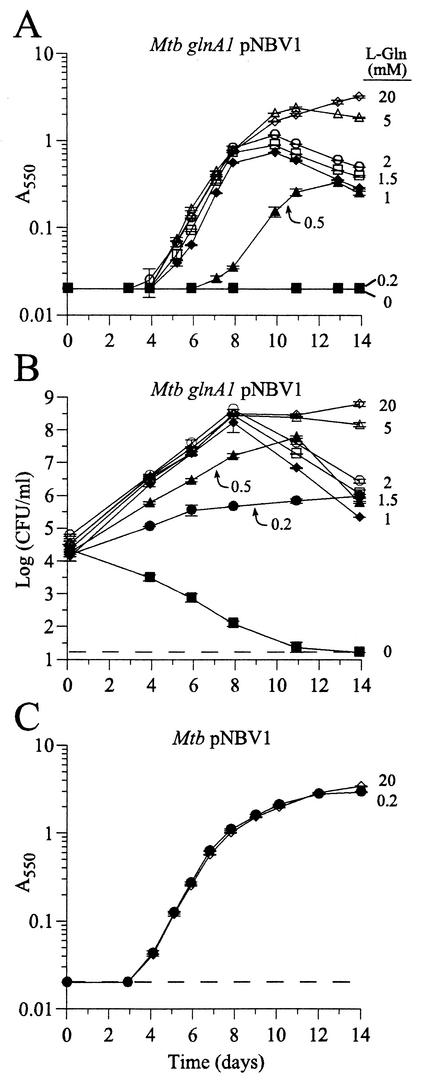
In broth culture, the initial growth of the mutant was essentially normal with an l-glutamine concentration as low as 1 mM (Fig. 3A and B; Table 2). However, at concentrations of 1 to 2 mM l-glutamine, the mutant did not reach as high a maximum density as it did when grown with 5 or 20 mM l-glutamine. In addition, the cell density (as measured by absorbance) of the 1 to 2 mM l-glutamine cultures dropped quickly after exponential growth, with an even more precipitous drop in viability. Growth was achieved with 0.2 and 0.5 mM l-glutamine, although the doubling times were substantially slower than normal, and the mutant lost viability rapidly when diluted into medium lacking l-glutamine. Growth of the wild-type strain was unaffected by l-glutamine concentration (Fig. 3C).
FIG.3.
Glutamine requirement of the M. tuberculosis glnA1 mutant in broth culture. Cultures of the glnA1 strain (A and B) and the wild-type strain (C) were inoculated into 7H9-OADC-TW containing various concentrations of l-glutamine (indicated next to the corresponding lines on the graphs) to an initial calculated A550 of 0.0001. Growth was monitored by assaying absorbance (A and C) and CFU (B). Data are the means ± standard errors for duplicate or triplicate cultures. In many instances, the error bars are smaller than the symbols. For all measurements, the standard error was <14% of the mean. The limits of detection were 0.02 absorbance units (A and C) and 1.22 log CFU/ml (16 CFU/ml) (B), as indicated by the dashed lines (measurements below the detection limit were scored as equal to the detection limit). The experiment was repeated once with similar results (only the 0 and 0.2 mM l-glutamine cultures were plated for CFU in the second experiment). Growth of the wild-type strain was unaffected by the l-glutamine concentration. Mtb, M. tuberculosis.
TABLE 2.
Growth rates of the M. tuberculosis glnA1 mutant in broth culture and in human THP-1 macrophages
| Culture type and l-glutamine concn (mM)a | Doubling time (h)b |
|---|---|
| Broth | |
| 0.5 | 33.1 ± 6.6 |
| 1 | 19.3 ± 3.0 |
| 1.5 | 18.4 ± 2.1 |
| 2 | 19.7 ± 2.1 |
| 5 | 19.6 ± 0.4 |
| 20 | 19.7 ± 1.2 |
| THP-1 cells | |
| 1 | 50.9 ± 9.7 |
| 2 | 31.2 ± 5.0 |
| 10 | 19.6 ± 0.9 |
For growth in THP-1 cells, l-glutamine concentration is extracellular.
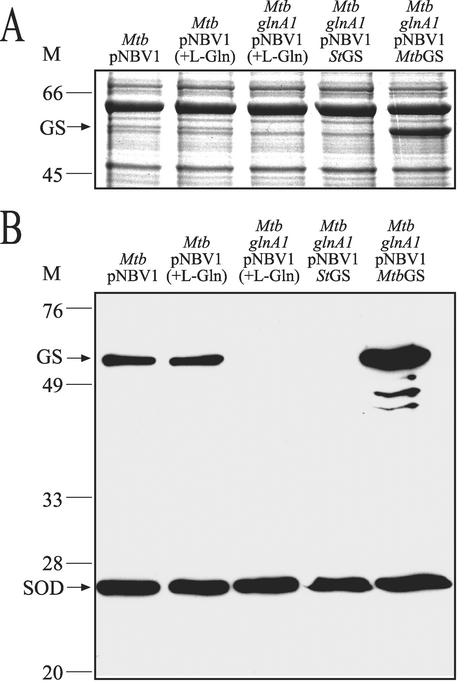
Complementation analysis was performed by transforming the mutant strain with plasmids containing the M. tuberculosis glnA1 gene (pNBV1-MtbGS) or the S. enterica serovar Typhimurium glnA gene (pNBV1-StGS). Both plasmids restored a wild-type growth phenotype to the mutant (data not shown). SDS-PAGE and immunoblot analysis of cell lysates of the glnA1 mutant, complemented strains, and the wild-type strain revealed the loss of a band corresponding to GS in the glnA1 strain (Fig. 4). Furthermore, no GS activity could be measured in cell lysates of the glnA1 mutant (Table 3). Growth of the wild-type strain in medium containing high levels of l-glutamine resulted in a moderate decrease in GS specific activity, as previously observed for M. smegmatis (39). Due to expression from a multicopy plasmid, the level of GS activity in the mutant strain complemented with the M. tuberculosis glnA1 gene (Mtb glnA1 pNBV1-MtbGS) was ∼three-fold higher than in the wild-type strain, and GS bands of greater intensity were observed by SDS-PAGE and immunoblot analysis (Table 3; Fig. 4). Despite using the strong M. tuberculosis glnA1 promoter, the S. enterica serovar Typhimurium GS was poorly expressed in the M. tuberculosis glnA1 mutant, as no GS band was detected on SDS-PAGE analysis and the GS specific activity of the strain was very low (1% of the wild-type level) (Table 3). Poor expression of the S. enterica serovar Typhimurium GS was previously observed in an M. smegmatis glnA1 mutant (39).
FIG. 4.
GS expression in M. tuberculosis wild-type, glnA1, and complemented strains. (A) SDS-PAGE analysis of cell lysates (∼18 μg of total protein per lane). (B) Immunoblot analysis of the cell lysates (∼18 μg of total protein per lane). Blots were probed with polyclonal rabbit anti-M. tuberculosis GS and, as a control, rabbit anti-M. tuberculosis superoxide dismutase antibody. The GS band (arrow) present in the wild-type lysates is absent in the M. tuberculosis glnA1 pNBV1 and M. tuberculosis glnA1 pNBV1-StGS lysates. GS is overexpressed in the M. tuberculosis glnA1 pNBV1-MtbGS strain due to its expression from a multicopy plasmid. The M. tuberculosis GS polyclonal antibodies used for immunodetection of GS were not capable of detecting <1 μg of purified S. enterica serovar Typhimurium GS (data not shown); therefore, the absence of a band for S. enterica serovar Typhimurium GS shows only that expression is <1 μg. +l-Gln, cultures grown in the presence of 20 mM l-glutamine; M, molecular mass markers in kilodaltons; Mtb, M. tuberculosis.
TABLE 3.
Expression of glutamine synthetase
| Strain | Specific activity (U/mg of protein)a | Relative activityc |
|---|---|---|
| M. tuberculosis pNBV1 | 1.52 ± 0.02 | 1.00 |
| M. tuberculosis pNBV1 (20 mM L-Gln) | 0.65 ± 0.02 | 0.43 |
| M. tuberculosis glnA1 pNBV1 (20 mM L-Gln) | ≤0.002b | 0.00 |
| M. tuberculosis glnA1 pNBV1-MtbGS | 4.17 ± 0.21 | 2.73 |
| M. tuberculosis glnA1 pNBV1-StGS | 0.014 ± 0.001 | 0.01 |
Data are means ± standard errors of results of three independent cultures.
Limit of detection, 0.002 U/mg of protein.
Values are relative to the activity of the wild-type strain transformed with the parental plasmid (Mtb pNBV1).
Although the mutant strain complemented with the S. enterica serovar Typhimurium glnA gene grew normally on our standard media (7H9 and 7H10), which contain 3.8 mM (NH4)2SO4 and 3.4 mM l-glutamate as the nitrogen sources, this strain did not grow on plates containing 10 mM NH4Cl as the sole nitrogen source when plated as single colonies and exhibited very poor growth when plated as a lawn (data not shown). Growth was normal on plates containing l-glutamine, l-glutamate, or l-glutamate plus NH4Cl (each nitrogen source at 10 mM). This phenotype can likely be explained by greater repression of GS expression and greater posttranslational adenylylation at 10 mM NH4Cl, which reduces the already very low level of active GS in the strain to a point where it no longer provides enough glutamine for growth. An S. meliloti mutant lacking its two primary GS genes and expressing only its third, minor GS gene exhibited a similar phenotype (35, 37).
The glnA1 mutant is attenuated for intracellular growth in human THP-1 macrophages.
Plate and broth cultures indicated that the glnA1 mutant required a fairly high l-glutamine concentration for robust growth. To assess whether the mutant could obtain enough l-glutamine for growth in an intracellular environment, we infected THP-1 macrophages with the glnA1 mutant and monitored bacterial growth under normal (2 mM) and low (0.2 mM) extracellular l-glutamine concentrations (Fig. 5). The wild-type and complemented strains served as controls. When 2 mM l-glutamine was present in the tissue culture medium (the standard amount in RPMI 1640), the glnA1 mutant was capable of intracellular growth, but it grew much more slowly than the wild-type strain. However, when only 0.2 mM l-glutamine was present in the tissue culture medium, the mutant was unable to grow intracellularly, and CFU slowly decreased over the course of the experiment (∼0.5 log reduction in 6 days). In contrast, the wild-type strain and both complemented mutant strains grew normally under these conditions. Intracellular growth of the mutant increased with increasing extracellular l-glutamine over a wide range of concentrations, and a growth rate similar to the wild-type growth rate was achieved at 10 mM extracellular l-glutamine (Fig. 6). A comparison of growth rates obtained in broth culture to those obtained in THP-1 cells is shown in Table 2. With an extracellular concentration of 2 mM l-glutamine, the standard amount in tissue culture medium, the mutant's intracellular growth rate (doubling time, 31.2 ± 5.0 h) is very similar to its broth culture growth rate in the presence of 0.5 mM l-glutamine (doubling time, 33.1 ± 6.6 h). In both settings, the growth rate is considerably slower (doubling time is 1.6 to 1.7 times longer) than the maximum growth rate (i.e., growth in broth with ≥1 mM l-glutamine or growth in THP-1 cells with 10 mM extracellular l-glutamine).
M. tuberculosis has limited access to the intracellular l-glutamine pool in human THP-1 macrophages.
l-Glutamine is reported to be highly abundant in some cell types, such as astrocytes and fibroblasts (5, 6, 29, 43), but its concentration in human macrophages is, to the best of our knowledge, unknown. Therefore, we determined the intracellular l-glutamine concentration in differentiated THP-1 cells incubated in the presence of 0.2 or 2 mM l-glutamine (Table 4). Although not as high as in astrocytes and fibroblasts, the intracellular l-glutamine concentration was found to be 4.5 mM when the cells were incubated in the presence of 2 mM extracellular l-glutamine, a concentration that is similar to that determined in HeLa and MDCK cells (32, 34). This concentration is approximately five times greater than that needed to achieve normal growth in broth culture, yet the mutant grows poorly intracellularly under these conditions, strongly suggesting that M. tuberculosis has limited access to the host's intracellular pool of l-glutamine.
TABLE 4.
Intracellular amino acid pool of human THP-1 macrophagesa
| Amino acid | THP-1 cells cultured in the presence of 2 mM l-glutamined
|
THP-1 cells cultured in the presence of 0.2 mM l-glutaminee
|
||||
|---|---|---|---|---|---|---|
| Intracellular concn (mM) | Concn in medium (mM)b | Concn (fold)c | Intracellular concn (mM) | Concn medium (mM)b | Concn (fold)c | |
| Asp | 7.16 ± 0.45 | 0.08 ± 0.08 | 88.9 | 5.33 ± 0.09 | 0.01 ± 0.01 | 356.9 |
| Thr | 0.78 ± 0.21 | 0.18 ± 0.01 | 4.4 | 1.25 ± 0.14 | 0.18 ± 0.02 | 6.8 |
| Ser | 0.34 ± 0.05 | 0.11 ± 0.05 | 3.2 | 0.53 ± 0.21 | 0.10 ± 0.04 | 5.5 |
| Glu | 18.56 ± 2.79 | 0.47 ± 0.01 | 39.2 | 10.53 ± 2.47 | 0.34 ± 0.03 | 30.9 |
| Gln | 4.45 ± 0.95 | 1.17 ± 0.19 | 3.8 | 0.06 ± 0.06 | 0.05 ± 0.01 | 1.2 |
| Asn | 1.28 ± 0.17 | 0.31 ± 0.02 | 4.2 | 2.55 ± 0.10 | 0.30 ± 0.03 | 8.4 |
| Pro | 1.18 ± 0.35 | 0.18 ± 0.01 | 6.6 | 1.92 ± 0.28 | 0.17 ± 0.02 | 11.1 |
| Gly | 3.82 ± 0.68 | 0.34 ± 0.03 | 11.1 | 5.96 ± 0.52 | 0.36 ± 0.02 | 16.5 |
| Ala | 0.95 ± 0.23 | 0.18 ± 0.02 | 5.3 | 0.85 ± 0.03 | 0.11 ± 0.03 | 8.0 |
| Val | 0.32 ± 0.32 | 0.17 ± 0.01 | 1.9 | 0.49 ± 0.09 | 0.17 ± 0.02 | 2.9 |
| Met | 0.21 ± 0.08 | 0.07 ± 0.00 | 3.1 | 0.27 ± 0.06 | 0.07 ± 0.00 | 4.0 |
| Ile | 0.67 ± 0.18 | 0.29 ± 0.01 | 2.3 | 0.73 ± 0.07 | 0.26 ± 0.02 | 2.8 |
| Leu | 0.77 ± 0.16 | 0.32 ± 0.02 | 2.4 | 0.84 ± 0.06 | 0.30 ± 0.03 | 2.8 |
| Tyr | 0.27 ± 0.18 | 0.11 ± 0.01 | 2.5 | 0.43 ± 0.06 | 0.11 ± 0.01 | 3.9 |
| Phe | 0.19 ± 0.19 | 0.10 ± 0.01 | 1.9 | 0.38 ± 0.04 | 0.10 ± 0.01 | 3.8 |
| Lys | 0.16 ± 0.07 | 0.21 ± 0.02 | 0.8 | 0.19 ± 0.02 | 0.21 ± 0.03 | 0.9 |
| His | 0.22 ± 0.05 | 0.08 ± 0.00 | 2.6 | 0.27 ± 0.03 | 0.08 ± 0.00 | 3.2 |
| Arg | 0.74 ± 0.14 | 0.91 ± 0.04 | 0.8 | 0.75 ± 0.04 | 0.91 ± 0.06 | 0.8 |
Results are the means ± standard errors from two independent experiments.
Measurements were performed on samples of the growth medium from the same flasks that were used for the intracellular amino acid analyses.
Results are the intracellular concentration divided by the concentration in the medium.
The concentration of l-glutamine in the tissue culture medium at time 0 (immediately after fresh medium containing 10% serum was added to the THP-1 cells) in one experiment was assayed and found to be 2.0 mM.
The concentration of l-glutamine in the tissue culture medium at time 0 (immediately after fresh medium containing 10% serum was added to the THP-1 cells) in one experiment was assayed and found to be 0.26 mM.
The glnA1 mutant is avirulent in the highly susceptible guinea pig model of pulmonary tuberculosis.
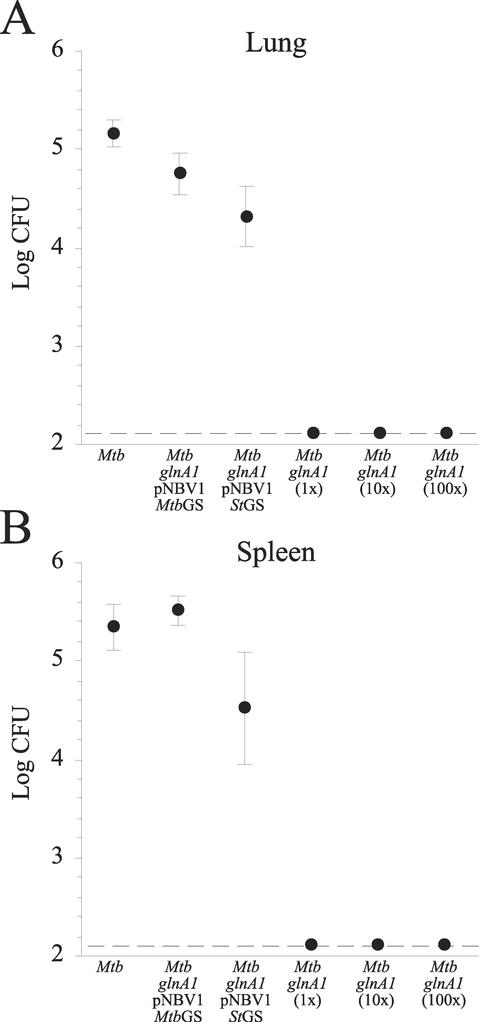
The virulence of the glnA1 mutant and complemented strains was assessed by exposing guinea pigs to an aerosolized inoculum of the strains. Guinea pigs are very susceptible to M. tuberculosis infection, with as few as 3 to 5 organisms causing disease (36). Although humans are far more resistant to M. tuberculosis, the disease in guinea pigs closely resembles the disease in humans (19). Our standard aerosol dose delivers ∼20 CFU to the lungs of each animal and results in progressive infection in 100% of the animals (18, 19). In our experiments, the animals infected with the wild-type strain as well as the two complemented strains had an average of >104 CFU of M. tuberculosis in both their lungs and spleens (Fig. 7). However, even when the glnA1 strain was administered at 100 times the standard dose, none of the animals exposed to the mutant had CFU in plated aliquots of the lung and spleen (>3 log less CFU in both the lung and spleen than with the wild-type strain; P < 0.0001 with analysis of variance). Animals infected with the mutant strain complemented with the S. enterica serovar Typhimurium GS exhibited a phenotype intermediate between those of the wild-type and the glnA1 mutant strains. The strain multiplied in guinea pig lung and disseminated to the guinea pig spleen, but growth was significantly less than that of the wild-type (0.86 log fewer CFU in the lung [P < 0.0001]; 0.86 log fewer CFU in the spleen [P < 0.01]).
FIG. 7.
The M. tuberculosis glnA1 mutant is avirulent in guinea pigs. Guinea pigs were infected by aerosol with M. tuberculosis strains as indicated, and 10 weeks later, bacterial load in the right lung (A) and spleen (B) was quantified. Guinea pigs were infected with the M. tuberculosis glnA1 mutant at the standard dose (1×) used for the other strains and at 10× and 100× the standard dose. Data are the means ± standard errors for all animals in a group (n = 5). No CFU were detected in plated aliquots of the lung and spleen for any of the M. tuberculosis glnA1-infected animals, and all of these organs were scored as 2.1 log CFU for statistical purposes (2.1 log CFU/organ, the limit of detection, is indicated by the dashed lines). Mtb, M. tuberculosis.
DISCUSSION
We have shown that an M. tuberculosis glnA1 mutant possesses no detectable GS protein or GS activity, is auxotrophic for l-glutamine, is attenuated for intracellular growth in human THP-1 macrophages, and is avirulent in highly susceptible guinea pigs. With regard to auxotrophy, the mutant is similar to glnA null mutants of enteric bacteria that possess a single glnA gene encoding a GSI enzyme (33). In addition to glnA1, M. tuberculosis has three other glnA homologs (glnA2, glnA3, and glnA4) that are predicted to encode GSI type enzymes; however, the predicted proteins are only somewhat similar to GlnA1 (15 to 25% identity). Although these genes are transcribed by M. tuberculosis in broth culture (our unpublished results), our results clearly show that they do not provide sufficient l-glutamine to support the growth of the bacterium, and their role remains to be elucidated. Further support for a lack of a role for glnA2, glnA3, and glnA4 in the biosynthesis of l-glutamine is the fact that both M. smegmatis and Corynebacterium glutamicum glnA1 mutants are l-glutamine auxotrophs with no detectable GS activity (22, 39) despite possessing glnA2 (C. glutamicum [27]) or glnA2, glnA3, and glnA4 genes (M. smegmatis; preliminary sequence data for M. smegmatis was obtained from The Institute for Genomic Research through the website at http://www.tigr.org).
Although much progress has been achieved in recent years in generating defined mutants of M. tuberculosis via allelic exchange, there is still often the need for extensive screening to identify correct clones (9, 16, 28, 30, 31). Both XylE and LacZ have been used as aids in screening to identify undesired clones that retained the vector after counterselection (28, 31). Because colonies of M. tuberculosis expressing GFPuv are intensely green fluorescent, we considered that GFPuv might also serve as a useful marker. While this proved true, discriminating fluorescent from nonfluorescent colonies after counterselection was more difficult than expected as fluorescence was greatly reduced compared with fluorescence of M. tuberculosis pNBV1-GFPuv, which expresses GFPuv at a fairly high level (39). Despite these technical problems, we were able to successfully generate an M. tuberculosis glnA1 mutant using a two-step protocol.
High levels of l-glutamine (10 to 20 mM) were required in solid medium for the mutant to grow normally. Growth was poor at 2 mM l-glutamine, as only small colonies were visible, and growth was extremely poor at 1 mM l-glutamine. In liquid medium, the mutant grew at a normal growth rate (similar to that of the wild-type strain) at ≥ 1 mM l-glutamine. Although initially growth was normal at 1 to 2 mM l-glutamine, these cultures did not reach as high a density as the 5 mM and 20 mM l-glutamine cultures, and they exhibited a sharp drop in viability shortly after log phase, presumably due to nearly complete depletion of l-glutamine in the culture. The high level of l-glutamine required for optimal growth might be due to an inherently high requirement for this amino acid by the organism, poor uptake, instability of l-glutamine, or a combination of these factors. It is known that l-glutamine is not as stable as most other amino acids in aqueous solution (23), and given the long incubation time necessary for the growth of M. tuberculosis (particularly for plate-grown organisms), a substantial amount of the initial l-glutamine in the culture medium may be degraded. However, over the relatively short time period of initial growth in broth culture and in THP-1 macrophages, hydrolysis of l-glutamine is likely a minor issue.
M. tuberculosis appears to require substantially more l-glutamine than S. enterica serovar Typhimurium, as an S. enterica serovar Typhimurium glnA strain was able to grow normally with as little as 0.2 mM supplemental l-glutamine in minimal medium (24). S. enterica serovar Typhimurium possesses both a high-affinity glutamine transport system (Km = 0.2 μM, Vmax = 2 nmol min−1 mg dry weight−1), encoded by glnHPQ, and a low-affinity transport system (Km = 10 μM, Vmax = 3.5 nmol min−1 mg dry weight−1) (1, 24). M. tuberculosis possesses glnH and glnQ homologs but lacks a glnP homolog (3). The lack of the permease encoded by glnP may account for the greater requirement of M. tuberculosis for l-glutamine. However, as the M. tuberculosis glnH and glnQ genes are >30% larger than their S. enterica serovar Typhimurium homologs, it is possible that one or both genes encode a permease function that replaces the missing glnP or that another M. tuberculosis protein lacking homology with GlnP functions as a glutamine permease. An S. enterica serovar Typhimurium glnA glnH strain (defective in both GS and high-affinity glutamine transport) had a doubling time 2.7 times greater than that of the S. enterica serovar Typhimurium glnA strain at 0.2 mM l-glutamine and 1.8 times greater at 2 mM l-glutamine (24). As the low-affinity transport system should be (nearly) saturated at both concentrations, it appears that this strain simply cannot transport l-glutamine fast enough to achieve a wild-type growth rate. In contrast, in the presence of high l-glutamine concentrations, the M. tuberculosis glnA1 mutant can transport l-glutamine fast enough (a sufficiently high glutamine transport Vmax) to achieve a wild-type growth rate. Therefore, M. tuberculosis either has a high Km for glutamine transport that limits growth in the presence of low l-glutamine concentrations or it has a higher metabolic requirement for l-glutamine than S. enterica serovar Typhimurium. In support of the latter, a study in C. glutamicum (a phylogenetically close relative of mycobacteria) showed that 28% of the total nitrogen was assimilated via glutamine, which is approximately two times the glutamine requirement of E. coli (38). The authors suggested that this higher l-glutamine requirement may be due to increased amounts of peptidoglycan synthesized by gram-positive bacteria. In addition to peptidoglycan, M. tuberculosis produces a poly-l-glutamate/glutamine cell wall structure that accounts for ∼10% of the cell wall mass (17, 40). Synthesis of this polymer might also contribute to a greater l-glutamine requirement.
Growth of the glnA1 mutant in human macrophages is poor when macrophages are cultured in standard tissue culture medium containing 2 mM l-glutamine, a concentration greater than that found in human plasma (0.6 mM [4]). However, intracellular growth similar to that of the wild-type strain was achieved by adding a large excess of l-glutamine (10 mM) to the tissue culture medium. No growth of the mutant occurs when the infected macrophages are cultured in the presence of 0.2 mM l-glutamine, a condition under which the wild-type strain grows normally. Comparison of the growth rates of the mutant intracellularly and in broth culture suggests that at an extracellular concentration of 2 mM l-glutamine, the mutant grows as if it has access to an effective concentration of only ∼0.5 mM l-glutamine. At 10 mM extracellular l-glutamine, the mutant grows normally and so must have access to ≥1 mM l-glutamine.
Because the mutant requires such a high concentration of extracellular l-glutamine for normal intracellular growth, we determined the intracellular l-glutamine concentration in THP-1 macrophages. When THP-1 cells were cultured in medium containing 2 mM l-glutamine, the intracellular l-glutamine concentration was 4.5 mM, nearly 10 times more than the estimated concentration in the phagosome. This strongly suggests that the M. tuberculosis phagosome is not permeable to even small molecules in the host cytoplasm. Recent work from this laboratory has demonstrated that the M. tuberculosis phagosome is not permeable to molecules of ≥50,000 Da (2). As the M. tuberculosis phagosome appears impermeable to l-glutamine, any putative pore in the M. tuberculosis phagosomal membrane evidently would be capable of excluding molecules as small as a few hundred daltons (the mass of l-glutamine is 146 Da). When the THP-1 cells were cultured in medium containing 0.2 mM l-glutamine, the intracellular l-glutamine concentration was <0.1 mM. As this is below the concentration of l-glutamine at which the mutant can grow to any appreciable extent extracellularly in broth culture, it is not surprising that the mutant was unable to grow intracellularly in THP-1 cells under this condition.
Our results thus show that M. tuberculosis in a host phagosome has limited access to both intracellular and extracellular sources of l-glutamine, at least in vitro. That the glnA1 mutant was highly attenuated in guinea pigs suggests that the concentration of l-glutamine in the M. tuberculosis phagosome is limited in vivo as well. In contrast to M. tuberculosis, an S. enterica serovar Typhimurium glnA strain auxotrophic for l-glutamine was as virulent in vivo as the wild-type strain due to the organism's ability to acquire l-glutamine efficiently from its phagolysosome utilizing its high-affinity glutamine transport system (24). However, S. enterica serovar Typhimurium mutants defective in both GS and glutamine transport were highly attenuated.
M. tuberculosis is quite sensitive to growth inhibition by the GS inhibitor MSO, especially in comparison with the nonpathogenic M. smegmatis, and MSO is effective in reducing bacterial load in infected guinea pigs (11, 14). Antisense oligonucleotides to glnA1 also inhibit the growth of M. tuberculosis (15). In this study, we have shown that glnA1 is essential for growth of M. tuberculosis in vitro and in vivo. This provides further support for the concept that the mechanism by which MSO inhibits M. tuberculosis growth is that of inhibition of GS and not inhibition of another unidentified cellular target. In this regard, it is interesting that M. tuberculosis glnA1 pNBV1-StGS can grow normally in vitro and multiply in vivo, albeit suboptimally compared with the wild-type strain, even though it expresses only 1% of normal GS activity. This implies that any drug targeting M. tuberculosis GS must, like MSO, be capable of essentially complete inhibition of the enzyme in order to halt bacterial growth.
Our findings raise this question: why does M. tuberculosis produce so much GS when the organism seems to grow normally with much less enzyme? As in the case of M. smegmatis (39), the M. tuberculosis glnA1 pNBV1-StGS strain grew normally in 7H9 medium lacking l-glutamine despite greatly reduced GS activity. The M. tuberculosis glnA1 pNBV1-StGS strain also grew normally in THP-1 macrophages. However, the strain exhibited glutamine auxotrophy when NH4Cl was the sole nitrogen source and was not as virulent as the wild-type strain in guinea pigs. Perhaps high GS levels allow for enhanced growth under more restrictive conditions found in vivo. Along this line, it is possible that high GS levels are needed for efficient regulation of nitrogen metabolism under in vivo conditions.
Acknowledgments
This work was supported by grants AI 31338 and AI 42925 from the National Institutes of Health.
We are grateful to Sasa Maslesa-Galic and Barbara Jane Dillon for technical assistance and to Harindarpal Gill for providing purified, recombinant S. enterica serovar Typhimurium GS.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Betteridge, P. R., and P. D. Ayling. 1976. The regulation of glutamine transport and glutamine synthetase in Salmonella typhimurium. J. Gen. Microbiol. 95:324-334. [DOI] [PubMed] [Google Scholar]
- 2.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2002. The Mycobacterium tuberculosis phagosome in human macrophages is isolated from the host cell cytoplasm. Infect. Immun. 70:5800-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Cynober, L. 2002. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition 18:761-766. [DOI] [PubMed] [Google Scholar]
- 5.Dall'Asta, V., P. A. Rossi, O. Bussolati, and G. C. Gazzola. 1994. Regulatory volume decrease of cultured human fibroblasts involves changes in intracellular amino-acid pool. Biochim. Biophys. Acta 1220:139-145. [DOI] [PubMed] [Google Scholar]
- 6.Darmaun, D., D. E. Matthews, J. F. Desjeux, and D. M. Bier. 1988. Glutamine and glutamate nitrogen exchangeable pools in cultured fibroblasts: a stable isotope study. J. Cell. Physiol. 134:143-148. [DOI] [PubMed] [Google Scholar]
- 7.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la difference! Mol. Microbiol. 32:223-232. [DOI] [PubMed] [Google Scholar]
- 8.Gallin, E. K., T. M. Mason, and A. Moran. 1994. Characterization of regulatory volume decrease in the THP-1 and HL-60 human myelocytic cell lines. J. Cell. Physiol. 159:573-581. [DOI] [PubMed] [Google Scholar]
- 9.Gordhan, B. G., D. A. Smith, H. Alderton, R. A. McAdam, G. J. Bancroft, and V. Mizrahi. 2002. Construction and phenotypic characterization of an auxotrophic mutant of Mycobacterium tuberculosis defective in l-arginine biosynthesis. Infect. Immun. 70:3080-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harth, G., D. L. Clemens, and M. A. Horwitz. 1994. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc. Natl. Acad. Sci. USA 91:9342-9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harth, G., and M. A. Horwitz. 2003. Inhibition of Mycobacterium tuberculosis glutamine synthetase as a novel antibiotic strategy against tuberculosis: demonstration of efficacy in vivo. Infect. Immun. 71:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harth, G., and M. A. Horwitz. 1999. Export of recombinant Mycobacterium tuberculosis superoxide dismutase is dependent upon both information in the protein and mycobacterial export machinery. A model for studying export of leaderless proteins by pathogenic mycobacteria. J. Biol. Chem. 274:4281-4292. [DOI] [PubMed] [Google Scholar]
- 13.Harth, G., and M. A. Horwitz. 1997. Expression and efficient export of enzymatically active Mycobacterium tuberculosis glutamine synthetase in Mycobacterium smegmatis and evidence that the information for export is contained within the protein. J. Biol. Chem. 272:22728-22735. [DOI] [PubMed] [Google Scholar]
- 14.Harth, G., and M. A. Horwitz. 1999. An inhibitor of exported Mycobacterium tuberculosis glutamine synthetase selectively blocks the growth of pathogenic mycobacteria in axenic culture and in human monocytes: extracellular proteins as potential novel drug targets. J. Exp. Med. 189:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harth, G., P. C. Zamecnik, J. Y. Tang, D. Tabatadze, and M. A. Horwitz. 2000. Treatment of Mycobacterium tuberculosis with antisense oligonucleotides to glutamine synthetase mRNA inhibits glutamine synthetase activity, formation of the poly-L-glutamate/glutamine cell wall structure, and bacterial replication. Proc. Natl. Acad. Sci. USA 97:418-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinds, J., E. Mahenthiralingam, K. E. Kempsell, K. Duncan, R. W. Stokes, T. Parish, and N. G. Stoker. 1999. Enhanced gene replacement in mycobacteria. Microbiology 145:519-527. [DOI] [PubMed] [Google Scholar]
- 17.Hirschfield, G. R., M. McNeil, and P. J. Brennan. 1990. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J. Bacteriol. 172:1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2000. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz, M. A., B. W. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard, N. S., J. E. Gomez, C. Ko, and W. R. Bishai. 1995. Color selection with a hygromycin-resistance-based Escherichia coli-mycobacterial shuttle vector. Gene 166:181-182. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, T. P., A. E. Shauger, and S. Kustu. 1996. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J. Mol. Biol. 259:589-607. [DOI] [PubMed] [Google Scholar]
- 22.Jakoby, M., M. Tesch, H. Sahm, R. Kramer, and A. Burkovski. 1997. Isolation of the Corynebacterium glutamicum glnA gene encoding glutamine synthetase I. FEMS Microbiol. Lett. 154:81-88. [DOI] [PubMed] [Google Scholar]
- 23.Khan, K., G. Hardy, B. McElroy, and M. Elia. 1991. The stability of L-glutamine in total parenteral nutrition solutions. Clin. Nutr. 10:193-198. [DOI] [PubMed] [Google Scholar]
- 24.Klose, K. E., and J. J. Mekalanos. 1997. Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect. Immun. 65:587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrick, M. J., and R. A. Edwards. 1995. Nitrogen control in bacteria. Microbiol. Rev. 59:604-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuhoff, V., N. Arold, D. Taube, and W. Ehrhardt. 1988. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9:255-262. [DOI] [PubMed] [Google Scholar]
- 27.Nolden, L., M. Farwick, R. Kramer, and A. Burkovski. 2001. Glutamine synthetases of Corynebacterium glutamicum: transcriptional control and regulation of activity. FEMS Microbiol. Lett. 201:91-98. [DOI] [PubMed] [Google Scholar]
- 28.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146:1969-1975. [DOI] [PubMed] [Google Scholar]
- 29.Pasantes-Morales, H., S. Alavez, R. Sanchez Olea, and J. Moran. 1993. Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem. Res. 18:445-452. [DOI] [PubMed] [Google Scholar]
- 30.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piez, K. A., and H. Eagle. 1958. The free amino acid pool of cultured human cells. J. Biol. Chem. 231:533-545. [PubMed] [Google Scholar]
- 33.Reitzer, L. J. 1996. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, l-alanine, and d-alanine, p. 391-407. In F. C. Neidhardt, and R. Curtiss (ed.), Escherichia coli and Salmonella typhimurium, 2nd ed. ASM Press, Washington, D.C.
- 34.Sanchez Olea, R., H. Pasantes-Morales, A. Lazaro, and M. Cereijido. 1991. Osmolarity-sensitive release of free amino acids from cultured kidney cells (MDCK). J. Membr. Biol. 121:1-9. [DOI] [PubMed] [Google Scholar]
- 35.Shatters, R. G., Y. Liu, and M. L. Kahn. 1993. Isolation and characterization of a novel glutamine synthetase from Rhizobium meliloti. J. Biol. Chem. 268:469-475. [PubMed] [Google Scholar]
- 36.Smith, D. W. 1984. Diseases in guinea pigs, p. 925-946. In G. P. Kubica and L. G. Wayne (ed.), The mycobacteria: a sourcebook. Dekker, New York, N.Y.
- 37.Somerville, J. E., R. G. Shatters, and M. L. Kahn. 1989. Isolation, characterization, and complementation of Rhizobium meliloti 104A14 mutants that lack glutamine synthetase II activity. J. Bacteriol. 171:5079-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tesch, M., A. A. de Graaf, and H. Sahm. 1999. In vivo fluxes in the ammonium-assimilatory pathways in Corynebacterium glutamicum studied by 15N nuclear magnetic resonance. Appl. Environ. Microbiol. 65:1099-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tullius, M. V., G. Harth, and M. A. Horwitz. 2001. High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism. Infect. Immun. 69:6348-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wietzerbin, J., F. Lederer, and J. F. Petit. 1975. Structural study of the poly-l-Glutamic acid of the cell wall of Mycobacterium tuberculosis var hominis, strain Brevannes. Biochem. Biophys. Res. Commun. 62:246-252. [DOI] [PubMed] [Google Scholar]
- 41.Woolfolk, C. A., B. Shapiro, and E. R. Stadtman. 1966. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch. Biochem. Biophys. 116:177-192. [DOI] [PubMed] [Google Scholar]
- 42.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 43.Yudkoff, M., I. Nissim, J. Stern, and D. Pleasure. 1989. Effects of palmitate on astrocyte amino acid contents. Neurochem. Res. 14:367-370. [DOI] [PubMed] [Google Scholar]