Abstract
Outer membrane proteins (OMPs) are incorporated into the outer plasma membrane of Helicobacter pylori and are important for, e.g., ion transport, adherence, structural and osmotic stability, and bacterial virulence but may also be antigenic due to their surface exposure. Previous proteome-based approaches with H. pylori lysates determined a strong serological reaction towards two H. pylori OMPs, HpaA (TIGR HP0797) and Omp18 (TIGR HP1125). PCR was used to detect DNA encoding the two proteins, and a positive signal was found in all H. pylori strains tested. Proteins were cloned and expressed in the human kidney cell line HK293 with the QiaExpressionist system with a C-terminal His tag. Only sera from infected persons showed a positive reaction with the recombinant proteins. Recombinant HpaA (rHpaA) and rOmp18 were incubated with human peripheral blood mononuclear cells and induced secretion of interleukin-12 (IL-12) and IL-10 from these cells. To determine the effect on antigen-presenting cells, human blood monocytic and dendritic cells (DCs) were isolated by magnetic cell separation. rOmp18 and rHpaA strongly stimulated major histocompatibility class II and CD83 expression 7- to 10-fold on isolated DCs. rHpaA and rOmp18 failed to stimulate IL-8 secretion from monocytes but increased secretion of IL-12 and IL-10 from DCs significantly. In summary, HpaA and Omp18 are recognized by human dendritic cells and induce their maturation as well as antigen presentation. HpaA and Omp18 of H. pylori thereby appear to have a specific antigenic potential in humans.
Helicobacter pylori is a gram-negative bacterium which colonizes the gastric epithelium and causes chronic gastritis, which may progress to peptic ulcer disease or gastric adenocarcinoma in a subset of patients (3, 4). Since the number of infected individuals is too large, antibiotic regimes to prevent gastric cancer are not cost-effective in most countries. Vaccination against this pathogen has been demonstrated in animal models but not in humans so far (6). Proteome-based techniques have therefore been used to identify potential antigens. These assays were based on two-dimensional gel electrophoresis of H. pylori proteins obtained from whole bacteria with subsequent immunoblotting with sera from H. pylori-infected patients (15, 19, 31). Studies of our own group showed that only the serological response towards low-molecular-weight proteins decreased after successful elimination. Furthermore, these studies indicated that two membrane-associated proteins, HP1125 (Omp18) and HP0797 (HpaA), when expressed in Escherichia coli, reacted specifically with sera obtained from H. pylori-infected patients. These two outer membrane proteins (OMPs) were specific for H. pylori, and no sequence homology was detected with OMPs from other species (31).
Bacterial OMPs are important for ion transport, bacterial adherence, and osmotic and structural stability but may also be antigenic due to their surface exposure. For example, OMPs from Klebsiella pneumoniae, Brucella abortus, and Neisseria meningitidis serve as powerful antigens in the induction of a human immune response (6). Recognition of bacterial OMPs by dendritic cells (DCs) has been reported for the Klebsiella pneumoniae OmpA (KpOmpA) (17). KpOmpA specifically binds to professional antigen-presenting cells and is endocytosed by immature myeloid DCs via a receptor-dependent mechanism. KpOmpA signaled through Toll-like receptor 2 and induced maturation of premyeloid DCs (pmDCs) by expression of major histocompatibility complex (MHC) class I molecules (17).
HpaA and Omp18 both belong to a group of outer membrane proteins of H. pylori. HpaA has been described as an adherence factor for blood cells, but this was controversial (26). HpaA mediates binding to sialic acid in vitro (9, 10), is a putative neuraminyllactose-binding hemagglutinin, and has also been described as a flagellar sheath protein (18). HpaA is a surface-localized antigen recognized by human antibodies (22). Omp18 is a peptidoglycan-associated lipoprotein precursor which is structurally related to Omp18 of Campylobacter jejuni and to Omp22 (HP0923) of H. pylori. In C. jejuni, Omp18 is an immunodominant antigen which is used for vaccination trials in chickens (20, 27). Nevertheless, the sequence homology to H. pylori Omp18 is low. These experiments, however, suggest that outer membrane proteins of H. pylori may be suitable for use in serological assays as well as vaccination trials.
The specific serological response towards these proteins implies that these structures are recognized, processed, and presented by human antigen-presenting cells in order to stimulate the function and/or the proliferation of T as well as B lymphocytes. We therefore used peripheral blood mononuclear cells (PBMC) isolated from the blood of healthy volunteers and separated DCs from those PBMC to determine the antigenic potential of the OMPs independent of the influence of cross-reacting immune cells. Blood DCs are antigen-presenting cells with the unique capacity to initiate primary immune responses (28). Maturation and antigen presentation in DCs in response to bacterial antigens can be monitored by fluorescence-activated cell sorting (FACS) analysis of the expression of MHC and costimulatory molecules, such as CD83, and by determination of cytokine secretion from these cells.
The current study therefore determined the antigenic potential of two specific H. pylori proteins in human PBMC and human DCs. Our studies show that the two H. pylori outer membrane proteins Omp18 and HpaA have potent and specific antigenic potential in vitro and induce maturation of isolated dendritic cells.
MATERIALS AND METHODS
PCR screening of different H. pylori strains for HpaA and Omp18.
Biopsies were randomly chosen from H. pylori-infected patients with different clinical symptoms (gastritis, gastric ulcer, duodenal ulcer, and gastric cancer). Patients underwent upper gastrointestinal endoscopy at the medical department of our hospital because of abdominal complaints, and five (four in the case of gastritis) patients with different diagnoses were chosen from each group; the median age in these groups was 62, 64, 59, and 66 years. Patients taking nonsteroidal antiinflammatory drugs were excluded. All patients lived in the immediate vicinity of Munich, Germany, and had German nationality.
H. pylori DNA was isolated for PCR analysis with the QIAamp tissue kit (Qiagen, Munich, Germany) after the biopsies were snap frozen in liquid nitrogen immediately after endoscopy, homogenized in liquid nitrogen, and lysed with proteinase K. PCR of the hpaA and omp18 gene loci was performed. Primer sequences were 5′-CTTTTAGGTGCGAGCGTGGTG-3′ (sense and 5′-AATTCCTTGGCGTCTTTTTGATAA-3′ (antisense) (positions 46 to 761; product length, 716 bp) for hpaA and 5′-ATTGGTAGCTGGCTGTAGTCATAA-3′ (sense) and 5′-GGGCGCATTTGGGTTTG-3′ (antisense) (positions 42 to 481; product length, 440 bp) for omp18. Amplification was done in a total volume of 20 μl with Taq PCR master mix (Qiagen), 1 μl of template DNA, and 0.5 μl of each primer (20 μM) in a Primus PCR cycler (MWG Biotech, Ebersberg, Germany) under standard conditions (30 cycles) with annealing temperatures of 54.2°C for hpaA and 53.7°C for omp18. PCR products were analyzed on 1% agarose gels stained with ethidium bromide.
Expression of recombinant HpaA and Omp18 in HK293 cells.
The antigens were expressed in HK293 cells by transient transfection and purified with the QIAexpressionist system (Qiagen, Hilden, Germany). This cell line was used for expression to avoid possible contamination with bacterial lipopolysaccharides frequently found in bacterial expression systems. Briefly, the coding sequence of the two proteins was cloned into vector pQE-Tri, providing a C-terminal 8×His tag excluding the signal peptide. HK293 cells (human kidney embryonic cell line ATCC CRL-1573) were cultured in RPMI 1640 medium supplemented with 10% (vol/vol) fetal bovine serum (FCS), 2 mM l-glutamine, 1% (vol/vol) streptomycin, and 1% (vol/vol) penicillin. Then 16 × 106 cells were transiently transfected with 40 μg of plasmid DNA in 125 mM CaCl2-25 mM BES (N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid)-140 mM NaCl-0.75 mM Na2HPO4 (pH 6.95) overnight. After changing the medium the next day, cells were cultured for another 24 h, finally harvested by scraping, and washed once with phosphate-buffered saline (pH 7.5).
Purification of the recombinant proteins was performed under denaturing conditions with Ni-nitrilotriacetic acid-agarose according to the manufacturer's protocol (Qiagen). The protein preparations were tested for endotoxin content by the Limulus amoebocyte lysate assay according to the manufacturer's protocol (QCL-1000; BioWhittaker, Walkersville, Md.). The preparations were treated with TX-114 over three cycles to ensure endotoxin extraction as described by Liu et al. (21). TX-114 had no effect on cytokine release or antigen expression. Endotoxin concentrations as determined by the Limulus amoebocyte lysate assay were below 0.1 U in each experiment. The antigenic reactivity of the recombinant proteins was determined by Western blot analysis with sera from H. pylori-infected patients and noninfected volunteers. The H. pylori status of the individuals was confirmed by a [13C]urea breath test and by histological evaluation, which showed identical results.
SDS-PAGE and Western blot analysis.
For Western blot analysis, the purified recombinant proteins and lysate of untransfected HK293 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% or 4 to 12% Tricine-SDS minigels and subsequently electrotransferred onto nitrocellulose membranes (Bio-Rad, Munich, Germany) with 192 mM glycine-25 mM Tris-20% methanol as the transfer buffer. After transfer, proteins were visualized with Ponceau S (0.2% Ponceau S in 3% trichloroacetic acid) to allow cutting of the membrane into individual strips for incubation with different antisera. Nonspecific binding sites were blocked for 1 h in 5% nonfat milk in Tris-buffered saline-0.1% Tween 20 (TBST). Incubation with human sera (1:500) and anti-penta-His (1:5,000, Qiagen) in 5% (wt/vol) nonfat milk in TBST was performed overnight at 4°C. After washing with TBST, the membranes were incubated with a peroxidase-labeled anti-human/anti-mouse immunoglobulin G (IgG) antibody (1:20,000; Dianova, Hamburg, Germany). After further washing with TBST, the antigens were visualized by chemiluminescence with the Uptima System (Interchim, Montlucon Cedex, France) according to the manufacturer's protocol.
Preparation of PBMC, monocytes, and blood DCs.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood of healthy adult volunteers by Ficoll-Hypaque (Pharmacia BiotechAB, Uppsala, Sweden) density gradient centrifugation as recommended by the manufacturer.
Monocytes were isolated from PBMC by negative selection with magnetic cell separation and the monocyte isolation kit from Miltenyi Biotec (Bergisch Gladbach, Germany) according to the manufacturer's protocol. The purity of the cells was determined by FACS analysis with specific antibodies directed against the CD14 epitope (Dianova, Hamburg, Germany).
DCs were isolated from PBMC by sequential negative and positive selection with magnetic cell separation and the DC isolation kit from Miltenyi Biotec (Product 130-046-801) according to the manufacturer's protocol. In this isolation protocol, negative selection is carried out with CD14+, CD56+, and CD3/9+ microbeads, followed by positive selection with CD4+ microbeads. Enrichment of DCs was determined by FACS analysis. A total of 3.5 × 105 to 1 × 106 cells were cultured in 700 μl of RPMI 1640 supplemented with 10% FCS, 20 ng of recombinant human IL-3 (BD Bioscience, Heidelberg, Germany) per ml, 1% l-glutamine, 1% streptomycin, and 1% penicillin in 48-well plates.
Quantification of cytokines by immunoassay.
PBMC (1 × 106 to 2 × 106 cells in 48-well plates), monocytes (5 × 106 cells in 48-well plates), and DCs (3.5 × 105 to 1 × 106 cells in 48-well plates) were incubated for 24 h with purified recombinant H. pylori proteins rHpaA and rOmp18 (1 to 20 μg/ml). The culture supernatants were collected and stored at −20°C until assayed. Interleukin-8 (IL-8), IL-12 (p40), IL-10, and alpha interferon (IFN-α) in the culture supernatant were measured by specific enzyme-linked immunosorbent assay (ELISA) with commercially available assay kits and according to standard procedures. In these assays, the lower limits of detection were 12 pg/ml for IL-8, 7.5 pg/ml for IL-10, 7.5 pg/ml for IL-12 (p40) (Biosource International, Nivelles, Belgium), and 8 pg/ml for IFN-α (Biozol, Eching, Germany).
FACS analysis.
For FACS analysis, ∼106 dendritic cells in 100 μl of PBS-1% bovine serum albumin (FACS buffer) were incubated with 10 μl of the appropriate fluorescein isothiocyanate- and/or phycoerythrin-labeled antibody (Becton Dickinson, Heidelberg, Germany) for 30 min on ice in the dark. After the incubation the cells were washed with 880 μl of FACS buffer and then suspended in 500 μl of FACS buffer before automated analysis with the FACSCalibur (Becton Dickinson).
Statistical analysis.
Results are expressed as mean ± standard error of the mean. Data were analyzed by one-way analysis of variance followed by the Newman-Keuls test. P values of ≤0.05 were considered significant.
RESULTS
PCR of various H. pylori strains for hpaA and omp18.
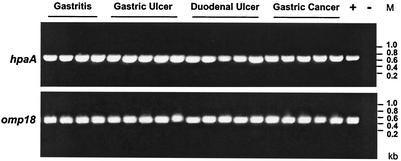
PCR was performed with DNA isolated from various H. pylori strains obtained from gastric biopsies of patients with different gastric diseases, such as gastritis, gastric ulcer, duodenal ulcer and gastric cancer. PCR revealed that the genes encoding HpaA and Omp18 were highly conserved and were present in all 19 H. pylori strains tested (Fig. 1) independent of the clinical outcome of the infection.
FIG. 1.
PCR analysis of different H. pylori strains. PCR screening for hpaA and omp18 was done by isolating H. pylori DNA from biopsies obtained from H. pylori-infected patients with different clinical symptoms (gastritis, gastric ulcer, duodenal ulcer, and gastric cancer). PCR of the hpaA and omp18 gene loci was performed with primer positions 46 to 761 for hpaA (product length, 716 bp) and primer positions 42 to 481 for omp18 (product length, 440 bp). After amplification, the PCR products were analyzed on 1% agarose gels stained with ethidium bromide. Lane M, size markers.
Immunoreactivity of recombinant proteins with human sera.
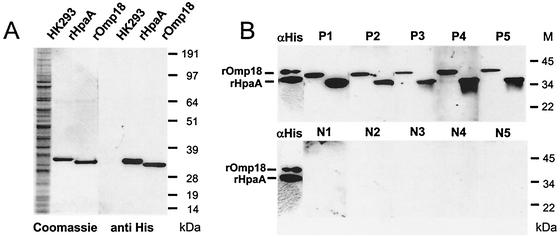
The sequences encoding HpaA and Omp18 were cloned and expressed full-length in the human kidney cell line HK293. Proteins from the transfected cells were purified by affinity chromatography via the His tag through nickel-agarose columns. The purified recombinant proteins were separated by SDS-PAGE. Figure 2A shows the Coomassie blue staining as well as immunoblotting of the His-tagged purified proteins obtained from HK293 cell lysates.
FIG. 2.
Western blot analysis of recombinant HpaA and Omp18 expressed in HK293 cells. (A) The purified recombinant antigens and lysate from untransfected HK293 cells were separated on a 4 to 12% Tricine-SDS-polyacrylamide gel and detected either by Coomassie blue staining (left lanes) or with a murine antibody against the His tag (αHis, 1:5,000) (right lanes). The secondary antibody was peroxidase-labeled anti-mouse IgG (1:20,000). The immunoblots were developed with a chemiluminescence system. The molecular masses are indicated on the right. (B) The purified recombinant antigens were separated on 10% tricine-SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with five H. pylori-positive sera (P1 to P5, 1:500) or five sera from H. pylori-negative individuals (N1 to N5, 1:500). As controls, both recombinant proteins were detected with a murine antibody against the His tag (αHis, 1:5,000). The secondary antibody was peroxidase-labeled anti-human/anti-mouse IgG (1:20,000). The immunoblots were developed with a chemiluminescence system. The molecular masses are indicated on the right.
The purified recombinant proteins were also analyzed by immunoblotting after incubation with sera from infected and uninfected patients. Two bands were detected, indicating reaction of the sera with rHpaA (upper band, 32 kDa) and rOmp18 (lower band, 30 kDa) (Fig. 2B). As illustrated in Fig. 2B, sera from all patients infected with H. pylori recognized both recombinant proteins. As a control, the recombinant proteins were also incubated with an anti-penta-His antibody, showing strong reaction with the two proteins. In contrast, sera from uninfected patients (lower panel) did not show any detectable reaction.
Cytokine production from PBMC after stimulation with recombinant proteins.
PBMC were isolated and stimulated with recombinant HpaA and Omp18 over a 24-h period at a concentration of 20 μg/ml. After incubation, supernatants were collected and cytokine concentrations were determined by ELISA. IL-10 release from PBMC was significantly increased after stimulation with rHpaA (245.5 ± 72.2 pg/ml) and rOmp18 (311.8 ± 64.3 pg/ml) compared to basal IL-10 production (116.2 ± 23.1 pg/ml) (n = 4, p < 0.05). Production of IL-12 was found to be significantly increased only by rHpaA (88.6 ± 18.2 pg/ml) compared to basal secretion (34.3 ± 9.1 pg/ml) (n = 4, p < 0.05), while Omp18 induced only a slight increase (46.8 ± 11.3 pg/ml) (n = 4) in the production of this cytokine.
Stimulation of monocytes and blood DCs with recombinant HpaA and Omp18.
To investigate the importance of monocytes for the presentation of these potential antigens, monocytes were isolated from human peripheral blood and stimulated with the recombinant proteins at a concentration of 20 μg/ml. While there was a high release of IL-8 under basal conditions (21 ± 10 ng/ml), rHpaA and rOmp18 did not alter the secretion significantly (24 to 32 ± 3 ng/ml) in five different experiments (not shown).
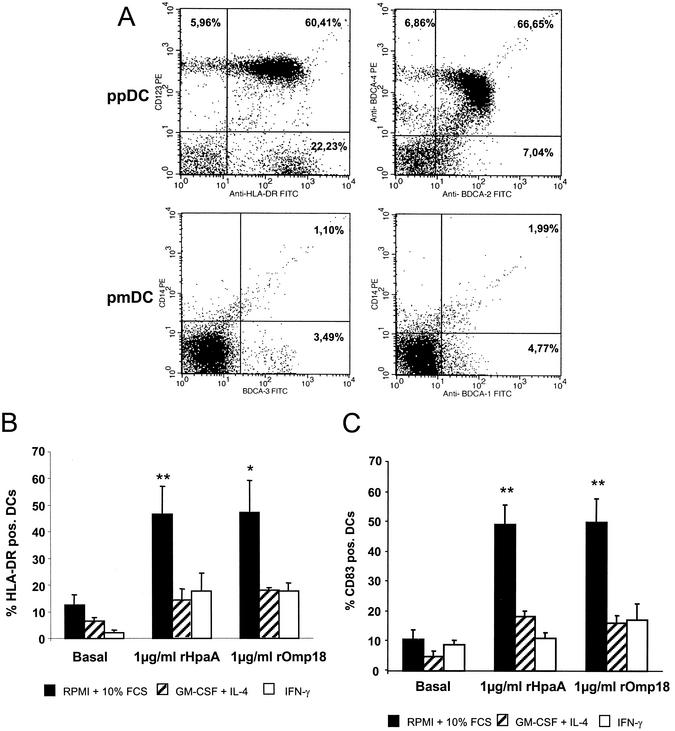
Human DCs were obtained from PBMC with a commercially available magnetic cell separation kit (Miltenyi blood dendritic cell isolation kit). Expression of BDCA-2 and -4 (markers for preplasmacytoid DCs [pp DCs] as well as BDCA-1 and -3 (markers for premyeloid DCs) was determined by FACS analysis in the enriched cell fraction. Figure 3A outlines the results obtained by FACS and shows the enrichment of ppDCs as indicated by the numbers in the quadrants of each individual figure. In the upper left panel, cells that stained positive for CD123 as well as HLA-DR can be considered ppDCs (total of 88.7%). As illustrated in the upper right panel, 80.5% of the cells in this preparation were also BDCA-2 and/or BDCA-4 positive (calculated as follows: 66.6 + 6.86 + 7.04%), i.e., ppDCs, and 6.7% of the cells were BDCA-1 positive (lower right panel, 4.7 + 1.99%) and 4.6% were BDCA-3 positive (3.49 ± 1.1%), indicating the percentage of pmDCs in the preparation.
FIG. 3.
Enrichment, maturation, and activation of blood dendritic cells after stimulation with recombinant HpaA and Omp18. (A) Enrichment of blood DCs after magnetic cell separation was determined by FACS analysis with two antibody combinations to identify ppDCs, anti-BDCA-4 plus anti-BDCA-2 and anti-CD123 plus anti-HLA-DR (upper panels). To identify pmDCs, two other antibody combinations were used, anti-BDCA-1 plus CD14 and anti-BDCA3 plus CD14 (lower panels). (B) Stimulation of MHC class II expression on isolated DCs after incubation with rHpaA or rOmp18 (1 μg/ml) for 24 h shown by the percentage of HLA-DR-positive cells. (C) Stimulation of expression of CD83 costimulatory molecules on DCs after incubation with rHpaA or rOmp18 (1 μg/ml) for 24 h shown by the percentage of CD83-positive cells. N = 6; *, P < 0.05, and **P < 0.01, as determined by one-way analysis of variance.
With this preparation of isolated DCs, the expression of MHC class II and costimulatory molecules, such as CD83, on these cells was subsequently investigated. As shown in Fig. 3B and C, rHpaA and rOmp18 increased the expression of MHC class II and CD83 significantly, indicating the maturation of these antigen-presenting cells. A difference was noted with varions additions to the culture medium. In both experiments, the maximal response was observed in the presence of 10% FCS but the absence of IL-4 or IFN.
Cytokine secretion from blood DCs after incubation with recombinant HpaA and Omp18.
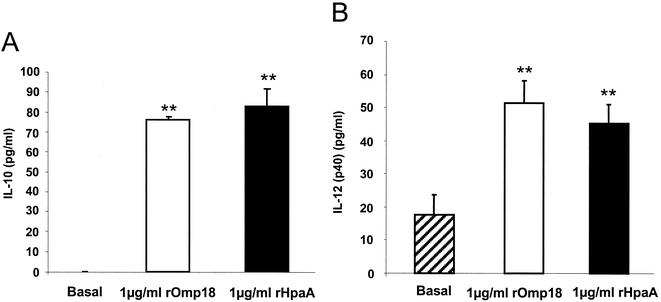
Secretion of cytokines from DCs was determined by ELISA. IL-12, IL-10, and IFN-α were determined under basal conditions or after incubation with the recombinant proteins for 24 h at a concentration of 1 μg/ml (Fig. 4). IL-10 secretion from ppDCs, as determined by ELISA revealed that basal secretion was absent, but both recombinant proteins induced a statistically significant increase (Fig. 4A). Secretion of IL-12 was observed under basal conditions, and rHpaA and rOmp18 both induced a statistically significant increase of IL-12 secretion from pmDCs (Fig. 4B). IFN-α concentrations were also determined in the cell culture supernatant by ELISA, but no secretion was detectable, assuming that the IFN-α concentration was below 3 pg/ml (data not shown). In contrast, the human immunostimulatory CpG oligonucleotide 2616 (5′-TCGTCGTTTTGTCGTTTTGCTGTT-3′) (MWG Biotech AG, Ebersberg, Germany), used as a positive control, induced a strong secretion of IFN-α from isolated DCs (basal, 0 pg/ml versus 94.9 pg/ml with 3 μmol/ml of CpG).
FIG. 4.
Secretion of IL-12 (p40) and IL-10 by DCs is stimulated by recombinant HpaA and Omp18. Blood DCs (maximum 88.6% ppDC, maximum 6.76% pmDCs) were incubated with rHpaA or rOmp18 (1 μg/ml) for 24 h in RPMI-10% FCS. (A) IL-10 secretion from human DCs as determined by ELISA. No secretion of IL-10 was observed under basal conditions (n = 3). (B) IL-12 (p40) concentrations were also determined in the cell culture supernatant by ELISA. Both proteins showed a statistically significant stimulation of IL-10 or IL-12 release (n = 5). Mean values I standard error of the mean are shown. **P < 0.01, as determined by one-way analysis of variance.
DISCUSSION
H. pylori is a gram-negative bacterium that elicits a strong humoral response in the human immune system. Previous studies with proteome-based techniques and subsequent immunoblotting have identified several proteins as potential antigens suitable for diagnosis, therapy, and vaccine development. Of the most frequently recognized H. pylori antigens, approximately 20 showed a consistent and immunodominant response in different studies and were recognized independently of the nature of the associated diseases (15, 19, 31). Among these candidates, the two outer membrane proteins HpaA (HP0797) and Omp18 (HP1125) showed a specific and reversible immune response after eradication (31). These proteins may therefore be of special interest.
In the current study, we determined that the genes encoding HpaA and Omp18 are present in virtually all H. pylori strains tested. Previous studies have investigated the expression and surface localization of HpaA on different bacteria in detail (22). The highest transcriptional activity of the hpaA gene was observed after 3 to 4 days of culture. Interestingly, hpaA was cotranscribed with the downstream omp18 gene. The highest total HpaA protein production in bacteria occurred between days 3 and 7 and was similar in the different strains. By electron microscopy, HpaA protein was detected in all strains both on the bacterial surface and on the flagellar sheath. These studies further confirm our findings that these proteins appear to be expressed in all strains tested and have a surface localization (22). The functions of HpaA and Omp18 remain unclear.
Based on our current data, it is evident that the serological response towards the two proteins is continuously present in H. pylori-infected patients but is absent in persons who are not infected. Recent proteome-based studies have shown that the serological response towards three low-molecular-weight proteins in the range of 34 to 22 kDa (HpaA, Omp18, and HP0596) is decreased following eradication of the bacteria (31). The previous studies, however, used recombinant proteins expressed in bacterial systems. Expression in E. coli may be contaminated by endotoxins, limiting the definition of the true antigenic nature of proteins in in vitro studies. We therefore used recombinant proteins expressed in a human cell line in the current study to determine their antigenic potential. Endotoxin contaminations were excluded by Limulus amoebocyte lysate assays. The recombinant proteins obtained from cell lines also reacted with human sera from H. pylori-infected individuals, confirming the specific reaction of these antigens with human sera.
The strong humoral response suggests that the proteins serve as immunodominant antigens and may be recognized by human DCs, which are equipped with special sensors and receptors to recognize bacterial structures such as lipopolysaccharides, bacterial DNA and outer membrane proteins (2, 29). Dendritic cells can be divided into premyeloid DCs and preplasmacytoid DCs. The latter express BDCA-2 and BDCA-4 on the surface and usually secrete cytokines such as IL-10, IFN-α, and IL-12 to a smaller extent (24, 25). Premyeloid DCs express BDCA-1 and BDCA-3 and secrete IL-12 (29). Our current preparation of DCs showed an enrichment of more than 80% ppDCs and 4 to 6% pmDCs, indicating a direct effect of the proteins on these two cell types after 24 h of incubation. When stimulated with the two recombinant proteins, isolated blood DCs reacted with expression of MHC class II molecules as well as expression of CD83 costimulatory molecules, underlining the specific recognition of the proteins. Other cells detected in the preparation, such as monocytes, were also isolated from blood but did not react with the recombinant proteins.
Stimulation and maturation of DCs are typically associated with the expression of bacterial epitopes on so-called major histocompatibility complexes (MHC) on the surface together with costimulatory molecules (1). MHC class I molecules react with cytotoxic CD8+ T cells; the epitopes are mostly derived from cytosolic proteins which have been ubiquitinylated and degraded in the cytosol of dendritic cells. Expression of MHC class II molecules and costimulatory molecules, e.g., CD83, in contrast, is associated with activation of CD4+ cells and differentiation of T cells into Th1 or Th2 helper cells (1); MHC class II expression can also induce activation of natural killer (NK) cells.
In the current experiment, the two recombinant proteins significantly stimulated the expression of MHC class II and CD83 molecules. These studies suggest that the proteins are indeed processed and presented on the surface of ppDCs via the MHC class II molecule, thereby activating a CD4+ polarized T-cell response. Increased expression of CD83 molecules appears to be effective in potentiating the innate immune response in order to signal the human immune system of infectious danger (5). Interestingly, stimulation of MHC class II expression was only observed in medium containing FCS, not in medium containing granulocyte-macrophage colony-stimulating factor and IL-4. Since ppDC do not express IL-4 receptors, these data emphasize that the ppDCs are in fact the cells in this preparation which react strongly to stimulation with the H. pylori proteins.
Two key cytokines that are released from DCs are IL-12 and IL-10 (14). IL-12 appears to be a requisite for generating optimal Th1 responses and plays a pivotal role in promoting cell-mediated immunity against predominantly intracellular microbial pathogens (14); IL-10, in contrast, appears to promote a Th2 response (12). Here, we show that two recombinant OMPs of H. pylori stimulate IL-12 and more prominently IL-10 secretion of human DCs (13, 23, 25). Haeberle et al. (16) determined that incubation of PBMC with live H. pylori favors the secretion of IL-12 and selection of Th1 cells, while incubation with preparations used in oral vaccines favors IL-10 secretion and a Th2 response. The strong induction of IL-10 release observed in our study suggests interaction of the proteins with ppDCs, underlining the importance of ppDCs for the Th2 and B-cell responses. However, the proteins also induced an increase in IL-12 which was less pronounced but significant. Induction of IL-12, on the other hand, suggests that these proteins also interact with the smaller percentage of pmDC present in the preparation, indicating that the proteins might also lead to a Th1 response.
Experiments in knockout mice have indicated that immunization of mice can only be performed in animals with intact Th1 function and IFN-γ secretion, while knockout of the Th2 response does not impair immunization studies, implicating that Th1-polarized T cells or NK cells are of crucial importance for successful vaccination (6, 8, 11, 16, 30, 32). Gastric T cells from infected mice and humans produce predominantly IFN-γ and tumor necrosis factor alpha, typical for a Th 1-polarized T-cell response (7). It should be noted, however, that the polarization of T cells is affected not only by the nature of the recombinant protein but also by the addition of various adjuvants. As observed here, the proteins appear to induce a mixed Th1/Th2 response, and addition of suitable, i.e., Th1-promoting, adjuvants might help to achieve vaccination in vivo.
In summary, our current results indicate that HpaA and Omp18 may serve as powerful antigens by interacting with human DCs. ppDC and subsequent IL-10 secretion may be crucial players in the process of recognition of H. pylori via OMPs, leading to a strong subsequent humoral immune response. In addition, recognition via pmDC and IL-12 secretion may also activate a Th1-polarized response. These mechanisms may be of special importance for the development of serological assays as well as vaccination strategies.
Acknowledgments
Sponsored by a grant from the Deutsche Forschungsgemeinschaft, DFG 411/7-1, to C.P. and by Fresenius Stiftung, Germany.
Editor: F. C. Fang
REFERENCES
- 1.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 2.Barton, G. M., and R. Medzhitov. 2002. Toll-like receptors and their ligands. Curr. Top. Microbiol. Immunol. 270:81-92. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, M. J. 1996. Role of vacA and the cagA locus of Helicobacter pylori in human disease. Aliment. Pharmacol. Ther. 10(Suppl. 1):73-77. [DOI] [PubMed] [Google Scholar]
- 4.Covacci, A., J. L. Telford, G. G. Del, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 5.Czerniecki, B. J., P. A. Cohen, M. Faries, S. Xu, J. G. Roros, and I. Bedrosian. 2001. Diverse functional activity of CD83+ monocyte-derived dendritic cells and the implications for cancer vaccines. Crit. Rev. Immunol. 21:157-178. [PubMed] [Google Scholar]
- 6.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 7.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 8.Ernst, P. B., and J. Pappo. 2000. Preventive and therapeutic vaccines against Helicobacter pylori: current status and future challenges. Curr. Pharm. Des. 6:1557-1573. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. G., D. J. Evans, Jr., J. J. Moulds, and D. Y. Graham. 1988. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect. Immun. 56:2896-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, D. G., T. K. Karjalainen, D. J. Evans, Jr., D. Y. Graham, and C. H. Lee. 1993. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J. Bacteriol. 175:674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan, X., S. E. Crowe, S. Behar, H. Gunasena, G. Ye, H. Haeberle, H. N. Van, W. K. Gourley, P. B. Ernst, and V. E. Reyes. 1998. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J. Exp. Med. 187:1659-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 13.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 14.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 15.Haas, G., G. Karaali, K. Ebermayer, W. G. Metzger, S. Lamer, U. Zimny-Arndt, S. Diescher, U. B. Goebel, K. Vogt, A. B. Roznowski, B. J. Wiedenmann, T. F. Meyer, T. Aebischer, and P. R. Jungblut. 2002. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2:313-324. [DOI] [PubMed] [Google Scholar]
- 16.Haeberle, H. A., M. Kubin, K. B. Bamford, R. Garofalo, D. Y. Graham, F. El Zaatari, R. Karttunen, S. E. Crowe, V. E. Reyes, and P. B. Ernst. 1997. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect. Immun. 65:4229-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeannin, P., T. Renno, L. Goetsch, I. Miconnet, J. P. Aubry, Y. Delneste, N. Herbault, T. Baussant, G. Magistrelli, C. Soulas, P. Romero, J. C. Cerottini, and J. Y. Bonnefoy. 2000. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat. Immunol. 1:502-509. [DOI] [PubMed] [Google Scholar]
- 18.Jones, A. C., R. P. Logan, S. Foynes, A. Cockayne, B. W. Wren, and C. W. Penn. 1997. A flagellar sheath protein of Helicobacter pylori is identical to HpaA, a putative N-acetylneuraminyllactose-binding hemagglutinin, but is not an adhesin for AGS cells. J. Bacteriol. 179:5643-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmel, B., A. Bosserhoff, R. Frank, R. Gross, W. Goebel, and D. Beler. 2000. Identification of immunodominant antigens from Helicobacter pylori and evaluation of their reactivities with sera from patients with different gastroduodenal pathologies. Infect. Immun. 68:915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konkel, M. E., D. J. Mead, and W. Cleplak, Jr. 1996. Cloning, sequencing, and expression of a gene from Campylobacter jejuni encoding a protein (Omp18) with similarity to peptidoglycan-associated lipoproteins. Infect. Immun. 64:1850-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, S., R. Tobias, S. McClure, G. Styba, Q. Shi, and G. Jackowski. 1997. Removal of endotoxin from recombinant protein preparations. Clin. Biochem. 30:455-463. [DOI] [PubMed] [Google Scholar]
- 22.Lundstrom, A. M., K. Blom, V. Sundaeus, and I. Bolin. 2001. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb. Pathog. 31:243-253. [DOI] [PubMed] [Google Scholar]
- 23.Manetti, R., P. Parronchi, M. G. Giudizi, M. P. Piccinni, E. Maggi, G. Trinchieri, and S. Romagnani. 1993. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 177:1199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 25.Oleksowicz, L., and J. P. Dutcher. 1994. A review of the new cytokines: IL-4, IL-6, IL-11, and IL-12. Am. J. Ther. 1:107-115. [DOI] [PubMed] [Google Scholar]
- 26.O'Toole, P. W., L. Janzon, P. Doig, J. Huang, M. Kostrzynska, and T. J. Trust. 1995. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pylori CCUG 17874 is a lipoprotein. J. Bacteriol. 177:6049-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawelec, D. P., D. Korsak, A. K. Wyszynska, E. Rozynek, J. Popowski, and E. K. Jagusztyn-Krynicka. 2000. Genetic diversity of the Campylobacter genes coding immunodominant proteins. FEMS Microbiol. Lett. 185:43-49. [DOI] [PubMed] [Google Scholar]
- 28.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-256. [DOI] [PubMed] [Google Scholar]
- 29.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 30.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 31.Voland, P., D. L. Weeks, D. Vaira, C. Prinz, and G. Sachs. 2002. Specific identification of three low molecular weight membrane-associated antigens of Helicobacter pylori. Aliment. Pharmacol. Ther. 16:533-544. [DOI] [PubMed] [Google Scholar]
- 32.Wang, J., X. Fan, C. Lindholm, M. Bennett, J. O'Connoll, F. Shanahan, E. G. Brooks, V. E. Reyes, and P. B. Ernst. 2000. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect. Immun. 68:4303-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]