Abstract
Zinc plays a critical role in the normal functioning of the immune system. We investigated whether zinc sulfate administered orally to adult zinc-replete volunteers modulates systemic and intestinal immune responses to an oral killed cholera toxoid B subunit (CTB) whole-cell cholera vaccine. The 30 participants were immunized twice, with a 17-day interval. The vaccinees in the intervention group ingested 45 mg of elemental zinc thrice daily for 9 days starting 2 days before each vaccine dose. The median serum anti-CTB immunoglobulin A (IgA) and IgG responses from day 0 to day 30, i.e. after two vaccine doses, were 13-fold lower (P value for identical distribution, <0.005) in the zinc-supplemented compared to the nonsupplemented vaccinees. The median serum vibriocidal responses from baseline to after one (day 0 to day 17) and two (day 0 to day 30) vaccine doses were at least sixfold (P = 0.033) and fourfold (P = 0.091) higher, while the median fecal anti-CTB IgA response after two doses was estimated to be fourfold higher (P = 0.084) in the zinc-supplemented vaccinees. These observations show that zinc reduces the antitoxin and may enhance the antibacterial responses in serum. Zinc may also improve the intestinal antitoxin immune response. Oral zinc administration has the potential to modify critical immune responses to antigens applied to mucosal surfaces.
As a constituent of many biological systems, zinc is an essential nutrient and plays a critical role in the normal functioning of the immune system (4, 25, 31, 38). Several in vitro studies have shown that the immunomodulatory effects of zinc closely resemble the actions of several well-characterized adjuvants (e.g., aluminum salts) that selectively activate specific T-cell subpopulations controlling immune responses (3, 13).
In vitro studies indicate that there is a delicate zinc concentration dependency for lymphocyte and monocyte activation (22, 31, 38). Zinc dosage regimens for achieving modulation of these processes in vivo are not established and may differ depending on the desired outcome. To achieve adequate immunomodulation, zinc supplementation should probably coincide with the 7 to 10 days of maximum clonal expansion of lymphocytes following immunization (14, 15, 22, 36). The dose administered has to induce adequate zinc concentrations in the appropriate lymphoid tissues (8, 34).
The clinical effects of zinc are known from several studies in developing countries, where zinc supplementation substantially reduces the severity and duration of diarrhea in children and also reduces the incidence of diarrhea (6). Somewhat surprisingly, these effects do not seem to be limited to those who are malnourished or zinc deficient; zinc also has substantial preventive effects in children with normal plasma zinc levels (5).
In order to be efficacious, vaccines against enteric infections should induce a specific secretory immunoglobulin A (S-IgA) as well as an adequate systemic immune response (24). To exploit the practicality of oral immunization and the superiorability of this route to induce protective immune responses, the effects of substances that can modulate the responses to orally administered antigens must be determined. Concomitant parenteral immunization and moderately high-dose zinc supplementation have been undertaken, with inconclusive results (26, 29, 37). To our knowledge, no study has addressed the effect of zinc supplementation on mucosal or systemic immune responses to orally administered antigens in zinc-replete individuals. This trial was conducted to investigate the hypothesis that zinc may exert a pharmacological action on immune responses to antigens in the intestine.
MATERIALS AND METHODS
Study design and subjects.
We performed an open randomized trial to examine whether a short-term, moderately high dose of zinc sulfate given orally to healthy adult volunteers alters the gut mucosal and/or systemic immune responses to a killed cholera toxoid B subunit (CTB) whole-cell cholera vaccine. The study was approved by the Human Research Ethical Committee of the Medical Faculty at the University of Bergen, Bergen, Norway.
We obtained written informed consent from 30 medical students aged 20 to 29 years at the University of Bergen. The students were block randomized into two equal groups. None of the subjects had been immunized against cholera previously or traveled outside Scandinavia during the last 6 months.
Zinc administration and immunization.
The 15 vaccinees in the intervention group were instructed to ingest one effervescent tablet containing 45 mg of elemental zinc in 200 mg of zinc sulfate (Solvezink; Tika Läkemedel AB, Lund, Sweden) thrice daily for two periods of 9 days each starting 2 days before each vaccine dose. The 15 vaccinees in the control group received no zinc sulfate.
All 30 participants were immunized with two doses of the killed CTB whole-cell cholera vaccine (Dukoral; SBL Vaccin AB, Stockholm, Sweden) with a 17-day interval. Vaccination was performed at least 1 h before or 2 h after any meal and not in conjunction with the intake of zinc sulfate. Each vaccine dose, which contained 1 mg of recombinantly produced CTB and 1011 killed O1 vibrios, was administered in 150 ml of a sodium bicarbonate solution (18).
Specimen collection and processing and registration of side effects.
Blood for biochemical and immunological analyses was obtained 3 days before the first vaccine dose, i.e., the day before initiation of zinc supplementation (day 0), and on days 10, 17, and 30. To minimize the effects of diurnal variation on serum zinc concentration, we collected all serum samples at the same time of day.
Approximately 2 g of freshly voided feces was collected on days 0 and 30 by the vaccinees at home less than 12 h prior to the collection of blood samples and immediately stored in their home freezers or refrigerators. The samples were transferred to −70°C within the next 12 h. Fecal extracts (FEs) were made as described previously (12).
Subjective side effects to the vaccine and the zinc sulfate and any serious illness during a 3-month period following completion of the trial were recorded.
Determination of systemic and intestinal immune responses.
Levels of serum antibodies against the bacterial whole-cell components were determined with a vibriocidal assay (23). Samples were twofold serially diluted from an initial dilution of 1:5, and titers, i.e., the reciprocal serum dilution giving complete bactericidal activity, were determined. Titers of <5 U/ml were assigned a value of 2.5 U/ml. In each vaccinee, the log vibriocidal response was calculated as the postimmunization minus the preimmunization log titer.
Anti-CTB IgA and IgG in serum and IgA in FEs were determined by a GM1 enzyme-linked immunosorbent assay (ELISA) (35). Endpoint titers were determined as the reciprocal dilution of the samples giving an optical density at 490 nm (OD490) of 0.4 above the background. Fecal specimens with OD490 values of up to 0.2 above background in the well with the lowest dilution (i.e., 1:2) were assigned the log titer 0 in the anti-CTB IgA ELISA. Similarly, FEs with first-well OD490 values between 0.2 and 0.35 above background were assigned 0.15 in log titer, while samples between 0.35 and 0.4 were given a log titer of 0.3. These ordered estimates for samples with first-well OD490 values between 0.2 and 0.4 above background allowed us to undertake ranked data analysis and, after assessing the shape of the distributions, analyses based on the normal distribution. A high-titered reference serum or FE was included in each microtiter plate to compensate for any variation between analyses on different occasions. All serum samples from each vaccinee were analyzed on the same plate, as were the FEs. The antibody titer assigned to each sample was the mean from duplicate determinations.
Concentrations of total IgA in the FEs were determined by a modified microplate ELISA (2, 12). Vaccinees with a total IgA concentration of <10 μg/ml in either the day 0 or the day 30 FE were excluded from the analyses because determination of specific antibody titers in such specimens is unreliable (2). The specific IgA antibody titers were divided by the total IgA concentrations in the FEs to obtain the fecal anti-CTB IgA indices, thereby adjusting for variations in the IgA content in fecal samples collected from the same vaccinee on different days (2, 13). All vaccinees had a less than 10-fold change in the total FE IgA concentration from day 0 to day 30, a prerequisite for using the anti-CTB index (2).
Determination of serum zinc concentration.
Serum zinc concentration was determined at 206.2 nm by inductively coupled plasma atomic emission spectrometry (ICP-AES) on an IRIS/AP spectrometer (Thermo Jarell-Ash, Franklin, Mo.) (28). Serum C-reactive protein (CRP) concentration was determined by an immunoturbidometric, micro-CRP method (Tina-Quant; Roche) on a Hitachi 917 (10).
Sample size calculations.
Sample sizes were calculated to obtain a power of 80% with the conventional 5% significance level. We intended to identify a fivefold difference (corresponding to a log difference of 0.7) in the mean serum vibriocidal and anti-CTB responses as well as in the mean intestinal anti-CTB response among the zinc-supplemented compared to the nonsupplemented vaccinees. Assuming that the standard deviations of the log-transformed serum vibriocidal and anti-CTB responses and fecal anti-CTB IgA indices would be no more than 0.65 (1, 2, 17, 18), we would need 14 individuals in each group to identify the desired effect of zinc. We accordingly recruited 15 individuals to each study group.
Statistical analyses.
We used the Wilcoxon rank sum test to compare the immune responses in each vaccinee, i.e., the post- minus the preimmunization serum anti-CTB and vibriocidal titers and the fecal anti-CTB IgA indices, between the zinc-supplemented and the control group. To adjust for differences in preimmunization vibriocidal antibody titers, we used the Wilcoxon rank sum test stratified on the proportion of vaccinees that had baseline vibriocidal antibody titers of ≥40 because such high titers prior to vaccination have been shown to impinge on vibriocidal responses (18). Similarly, to control for differences in preimmunization fecal anti-CTB indices, we used the Wilcoxon rank sum test stratified on the proportion of vaccinees that had baseline anti-CTB indices of greater than the 75th percentile of the vaccinees included in this analysis. Independent-sample t tests were used to compare differences in serum zinc and CRP between the zinc group and the control group.
We computed the Spearman's rank correlation coefficient, rho, to examine the association between anti-CTB responses from day 0 to day 30 in serum and in feces. The distributions of the log-transformed post- versus preimmunization differences in anti-CTB titers in serum and in fecal anti-CTB indices were reasonably symmetrical.
In order to examine the relationship between serum responses on one hand and zinc supplementation and fecal responses on the other, we accordingly used linear regression of differences in log-transformed serum anti-CTB titers on zinc supplementation and differences in log-transformed fecal anti-CTB indices.
RESULTS
Preimmunization status.
Based on the predefined criteria, three zinc-supplemented vaccinees were excluded from the analyses of fecal immune responses. Preimmunization titers are shown in Table 1. Prior to the intervention, there was no substantial difference in serum zinc levels between the zinc group (mean, 11.3 μM; standard deviation [SD], 1.71 μM) and the control group (mean, 12.4 μM; SD, 1.77 μM).
TABLE 1.
Preimmunization serum antitoxin titers, serum vibriocidal titers, and fecal anti-CTB IgA indices in nonsupplemented and zinc-supplemented adult volunteers receiving an oral killed CTB whole-cell cholera vaccine
| Group | Median titer (interquartile range) in serum
|
No. of vaccinees with preimmunization titer of ≥40a (n = 15/group) | Fecal anti-CTB IgA indexb | No. of vaccinees with preimmunization index of ≥0.77/ no. in groupc | ||
|---|---|---|---|---|---|---|
| Anti-CTB IgA | Anti-CTB IgG | Vibriocidal antibody | ||||
| Control | 3.9 (2.1, 12.5) | 6.7 (4.7, 9.4) | 2.5 (2.5, 5) | 1 | 0.48 (0.099, 1.23) | 5/15 |
| Zinc supplemented | 3.1 (1.7, 10.2) | 5.7 (2.8, 7.7) | 2.5 (2.5, 40) | 4 | 0.12 (0.061, 0.24) | 2/12 |
Cutoff was 20.
For the 15 vaccinees in the control group and the 12 vaccinees included in the zinc group.
Cutoff, 0.77, the 75th percentile of the 27 vaccinees included in the analyses of fecal responses.
Systemic and intestinal immune responses.
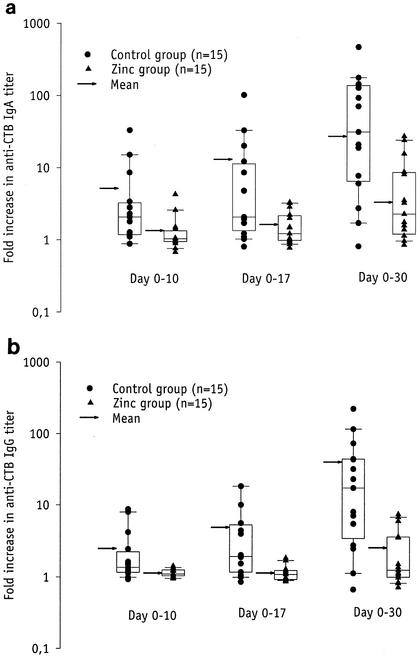
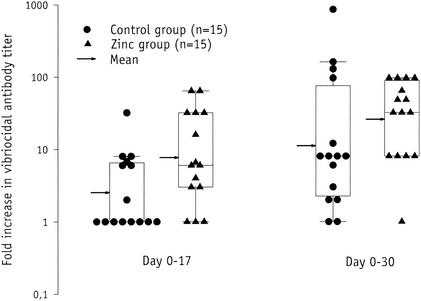
The rise in serum anti-CTB IgA and IgG titers from day 0 to day 30 (Fig. 1a and 1b) were significantly lower in the vaccinees receiving zinc (median, 2.3-fold and 1.3-fold, respectively) than among the controls (median, 31-fold and 17-fold, respectively) (P = 0.004 and P = 0.001, respectively). The rise in vibriocidal titers from day 0 to day 17 and from day 0 to day 30 (Fig. 2) was higher in the zinc group (median, 6-fold and 32- fold, respectively) than in the control group (median, 1-fold and 8-fold, respectively) (P = 0.061 and P = 0.16, respectively). The corresponding P values obtained when stratifying the analysis on the proportion of vaccinees that had baseline vibriocidal antibody titers of ≥40 were 0.033 and 0.091, respectively, indicating that the effect of zinc supplementation on vibriocidal responses was negatively confounded by the baseline titers.
FIG. 1.
Serum anti-CTB IgA (a) and IgG (b) titer rises 7 (day 10) and 14 (day 17) days after the first dose and 10 (day 30) days after the second dose of an oral killed CTB whole-cell cholera vaccine in nonsupplemented (•) and zinc-supplemented (▴) vaccinees. The box provides the interquartile range, the horizontal line within the box gives the median, and the whisker caps enclose values between the 10th and 90th percentiles. The arrow indicates the geometric mean.
FIG. 2.
Serum vibriocidal antibody titer rise 14 (day 17) days after the first and 10 (day 30) days after the second dose of an oral killed CTB whole-cell cholera vaccine in nonsupplemented (•) and zinc-supplemented (▴) vaccinees. The box provides the interquartile range, the horizontal line within the box gives the median, and the whisker caps enclose values between the 10th and 90th percentiles. The arrow indicates the geometric mean.
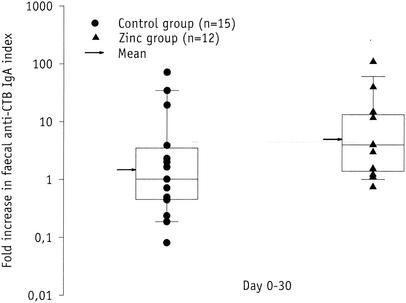
The rise in the fecal anti-CTB IgA index from day 0 to day 30 (Fig. 3) was higher in the zinc group (median, fourfold) than in the control group (median, onefold) (P = 0.045). When stratifying the analysis on the proportion of vaccinees that had baseline anti- CTB indices of ≥0.77, corresponding to the 75th percentile of the 27 vaccinees included in this analysis, the adjusted P value was 0.084.
FIG. 3.
Fecal anti-CTB IgA index rise in nonsupplemented (•) and zinc- supplemented (▴) volunteers immunized with an oral killed CTB whole-cell cholera vaccine. The box provides the interquartile range, the horizontal line within the box gives the median, and the whisker caps enclose values between the 10th and 90th percentiles. The arrow indicates the geometric mean.
Association between zinc supplementation, fecal antibody response, and serum antibody response.
There was a negative correlation between the anti-CTB response in feces and the anti-CTB IgA response in serum (rho = −0.40, P = 0.041). Linear regression with the log-transformed anti-CTB IgA serum response as the dependent and the log- transformed fecal anti-CTB response as the independent variable yielded a regression coefficient (β) of −0.36 (95% confidence intervals [CI], −0.77, 0.042) and an R2 of 0.12 (P = 0.077). In the zinc group, β was found to be −0.12 (95% CI, −0.71, 0.47; P = 0.66) while in the control vaccinees, β was −0.24 (95% CI, −0.80, 0.33; P = 0.39). As these regression coefficients were similar and close to 0, we included zinc supplementation as well as the log-transformed fecal anti-CTB response as covariates in the regression model to examine whether zinc induced the increased intestinal and the decreased serum response in the same individuals. The model yielded an R2 of 0.32 (P = 0.0091), while the β for fecal responses was −0.20 (95% CI, −0.58, 0.19; P = 0.30) and for zinc supplementation it was −0.80 (95% CI, −1.4, −0.19; P = 0.013).
Serum zinc and CRP concentrations.
On day 10, the serum zinc levels were somewhat higher in the zinc-supplemented than in the control vaccinees (mean, 12.5; [95% CI, 11.3, 13.8] versus 11.1 [95% CI, 10.1, 12.1] μM, P = 0.064), with only a single participant having a level above the upper normal limit of 16 μM, at 16.9 μM. The difference between the groups had disappeared by day 17. There were no important or significant differences in CRP levels between the zinc-supplemented and the control group at any point.
Reported side effects from vaccination and zinc administration.
One of 30 vaccinees experienced mild upper gastrointestinal disturbance following vaccine intake. Two of 15 did not like the taste of the Solvezink solution, but there were no defaulters. No serious illness was reported during a 3-month follow-up period.
DISCUSSION
This is, to our knowledge, the first randomized trial to demonstrate that short-term supplementation with moderately high doses of zinc substantially modulates immune responses to an oral vaccine in zinc-replete individuals.
In the control group, the proportion of vaccinees with more than a twofold increase in serum anti-CTB IgA and IgG titers (87%) and more than a fourfold increase in vibriocidal antibody titers (67%) was in line with previous observations (1, 16, 18). The proportion of vaccinees with more than a twofold increase in fecal anti-CTB index (47%) was somewhat lower than that reported previously (2). This may be explained by the difference in sample collection procedures, as the fecal specimens in the previous studies were collected after the ingestion of intestinal lavage fluids. The determination of fecal antibody titers is generally subject to a higher degree of methodological error than that of serum titers (11), and three individuals had to be excluded from the analyses of fecal anti-CTB IgA index. Thus, our estimate of the intestinal anti-CTB IgA response is likely to have a lower precision than that of the anti-CTB IgA and IgG and the vibriocidal responses in serum.
The anti-CTB IgA responses in feces and in serum were negatively correlated, indicating that the somewhat stronger intestinal response was associated with a substantially poorer response in serum. The linear regression analysis showed that while intestinal response alone explained only 12%, the combined and adjusted effects of zinc supplementation and intestinal response explained 32% of the variation in serum anti-CTB responses. Adjusting for zinc supplementation, which had a strong negative impact on the serum response, the intestinal response was weakly and nonsignificantly associated with a decreased serum response. The baseline anti-CTB index did not confound the observed associations (data not shown). These findings indicate that zinc may suppress the systemic and enhance the intestinal antitoxin response in the same individual. Larger studies with more precise methods for measuring intestinal immunity (2, 11) are required to examine whether and to what extent the negative impact of zinc on the serum immune response to CTB is mediated by an enhanced intestinal immune response (7, 33).
The enhanced vibriocidal and decreased antitoxin responses in serum mirror the changes in serum responses reported to have occurred in young Bangladeshi children supplemented with zinc and vitamin A (27). Since all our study subjects were well nourished and zinc replete, the similarity in the responses noted in the two populations may suggest that at least part of the effect among the children could have been pharmacological rather than a result of replenishing their zinc and vitamin A stores. Such a mechanism could explain some of the therapeutic and preventive action of zinc against diarrhea observed in zinc-replete individuals (5).
The vibriocidal response in the zinc group from day 0 to day 17, i.e., after the first vaccine dose, resembled that in the control group 2 weeks later, i.e., 10 days after the booster vaccination. If these findings are confirmed, the killed CTB whole-cell cholera vaccine in combination with zinc could prove useful for controlling cholera outbreaks, giving a substantial vibriocidal response after just a single dose.
Because of the substantially larger impact on the systemic than on the intestinal response and the less precise estimates of the latter, a search for alternative explanations is warranted. The reduced serum antitoxin responses in the zinc group could be a consequence of a generalized decrease in T-cell responsiveness (8, 9, 22, 38). However, the enhanced systemic vibriocidal response makes global systemic immunosuppression an unlikely explanation for the reduced serum anti-CTB responses.
Immunomodulating substances are important for both immunogenic and tolerogenic mucosal vaccines currently under development. A major challenge is to achieve acceptable safety while maintaining the desired immunomodulation. Humans appear to have the capacity to effectively regulate whole-body zinc content over a 10-fold change in intake (19, 20, 21, 30). Short-term intake of zinc even severalfold above the recommended daily allowance is considered safe in children and adults (30, 32). In the present study, there were no substantial differences in serum zinc or CRP levels between the zinc and the control group at any point, and subjective side effects were negligible. One day after completion of the first zinc supplementation period, serum zinc was somewhat higher in the subjects that had received zinc than among the controls, a difference that disappeared 1 week later, reflecting effective homeostatic control (20).
The distribution of the vibriocidal responses was not sufficiently symmetric to use methods based on the normal distribution when estimating the effect of zinc on the antibacterial immune responses to the vaccine. Moreover, as with many trials of limited size, randomization failed to distribute relevant baseline characteristics, notably the preimmunization serum vibriocidal titers and fecal anti-CTB indices, equally between the intervention groups. Although we did adjust for the baseline differences in the statistical analyses, larger studies must be undertaken to increase the chance that randomization yields a balance between the intervention groups, to be able to analyze the data by methods based on the normal distribution and to yield more precise effect measures.
While the present study clearly demonstrates a negative impact of the zinc supplementation regimen on the serum anti-CTB responses, the more uncertain enhancement of vibriocidal responses finds support in the findings from Bangladesh (27). The possible increase in fecal anti-CTB response has not been reported earlier. These findings provide an impetus to undertake further studies of the effect of zinc supplementation on immune responses to intestinally delivered antigens, not only in populations expected to be zinc deficient but also in well-nourished individuals. The impact of oral zinc administration on immune responses to individual cholera antigens (both toxins and somatic components) and to components of other mucosal vaccines must be established. The present zinc supplementation regimen must be optimized and, if possible, simplified. Ultimately, phase III trials are required to measure if any adjuvant effect is reflected in higher vaccine efficacy.
Acknowledgments
We thank the study participants for their cooperation throughout the study. Technical assistance from Kai Günther Brandt and Camilla Johansson is appreciated. We thank Ann-Mari Svennerholm at the Department of Medical Microbiology and Immunology, University of Gothenburg, for the vibriocidal antibody assays, Tor-Arne Hagve at the Department of Clinical Chemistry, Rikshospitalet University Hospital, Oslo, Norway, for the micro-CRP analysis, and Michael Hambidge and Nancy Krebs, University of Colorado Health Sciences Center, Denver, Colo., for quality control of serum zinc analyses.
The study was supported in part by ForInnova A/S through their grant to H. M. S. Grewal. The activities of the Danish Epidemiology Science Centre were supported by a grant from the Danish National Research Foundation.
Editor: J. D. Clements
REFERENCES
- 1.Ahrén, C., C. Wenneras, J. Holmgren, and A. M. Svennerholm. 1993. Intestinal antibody response after oral immunization with a prototype cholera B subunit-colonization factor antigen enterotoxigenic Escherichia coli vaccine. Vaccine 11:929-934. [DOI] [PubMed] [Google Scholar]
- 2.Ahrén, C., M. Jertborn, and A. M. Svennerholm. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect. Immun. 66:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audibert, F. M., and L. D. Lise. 1993. Adjuvants: current status, clinical perspectives and future prospects. Immunol. Today 14:281-284. [DOI] [PubMed] [Google Scholar]
- 4.Beck, F. W., A. S. Prasad, J. Kaplan, J. T. Fitzgerald, and G. J. Brewer. 1997. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 272:E1002-E1007. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari, N., R. Bahl, S. Taneja, T. Strand, K. Molbak, R. J. Ulvik, H. Sommerfelt, and M. K. Bhan. 2002. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics 109:e86. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta, Z. A., R. E. Black, K. H. Brown, J. M. Gardner, S. Gore, A. Hidayat, F. Khatun, R. Martorell, N. X. Ninh, M. E. Penny, J. L. Rosado, S. K. Roy, M. Ruel, S. Sazawal, and A. Shankar. 1999. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigator's Collaborative Group. J. Pediatr. 135:689-697. [DOI] [PubMed] [Google Scholar]
- 7.Challacombe, S. J., and T. B. Tomasi, Jr. 1980. Systemic tolerance and secretory immunity after oral immunization. J. Exp. Med. 152:1459-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driessen, C., K. Hirv, L. Rink, and H. Kirchner. 1994. Induction of cytokines by zinc ions in human peripheral blood mononuclear cells and separated monocytes. Lymphokine Cytokine Res. 13:15-20. [PubMed] [Google Scholar]
- 9.Duchateau, J., G. Delespesse, and P. Vereecke. 1981. Influence of oral zinc supplementation on the lymphocyte response to mitogens of normal subjects. Am. J. Clin. Nutr. 34:88-93. [DOI] [PubMed] [Google Scholar]
- 10.Eda, S., J. Kaufmann, W. Roos, and S. Pohl. 1998. Development of a new microparticle-enhanced turbidimetric assay for C-reactive protein with superior features in analytical sensitivity and dynamic range. J. Clin. Lab. Anal. 12:137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson, A., K. A. Humphreys, and N. M. Croft. 1995. Technical report: results of immunological tests on faecal extracts are likely to be extremely misleading. Clin. Exp. Immunol. 99:70-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal, H. M., T. H. Karlsen, H. Vetvik, C. Ahren, H. K. Gjessing, H. Sommerfelt, and B. Haneberg. 2000. Measurement of specific IgA in faecal extracts and intestinal lavage fluid for monitoring of mucosal immune responses. J. Immunol. Methods 239:53-62. [DOI] [PubMed] [Google Scholar]
- 13.Hadden, J. W. 1994. T-cell adjuvants. Int. J. Immunopharmacol. 16:703-710. [DOI] [PubMed] [Google Scholar]
- 14.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1984. Gut mucosal, salivary and serum antitoxic and antibacterial antibody responses in Swedes after oral immunization with B subunit-whole cell cholera vaccine. Int. Arch. Allergy Appl. Immunol. 75:38-43. [DOI] [PubMed] [Google Scholar]
- 15.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1988. Five-year immunologic memory in Swedish volunteers after oral cholera vaccination. J. Infect. Dis. 157:374-377. [DOI] [PubMed] [Google Scholar]
- 16.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1993. Evaluation of different immunization schedules for oral cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine 11:1007-1012. [DOI] [PubMed] [Google Scholar]
- 17.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1994. Immunological memory after immunization with oral cholera B subunit- whole-cell vaccine in Swedish volunteers. Vaccine 12:1078-1082. [DOI] [PubMed] [Google Scholar]
- 18.Kilhamn, J., M. Jertborn, and A. M. Svennerholm. 1998. Kinetics of local and systemic immune responses to an oral cholera vaccine given alone or together with acetylcysteine. Clin. Diagn. Lab. Immunol. 5:247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King, J. C. 1990. Assessment of zinc status. J. Nutr. 120(Suppl. 11):1474-1479. [DOI] [PubMed] [Google Scholar]
- 20.King, J. C., D. M. Shames, and L. R. Woodhouse. 2000. Zinc homeostasis in humans. J. Nutr. 130:1360s-1366s. [DOI] [PubMed] [Google Scholar]
- 21.Krebs, N. F. 2000. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 130:1374s-1377s. [DOI] [PubMed] [Google Scholar]
- 22.Malave, I., S. Claverie Benureau, and I. R. Benaim. 1983. Modulation by zinc of the in vitro antibody response to T-dependent and T-independent antigens. Immunol. Commun. 12:397-406. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre, O. R., and J. C. Feeley. 1964. Passive serum protection of the infant rabbit against experimental cholera. J. Infect. Dis. 114:468-475. [DOI] [PubMed] [Google Scholar]
- 24.Mestecky, J., R. Abraham, and P. L. Ogra. 1994. Common mucosal immune system and strategies for the development of vaccines effective at the mucosal surfaces, p. 357-371. In P. L. Ogra, W. Strober, J. Mestecky, J. McGhee, M. E. Lamm, and J. Bienstock (ed.), Handbook of mucosal immunology. Academic Press Inc., New York, N.Y.
- 25.Prasad, A. S. 1995. Zinc: an overview. Nutrition 11:93-99. [PubMed] [Google Scholar]
- 26.Provinciali, M., A. Montenovo, G. Di Stefano, M. Colombo, L. Daghetta, M. Cairati, C. Veroni, R. Cassino, F. Della Torre, and N. Fabris. 1998. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: a randomized controlled trial. Age Ageing 27:715-722. [DOI] [PubMed]
- 27.Quadri, F., M. J. Albert, M. A. Wahed, A. H. Baqui, G. J. Fuchs, J. Clemens, and R. E. Black. 2000. Effect of vitamin A and zinc supplementation on immune responses to oral cholera vaccination. Yearly Report from the International Centre for Diarrheal Disease Research, Dacca, Bangladesh. [Online.] http://www.icddrb.org/ar2000/ar2000_isd.html.
- 28.Rahil Khazen, R., B. J. Bolann, and R. J. Ulvik. 2000. Trace element reference values in serum determined by inductively coupled plasma atomic emission spectrometry. Clin. Chem. Lab. Med. 38:765-772. [DOI] [PubMed] [Google Scholar]
- 29.Rawer, P., W. R. Willems, T. Breidenbach, W. Guttmann, W. Pabst, and G. Schutterle. 1987. Seroconversion rate, hepatitis B vaccination, hemodialysis, and zinc supplementation. Kidney Int. Suppl. 22:149-152. [PubMed] [Google Scholar]
- 30.Rimbach, G., A. Markant, J. Pallauf, and K. Kramer. 1996. Zink—Update eines essentiellen Spurenelements. Z. Ernaehrwiss. 35:123-142. [DOI] [PubMed] [Google Scholar]
- 31.Rink, L., and H. Kirchner. 2000. Zinc-altered immune function and cytokine production. J. Nutr. 130:1407s-1411s. [DOI] [PubMed] [Google Scholar]
- 32.Shankar, A. H., and A. S. Prasad. 1998. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68:447s-463s. [DOI] [PubMed] [Google Scholar]
- 33.Strobel, S., and A. M. Mowat. 1998. Immune responses to dietary antigens: oral tolerance. Immunol. Today 19:173-181. [DOI] [PubMed] [Google Scholar]
- 34.Sturniolo, G. C., C. Mestriner, P. Irato, V. Albergoni, G. Longo, and R. D'Inca. 1999. Zinc therapy increases duodenal concentrations of metallothionein and iron in Wilson's disease patients. Am. J. Gastroenterol. 94:334-338. [DOI] [PubMed] [Google Scholar]
- 35.Svennerholm, A. M., J. Holmgren, R. Black, M. Levine, and M. Merson. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J. Infect. Dis. 147:514-522. [DOI] [PubMed] [Google Scholar]
- 36.Svennerholm, A. M., M. Jertborn, L. Gothefors, A. M. Karim, D. A. Sack, and J. Holmgren. 1984. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J. Infect. Dis. 149:884-893. [DOI] [PubMed] [Google Scholar]
- 37.Turk, S., S. Bozfakioglu, S. T. Ecder, T. Kahraman, N. Gurel, R. Erkoc, N. Aysuna, A. Turkmen, N. Bekiroglu, and E. Ark. 1998. Effects of zinc supplementation on the immune system and on antibody response to multivalent influenza vaccine in hemodialysis patients. Int. J. Artif. Organs 21:274-278. [PubMed] [Google Scholar]
- 38.Wellinghausen, N., H. Kirchner, and L. Rink. 1997. The immunobiology of zinc. Immunol. Today 18:519-521. [DOI] [PubMed] [Google Scholar]