Abstract
Early after the intranasal instillation of Bordetella bronchiseptica into mice, not only are mature dendritic leukocytes recovered from lung parenchyma and bronchoalveolar lavage fluid but their numbers are also increased in the mediastinal lymph nodes and the nasal mucosa-associated lymphoid tissue. Later during the infectious process, the bacteria persist mainly in the nasal cavity.
Bordetella bronchiseptica can be isolated from many mammals suffering from respiratory disease (3, 7, 40). In rodents, rabbits, dogs, cats, and pigs, typically B. bronchiseptica can also establish asymptomatic infections, with the bacteria persisting indefinitely in the upper respiratory tract (34). These data correspond with the observation that B. bronchiseptica-reactive antibodies (Abs) and T lymphocytes have a long-lasting presence in mice (9). B. bronchiseptica may also colonize the human respiratory tract and may be isolated as the pathogenic agent of respiratory symptoms diagnosed in immunocompromised hosts (2, 5, 7, 10). B. bronchiseptica is closely related to Bordetella pertussis, the agent of whooping cough (1, 8). The bacterium expresses several adhesins and toxins related to its disease-causing potential: filamentous hemagglutinin, fimbriae, pertactin, tracheal cytotoxin, dermonecrotic toxin, adenylate cyclase-hemolysin, and type III secreted polypeptides. The expression of the genes encoding these factors, with the exception of tracheal cytotoxin, is positively regulated by the regulatory BvgA and BvgS proteins. These proteins belong to the large family of environment-sensing regulatory proteins, a family that comprises two-component regulatory systems (24). In addition to positively regulating the vag (virulence-activated genes) genes encoding adhesins and toxins typical of a phase I phenotype bacterium, B. bronchiseptica harbors a number of important repressed genes (virulence-repressed genes, or vrg) encoding proteins such as iron scavengers, flagella, urease, and phosphatase (for a review, see reference 24). The expression of vrg, typical of a phase IV phenotype, occurs only under modulating conditions (e.g., a temperature change or the presence of modulators). A second two-component regulatory system, encoded by the ris locus, is essential for bacterial resistance to oxidative stress, production of acid phosphatase, and in vivo persistence (16). Of note, a type III secretion system has also been shown to play a role in mediating B. bronchiseptica persistence in the lower respiratory tract (41).
Dendritic cells (DCs), once they are mature and located in the T-cell areas of secondary lymphoid tissues, are potent antigen-presenting cells that are essential for the development of primary immune effector and regulatory cells that are reactive to microbial peptides and lipids (21, 26, 28). Airway DCs form a highly developed network of leukocytes that reside in the lateral intercellular spaces formed by basal epithelial cells and are thus ideally positioned to capture and process potentially immunogenic material that is deposited in the conducting airways (6, 14, 19, 29, 35, 38). Airway DCs originate from either myeloid or lymphoid precursors, and in these nonlymphoid spaces, they are functionally immature under steady-state conditions (33, 36, 37). When they sense, process, and display immunostimulating signals, DCs move to the T-cell-dependent areas of secondary lymphoid organs, where they become mature DCs and display the counterligands of T lymphocytes, including their T-cell receptors (27). Two main secondary lymphoid organs may be the sites of delivery of either immunogenic or tolerance-producing signals following the intranasal deposition of bacteria, depending on the extent of dissemination from the nasal cavity towards the lower respiratory tract; while the nasal mucosa-associated lymphoid tissue (NALT) drains the nasal cavity (4, 17), the cervical and mediastinal lymph nodes drain the downstream respiratory tract (31).
In the present study, our aims were to analyze the interactions between B. bronchiseptica and DCs in vivo by using a model relying on the intranasal delivery of the bacteria triggered to express the phase I program and to build on previous data (8) by considering the unique features of the upper respiratory tract that distinguish the NALT-free nasal epithelium and subepithelium and the NALT. The main lineage under study was the CD11c-positive DC lineage. For the lower respiratory tract, both the lung parenchyma and the mediastinal lymph nodes, in addition to the classical biological sample, namely bronchoalveolar lavage (BAL) fluid, were monitored.
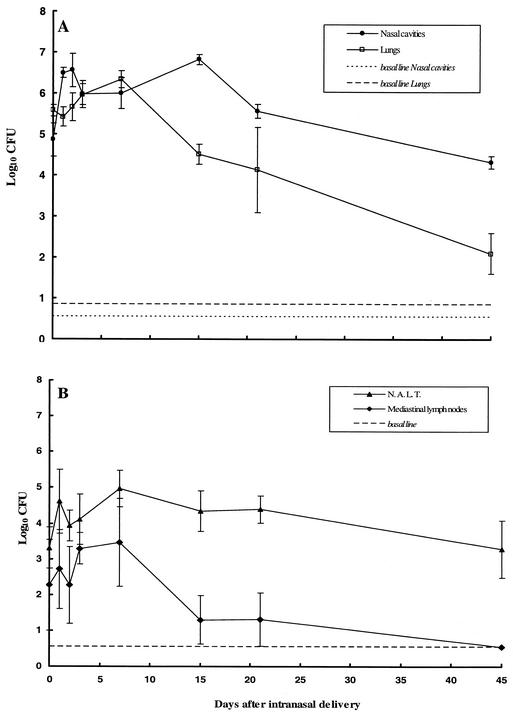
Persistence of B. bronchiseptica in the nasal cavity and NALT of infected mice.
The isolate used in this study was B. bronchiseptica 9.73H+, a phase I isolate expressing adhesins and toxins collected from a hare. The isolate was always freshly cultured from the lungs of an infected mouse 3 to 5 days after intranasal delivery, as described previously (11). Groups of 4-week-old female BALB/c mice (Charles River, Saint-Aubin-les-Elbeuf, France) were lightly anesthetized with ether and then infected with bacterial suspensions containing 5.5 × 105 bacteria; these bacterial suspensions were delivered into the nasal cavity in a volume of 50 μl. The colonization of both lymphoid tissues, especially NALT and the posterior mediastinal lymph nodes, and nonlymphoid tissues (the entire lungs and the nasal cavity epithelium and subepithelium after the excision of NALT) was quantified by homogenizing each tissue in saline, plating aliquots onto Bordet-Gengou blood agar, and counting the colonies present after 2 days of incubation at 36°C. To collect NALTs, we dissected mice by the method described previously, but with some modifications, using a dissection microscope (Fisher Bioblock Scientific, Illkirch, France) (13). The collected NALTs were gently teased into saline. The epithelium and subepithelium lining the nasal cavity were excised and collected in saline after the nasal septum was sectioned with the use of a dissection microscope. Posterior mediastinal lymph nodes localized along the trachea, under the thymus, were excised and collected in saline, and then bacterial colonization was studied. Figure 1 illustrates that in the lower respiratory tract, a B. bronchiseptica phase I isolate was able to colonize the lungs (Fig. 1A) and the mediastinal lymph nodes (Fig. 1B) but did not persist in these tissues. However, the bacteria persisted in the nasal cavity (Fig. 1A), a finding that is consistent with previous data (12), and in the NALT (Fig. 1B) until 232 days after infection (data not shown). Thus, in mice, B. bronchiseptica bacteria persist not only at the level of the epithelium and subepithelium lining the nasal cavity but also within the NALT.
FIG. 1.
Colonization of lymphoid and nonlymphoid tissues after intranasal delivery of B. bronchiseptica. A total of 5.5 × 105 CFU of freshly collected bacteria was delivered intranasally to mice. B. bronchiseptica colonization of nonlymphoid tissues (lungs [□] and nasal cavities [•)]) and lymphoid tissues (NALT [(▴)] and mediastinal lymph nodes [(⧫)]) was analyzed. The plots show means ± standard errors of the means (bars) for 3 to 22 tested mice at each time point in nine separate experiments.
Early changes in CD11c-positive leukocyte numbers in BAL fluids and lung tissues after intranasal B. bronchiseptica delivery.
The recruitment of granulocytes and DCs in the BAL fluids and lungs from infected mice was analyzed. Infected mice were injected intraperitoneally with 2% xylazine (Rompum; 12.7 mg/kg of body weight) and ketamine (Imalgen 1000; 79.5 mg/kg) (Centravet, Pludunou, France). Bronchoalveolar cells were obtained by inserting a catheter into the trachea and washing the lungs with eight 0.5-ml aliquots of Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 0.06 mM EDTA. The BAL fluids were centrifuged (10 min, 4°C,400 × g) and resuspended in PBS supplemented with 5% fetal calf serum (DAP, Vogelgrun, France). After the washing, flow cytometry was carried out with aliquots containing 5 × 105 to 1 × 106 cells. The pulmonary vasculature was then perfused with sterile Ca2+- and Mg2+-free PBS to remove peripheral blood cells. The lavaged, perfused lungs were removed, minced, and digested in 5 ml of type IV collagenase (400 U/ml in RPMI 1640; Boehringer Mannheim) containing DNase I (50 μg/ml; Boehringer Mannheim) for 30 min at 37°C and further dissociated in Ca2+- and Mg2+-free PBS in the presence of 2 mM EDTA. Particulate matter was removed by rapid filtration through a nylon cell strainer, and the cells were washed twice in RPMI 1640 (GIBCO Laboratories).
The lung cell populations were then stained and subjected to fluorescence-activated cell sorter analysis. We used a combination of purified, biotinylated, fluorescein isothiocyanate-, Cy-Chrome-, or phycoerythrin-labeled anti-CD8α {Ly-2, 53-6.7, rat immunoglobulin G2a(κ) [IgG2a(κ)]}, anti-CD11b [M1/70, rat IgG2b(κ)], anti-CD11c (HL3, Armenian hamster IgG1), anti-major histocompatibility complex class II (anti-MHC II; I-Ab)[M5/114.15.2, rat IgG2b(κ)], anti-CD40 [3/23, rat IgG2a(κ)], anti-CD80 [16-10A1, Armenian hamster IgG1(κ)], and anti-CD86 [GL1, rat IgG2a(κ)] Abs. Streptavidin, anti-rat or anti-mouse IgG conjugated to fluorescein isothiocyanate, phycoerythrin, or Cy-Chrome was used as a secondary Ab when necessary. All Abs and isotypic controls were from Becton Dickinson. Neutrophil-specific monoclonal Abs were purified from the supernatants of hybridoma cell line NIMPR14 (22). The analysis was performed on freshly isolated or stabilized (StabilCyte; Clinisciences, Montrouge, France) BAL fluids or lung cell populations that had been incubated with normal mouse serum to block the FcR-mediated binding of staining Abs. Cell labeling was performed as described previously (18). DCs were subjected to three-color immunophenotyping. A Becton Dickinson FACScan flow cytometer was used for data acquisition. We collected data for 75,000 events and analyzed them with CellQuest software (Becton Dickinson).
At a very early time point after intranasal delivery of B. bronchiseptica, i.e., 5 h, cellular recruitment was observed in the BAL fluids and lung tissues from the infected mice (Fig. 2A and B); the increase in cells was accounted for mostly by an increase in granulocyte counts (Fig. 2C and D). Morphological analysis of granulocytes in BAL fluid cytospin preparations showed that 100% of the recruited granulocytes were neutrophils. Five hours after infection, the number of CD11c-positive, CD11b-positive, NIMPR14-negative cells decreased in BAL fluids, compared to that of the control mice. Two days after infection, the number of neutrophils decreased, whereas the number of leukocytes that expressed CD11c and CD11b membrane markers and that were negative for the NIMPR14 granulocyte marker increased significantly in the BAL fluids (5.1% of total cells) and lung tissues (8.4% of total cells) (Fig. 2E and F). Interestingly, the cellular infiltrate driven by B. bronchiseptica was still significant 7 days after infection, both in respiratory epithelia and alveolar spaces. This is suggestive of a rapid and permanent turnover of DCs at these sites. Two days after infection, most CD11c-positive, CD11b-positive, NIMPR14-negative cells recruited to the lungs were MHC-II positive (60% I-Ab positive) and expressed CD80 (95%), CD86 (84%), and CD40 (90%) costimulatory molecules. The recruited CD11c-positive, CD11b-positive, NIMPR14-negative population was identified as being a myeloid DC population expressing the myeloid-related DC marker CD11b. These cells were also recruited to the respiratory epithelium during the early phase of cellular inflammatory processes (26). The CD80, CD86, and CD40 molecules expressed on mature DCs in the lungs also acted as costimulators of T cells to allow differentiation of effector T lymphocytes (23, 25).
FIG. 2.
Dendritic leukocyte and neutrophil contents of BAL fluids and lung tissues at early time points after intranasal delivery of B. bronchiseptica. BAL fluids (A, C, and E) and lung samples (B, D, and F) were collected from mice infected intranasally with 5.5 × 105 CFU of freshly collected bacteria or 50 μl of Stainer-Scholte liquid medium (control mice). The lavaged, perfused lungs were removed, minced, and digested with collagenase. Cells were collected and stained with anti-CD11c and anti-CD11b monoclonal Abs and granulocyte-reactive NIMPR14 monoclonal Ab and analyzed by flow cytometry to determine the numbers of total cells (A and B), neutrophils (C and D), and CD11c-positive, Cd11b-positive, NIMPR14-negative cells (E and F) at the indicated time points. The plots show data from three representative experiments; each point is a mean value (± the standard error of the mean) derived from results for five or six animals. One-way analysis of variance was used to identify differences between the groups. If the analysis of variance was significant, a nonparametric Mann-Whitney test was used to compare the means. A P value of <0.05 was considered significant.
CD11c-positive leukocytes in lung and lymph node tissues of B. bronchiseptica-infected mice: an in situ analysis.
Samples of NALT, mediastinal lymph node tissues, and lung tissue were processed, embedded, and cut for further immunohistochemical studies. After the mice were anesthetized, perfused lungs, mediastinal lymph nodes, and NALT samples were removed and stored at 4°C in a zinc fixative solution for 2 or 3 days. The tissue blocks were embedded in low-temperature paraffin (melting point, 37°C) (20). Sections (4-μm thick) were stained with hematoxylin-eosin and Giemsa stains and then studied at a ×100 magnification with an Eclipse E-800 microscope (Nikon, Champigny-sur-Marne, France). Some tissue sections that had been fixed were used for immunohistochemistry analysis. Paraffin sections were deparaffinized in absolute ethanol and then incubated in PBS for 10 min. They were incubated in a commercial blocking agent (DuPont, NEN Research Products, Boston, Mass.) to diminish nonspecific binding. Primary Abs dissolved in PBS-0.05% saponin (Sigma) were added for 2 h. The following primary Abs were used: biotinylated monoclonal Ab against murine CD11c (HL3, Armenian hamster IgG1; Becton Dickinson France, Le Pont-De-Claix, France), rat monoclonal anti-CD45 Ab (RA3-6B2, B220; Tebu, Le Perray-en-Yvelines, France), rabbit polyclonal anti-CD3 Ab (Dako, Trappes, France), and anti-MHC II I-Ab Ab [M5/114.15.2, rat IgG2b(κ); Becton Dickinson France]. Endogenous peroxidase activity was quenched by immersing the slides in PBS containing 0.03% H2O2. The slides were further incubated with either host-specific biotinylated Ab (rabbit anti-rat; Dako) or nonbiotinylated secondary Abs (anti-rabbit EnVision, rabbit anti-rat; Dako) or streptavidin-peroxidase conjugate. Bound peroxidase activity was detected by using the aminoethylcarbazole substrate (Sigma), and alkaline phosphatase activity was detected by using the alkaline phosphatase anti-alkaline phosphatase (APAAP) complex (rat; Dako) and fast blue as the substrate. Double labeling with CD11c and CMH II was performed by using the streptavidin-peroxidase method and the aminoethylcarbazole substrate, followed by the APAAP method and fast blue. All tissues except double-labeled tissues were counterstained with hematoxylin and mounted in Aquamount (LaboNord, Templemars, France).
The lungs of B. bronchiseptica-infected mice showed extensive perivascular and peribronchiolar inflammatory processes that extended progressively (Fig. 3B and C), mostly caused by neutrophils and mononuclear cells (particularly obvious at 48 h). A few CD11c-positive leukocytes were present in the airway epithelia of naive mice, close to the alveolar walls, interstitial spaces, and lung alveoli (Fig. 3A). After delivery of the bacteria, the number of CD11C-positive cells rapidly increased and localized first in the peribronchial spaces (Fig. 3B) and later within the pulmonary parenchyma (Fig. 3C). Different-sized CD11c-positive leukocytes were observed. At early time points, CD11c-positive cells were small, with polar labeling. At later time points (especially 48 h), CD11c-positive leukocytes were large cells with lobulated nuclei, abundant cytoplasm, and small cytoplasmic projections, demonstrating the classical dendritic pattern of more mature and differentiated cells. At this time point, some cells coexpressed CD11c and MHC II molecules within the infiltrates and in the peribronchial spaces (Fig. 3D), findings which are consistent with the flow cytometry data. These data demonstrate the properties of DC precursors to not only migrate toward but also to mature within the airway epithelium once the airway epithelium is invaded by bacteria (23, 30). Of note, we do not exclude the possibility that monocytes may be the precursors of the DCs we detected in these sites (32).
FIG.3.
Histological examination results for lung, mediastinal lymph node, and NALT tissues at early time points after intranasal delivery of B. bronchiseptica to BALB/c mice. (A to D) Photomicrographs of lung sections stained with monoclonal anti-CD11c Abs are shown. (A) Normal lung tissue from BALB/c mice. Positive cells are visible close to the alveolar walls, interstitial spaces, and alveoli. (B to D) Peribronchial and perivascular pleomorphic infiltrates at 24 (B) and 48 (C and D) h after infection with B. bronchiseptica by intranasal delivery. (B) Peribronchial localization of CD11c-positive leukocytes. (C) Diffuse localization of CD11c-positive leukocytes within the pulmonary parenchyma. (D) Double immunostaining with monoclonal anti-CD11c and anti-M5-114 (MHC II) Abs. CD11c-positive leukocytes (arrow labeled 2) stained red, MHC-II-positive leukocytes (arrow labeled 3) stained blue, and doubly positive (CD11c-positive, MHC-II-positive) leukocytes stained purple (perivascular location indicated by arrow labeled 1). Scale bar, 100 μm. (E to H) Photomicrographs of mediastinal lymph node sections stained with monoclonal anti-CD11c (E and H) and anti-CD45 (F) Abs and polyclonal anti-CD3 (G) Abs are shown. (E, F, and G) Lymph node tissue from naive BALB/c mice. (H) Lymph node tissue from mice 5 h after B. bronchiseptica infection. Scale bar, 100 μm. (I to N) Photomicrographs of NALT sections. (I) Anatomic locations of NALT in naive BALB/c mice after hematoxylin-eosin staining (unlabeled arrows). A frontal, horizontal section is shown. The nasal cavities (nc) are lined by a stratified, nonciliated, and squamous epithelium in the anterior and external areas (arrow labeled 1) and by a columnar, ciliated epithelium (arrow labeled 2) with goblet cells in the internal and posterior areas. The NALT is found beneath the columnar epithelium. mg, mucous gland; lb, lamellar bone. Scale bar, 500 μm. (J, K, L, M, and N) Photomicrographs of NALT sections stained with monoclonal anti-CD45 (J), polyclonal anti-CD3 (K) and monoclonal anti-CD11c (L, M, and N) Abs are shown. (J, K, and L) Lymph node tissue from naïve BALB/c mice. (M and N) Lymph node tissue from mice at 5 (M) and 24 (N) h after intranasal delivery of B. bronchiseptica. Scale bar, 100 μm.
To determine whether these mature DCs are still able to migrate via the lymphatic system to T-cell-dependent areas of the draining lymph tissues associated with the respiratory system, we analyzed the presence of CD11c-positive leukocytes on sections of posterior mediastinal lymph nodes and of NALT samples. Lymph node tissue sections from naive and infected BALB/c mice were stained with monoclonal anti-CD11c and anti-CD45 Abs and with polyclonal anti-CD3 Abs. CD11c-positive leukocytes were easily detected at an early time point after bacterial inoculation on mediastinal lymph node tissue sections (5 h) (Fig. 3H) and were exclusively localized in the T-cell area (Fig. 3G). This localization may initiate T-cell stimulation in lymphoid tissues draining the lungs. NALT is an immunocompetent inductive tissue for the nasal respiratory tract. After microdissection, paired NALT aggregates were clearly visible on the palate near the entrance of the nasopharyngeal duct (Fig. 3I). This tissue is important for the activation of immune effectors (IgA induction and generation of cytotoxic activity) in the nose (15, 31, 39, 42). CD11c-positive leukocytes were present in the NALT subepithelium of naive mice, but they were not homogeneously distributed (Fig. 3L). At 5 and 24 h after B. bronchiseptica delivery (Fig. 3M and N, respectively), an increased number of cells expressing CD11c was detected. These cells were located exclusively along the epithelial side of each NALT aggregate without specific localization to the T area (Fig. 3J and K). This unique pattern suggests that the NALT DC population may demonstrate its ability to trigger the production of bacterium-reactive effectors (Abs as well as helper T lymphocytes) during the early stages of B. bronchiseptica infection (9).
Having documented that the nasal cavity is the main site of bacterial persistence, we can hypothesize that DCs of the myeloid or lymphoid lineages may be mobilized, primed, and turned over in this site and that these cells may play a unique role in sustaining the turnover of bacterium-reactive T-cell effectors as well as plasmacyte precursors in B. bronchiseptica-loaded tissues. The following are preliminary data on these issues. Thirty days after intranasal delivery of B. bronchiseptica, we could isolate only a small percentage of cultivable intracellular bacteria from CD11c-positive leukocytes purified from the NALT-free nasal epithelia of mice (data not shown). These cultivable bacteria displayed a phase I phenotype—i.e., the phenotype of bacteria transmissible to another host. As the nasal cavity is the site of bacterial persistence (a permanent extracellular source of bacteria), the low number of bacteria still detectable within the CD11c-positive leukocyte population may be important in sustaining the production of unique immune effectors and regulators on which the persistence of a transmissible bacterial population of a stable size relies. In addition, if there is a rupture in the sustained delivery of these unique regulators and effectors, the bacteria could again be switched on for further uncontrolled multiplication leading to acute inflammatory symptomatic processes.
Acknowledgments
We are grateful to Gilles Marchal for the gift of the CellTracker green fluorescent BODIPY dye (5 [and 6]-carboxyfluorescein diacetate, succinimidyl ester) and to Mai Lebastard for the gift of the NIMPR14 Ab. We are also grateful to Micheline Lagranderie and Gilles Marchal for helpful discussions.
Financial support for this work was provided by the Institut Pasteur Foundation and CNRS (Appel d'offres puces à ADN-GM).
Editor: J. N. Weiser
REFERENCES
- 1.Arico, B., R. Gross, J. Smida, and R. Rappuoli. 1987. Evolutionary relationships in the genus Bordetella. Mol. Microbiol. 1:301-308. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin, M. S., P. S. Sullivan, S. E. Buskin, R. D. Harrington, J. Oliffe, R. D. MacArthur, and C. E. Lopez. 1999. Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 28: 1095-1099. [DOI] [PubMed] [Google Scholar]
- 3.Ferry, N. S. 1912. A preliminary report of the bacterial findings in canine distemper. Am. Vet. Rev. 37:449-504. [Google Scholar]
- 4.Fukuyama, S., T. Hiroi, Y. Yokota, P. D. Rennert, M. Yanagita, N. Kinoshita, S. Terawaki, T. Shikina, M. Yamamoto, Y. Kurono, and H. Kiyono. 2002. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3−CD4+CD45+ cells. Immunity 17:31-40. [DOI] [PubMed] [Google Scholar]
- 5.Garcia, S. M., C. Quereda, M. Martinez, P. Martin-Davila, J. Cobo, and A. Guerrero. 1998. Bordetella bronchiseptica cavitary pneumonia in a patient with AIDS. Eur. J. Clin. Microbiol. Infect. Dis. 17:675-676. [DOI] [PubMed] [Google Scholar]
- 6.Gong, J. L., K. M. McCarthy, J. Telford, T. Tamatani, M. Miyasaka, and E. Schneeberger. 1992. Intraepithelial airway dendritic cells: a distinct subset of pulmonary dendritic cells obtained by microdissection. J. Exp. Med. 175:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gueirard, P., and N. Guiso. 1993. Virulence of Bordetella bronchiseptica: role of adenylate cyclase-hemolysin. Infect. Immun. 61:4072-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gueirard, P., P. Minoprio, and N. Guiso. 1996. Intranasal inoculation of Bordetella bronchiseptica in mice induces long-lasting antibody and T-cell mediated immune responses. Scand. J. Immunol. 43:181-192. [DOI] [PubMed] [Google Scholar]
- 10.Gueirard, P., C. Weber, A. Le Coustumier, and N. Guiso. 1995. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J. Clin. Microbiol. 33:2002-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiso, N., M. Szatanik, and M. Rocancourt. 1991. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb. Pathog. 11:423-431. [DOI] [PubMed] [Google Scholar]
- 12.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heritage, P. L., B. J. Underdown, A. L. Arsenault, D. P. Snider, and M. R. McDermott. 1997. Comparison of murine nasal-associated lymphoid tissue and Peyer's patches. Am. J. Respir. Crit. Care Med. 156:1256-1262. [DOI] [PubMed] [Google Scholar]
- 14.Holt, P. G., M. A. Schon-Hegrad, and J. Oliver. 1988. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat: regulation of antigen presentation activity by endogenous macrophage population. J. Exp. Med. 167:262-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou, Y., W. G. Hu, T. Hirano, and X. X. Gu. 2002. A new intra-NALT route elicits mucosal and systemic immunity against Moraxella catarrhalis in a mouse challenge model. Vaccine 20:2375-2381. [DOI] [PubMed] [Google Scholar]
- 16.Jungnitz, H., N. P. West, M. J. Walker, G. S. Chhatwal, and C. A. Guzman. 1998. A second two-component regulatory system of Bordetella bronchiseptica required for bacterial resistance to oxidative stress, production of acid phosphatase, and in vivo persistence. Infect. Immun. 66:4640-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraal, G. Nasal associated lymphoid tissue. In J. Mestecki et al. (ed.), Mucosal immunology, 2nd ed., in press.
- 18.Lagranderie, M., M. A. Nahori, A. M. Balazuc, H. Kieffer-Biasizzo, J. R. Lapa E Silva, G. Milon, G. Marchal, and B. B. Vargaftig. 2003. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology 108:352-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambrecht, B. N., H. C. Hoogsteden, and R. A. Pauwels. 2001. Dendritic cells as regulators of the immune response to inhaled allergen: recent findings in animal models of asthma. Int. Arch. Allergy Immunol. 124:432-436. [DOI] [PubMed] [Google Scholar]
- 20.Lang, T., P. Ave, M. Huerre, G. Milon, and J. C. Antoine. 2000. Macrophage subsets harbouring Leishmania donovani in spleens of infected BALB/c mice; localization and characterization. Cell. Microbiol. 2:415-430. [DOI] [PubMed] [Google Scholar]
- 21.Lipscomb, M. F., and B. J. Masten. 2002. Dendritic cells: immune regulators in health and disease. Physiol. Rev. 82:97-130. [DOI] [PubMed] [Google Scholar]
- 22.Lopez, A. F., M. Strath, and C. J. Sanderson. 1984. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br. J. Haematol. 57:489-494. [DOI] [PubMed] [Google Scholar]
- 23.Masten, B. J., J. L. Yates, A. M. Pollard Koga, and M. F. Lipscomb. 1997. Characterization of accessory molecules in murine lung dendritic cell function: roles for CD80, CD86, CD54, and CD40L. Am. J. Respir. Cell Mol. Biol. 16:335-342. [DOI] [PubMed] [Google Scholar]
- 24.Mattoo, S., A. K. Foreman-Wykert, P. A. Cotter, and J. F. Miller. 2001. Mechanisms of Bordetella pathogenesis. Front. Biosci. 6: E168-E186. [DOI] [PubMed] [Google Scholar]
- 25.McLellan, A., M. Heldmann, G. Terbeck, F. Weih, C. Linden, E. B. Bröcker, M. Leverkus, and E. Kämpgen. 2000. MHC class II and CD40 play opposing roles in dendritic cell survival. Eur. J. Immunol. 30:2612-2619. [DOI] [PubMed] [Google Scholar]
- 26.McWilliam, A. S., S. Napoli, A. M. Marsh, F. L. Pemper, D. J. Nelson, C. L. Pimm, P. A. Stumbles, T. N. C. Wells, and P. G. Holt. 1996. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J. Exp. Med. 184:2429-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McWilliam, A. S., D. Nelson, J. A. Thomas, and P. G. Holt. 1994. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J. Exp. Med. 179:1331-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McWilliam, A. S., P. A. Stumbles, and P. G. Holt. 1999. Dendritic cells in the lung, p. 123-140. In M. T. Loyze and A. W. Thomson (ed.), Dendritic cells: biology and clinical applications. Academic Press, Inc., New York, N.Y.P. A.
- 29.Nelson, D. J., C. McMenamin, A. S. McWilliam, M. Brenan, and P. G. Holt. 1994. Development of the airway intraepithelial dendritic cell network in the rat from class II major histocompatibility (Ia)-negative precursors: differential regulation of Ia expression at different levels of the respiratory tract. J. Exp. Med. 179:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajak, B., T. De Smedt, V. Moulin, C. De Trez, R. Maldonado-Lopez, G. Vansanten, E. Briend, J. Urbain, O. Leo, and M. Moser. 2000. Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualisation of dendritic cells in peripheral organs. J. Clin. Pathol. 53:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porgador, A., H. F. Staats, Y. Itoh, and B. L. Kelsall. 1998. Intranasal immunization with cytotoxic T-lymphocyte epitope peptide and mucosal adjuvant cholera toxin: selective augmentation of peptide-presenting dendritic cells in nasal mucosa-associated lymphoid tissue. Infect. Immun. 66:5876-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 33.Rao, A. S., J. A. Roake, C. P. Larsen, D. F. Hankins, P. J. Morris, and J. M. Austyn. 1993. Isolation of dendritic leukocytes from non-lymphoid organs. Adv. Exp. Med. Biol. 329:507-512. [DOI] [PubMed] [Google Scholar]
- 34.Sekiya, K., M. Kawahira, and Y. Nakase. 1983. Protection against experimental Bordetella bronchiseptica infection in mice by active immunization with killed vaccine. Infect. Immun. 41:598-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sertl, K., T. Takemura, E. Tschachler, V. J. Ferrans, M. A. Kaliner, and E. M. Shevach. 1986. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J. Exp. Med. 163:436-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman, R. M., and M. C. Nussenzweig. 1980. Dendritic cells: features and functions. Immunol. Rev. 53:127-147. [DOI] [PubMed] [Google Scholar]
- 37.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cell development and maturation. Adv. Exp. Med. Biol. 417:1-6. [DOI] [PubMed] [Google Scholar]
- 38.Stockwin, L. H., D. McGonagle, I. G. Martin, and G. E. Blair. 2000. Dendritic cells: immunological sentinels with a central role in health and disease. Immunol. Cell Biol. 78:91-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiley, J. A., R. J. Hoggan, D. L. Woodland, and A. G. Harmsen. 2001. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol. 167:3293-3299. [DOI] [PubMed] [Google Scholar]
- 40.Woolfrey, B. F., and J. A. Moody. 1991. Human infections associated with Bordetella bronchiseptica. J. Clin. Microbiol. Rev. 4:243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuk, M. H., E. T. Harvill, P. A. Cotter, and J. F. Miller. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-KB activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991-1004. [DOI] [PubMed] [Google Scholar]
- 42.Zuercher, A. W., S. E. Coffin, M. C. Thurnheer, P. Fundova, and J. J. Cebra. 2002. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J. Immunol. 168:1796-1803. [DOI] [PubMed] [Google Scholar]