Abstract
Antibody specificities of pre- and postvaccination serum samples from 40 (53%) teenagers who received three doses of the Norwegian Neisseria meningitidis serogroup B vaccine (B:15:P1.7,16) during a previous trial in Iceland (Perkins et al., J. Infect. Dis. 177:683-691, 1998) were analyzed with serum bactericidal activity (SBA) and immunoblotting assays with reference and isogenic meningococcal H44/76 vaccine strains. The H44/76 variants demonstrated significant vaccine-induced SBA to P1.7,16 PorA and Opc but not to PorB, Opa5.5, and a heterologous PorA protein. On blots, immunoglobulin G levels to all these proteins increased significantly after vaccination. Measurement of SBA to the two main variable regions (P1.7 and P1.16) on the P1.7,16 PorA with PorA deletion mutants revealed significantly higher activity to the P1.7,− and P1.−,16 mutants compared to the P1.7,16 strain, indicating exposure of new accessible epitopes. Only 12 (30%) serum samples showed distinct decreases with these or the P1.−,− mutant, with most samples containing SBA to the P1.7 and P1.16 combination. In contrast, P1.16-specific antibodies were mainly found on blots. Thirteen of the vaccinees (32.5%) were carriers of meningococci at the time of the third dose, of whom four (30.8%) harbored strains of the ET-5 complex. Carriage of P1.15 strains was generally reflected in ≥4-fold increases in SBA and distinct immunoglobulin G binding to the P1.19,15 PorA on blots. Although vaccination did not elicit bactericidal activity to the serotype 15 PorB, most carriers of serotype 15 strains showed ≥4-fold increases in SBA to this antigen.
Neisseria meningitidis serogroup B is a major cause of bacterial meningitis and septicemina, primarily affecting young children and teenagers with high mortality and morbidity. The poor immunogenicity of the group B capsular polysaccharide in humans has led to the development of meningococcal vaccines based on outer membrane proteins. Two such vaccines were protective in trials conducted in Cuba and Norway (2, 38). Both consisted of purified outer membrane vesicles (OMVs) derived from epidemic strains, i.e., CU385 (B:4:P1.19,15) for the Cuban vaccine (38) and 44/76 (B15:P1.7,16), also designated H44/76 (41), for the Norwegian vaccine (11). The immunogenicity of these two vaccines has been compared in clinical trials among teenagers in Iceland (28) and among infants, toddlers, and adults in Chile (40).
Since the pioneering work by Artenstein's group on group C disease in the late 1960s (12), serum bactericidal activity (SBA) has been used as the primary serological correlate in the development of meningococcal vaccines. Although the importance of SBA for protection against group B meningococci is not fully known, evidence has been presented that it correlates with protection (3, 25, 26). Both the Cuban and Norwegian OMV vaccines induce bactericidal antibodies in small children and adults (14, 28, 40). With the latter vaccine, the majority of the bactericidal antibodies were directed to the P1.7,16 PorA and Opc proteins (33). Following a third dose of this vaccine, both the level and persistence of SBA against the homologous vaccine strain increased compared to a two-dose regimen, as did the level of cross-reacting antibodies (28, 33, 40; J. Fuglesang, E. A. Høiby, J. Holst, E. Rosenqvist, and H. Nøkleby, Abstr. 11th Int. Pathogenic Neisseria Conf., 1998, p. 174).
In the Icelandic trial, the immune responses were assayed as geometric mean SBA titers against both the vaccine and three heterologous strains (28). The aim of our study was to examine in more detail the specificity and cross-reactivity of antibodies elicited after three doses of the Norwegian OMV vaccine in this trial. For this purpose, different isogenic variants of the vaccine strain H44/76 (B:15:P1.7,16) were used to measure SBA. The mutants lacked either the serotype 15 PorB, the serosubtype P1.7,16 PorA, or one or both of the Opc and Opa5.5 proteins. The ability of the various PorA domains in raising bactericidal antibodies was analyzed with variants that lacked either one or both of the two major variable regions (VR) of P1.7,16 PorA or with a variant expressing a heterologous PorA. The SBA results against each of the antigens were compared with the corresponding antibody levels measured on immunoblots with the vaccine strain and other reference strains as antigens. In addition, a carrier study performed among the participants at the time of the third dose (28) offered the possibility to study the effect of carriage on specific antibody responses.
(A preliminary report of a smaller subset of serum samples was presented previously [E. Wedege, E. Rouppe van der Voort, B. Kuipers, K. Bolstad, H. van Dijken, and J. T. Poolman, Abstr. 11th Int. Pathogenic Neisseria Conf., 1998, p. 176], and part of the present work was shown at the Twelfth International Pathogenic Neisseria Conference in Galveston, Tex. [E. Wedege, K. Bolstad, H. van Dijken, G. van den Dobbelsteen, B. Kuipers, and L. van Alphen, Abstr. 12th Int. Pathogenic Neisseria Conf., 2000, abstr. no. 142, p. 52]).
MATERIALS AND METHODS
Serum samples.
Pre- and postvaccination serum samples from a random selection of 40 of 75 (53%) Icelandic teenagers who received three doses of the Norwegian B:15:P1.7,16 OMV vaccine during the clinical trial in Iceland in 1992 to 1993 (28) were studied. Postvaccination samples were drawn 5 weeks after the third dose (bleed 5). The serum samples were obtained through B. Perkins and G. Carlone, Centers for Disease Control and Prevention, Atlanta, Ga., and stored at −80°C.
Carriage of meningococci.
Pharyngeal swabs were taken from volunteers at the time of the fourth and fifth blood collection as described previously (28). The isolates were characterized by serotyping on dot blots with a limited number of monoclonal antibodies (28) and for electrophoretic types (ETs) by multilocus enzyme electrophoresis (4).
Strains.
Descriptions of the strains in our study are detailed in Table 1. The mother strain (44/76) for the Norwegian OMV vaccine (11) was first isolated by Holten (15). This strain was later designated H44/76 and used for preparation of recombinant isogenic variants (27, 34, 41). H44/76 and its variants were characterized in whole-cell enzyme-linked immunosorbent assay (ELISA) with various monoclonal antibodies as shown below. Isogenic strains, expressing similar levels of each of the Opc and Opa5.5 proteins, were obtained by colony blot as described below. All isogenic variants used in the bactericidal assays were L3 positive and were lysed to the same degree as H44/76 with a lipopolysaccharide-specific monoclonal antibody (34). The Cuban vaccine strain (CU385) and the other reference strains, M1080 and S3032 (10), were characterized on dot blots with serotype- and subtype-specific monoclonal antibodies (45).
TABLE 1.
Characteristics of the strain panel used for bactericidal and immunoblot assays
| Strain type | Strain no. | Serogroup | PorB | PorA | Opc | Opa 5.5 | Reference(s) |
|---|---|---|---|---|---|---|---|
| Vaccine strain | H44/76 | B | 15 | P1.7,16 | + | + | 11, 14 |
| Isogenic variants | H44/76 | B | P1.7,16 | − | − | 35, 36 | |
| H44/76 | B | 15 | P1.7,− | − | − | 35, 36 | |
| H44/76 | B | 15 | P1.−,16 | − | − | 35, 36 | |
| H44/76 | B | 15 | P1.−,− | − | − | 35, 36 | |
| H44/76 | B | 15 | P1.19,15-1 | − | − | 27 | |
| H44/76 | B | P1.19,15-1 | − | − | This study | ||
| H44/76 | B | P1.19,15-1 | + | − | This study | ||
| H44/76 | B | P1.19,15-1 | − | + | This study | ||
| HI-5a | B | 15 | − | − | 41 | ||
| HI-5a | B | 15 | + | − | This study | ||
| Other reference strains | M1080 | B | 1 | P1.7-1,1 | − | − | 10 |
| S3032 | B | 19b | P1.12,16 | + | − | 10 | |
| CU385 | B | 4 | P1.19,15 | (+) | NDc | 38 |
Monoclonal antibodies.
Isogenic H44/76 strains were characterized with MN5C11G (anti-P1.16), MN14C11.6 (anti-P1.7), and MN15A14H6 (anti-serotype 15) as reported (34) in addition to MN3C5C (anti-P1.15), MN4A8B2 (anti-L3 lipopolysaccharide), B306 (anti-Opc), and 15-1-P5.5 (anti-Opa5.5). The remaining strains were probed with 279-5c (anti-Opc) and 15-1-P5.5 (anti-Opa5.5) in addition to PorA- and PorB-specific monoclonal antibodies as reported previously (45).
Colony blotting.
Colony blots were used to select Opc and Opa5.5 positive and negative colonies as described (18). Briefly, agar plates with colonies were covered with nitrocellulose membranes to transfer part of the bacterial colonies to the membrane. Double staining with monoclonal antibodies against two different antigens was employed to identify a negative colony among a large number of positive colonies. Single colonies were then picked from the reincubated master cultures after comparison with the corresponding colony blots. After streaking and retesting, pure cultures were obtained with the desired phenotypic characteristic.
SBA.
The bactericidal activity of pre- and postvaccination serum samples was determined as described (27, 34) with the H44/76 isogenic variants listed in Table 1. In short, twofold dilutions of heat-inactivated serum samples (30 min at 56°C) were incubated with human complement (final concentration, 10%, vol/vol) and 2.5 × 102 of CFU of bacteria for 60 min at 37°C. The human complement showed no SBA with a panel of meningococci. SBA is reported as log2 of the lowest reciprocal serum dilution that yielded ≥90% killing. A vaccine responder was defined as an individual with a ≥4-fold rise in SBA compared with the prevaccination titer.
Immunoblot analysis.
The antigens were deoxycholate extracted OMVs from strains H44/76 and CU385 (11). All serum samples (diluted 1:200) were incubated in the absence and presence of 0.15% Empigen BB (Albright and Wilson, Cumbria, United Kingdom) to detect antibody binding to conformation-dependent epitopes (48). Immunoglobulin G (IgG) binding to the different outer membrane proteins, given in arbitrary units, was measured by digital image analysis (44). When IgG binding was higher in the presence of detergent, this value was used for subsequent analyses. Visually distinct bands were differentiated from more weakly stained bands by a cutoff corresponding to 1,000 arbitrary units for PorA and PorB. The P1.7 and P1.16 specificities of the postvaccination serum samples were determined from the intensity of PorA signals on blots with whole-cell suspensions of M1080 (P1.7-1,1 PorA) and S3032 (P1.12,16 PorA), respectively, in comparison with those for the PorA of 44/76 and CU385 (Table 1).
Statistical analyses.
Differences between pre- and postvaccination serum samples for each strain in SBA or in antigen-specific IgG binding were compared with the Wilcoxon signed rank test. Differences in SBA between various strains were compared by the Mann-Whitney rank sum test. P values of ≤0.05 were considered significant. The analyses were performed with a SigmaStat program from Jandel GmbH, Erkrath, Germany.
RESULTS
Antigen specificity of vaccine-induced SBA.
The specificities of paired serum samples from the 40 vaccinees were analyzed in bactericidal assays with isogenic H44/76 strains that differed in the expression of the PorA, PorB, Opc, and Opa5.5 proteins (Table 2). The prevaccination serum samples had low SBA (mean log2 titer < 2) to all test strains. Significantly higher SBA was measured after the third vaccine dose (P = 0.001 to 0.034). The mean log2 titers of the postvaccination serum samples increased 1.4- to 10.6-fold; the highest increases were found with variants that expressed the P1.7,16 and Opc proteins. A titer of 3.8 was obtained with the P1.7,16 mutant lacking the PorB, Opc, and Opa5.5 proteins. When P1.7,16 PorA was substituted with the heterologous P1.19,15-1 PorA, SBA decreased significantly (mean log2 titer 2.2; P = 0.001). SBA with the P1.19,15-1+ PorB+ Opc− Opa5.5− strain (mean log2 titer 2.7) was not significantly different (P = 0.127) from that with the corresponding PorA− strain (mean log2 titer 1.8), implying that vaccine-induced bactericidal antibodies to the P1.7,16 PorA did not cross-react with the heterologous PorA.
TABLE 2.
Bactericidal activity of pre- and postvaccination samples from 40 vaccinees against isogenic H44/76 strains
| Strain phenotype | Mean log2 SBA titer
|
Pa | Increase (fold) | Responseb (% of vaccinees) | |
|---|---|---|---|---|---|
| Prevaccination | Postvaccination | ||||
| P1.7,16+ PorB− Opc− Opa5.5− | 1.2 | 3.8 | 0.001 | 6.1 | 65.0 |
| P1.19,15-1+ PorB− Opc− Opa5.5− | 1.5 | 2.2 | 0.005 | 1.6 | 22.5 |
| P1.19,15-1+ PorB+ Opc− Opa5.5− | 1.7 | 2.7 | 0.018 | 2.0 | 35.0 |
| P1.19,15-1+ PorB− Opc− Opa5.5+ | 1.5 | 2.4 | 0.004 | 1.9 | 25.0 |
| P1.19,15-1+ PorB− Opc+ Opa5.5− | 1.7 | 5.1 | 0.001 | 10.6 | 87.5 |
| PorA− PorB+ Opc+ Opa5.5− | 1.9 | 5.3 | 0.001 | 10.6 | 79.0 |
| PorA− PorB+ Opc− Opa5.5− | 1.3 | 1.8 | 0.034 | 1.4 | 15.0 |
Significance of differences between paired serum samples measured with Wilcoxon signed rank test.
Percentage of vaccinees with ≥4-fold increase in titer after vaccination.
The results also demonstrated that vaccination gave rise to negligible or no SBA to the PorB antigen (Table 2). With the PorA− PorB+ Opc− Opa5.5− mutant, titers increased only 1.4-fold to a mean log2 titer of 1.8 (P = 0.034). Furthermore, there was no significant difference (P = 0.48) in the postvaccination response to the P1.19,15-1+ PorB+ Opc− Opa5.5− strain (mean log2 titer 2.7) and the corresponding PorB− strain (mean log2 titer 2.2).
The large effect of Opc antibodies on SBA was demonstrated by a comparison of the postvaccination titers (Table 2) with the two P1.19,15-1 strains (mean log2 titers 5.1 and 2.4, respectively) as well as with the two PorA− strains (mean log2 titers 5.3 and 1.8, respectively; all P = 0.001), being either Opc+ or Opc−. A 10.6-fold increase in SBA was observed with both Opc-containing strains after vaccination. Our study showed no bactericidal effect of the Opa5.5 antibodies.
When the effect of vaccination was determined as ≥4-fold increases in SBA (Table 2), the highest number of responders was found with strains expressing the homologous P1.7,16 PorA (65.0%) and the Opc proteins (87.5% and 79%, respectively). Lower numbers were obtained with H44/76 variants without these dominant antigens, namely the P1.19,15-1, PorA−, and Opc− strains (range 15% to 35%). The lack of bactericidal antibodies to Opa5.5 was seen from the same low numbers of responders against the P1.19,15-1+ PorB− Opc− Opa5.5+ strain and the corresponding Opa5.5− variant (25% versus 22.5%).
Specificity of PorA-induced SBA.
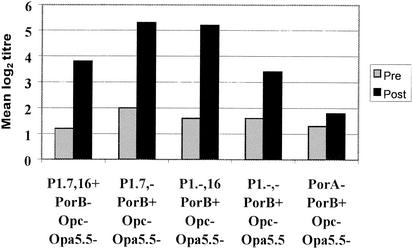
To examine the bactericidal responses against the two surface-exposed variable regions (VR1/P1.7 and VR2/P1.16) on the P1.7,16 PorA antigen, the serum samples were analyzed with isogenic H44/76 mutants that lacked either one (P1.7,− and P1.−,16) or both (P1.−,−) of these VR (Table 1). The results, shown in Fig. 1, are compared with those for the P1.7,16 and PorA− strains from Table 2. Vaccination induced significant titer increases with all three deletion mutants (P = 0.001). Mean log2 titers with the P1.7,−, P1.−,16, and P1.−,− target strains were 5.3, 5.2, and 3.4, respectively, and the corresponding number of SBA responders were 77%, 79%, and 37%, respectively. Based on the finding reported above that PorB did not induce SBA, the P1.7,− and P1.−,16 mutants gave significantly higher postvaccination titers (P = 0.003 and P = 0.021, respectively) than the P1.7,16 strain, indicating that these mutants were more sensitive to bactericidal antibodies.
FIG. 1.
Bactericidal activity (mean log2 titers) against PorA of paired serum samples from 40 volunteers measured with isogenic PorA deletion mutants of H44/76. Compared with the P1.7,16 strain, titers against the P1.7,− (P = 0.003) and P1.−,16 (P = 0.021) strains were significantly higher, that for the PorA− strain was significantly lower (P = 0.001), and no difference was found for the P1.−,− strain (P = 0.30).
The number of postvaccination serum samples containing antibodies to one or both of the VR was determined by comparing the titer of each serum against the P1.7,16 strain with those against the P1.7,−, P1.−,16, or P1.−,− deletion mutants. Only 12 of the 40 serum samples (29.2%) showed a distinct decrease in SBA of more than two log steps with one or more of the mutants, suggesting various VR-specific activities. The presence among all vaccinees of bactericidal antibodies to epitopes other than the VR was implicated from the similar SBA to the P1.7,16 and P1.−,− strains (mean log2 titers 3.8 and 3.4, respectively; P = 0.3); both were significantly higher than for the PorA− mutant (mean log2 titer 1.8; P < 0.005) (Fig. 1).
Immunoblot assays of vaccine-induced antibody levels.
Quantitation of IgG binding to the major outer membrane proteins of H44/76 and to P1.19,15 PorA of CU385 is shown in Table 3. After the third vaccine dose, IgG levels to all proteins increased significantly from 2.1-fold for the P1.19,15 protein to 31.4-fold for the Opc protein (P = 0.001). The majority of the vaccinees (75%) showed ≥4-fold increases in IgG binding to the P1.7,16 PorA after vaccination, whereas only 25% responded to the P1.19,15 PorA. Antibody levels to the P1.19,15 protein was about 10% of that elicited to the P1.7,16 protein (Table 3). Because similar amounts of the two PorA proteins were applied on the gels before blotting, these results indicated low levels of cross-reacting antibodies to the heterologous PorA. However, the blots showed that the postvaccination serum samples reacted with a wide range of nonporin antigens in CU385 and the other heterologous strains.
TABLE 3.
IgG binding to meningococcal outer membrane proteins in pre- and postvaccination serum samples from 40 vaccinees determined by digital scanning of immunoblots
| Protein | Median IgG binding (range), arbitrary units
|
Pa | Increase (fold) | Responseb (% of vaccinees) | |
|---|---|---|---|---|---|
| Prevaccination | Postvaccination | ||||
| P1.7,16 PorA | 236 (0-1,236) | 2,276 (400-10,394) | 0.001 | 9.6 | 75 |
| P1.19,15 PorA | 109 (0-1,087) | 234 (0-2,556) | 0.001 | 2.1 | 25 |
| P15 PorB | 182 (0-1,836) | 5,571 (584-14,001) | 0.001 | 30.6 | 90 |
| Opc | 50 (0-1,406) | 1,570 (120-6,643) | 0.001 | 31.4 | 75 |
| Opa5.5 | 50 (0-953) | 589 (0-5,002) | 0.001 | 11.8 | 60 |
See Table 2 footnote a.
Percentage of vaccinees with a fourfold or greater increase in IgG binding after vaccination.
Specificity of PorA responses in immunoblot assays.
All postvaccination serum samples were blotted against the reference strains S3032 (B:19:P1.12,16) and M1080 (B:1:P1.7-1,1) which each expresses one of the VR epitopes in the P1.7,16 vaccine antigen. The rationale for these experiments was to analyze antibodies directed to either the P1.7/VR1 or P1.16/VR2 domains irrespective of the contributions of the other. In our hands, the two extra amino acids in the P1.7-1 variant (19) did not affect antibody binding on blots of either the P1.7 reference monoclonal antibody or a human P1.7 reference serum.
IgG binding to PorA of strains S3032 and M1080, together with those to H44/76 and CU385, demonstrated two groups of PorA specificities. The majority of serum samples (26 of 40, 65%) had antibodies mainly directed to various epitopes on P1.7,16 PorA. Responses to the P1.16 region dominated (n = 14 serum samples), but binding to P1.7 (n = 5 serum samples) and other unknown epitopes on P1.7,16 PorA (n = 7 serum samples) was also detected. The remaining 14 (35%) serum samples showed no or other PorA reactions. Blotting of 20 paired serum samples with OMVs from the three VR deletion mutants of H44/76 gave VR specificities similar to those obtained with strains M1080 and S3032 (not shown).
Comparison of PorA responses measured in SBA and immunoblot assays.
As described above, 12 postvaccination serum samples showed distinct SBA decreases with the P1.7,16 deletion mutants. They all belonged to the group of serum samples that gave various antibody responses to the P1.7,16 PorA in the immunoblot assay. The results of the two methods are shown in Table 4. Most of the postvaccination serum samples had SBA directed to a combination of the P1.7 and P1.16 regions (P1.7 + P1.16), revealed as a decrease in SBA to the double deletion P1.−,− mutant, whereas a response to the P1.16 region dominated on blots. Both methods showed few serum samples with antibodies to the P1.7 region. Two serum samples had antibodies to epitopes different from the P1.7 and P1.16 regions on the P1.7,16 PorA (designated P1.7,16). Activity to the P1.19,15 PorA or its variant P1.19,15-1 was obtained with two serum samples; interestingly, they were from individuals carrying P1.19,15 strains (see Table 5). On blots, one serum reacted with all PorA, suggesting antibodies directed to common PorA epitopes (designated P1C). In conclusion, the two methods showed partially overlapping results for the PorA specific responses.
TABLE 4.
PorA antibody specificities of postvaccination sera determined in serum bactericidal activity (SBA) and immunoblot (IB) assaysa
| Vaccinee no. | SBA assayb | IB assayb | Concordance of assays |
|---|---|---|---|
| 459 | P1.7 + P1.16 | P1.16 | Partial |
| 314 | P1.7 + P1.16 | P1.16 | Partial |
| 384c | P1.7; P1.16; P1.19,15-1 | P1.7; P1.19,15 | Partial |
| 570 | P1.7; P1.7, 16 | P1.16; P1C | Partial |
| 371 | P1.16; P1.7 + P1.16 | P1.16 | Partial |
| 397c | P1.7 + P1.16; P1.19,15-1 | P1.7,16 | Partial |
| 474 | P1.7 + P1.16 | P1.16 | Partial |
| 298 | P1.7 + P1.16 | P1.16 | Partial |
| 320 | P1.7 + P1.16 | P1.16 | Partial |
| 530 | P1.7 | P1.16 | No |
| 485 | P1.16 | P1.16 | Yes |
| 562 | P1.7 + P1.16 | P1.16 | Partial |
Postvaccination sera (n = 12) showing two or more log step decrease in SBA for PorA deletion mutants compared with the P1.7,16 PorA strain (see text).
P1.7 + P1.16, antibodies to both VR demonstrated with the double H44/76 P1.−,− deletion mutant; P1.7,16, antibodies to non-VR epitopes on P1.7,16 PorA; P1C, antibodies to conserved epitopes on several PorA.
Carriers of nontypeable:P1.15 strains, see Table 5.
TABLE 5.
Antibody responses of sera from vaccinees who were identified as carriers of meningococcia
| Vaccinee no. | Carrier isolate | ET-5 complement | IgG bindingb
|
SBA responses | |||
|---|---|---|---|---|---|---|---|
| P15 PorB | P1.7,16 PorA | P4 PorB | P1.19,15 PorA | ||||
| 329 | B:15:P1.7,16 | + | + | + | − | + | Prevaccination ≥ postvaccination sera for all test strains |
| 317 | 15:P1.16 | + | + | − | − | − | >4-fold for P15 antibodies |
| 514 | 15:P1.7 | + | + | + | − | − | >4-fold for P15 antibodies |
| 568 | 15:− | − | + | + | + | − | >4-fold for P15 antibodies |
| 435 | 4:P1.15 | + | + | + | + | + | >4-fold for P1.19, 15-1 antibodies |
| 331 | 4:P1.14 | − | + | + | + | − | |
| 503 | NT:P1.15 | − | + | + | − | + | |
| 384 | NT:P1.15 | − | + | + | − | + | ≥4-fold for P1.19,15-1 antibodies |
| 397 | NT:P1.15 | − | − | + | − | − | >4-fold for P1.19,15-1 antibodies |
| 314 | NT:− | − | + | + | − | − | |
| 431 | NT:− | − | + | + | − | − | |
| 469 | B:NT:− | − | − | − | − | − | |
| 474 | NT:− | − | + | + | − | − | |
Thirteen of the 40 vaccinees were carriers of meningococci at the time of the third vaccine dose. NT, nontypeable.
Distinct binding of ≥1,000 arbitrary scan units to PorA and PorB on blots with OMV from H44/76 and CU385.
Characterization of meningococcal carriage strains.
Thirteen of the 40 volunteers (32.5%) were identified as carriers of meningococci at the time of the third dose or 5 weeks later. As shown in Table 5, four of them harbored strains belonging to the ET-5 complex. Four and five carrier strains expressed porins with epitopes present in the Norwegian (serotype 15 PorB, P1.7 and/or P1.16 PorA) and Cuban (serotype 4 PorB and/or P1.15 PorA) vaccine strain, respectively, whereas the remaining four strains were nontypeable (NT−).
The carriage rate among the 40 vaccinees was similar to that observed for the majority of the volunteers in the Icelandic trial, where 111 of the 358 teenagers (31%) carried N. meningitidis (28). In that study, the strains were designated by serogrouping only. By multilocus enzyme electrophoresis, we found that these isolates represented 55 different ETs, of which the ET-5 clone complex comprised 19 strains (17.1%). Twenty-six percent of the strains expressed serotype 15 PorB, P1.7, and/or P1.16 PorA epitopes, whereas 36% expressed serotype 4 PorB and/or P1.15 PorA epitopes. The majority of the remaining strains were nontypeable. The serotype distribution among the subset of 13 carrier strains thus reflected that found for all carrier strains.
Effect of meningococcal carriage on SBA and IgG levels.
Table 5 also shows the various antibody responses of the 13 carriers measured in SBA and immunoblot assays. Paired serum samples from three of the four individuals who harbored P1.15 strains showed ≥4-fold increases in SBA with the P1.19,15-1+PorB− Opc− Opa5.5− strain. In immunoblot assays, postvaccination serum samples from all but one of those carrying P1.19,15 or serotype 4 meningococci reacted distinctly with the corresponding PorA or PorB band of strain CU385 (B:4:P1.19,15). Thus, carriage of these heterologous strains generally led to antibody responses revealed by both SBA and immunoblot assays.
In the four individuals who harbored strains with porin epitopes similar to those in the Norwegian OMV vaccine (serotype 15, subtypes P1.7/P1.16), the effect of carriage could not be discriminated from that of vaccination by the immunoblot method. However, although the mean log2 titers with the P1.19,15-1+ PorB+ Opc− Opa5.5− strain and the corresponding PorB− strain were similar after vaccination (Table 2), three of these carriers showed ≥4-fold higher SBA with the PorB+ strain (Table 5). This result suggested the presence of PorB-specific SBA. The remaining carrier had high titers before and after vaccination against all strains tested. No specific reactions were found for carriers of nontypeable strains.
DISCUSSION
In this study, paired serum samples from about half of the individuals (53%) who received three doses of the Norwegian meningococcal OMV vaccine (B:15:P1.7,16) during the Icelandic trial (28) were examined with a panel of target strains to define the immunogenicity of the major vaccine components. The effect of meningococcal carriage during the vaccine trial on antibody responses was also analyzed. The strain panel consisted of isogenic recombinant H44/76 strains derived from the vaccine strain and wild-type reference strains (Table 1), and antibody levels were measured in SBA and immunoblot assays.
In agreement with previous results obtained with other methods (16, 31, 33, 40, 44), the isogenic H44/76 strains also demonstrated that the OMV vaccine induced SBA to the P1.7,16 PorA and Opc proteins (Table 2). However, we could not detect any SBA to the Opa5.5 protein as shown for Opa proteins of other vaccines (24, 50). Regarding PorB, its capacity for raising SBA has been questioned. A recent phylogenetic study indicated that PorB is under strong immune selection (42), and bactericidal PorB antibodies were reported after vaccination of rabbits (1) and mice (49). However, most murine PorB monoclonal antibodies have low or no SBA (37), and murine monoclonal antibodies as well as human antibodies to the class 3 PorB bind poorly to live meningococci (22).
Lack of PorB-specific SBA with the isogenic strains in our study, coupled with the high IgG-specific level on blots, corresponded to previous reports on the Norwegian and Cuban OMV vaccines in human volunteers (5, 6, 13, 14, 23, 33, 44, 46, 47). Interestingly, three of the four carriers of serotype 15 meningococcal strains in our study did show a ≥4-fold increase in SBA to this PorB (Table 5). Such activity could not be detected in the remaining carrier, whose prevaccination serum had high SBA with all target strains. On blots, this serum gave a strong lipopolysaccharide response that may explain its functional activity (W. D. Zollinger, E. E. Moran, and B. L. Brandt, Abstr. 11th Int. Pathogenic Neisseria Conf., 1998, p 187). It is also possible that carriage of serotype 15 strains in the period after vaccination may induce PorB antibodies with specificities different from those induced with the OMV vaccine.
Compared with the distinct P1.7,16 responses, levels to the heterologous P1.19,15 and P1.19,15-1 PorA variants in the immunoblot and SBA assays, respectively, were low after vaccination (Tables 2 and 3). That there was no significant difference in SBA to the H44/76 P1.19,15-1 strain and the corresponding PorA− variant indicated that bactericidal cross-reactive antibodies to common or conserved PorA epitopes were not raised by vaccination. However, the number of vaccinees with ≥4-fold responses in the SBA assays with the P1.19,15-1 strains, lacking the Opc and Opa5.5 proteins (23 and 35%; Table 2), was in the same range as reported previously with heterologous wild-type strains (28) and a PorA− mutant (33). This suggested that SBA may be elicited by other minor vaccine components or by acquisition of carrier strains in the period after vaccination.
The ability of the two major VR of PorA in inducing antibodies was compared with the SBA and immunoblot methods. Only 12 (30%) of the postvaccination serum samples demonstrated distinct decreases in SBA with the isogenic H44/76 mutants that lacked either the P1.7/VR1, the P1.16/VR2, or both VR. Most of these serum samples had SBA directed to a combination of the P1.7 and P1.16 regions (Table 4). This specificity was also observed for the P1.7,16 antigen in the Dutch hexavalent PorA vaccine (34, 35). On blots in contrast, the same serum samples mainly showed a P1.16 antibody response (Table 4) as previously found with this and other non-SBA methods for the Norwegian vaccine (7, 16, 33, 46). Thus, the PorA specificities obtained with the two assays corresponded only partially (Table 4).
The low P1.7 antibody levels detected in the immunoblot assay could be explained by the use of a PorA variant with a dipeptide insertion in VR1 (P1.7-1). However, this PorA reacted in the same way as P1.7,16 PorA on blots with the P1.7 reference monoclonal antibody (MN14C11.6), in accordance with structural studies (8). It also gave a strong signal on blots with a bactericidal human postvaccination serum demonstrated by other methods to possess P1.7 activity (7). That antibodies against the P1.16 region is important can be deduced from outbreaks in different countries by B:15:P1.7,16 strains with smaller mutations in the P1.16 epitope (21, 29, 32, 39). Such mutations often occur near the β-turn of the P1.16 loop being buried within the antigen-binding site of the antibody (8, 43). Recently, P1.16-specific antibodies were found to be more efficient than P1.7 antibodies in SBA assays (T. E. Michaelsen, Ø. Ihle, T. Herstad, A. Aase, J. Kolberg, and A. Høiby, Abstr. 13th Int. Pathogenic Neisseria Conf., Oslo, Norway, 2002, p. 137).
As shown in Fig. 1, mean log2 titers with the P1.7 and P1.16 deletion mutants were higher than for the P1.7,16 PorA strain. The close association between VR1 and VR2 of adjacent PorA monomers (9) could implicate that removal of one VR affected conformation of the remaining VR and so exposed otherwise nonaccessible epitopes to PorA antibodies. Increased sensitivity in SBA assays of such mutants has also been reported previously (20, 34, 35), which suggested that these mutants may not be ideal tools for analyses of bactericidal PorA epitopes. The probability that immunoblot assays will mainly detect antibodies against linear epitopes, indicates that none of the methods are perfect for characterization of specific PorA responses.
Among the 40 vaccinees, 13 were identified as carriers of meningococci. Antibody responses for the majority of these carriers, detected as SBA and specific porin bands on blots (Table 5), were consistent with their carrier strains as also described by others (17, 30). In addition to an increase in serotype 15 PorB-specific SBA in those harboring serotype 15 strains, as discussed above, a similar response to the P1.19,15-1 PorA was obtained with carriers of P1.19,15 strains. Six and three other vaccinees also showed SBA to the P1.19,15-1 PorA and serotype 15 PorB proteins, respectively. On blots, the porin responses resulting from carriage of strains similar to the vaccine strain cannot be differentiated from the vaccine response. However, carriage of 4:P1.15 strains was reflected in IgG binding to the serotype 4 and subtype P1.19,15 porins, as shown in Table 5. In addition, another 12 and 7 postvaccination serum samples also revealed distinct signals to the serotype 4 PorB and the P1.19,15 PorA, respectively. These results indicated that circulation of strains with phenotypes similar to the Cuban and Norwegian vaccine strains might have been frequent and so contributed to the bactericidal responses observed among the controls in the trial (28).
In conclusion, use of isogenic H44/76 vaccine strains confirmed the bactericidal responses of the P1.7,16 PorA and Opc antigens in the Norwegian OMV vaccine previously demonstrated with other methods. These strains furthermore showed that P1.7,16-specific SBA did not cross-react with a heterologous PorA and that carriage of meningococci among the vaccinees gave rise to specific SBA. As a tool for determination of immunogenic PorA domains, the isogenic PorA deletion mutants seem less suitable due to higher sensitivity in the SBA assays.
Acknowledgments
We are grateful to J. Poolman and E. Rouppe van der Voort, who initiated this study, to L. van Alphen, J. T. Poolman, and E. Rosenqvist for critical reading of the manuscript, to K. Jonsdottir for collecting the carrier isolates, to B. Perkins and G. Carlone for providing the serum samples, and to W. D. Zollinger for the gift of monoclonal antibodies.
Editor: J. T. Barbieri
REFERENCES
- 1.Bash, M. C., F. Lynn, N. F. Concepcion, J. W. Tappero, G. M. Carlone, and C. E. Frasch. 2000. Genetic and immunological characterization of a novel serotype 4,15 strain of Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 29:169-176. [DOI] [PubMed] [Google Scholar]
- 2.Bjune, G., E. A. Høiby, J. K. Grønnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A.-K. Lindbak, H. Nøkleby, E. Rosenqvist, L. K. Solberg, O. Closs, J. Eng, L. O. Frøholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of an outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 3.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, E. Moran, W. Hankins, J. Gilly, J. Mays, and the Chilean National Committee for Meningococcal Disease. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 4.Caugant, D. A., P. Bol, E. A. Høiby, H. C. Zanen, and L. O. Frøholm. 1990. Clones of serogroup B Neisseria meningitidis causing systemic disease in the Netherlands 1958 through 1986. J. Infect. Dis. 162:867-874. [DOI] [PubMed] [Google Scholar]
- 5.Delvig, A. A., E. Wedege, D. A. Caugant, R. Dalseg, J. Kolberg, A. Achtman, and E. Rosenqvist. 1995. A linear B-cell epitope on the class 3 outer-membrane protein of Neisseria meningitidis recognized after vaccination with the Norwegian group B outer-membrane vesicle vaccine. Microbiology 141:1593-1600. [DOI] [PubMed] [Google Scholar]
- 6.Delvig, A. A., T. E. Michaelsen, A. Aase, E. A. Høiby, and E. Rosenqvist. 1997. Vaccine-induced IgG antibodies to the linear epitope on the PorB outer membrane protein promote opsonophagocytosis of Neisseria meningitidis by human neutrophils. Clin. Immunol. Immunopathol. 84:27-35. [DOI] [PubMed] [Google Scholar]
- 7.Delvig, A., S. Jahn, B. Kusecek, J. H. Heckels, E. Rosenqvist, E. A. Høiby, T. E. Michaelsen, and M. Achtman. 1994. A comparison of human and murine monoclonal IgGs specific for the P1.7 PorA protein of Neisseria meningitidis. Mol. Immunol. 31:1257-1267. [DOI] [PubMed] [Google Scholar]
- 8.Derrick, J. P., M. Maiden, and I. M. Feavers. 1999. Crystal structure of an Fab fragment in complex with a meningococcal serosubtype antigen and a protein G domain. J. Mol. Biol. 293:81-91. [DOI] [PubMed] [Google Scholar]
- 9.Derrick, J. P., R. Urwin, J. Suker, I. M. Feavers, and M. Maiden. 1999. Structural and evolutionary interference from molecular variation in Neisseria porins. Infect. Immun. 67:2406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-510. [DOI] [PubMed]
- 11.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Frøholm, A. K. Lindbak, B. Møgster, E. Namork, U. Rye, G. Stabbetorp, R. Winsnes, B. Aase, and O. Closs. 1991. Production, characterization and control of MenB-Vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-80. [PubMed] [Google Scholar]
- 12.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guttormsen, H. K., L. M. Wetzler, and A. Næss. 1993. Humoral immune response to the class 3 outer membrane protein during the course of meningococcal disease. Infect. Immun. 61:4734-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Høiby, E. A., E. Rosenqvist, L. O. Frøholm, G. Bjune, B. Feiring, H. Nøkleby, and E. Rønnild. 1991. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 14:147-156. [PubMed] [Google Scholar]
- 15.Holten, E. 1978. Serotypes of Neisseria meningitidis isolated from patients in Norway during the fiarst six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idänpään-Heikkilä, I., E. A. Høiby, P. Chattopadhyay, U. Airaksinen, T. M. Michaelsen, and E. Wedege. 1995. Antibodies to meningococcal class 1 outer-membrane protein and its variable regions in patients with systemic meningococcal disease. J. Med. Microbiol. 43:335-343. [DOI] [PubMed] [Google Scholar]
- 17.Jones, G. R., M. Christodoulides, J. L. Brooks, A. R. O. Miller, K. A. V. Cartwright, and J. E. Heckels. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J. Infect. Dis. 178:451-459. [DOI] [PubMed] [Google Scholar]
- 18.Kuipers, B., G. van den Dobbelsteen, E. Wedege, and L. van Alphen. 2001. Serological characterisation, p. 131-145, In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal disease: methods in molecular medicine, vol. 67. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 19.Maiden, M. C., J. Suker, A. J. McKenna, J. A. Bygraves, and I. M. Feavers. 1991. Comparison of the class 1 outer membrane proteins of eight serological reference strains of Neisseria meningitidis. Mol. Microbiol. 5:727-736. [DOI] [PubMed] [Google Scholar]
- 20.Martin, S. L., R. Borrow, P. van der Ley, M. Dawson, A. J. Fox, and K. A. V. Cartwright. 2000. Effect of sequence variation in the meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine 18:2476-2481. [DOI] [PubMed] [Google Scholar]
- 21.McGuinness, B. T., I. N. Clarke, P. R. Lambden, A. K. Barlow, J. T. Poolman, D. M. Jones, and J. E. Heckels. 1991. Point mutations in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 22.Michaelsen, T. E., A. Aase, J. Kolberg, E. Wedege, and E. Rosenqvist. 2001. PorB3 outer membrane protein on Neisseria meningitidis is poorly accessible for antibody binding on live bacteria. Vaccine 19:1526-1533. [DOI] [PubMed] [Google Scholar]
- 23.Milagres, L. G., M. C. O. Gorla, C. T. Sacchi, and M. M. Rodrigues. 1998. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunization with an outer membrane vaccine. Infect. Immun. 66:4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milagres, L. G., M. C. O. Gorla, M. C. Rebelo, and D. E. Barroso. 2000. Bactericidal antibody response to Neisseria meningitidis serogroup B in patients with bacterial meningitis: effect of immunization with an outer membrane protein vaccine. FEMS Immunol. Med. Microbiol. 28:319-327. [DOI] [PubMed] [Google Scholar]
- 25.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moraes, J. C., B. A. Perkins, M. C. C. Camargo, N. T. R. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. L. Gral, V. L. Gattas, H. G. Vasconcelos, B. D. Plikaytis, J. D. Wenger, and C. V. Broome. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 27.Peeters, C. C. A. M., H. C. Rümke, L. C. Sundermann, E. M. Rouppe van der Voort, J. Meulenbelt, M. Schuller, A. J. Kuipers, P. van der Ley, and J. T. Poolman. 1996. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine 14:1009-1015. [DOI] [PubMed] [Google Scholar]
- 28.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Høiby, E. Rosenqvist, J. Holst, H. Nøkleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 29.Ristola, M., M. Jahkola, H. Käyhty, M. Sarvas, P. H. Mäkelä, D. Caugant, and R. Visakorpi. 1995. Meningococcal outbreak in Southern Finland. Eur. Commun. Dis. Bull. 1995:June2-3. [Google Scholar]
- 30.Robinson, K., K. R. Neal, C. Howard, J. Stockton, K. Atkinson, E. Scarth, J. Moran, A. Robins, I. Todd, E. Kaczmarski, S. Gray, I. Muscat, R. Slack, and D. A. A. Ala'Aldeen. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect. Immun. 70:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenqvist, E., E. A. Høiby, E. Wedege, B. Kusecek, and M. Achtman. 1993. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J. Infect. Dis. 167:1065-1073. [DOI] [PubMed] [Google Scholar]
- 32.Rosenqvist, E., E. A. Høiby, E. Wedege, D. A. Caugant, L. O. Frøholm, B. T. McGuinness, J. Brooks, P. R. Lambden, and J. E. Heckels. 1993. A new variant of serosubtype P1.16 in Neisseria meningitidis from Norway associated with increased resistance to bactericidal antibodies induced by a serogroup B outer membrane protein vaccine. Microb. Pathog. 15:197-205. [DOI] [PubMed] [Google Scholar]
- 33.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Rønnild, G. Bjune, and H. Nøkleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouppe van der Voort, E., P. van der Ley, J. van der Biezen, S. George, O. Tunnela, H. van Dijken, B. Kuipers, and J. Poolman. 1996. Specificity of human bactericidal antibodies induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect. Immun. 64:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouppe van der Voort, E., H. van Dijken, B. Kuipers, J. van der Biezen, P. van der Ley, J. Meylis, J. Claassen, and J. Poolman. 1997. Human B- and T-cell responses after immunization with a hexavalent PorA meningococcal outer membrane vesicle vaccine. Infect. Immun. 65:5184-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacchi, C. T., A. P. S. Lemos, A. M. Whitney, C. A. Solari, M. E. Brandt, C. E. A. Melles, C. E. Frasch, and L. W. Mayer. 1998. Correlation between serological and sequencing analyses of the PorB outer membrane protein in the Neisseria meningitidis serotyping system. Clin. Diagn. Lab. Immunol. 5:348-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saukkonen, K., H. Abdillahi, J. T. Poolman, and M. Leinonen. 1987. Protective efficacy of monoclonal antibodies to Class 1 and Class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model: new prospects for vaccine development. Microb. Pathog. 3:261-267. [DOI] [PubMed] [Google Scholar]
- 38.Sierra, G. V. G., H. C. Campa, N. M. Varacel., I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccines against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-210. [PubMed] [Google Scholar]
- 39.Taha, M. K., E. Bichier, A. Perrocheau, and J. M. Alonso. 2001. Circumvention of herd immunity during an outbreak of meningococcal disease could be correlated to escape mutation in the porA gene of Neisseria meningitidis. Infect. Immun. 69:1971-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Høiby, J. Holst, H. Nøkleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 41.Tommassen, J., P. Vermeij, M. Struyvé, R. Benz, and J. T. Poolman. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (PorA) and class 3 (PorB) outer membrane proteins. Infect. Immun. 58:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urwin, R., E. C. Holmes, A. J. Fox, J. P. Derrick, and M. C. J. Maiden. 2002. Phylogenetic evidence for frequent positive selection and recombination in the meningococcal surface antigen PorB. Mol. Biol. Evol. 19:1686-1694. [DOI] [PubMed] [Google Scholar]
- 43.van den Elsen, J. M. H., J. N. Herron, P. Hoogerhout, J. T. Poolman, E. Boel, T. Logtenberg, J. Wilting, D. J. A. Crommelin, J. Kroon, and P. Gros. 1997. Bactericidal antibody recognition of a PorA epitope of Neisseria meningitidis: crystal structure of a Fab fragment in a complex with a fluorecein-conjugate peptide. Proteins 29:113-125. [DOI] [PubMed] [Google Scholar]
- 44.Wedege, E., E. A. Høiby, E. Rosenqvist, and G. Bjune. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect. Immun. 66:3223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wedege, E., E. A. Høiby, E. Rosenqvist, and L. O. Frøholm. 1990. Serotyping and subtyping of Neisseria meningitidis isolates by coagglutination, dot-blotting and ELISA. J. Med. Microbiol. 31:195-201. [DOI] [PubMed] [Google Scholar]
- 46.Wedege, E., G. Bjune, L. O. Frøholm, E. A. Høiby, and E. Rosenqvist. 1991. Immunoblotting studies of vaccinee and patient serum samples from a Norwegian serogroup B meningococcal vaccination trial. NIPH Ann. 14:83-186. [PubMed] [Google Scholar]
- 47.Wedege, E., K. Bolstad, L. M. Wetzler, and H.-K. Guttormsen. 2000. IgG antibody levels to meningococcal porins in patient sera: comparison of immunoblotting and ELISA measurements. J. Immunol. Methods 244:9-15. [DOI] [PubMed] [Google Scholar]
- 48.Wedege, E., K. Bryn, and L. O. Frøholm. 1988. Restoration of antibody binding to blotted meningococcal outer membrane proteins using various detergents. J. Immunol. Methods 113:51-59. [DOI] [PubMed] [Google Scholar]
- 49.Wright, J. C., J. N. Williams, M. Christodoulides, and J. E. Heckels. 2002. Immunization with the recombinant PorB outer membrane protein induces a bactericidal immune response against Neisseria meningitidis. Infect. Immun. 70:4028-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zollinger, W. D., and E. Moran. 1991. Meningococcal vaccines—present and future. Trans. R. Soc. Trop. Med. Hyg. 85(Suppl. 1):37-43. [DOI] [PubMed] [Google Scholar]