Abstract
A subtle decline in episodic memory often occurs prior to the emergence of the full dementia syndrome in nondemented older adults who develop Alzheimer's disease (AD). The APOE-ε4 genotype may engender a more virulent form of AD that hastens this decline. To examine this possibility, we compared the rate of decline in episodic memory during the preclinical phase of AD in individuals with or without at least one APOE ε4 allele. Nondemented normal control (NC; n = 84) participants, nondemented older adults who subsequently developed dementia within 1 or 2 years (i.e., preclinical AD; n = 20), and patients with mild AD (n = 53) were examined with 2 commonly employed tests of episodic memory, the Logical Memory subtest of the Wechsler Memory Scale–Revised and the California Verbal Learning Test. Results revealed a precipitous decline in verbal memory abilities 1 to 2 years prior to the onset of the dementia syndrome, but there was little effect of APOE genotype on the rate of this memory decline. The presence of an APOE-ε4 allele, however, did have a differential effect on the sensitivity of the 2 types of memory tests for tracking progression and made an independent contribution to the prediction of conversion to AD.
Keywords: Episodic memory, Neuropsychology, Alzheimer's disease, Apolipoprotein E, Preclinical Alzheimer's disease
INTRODUCTION
Alzheimer's disease (AD) is an age-associated neurodegenerative disorder that results in the insidious onset and gradual progression of cognitive impairment (Katzman, 1986). A significant deficit in episodic memory is usually the earliest and most prominent manifestation of AD, with additional deficits in executive functions, language, attention, and visuospatial skills becoming evident as the disease progresses (for review, see Salmon & Bondi, 1999). The early occurrence of episodic memory impairment in AD is consistent with neuropathologic and neuroimaging evidence suggesting that the entorhinal cortex and hippocampus are initially affected in the earliest stage of the disease (Braak & Braak, 1991; Hyman et al., 1984; Jack et al., 1992; Killiany et al., 1993). Other cognitive abilities become affected as the neuropathological changes of AD (e.g., atrophy, loss of neurons and synapses, and the abnormal deposition of neuritic plaques and neurofibrillary tangles) spread from limbic structures (e.g., the hippocampus, entorhinal cortex, amygdala) to the association cortices of the temporal, parietal, and frontal lobes (Braak et al., 1998; Nagy et al., 1999; Terry et al., 1991).When these memory and other cognitive deficits become severe enough to interfere with everyday activities, criteria for dementia are met (e.g., DSM–IV, American Psychiatric Association, 1994) and a clinical diagnosis of possible or probable AD is warranted (McKhann et al., 1984).
A number of prospective studies of non-demented older adults who eventually developed AD have shown that a subtle decline in episodic memory often occurs prior to the emergence of the obvious cognitive and behavioral changes required for a clinical diagnosis of the disease (Bondi et al., 1994; Fuld et al., 1990; Grober & Kawas, 1997; Howieson et al., 1997; Jacobs et al., 1995). In some cases, this decline is evident many years prior to the development of dementia (Bäckman et al., 2001; Bondi et al., 1999; Linn et al., 1995; Small et al., 2000). This state of poor memory performance has been shown to predict the subsequent development of dementia in the elderly and is known variously as preclinical AD (Bondi et al., 1994) or questionable dementia (Albert et al., 2001). The importance of mild preclinical memory changes for the early detection of AD has led to considerable research and clinical interest (for review, see Collie & Maruff, 2000), including the formalization of criteria for Mild Cognitive Impairment (MCI; Peterson et al., 1995) and the recent development of practice parameters that recommend systematically monitoring patients with these changes for subsequent conversion to possible or probable AD (Petersen et al., 2001).
Despite this growing interest in subtle memory decline prior to the development of the dementia of AD, few studies have examined the course of episodic memory changes during the preclinical phase of the disease (Bäckman et al., 2001; Chen et al., 2001; Rubin et al., 1998; Small et al., 2000). In two studies that have addressed this issue, Small et al. (2000) and Bäckman et al. (2001) examined longitudinal changes in episodic memory in individuals who eventually developed probable AD and found that they had mild impairment of memory at an evaluation 6 years prior to diagnosis, but had little change in memory performance over the next 3 years. These investigators suggested that this long period of stable memory ability in individuals with preclinical AD is followed by a relatively precipitous decline in the period immediately preceding the development of the dementia syndrome. This speculation was borne out in a recent study that found a significant decline in episodic memory and executive functions in individuals with preclinical AD during the period from 3.5 years to 1.5 years prior to diagnosis (Chen et al., 2001). As Chen et al. (2001) point out, this finding suggests that imminent conversion to dementia might be better predicted by an abrupt decline in memory performance than by poor but stable memory abilities.
A factor that could influence the rate of decline in episodic memory abilities during the preclinical phase of AD is the presence or absence of a genetic risk factor for the disease, the ε4 allele of apolipoprotein E. Apolipoprotein E is a cholesterol-transporting protein coded on chromosome 19 by a polymorphic gene with three common allele types: ε2, ε3, and ε4, resulting in six possible genotypes: ε2/2, ε2/3, ε2/4, ε3/3, ε3/4, and ε4/4 (Corder et al., 1993; Saunders et al., 1993; Strittmatter et al., 1993). The presence of the ε4 allele confers an increased risk for late-onset AD in a dose-dependent manner (Corder et al., 1993) and may also have a significant impact on the neuropathologic and morphologic manifestations of the disease. Studies have shown that AD patients with the ε4 allele of the apolipoprotein E gene (APOE-ε4) have greater hippocampal atrophy and lower than expected hippocampal asymmetries evident on magnetic resonance imaging (MRI; Geroldi et al., 2000; Soininen et al., 1995; see also Soininen & Riekkinen, 1996, for review) than those without the allele, as well as more plaque and tangle pathology at autopsy (Gomez-Isla et al., 1996; Normann et al., 1995; Ohm et al., 1995; Schmechel et al., 1993; see also Strittmatter et al., 1994).
Because of these possible influences of the APOE-ε4 genotype on the pathologic and morphologic manifestations of AD, the nature of the association between the presence of an APOE-ε4 allele and episodic memory in the elderly has been the topic of a number of recent studies. With few exceptions (Craft et al., 1998; Dik et al., 2000; Mayeux et al., 2001), studies with nondemented older adults have shown that APOE-ε4 genotype has little effect on memory once individuals with preclinical AD are removed from the study sample (Basun et al., 1995; Bondi et al., 1999; Corder et al., 1995; Dal Forno et al., 1996; DeKosky et al., 1995; Gomez-Isla et al., 1996; Growdon et al., 1996; Kurz et al., 1996; Normann et al., 1995; Small et al., 1998; Smith et al., 1998). Studies of patients with possible or probable AD are less consistent, with some showing that patients with the APOE-ε4 allele have a greater memory deficit than equally demented patients without the ε4 allele (Smith et al., 1998), and others showing that there is little or no difference in memory performance (Tierney et al., 1996), or in the rate of memory deterioration (Frisoni et al., 1995; Growdon et al., 1996; Hyman et al., 1996) in these groups. Similarly, studies examining the effect of APOE-ε4 genotype on the memory performance of patients with preclinical AD have been mixed, with some showing worse memory in those with an ε4 allele than in those without (Bondi et al., 1999; Smith et al., 1998) and others showing no difference in these groups (Tierney et al., 1996). The potential impact of the presence of the APOE-ε4 allele on rate of decline in episodic memory during the preclinical phase of AD has not been examined.
Given the lack of consistent information from previous studies, the present study was designed to examine the effect of the APOE-ε4 genotype on rate of decline in verbal memory in nondemented healthy older adults, nondemented older adults with preclinical AD (i.e., those who later progressed to a diagnosis of AD), and patients with mild possible or probable AD. Memory decline was examined using two commonly employed tests of episodic memory, the Logical Memory subtest of the Wechsler Memory Scale–Revised (WMS–R) and the California Verbal Learning Test (CVLT). The tests had been administered on two occasions separated by approximately 1 year. The individuals with preclinical AD were tested 1 year prior to diagnosis and in the year that they received the clinical diagnosis of AD. A subset of the preclinical AD group had also been tested 2 years prior to receiving the AD diagnosis. If the APOE-ε4 genotype results in a more virulent form of AD, preclinical AD patients with the ε4 allele may exhibit an earlier and steeper decline in episodic memory than those patients without at least one ε4 allele, and this more rapid decline may carry over into the clinical phase of the disease.
METHODS
Research Participants
Eighty-four nondemented normal control (NC) subjects, 20 nondemented individuals who subsequently developed dementia within 1 or 2 years (i.e., preclinical AD), and 53 patients with mild Alzheimer's disease (AD) participated in the study. All participants were enrolled in the University of California, San Diego (UCSD) Alzheimer's Disease Research Center (ADRC). Informed consent was obtained from participants and/or their caregivers when appropriate. As part of their ADRC participation, each individual underwent an extensive annual assessment that included neurological, medical, neuropsychiatric, and neuropsychological evaluations that have been described in detail previously (Galasko et al., 1990; Salmon & Butters, 1992). Information concerning functional abilities was gathered from an informant using the Pfeffer Outpatient Disability scale (POD; Pfeffer et al., 1982) and items from the Blessed Functional Scale (Blessed et al., 1968). The APOE genotype of each participant was determined according to the methods described in Saunders et al. (1993). Individuals were classified as APOE-ε4-positive or APOE-ε4-negative based on the presence or absence of at least one APOE-ε4 allele. The number of participants in each group with each of the possible APOE genotypes is shown in Table 1.
Table 1.
Apolipoprotein E (APOE) genotype frequencies (and percent of the group total) in the normal control (NC) participants, patients with preclinical Alzheimer's disease (AD), and patients with AD
| Genotype | NC (n = 84) | Preclinical AD (n = 20) | AD (n = 53) |
|---|---|---|---|
| 2/3 | 11 (13%) | 3 (15%) | 3 (6%) |
| 3/3 | 47 (56%) | 3 (15%) | 17 (32%) |
| APOE-ε4 negative | 58 (69%) | 6 (30%) | 20 (38%) |
| 2/4 | 3 (4%) | 1 (5%) | 3 (6%) |
| 3/4 | 23 (27%) | 10 (50%) | 22 (41%) |
| 4/4 | 0 | 3 (15%) | 8 (15%) |
| APOE-ε4 positive | 26 (31%) | 14 (70%) | 33 (62%) |
At each annual evaluation, a clinical diagnosis was made by two senior neurologists following a review of a summary of the results from the entire evaluation. The diagnosing neurologists were blinded to specific neuropsychological test scores, but they were provided with the MMSE score and a general statement from the neuropsychologist as to whether or not two or more areas of cognition were abnormal. The diagnosis of dementia was based on DSM–III–R criteria, and the diagnosis of AD was based upon NINCDS/ADRDA criteria for possible or probable Alzheimer's disease (McKhann et al., 1984).
All participants completed at least two annual evaluations. All NC participants were classified as nondemented in both evaluations, and all participants with AD were diagnosed with possible or probable AD at both evaluations. The preclinical AD participants were all diagnosed with possible or probable AD at their last evaluation and had been classified as either normal, at-risk for AD, or with MCI in the immediately preceding annual evaluation. A subset of the Preclinical AD group (15/20 participants) had completed two preceding annual evaluations and received one of the normal, at-risk for AD, or MCI classifications in both. The at-risk for AD and MCI classifications were used for individuals who demonstrated evidence of mild cognitive decline without observed functional decline and therefore did not meet DSM–III–R criteria for dementia. These designations are essentially identical, but the at-risk for AD classification preceded the formalization and implementation of MCI criteria (Petersen et al., 1995).
The NC, preclinical AD, and mild AD groups were further divided into APOE-ε4-positive or APOE-ε4-negative subgroups. The mean age, education, Dementia Rating Scale (DRS; Mattis, 1988) score, Mini-Mental State Exam (MMSE; Folstein et al., 1975) score, and gender distribution for each subgroup at the time of the first evaluation are shown in Table 2. One-way analyses of variance (ANOVAs) showed no differences in age [F(5,151) = 0.98, p = .43] or education [F(5,151) = 1.06, p = .38] among the six subgroups. There was, however, an overall significant effect of group for both the MMSE and DRS (both p values <.01). Post-hoc pairwise comparisons with Tukey's test showed that, as expected, the two AD subgroups scored lower than the two NC and two preclinical AD subgroups on both the MMSE and DRS. In addition, the MMSE score of the APOE-ε4-negative preclinical AD subgroup was lower than that of the APOE-ε4-negative NC subgroup. The DRS score of the APOE-ε4-positive preclinical AD subgroup was lower than those of both NC subgroups, and the DRS score of the APOE-ε4-negative preclinical AD subgroup was lower than that of the APOE-ε4-positive NC subgroup. Within each diagnostic category (i.e., NC, preclinical AD, AD), there was no difference in the mental status scores of the ε4-positive and ε4-negative subgroups.
Table 2.
Demographic information and measures of general cognitive functioning at the initial time of testing for the normal control (NC) participants, patients with preclinical Alzheimer's disease (AD), and patients with AD
| NC |
Preclinical AD |
AD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APOE-ε4−(n = 58) |
APOE-ε4+(n = 26) |
APOE-ε4−(n = 6) |
APOE-ε4+(n = 14) |
APOE-ε4−(n = 20) |
APOE-ε4+(n = 33) |
|||||||
| Measure | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) |
| Demographics | ||||||||||||
| Age at test | 73.3 | (6.0) | 73.2 | (6.1) | 77.5 | (6.3) | 74.4 | (7.4) | 75.6 | (6.6) | 74.7 | (5.7) |
| Years of education | 14.7 | (3.2) | 14.8 | (2.5) | 15.2 | (2.8) | 14.9 | (3.2) | 13.6 | (4.5) | 13.5 | (3.6) |
| Sex (male/female) | 28/30 | 9/17 | 3/3 | 8/6 | 10/10 | 19/14 | ||||||
| Duration of cognitive complaints (years) | 3.7 | (2.4) | 3.8 | (2.3) | ||||||||
| Cognitive function | ||||||||||||
| Dementia Rating Scale | 138.8 | (3.5) | 139.9 | (3.4) | 130.8 | (7.2) | 131.3 | (7.0) | 116.8 | (9.7) | 116.5 | (9.1) |
| Mini-Mental State Exam | 29.5 | (0.7) | 29.4 | (0.9) | 26.5 | (2.3) | 27.5 | (1.4) | 23.0 | (4.4) | 23.0 | (3.5) |
Procedure
The California Verbal Learning Test (CVLT) and the WMS–R Logical Memory Test were administered to each participant as part of a larger battery of tests used at the annual evaluation carried out by the UCSD ADRC. Each participant was tested individually by a trained psychometrist in a quiet, well-lit room. The two memory tests are described below.
California Verbal Learning Test
The CVLT is a standardized memory test that was developed to assess a variety of memory processes identified through cognitive psychological studies of normal memory (Delis et al., 1987). The CVLT assesses rate of learning, retention after short- and long-delay intervals, semantic encoding ability, recognition memory (i.e., discriminability), intrusion and perseverative errors, and response biases. Five presentation/free recall trials for a list (List A) of 16 items (four items in each of four semantic categories) are administered followed by a single interference trial using a second, different list (List B) of 16 items. Immediately after the List B trial, free-recall and then cued-recall (utilizing the names of the four categories) of the items on the initial list (List A) are elicited. Twenty minutes later, free recall and cued recall of the List A items are again elicited, followed by a yes–no recognition test consisting of the 16 List A items and 28 randomly interspersed distractor items. A “savings” score was computed by dividing the number of items recalled on the Long Delay Free Recall trial by the number recalled on Trial 5 of the initial presentation/free recall trials.
Logical Memory Test
The Logical Memory (LM) Test is a standardized test that assesses free recall of two short stories, each consisting of 25 bits of information (Wechsler, 1987). The two stories are presented sequentially in the immediate recall condition, and free recall of the story is elicited after each presentation. Thirty minutes later, free recall of the two stories is again elicited. The total bits of information recalled immediately and after the delay interval is recorded, and a “savings” score is computed by dividing the total score achieved during delayed recall by the total score achieved during immediate recall.
Data Analysis
To examine the effects of disease state and APOE genotype on the rate of change in memory performance, separate 3 (group: NC, preclinical AD, AD) ×2 (genotype:APOE-ε4-positive, APOE-ε4-negative) × 2 (time: Year 1, Year 2) repeated measures ANOVAs were performed for each of the key memory measures from the CVLT and the LM Test. For the preclinical AD group, Year 1 was the evaluation preceding the diagnosis of dementia, and Year 2 was the evaluation in which the diagnosis was made. Significant main effects and interactions were explored with one-way ANOVA and/or t tests.
To examine the effect of APOE genotype on the rate of change in memory performance in the preclinical AD patients who had completed 3 years of testing, separate 2 (group: APOE-ε4-positive, APOE-ε4-negative) × 3 (time: Year 2, Year 1, Year zero) repeated measures ANOVAs were performed with each of the key memory measures from the CVLT and the LM Test. These analyses included data that had been obtained in annual evaluations 2 years prior (i.e., Year 2), 1 year prior (Year 1), and in the year (i.e., Year zero) in which the diagnosis was made. Significant main effects and interactions were explored with one-way ANOVA and/or t tests.
Bivariate logistic regression analyses were used to identify those variables that best predicted conversion to AD. The analyses included the NC and preclinical AD participants and used diagnosis (i.e., AD vs. NC) as the dependent—or outcome—measure. Only the subset of preclinical AD participants that had completed 3 years of testing were included so that memory measures collected in assessments prior to the year of diagnosis could be used. In this way, the predictive value of change in memory performance prior to the diagnosis of dementia could be assessed. APOE genotype (APOE-ε4-positive, APOE-ε4-negative), eight key learning and memory measures (CVLT: Trials 1–5 Total Recall, Short Delay Free Recall, Long Delay Free Recall, Recognition Discriminability, and Recall Savings; LM: Immediate Recall, Delayed Recall, Recall Savings), and age, years of education, and gender were entered as covariates in a stepwise forward selection method. The five CVLT and three LM Test variables were selected because of their sensitivity to the early stages of AD (see Delis et al., 1991) and to provide comparison to previous studies (see Bäckman et al., 2001). Each of the memory measures were z-transformed prior to analysis in order to frame relative risk (RR) ratios on a standard metric for comparison across tasks. Thus, reported odds ratios represent differences in risk of conversion to AD per standard deviation unit. Three logistic regression analyses were completed. The first analysis used memory data collected 2 years prior to conversion to AD for the preclinical AD group and in Year 1 for the NC group (i.e., Time 1). The second analysis used memory data collected one year prior to conversion to AD for the preclinical AD group and in Year 2 for the NC group (i.e., Time 2). The third analysis used a difference score between Time 1 and Time 2 for each memory measure in order to determine if rate of memory decline was a better predictor of subsequent dementia than overall level of memory performance.
RESULTS
Memory Decline in Normal Elderly, Preclinical AD, and Clinical AD: APOE-ε4 Allele Effects
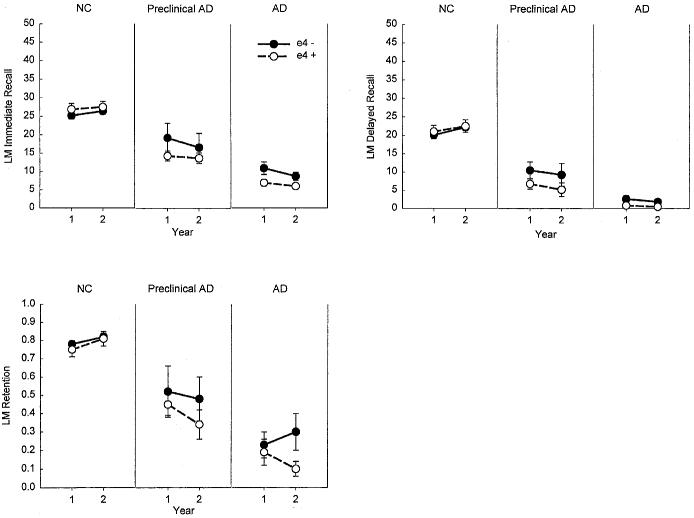
The performance of the NC, preclinical AD, and AD participants on each of the measures from the CVLT and LM Test is presented as a function of genotype and time in Figures 1 and 2. As expected, there was a significant main effect of group for each of the evaluated CVLT and LM Test measures [CVLT Trials 1–5: F(2,150) = 192.0, p < .001; CVLT Short Delay Free Recall: F(2,150) = 182.4, p < .001; CVLT Long Delay Free Recall: F(2,150) = 199.2, p < .001; CVLT Recognition Discriminability: F(2,149) = 199.9, p < .001; CVLT Retention: F(2,148) = 167.3, p < .001; LM Immediate Recall: F(2,150) = 133.2, p < .001; LM Delayed Recall: F(2,150) = 143.8, p < .001; LM Retention: F(2,149) = 109.5; p < .001]. In each case, the AD patients performed worse than the NC and preclinical AD groups, and the preclinical AD group performed worse than the NC group (all ps < .005). There was also a main effect of time for the CVLT Discriminability measure with participants performing worse in Year 2 than in Year 1 [F(1,149) = 4.9, p < .03]. There was a Group × Time interaction effect for all other CVLT and LM Test measures [CVLT Trials 1–5: F(2,150) = 6.1, p < .01; CVLT Short Delay Free Recall: F(2,150) = 5.6, p < .01; CVLT Long Delay Free Recall: F(2,150) = 4.4, p < .01; CVLT Retention: F(2,148) = 8.2, p < .001; LM Immediate Recall: F(2,150) = 4.4, p = .01; LM Delayed Recall: F(2,150) = 6.6, p < .01], except for the LM Retention score. The interactions were due, in each case, to a greater decline in performance from Year 1 to Year 2 in the preclinical AD and AD groups than in the NC group (all ps < .05). The preclinical AD and AD groups did not differ significantly in the rate of decline from Year 1 to Year 2 on any of the measures except for the CVLT Retention measure, where the preclinical AD group exhibited faster decline than the AD group (p < .05). There was no main effect of APOE genotype and no interactions involving that factor for any of the memory measures.
Fig. 1.
The mean scores achieved by normal control (NC), preclinical Alzheimer's disease (AD), and AD participants on key measures from the California Verbal Learning Test (CVLT). The scores are presented as a function of APOE genotype (ε4+ vs. ε4−) and time (Year 1 vs. Year 2).
Fig. 2.
The mean scores achieved by normal control (NC), preclinical Alzheimer's disease (AD), and AD participants on key measures from the Wechsler Memory Scale–Revised Logical Memory (LM) Test. The scores are presented as a function of APOE genotype (ε4+ vs. ε4−) and time (Year 1 vs. Year 2).
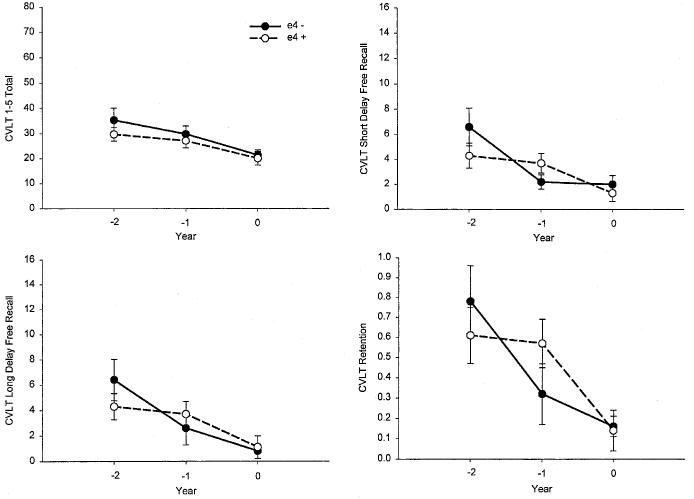
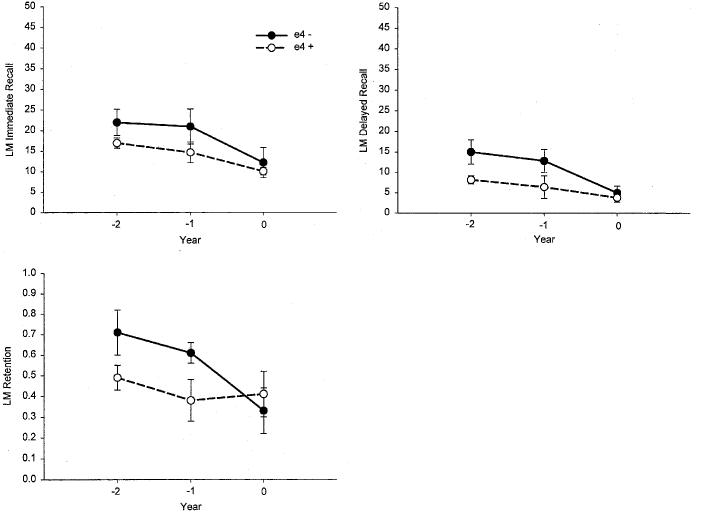
Memory Decline Over 2 Years in Preclinical AD: APOE-ε4 Allele Effects
The performance of the APOE-ε4-positive and APOE-ε4-negative preclinical AD participants on each of the memory measures from the CVLT and LM Test is presented as a function of time in Figures 3 and 4. Both groups declined significantly on all memory measures during the 2 years preceding the diagnosis of AD (all ps < .05 for the main effect of time). There was no overall effect of genotype on any measure (all ps > .10 for the main effect of genotype). There was, however, a significant Genotype × Time interaction for the CVLT Short Delay Free Recall measure [F(2,24) = 3.31, p = .05]. Follow-up analyses showed that the APOE-ε4-negative subgroup declined more rapidly than the APOE-ε4-positive subgroup on this measure between Years 1 and 2 (p = .05), but less rapidly between Years 2 and 3 (p = .05). No other Genotype × Time interaction effects were significant (all ps > .10).
Fig. 3.
The mean scores achieved by the APOE-ε4+ and APOE-ε4− preclinical Alzheimer's disease (AD) participants on key measures from the California Verbal Learning Test (CVLT). The scores are presented as a function of years prior to diagnosis of AD (−2 years vs. −1 year vs. zero years).
Fig. 4.
The mean scores achieved by the APOE-ε4+ and APOE-ε4− Preclinical Alzheimer's disease (AD) participants on key measures from the Wechsler Memory Scale–Revised Logical Memory (LM) Test. The scores are presented as a function of years prior to diagnosis of AD (−2 years vs. −1 year vs. zero years).
Comparison of APOE-ε4 Allele Effects on CVLT and LM Test Performance in Preclinical AD
The immediate recall and delayed recall scores achieved by the preclinical AD group on the CVLT and LM Test were converted to z scores relative to performance in the NC group in order to directly compare performance across the types of tests. The retention measures from each test were also directly compared. A series of Genotype (APOE-ε4-positive, APOE-ε4-negative) × Test Type (CVLT, LM) × Time (Year 1, Year 2) repeated measures ANOVAs were used to compare immediate memory (CVLT Trials 1–5 vs. LM Immediate Recall), delayed recall (CVLT Long Delay Free Recall vs. LM Delayed Recall), and retention (CVLT Retention vs. LM Retention) measures. There was a significant main effect of time for each of the comparisons [immediate memory: F(1,17) = 8.4, p < .01; delayed recall: F(1,17) = 15.0, p < .001; retention: F(1,17) = 6.2, p = .02], with Year 2 performance worse than performance in Year 1. There was also a main effect of test type for the immediate memory [F(1,17) = 8.1, p < .01] and delayed recall [F(1,17) = 9.2, p < .01] comparisons, with worse performance on CVLT than on LM Test measures. However, there was a significant Genotype × Test Type interaction for each of these comparisons [immediate memory: F(1,17) = 4.9, p = .04; delayed recall: F(1,17) = 4.9, p = .04]. In both cases, the interaction was due to better performance on the LM Test measures than on the CVLT measures in the APOE-ε4-negative group but not in the APOEε4-positive group.
Prediction of Conversion to AD by APOE Genotype and Memory Decline
The results of the three logistic regression analyses are shown in Table 3. The final model of the first analysis was highly significant (χ2 = 31.87, df = 2, p < .001) with CVLT Trials 1–5 Total Recall and LM Delayed Recall at Time 1 (i.e., 2 years prior to diagnosis for the preclinical AD participants and Year 1 for the NC participants) as independent predictors of conversion to possible or probable AD. Neither APOE genotype, the remaining CVLT or LM Test measures, nor the demographic variables were retained in the model. Specifically, each 1 standard deviation decrement in the Time 1 score on the CVLT Trials 1–5 Total Recall measure conferred a more than five-fold increase in risk for conversion to AD (RR = 5.29), and each 1 standard deviation decrement in the Time 1 score on the LM delayed recall measure conferred a more than twofold increase in risk for conversion to AD (RR = 2.71). Overall, the model provided 91% correct classification of individuals who would or would not go on to develop possible or probable AD (sensitivity = .50; specificity = .98).
Table 3.
Logistic regression analyses (with forward selection) predicting conversion to Alzheimer's disease using apolipoprotein (APOE) ε4 allele status and learning and memory measures from the CVLT and WMS–R logical memory
| Measure | β | Odds ratioa | 95% confidence interval | p |
|---|---|---|---|---|
| Time 1 (2 years before diagnosis) | ||||
| CVLT Trials 1–5 Total Recall | 1.67 | 5.29 | 1.79–15.61 | .003 |
| WMS-R Delayed Recall | 1.00 | 2.71 | 1.06–6.95 | .040 |
| Time 2 (1 year before diagnosis) | ||||
| Presence of APOE ε4 Allele | 2.49 | 12.02 | 1.49–97.19 | .02 |
| CVLT Short-Delay Free Recall | 2.08 | 7.98 | 1.47–43.29 | .02 |
| CVLT Long-Delay Free Recall | 1.14 | 3.14 | 0.84–11.76 | .09 |
| Time 2–Time 1 Difference | ||||
| Presence of APOE ε4 Allele | 1.76 | 5.79 | 1.41–23.83 | .02 |
| CVLT Delayed Recall Savings | 0.58 | 1.79 | 1.08–2.94 | .02 |
| WMS-R Delayed Recall Savings | 0.59 | 1.81 | 1.02–3.23 | .04 |
Odds ratios represent relative risk of conversion to disease per SD decrement independent of other variables.
The final model of the second logistic regression analysis was also highly significant (χ2 = 67.51, df = 3, p < .001) and correctly classified 95% of individuals who would or would not go on to develop possible or probable AD (sensitivity = .84; specificity = .98).The model retained APOE-ε4 allele status and two CVLT measures (Short Delay and Long Delay Free Recall) at Time 2 (i.e., 1 year prior to diagnosis for the preclinical AD participants and Year 2 for the NC participants) as significant and independent predictors of conversion to AD. Other CVLT measures, measures from the LM Test, and demographic variables were not retained in the model. Presence of the APOE-ε4 allele conferred a 12-times increase in risk for conversion to AD (RR = 12.02), and each 1 standard deviation decrement on the Time 2 scores for the CVLT Short Delay Free Recall (RR = 7.98) and Long Delay Free Recall (RR = 3.14) measures conferred approximately eightfold and threefold increases in risk for conversion to possible or probable AD, respectively.
The third logistic regression analysis produced a highly significant final model (χ2 = 15.76, df = 3, p = .001) that provided 89% correct classification of individuals who would or would not go on to develop possible or probable AD (sensitivity = .23; specificity = .99). The model retained APOE-ε4 allele status and difference scores (i.e., Time 1 minus Time 2 z scores) for the CVLT and LM Test retention measures as significant predictors of subsequent conversion to AD. None of the demographic variables or remaining memory-measure difference scores were retained in the final model. The presence of the APOE-ε4 allele conferred more than a fivefold increase in risk for conversion to AD (RR = 5.79), and each 1 standard deviation increment in the difference score for each of the two retention measures conferred an almost twofold increase in risk of developing AD (CVLT Retention: RR = 1.79; LM Retention: RR = 1.81).
DISCUSSION
Consistent with previous findings (Chen et al., 2001; Small et al., 1998), the results of the present study demonstrate that there is a precipitous decline in verbal memory abilities immediately prior to the onset of the dementia syndrome in patients with AD. The rate of decline in memory exhibited by preclinical AD patients during the year prior to the dementia diagnosis was similar to the 1-year rate of decline in mildly demented AD patients. This same rate of decline in memory was also apparent during a period between 2 years and 1 year prior to diagnosis in a subgroup of the preclinical AD patients. The presence or absence of the APOE-ε4 allele had little effect on the rate of decline in memory abilities of patients in the preclinical or clinical stages of AD. Furthermore, the decline was not a function of age as there was no decline in memory performance of older normal control subjects over a comparable period of time. The stability of the memory performance of normal older adults across a 1-year period was not influenced by the presence or absence of the APOE-ε4 allele.
The results obtained with AD patients are consistent with a number of previous studies that show little effect of APOE genotype on the rate of decline in memory in patients with clinically diagnosed AD (Frisoni et al., 1995; Growdon et al., 1996; Hyman et al., 1996). The failure to observe an effect of genotype in the present study was not simply due to floor performance in the AD patients, as the rate of decline in the ε4-positive and ε4-negative patients was similar on the recognition discriminability measure that was above floor for both AD groups in Years 1 and 2. The observed stability of episodic memory in normal older adults is also consistent with previous findings (e.g., Bondi et al., 1999; Growdon et al., 1996; Smith et al., 1998) demonstrating that there is little difference in the memory abilities of normal elderly with or without the APOE-ε4 allele once individuals who go on to develop AD are retrospectively removed from study samples. This result extends those of a previous crosssectional comparison by Bondi et al. (1999) which showed no difference in the memory performances of APOE ε4-positive and ε4-negative normal elderly groups that were free of preclinical AD patients. These findings are consistent with the notion that previously observed differences in the absolute level or rate of change of memory abilities in ε4-positive and ε4-negative normal older adult groups could be due to the over-representation of individuals with preclinical AD in the APOE ε4-positive group.
The results obtained with the preclinical AD patients are the first to demonstrate that the presence or absence of the APOE-ε4 allele has little effect on the rate of decline in episodic memory during the preclinical stage of AD. Although decline in memory during the preclinical stage of the disease was clearly documented, the rate was similar for ε4-positive and ε4-negative patients. Thus, while the presence of an APOE-ε4 allele may be associated with an increased risk of developing AD, or may hasten the onset of the disease (e.g., Corder et al., 1993), it does not appear to be associated with a phenotype of particularly rapid episodic memory deterioration once individuals are clearly segregated into those who are normal and those who are in the preclinical or clinical stages of AD. Unfortunately, Bäckman et al. (2001) did not examine the influence of APOE genotype on episodic memory during the earlier preclinical stage of AD, so possible genotype effects on rate of decline at the initiation of memory deterioration remain unknown.
The present results stand in contrast to those of Smith et al. (1998) who found that the presence of the APOE-ε4 allele was associated with poorer episodic memory performance in MCI patients who subsequently converted to dementia. These discrepant results may arise from the use of a cross-sectional approach by Smith et al. (1998) and a longitudinal approach in the present study. Although duration of illness was statistically controlled in the study by Smith et al. (1998), global level of cognitive functioning (i.e., MMSE score) was not, and it remains possible that the ε4-positive patients were further along in the course of their cognitive decline than the ε4-negative patients. This, rather than a specific APOE-ε4 phenotype, may be the cause of the ε4-positive MCI patients' particularly poor episodic memory performance. The use of a longitudinal procedure in the present study allowed a direct examination of the effect of APOE genotype on decline in memory functions in ε4-positive and ε4-negative patients who were at a similar initial level of memory performance.When initial memory performance is matched, there appears to be little or no effect of APOE-ε4 genotype on the rate of memory decline.
The logistic regression analyses demonstrated that the integrity of episodic memory ability and APOE-ε4 genotype make significant and independent contributions to the prediction of conversion to a clinical diagnosis of possible or probable AD. The predictive value of poor performance on measures of learning and delayed recall in the preclinical AD patients was evident both 1 year and 2 years prior to diagnosis and was not enhanced by employing the rate of change in memory performance in the year prior to the year of diagnosis. The predictive value of APOE-ε4 genotype was overshadowed by the value of episodic memory measures obtained 2 years prior to diagnosis, but was a strong predictor of the imminent development of dementia when considered with delayed recall scores obtained 1 year prior to diagnosis. This was also the case when APOE-ε4 genotype was considered with the degree of decline in these episodic memory abilities in the year preceding the year of diagnosis.
The independent predictive value of poor memory performance (particularly on delayed recall tasks) and APOE-ε4 genotype supports similar previous findings (Bondi et al., 1995, 1999), but contrasts with the findings of Albert et al. (2001) who observed no additional predictive value from the APOE-ε4 genotype. This discrepancy may arise from differences in the representation of the ε4 allele in the two preclinical AD samples. While only 39% of the patients who converted to AD in the Albert et al. sample had at least one ε4 allele, 70% of the present sample had the allele. The finding that APOE-ε4 genotype is a significant predictor of the impending development of dementia even though it has little effect on the rate of progression of episodic memory decline invites the hypothesis that the primary effect of the presence of the APOE-ε4 allele is to initiate an earlier onset of AD without altering the disease's course. Thus, at a given age, the APOE ε4 allele will be overrepresented in patients with AD, including those with preclinical disease, leading to its predictive value. However, when patients with verified clinical or preclinical AD are tracked longitudinally, the APOE-ε4 allele appears to have little effect on the disease's course.
Although APOE-ε4 genotype had little effect on the rate of decline in episodic memory abilities in patients with preclinical AD, it did have an effect on the sensitivity of the two types of memory tests for measuring the memory impairment. While APOE-ε4-positive preclinical AD patients performed similarly on the CVLT and LM Test immediate memory and delayed recall measures, APOE-ε4-negative preclinical AD patients performed significantly better on the LM Test measures than on the CVLT measures. This finding may indicate that the APOE-ε4-negative preclinical AD patients are better able than the ε4-positive patients to benefit from the additional semantic and contextual support provided by the story-learning format of the LM Test relative to the list-learning format of the CVLT. The inability of the APOE-ε4-positive patients to take advantage of the additional support (see also Bäckman & Small, 1998; Small et al., 1998) suggests that certain non-episodic-memory functions, such as semantic knowledge or executive abilities, may be affected sooner in these patients than in those who are APOE-ε4 negative. These non-episodic-memory functions may reflect compensatory mechanisms that are invoked in the early stages of AD (e.g., Becker et al., 1996; Desgranges et al., 1998). Thus, the APOE-ε4 genotype may be associated with more widespread dysfunction during the preclinical stage of AD than the non-ε4 genotype and, in that sense, it may engender a more virulent form of AD. However, the rate of change in the performance of ε4-positive and ε4-negative preclinical AD patients on the LM Test measures was not different, suggesting that the presence of the ε4 allele does not accelerate decline in semantic memory or executive abilities, just as it has little effect on rate of decline in episodic memory.
The episodic memory deterioration observed during the late preclinical stage of AD in the present study and in the study by Chen et al. (2001) stands in contrast to demonstrations of the stability of the memory impairment during the earlier preclinical stage 6 to 3 years prior to diagnosis (Bäckman et al., 2001; Small et al., 1998). Taken together, the results of these studies suggest that the decline in episodic memory associated with AD does not occur in a linear fashion, but may be characterized by an initial decline many years prior to the onset of dementia, followed by a period of stable but poor memory abilities that lasts several years, and finally a sharp decline in the period immediately prior to the development of the dementia syndrome.
A possible mechanism for this nonlinear pattern of episodic memory decline during the preclinical stage of AD lies in recent results from functional neuroimaging studies that suggest that there may be compensatory mechanisms in brain activity early in the course of the disease (Becker et al., 1996; Bookheimer et al., 2000; Burggren et al., 2002). Several studies have shown that brain activity associated with the performance of memory tasks is more diffuse in patients with early AD than in normal older individuals, apparently due to the need to recruit areas outside of the usual structures that mediate memory in order to maintain performance (for review, see Desgranges et al., 1998). It may be the case that after an initial decline in memory following damage to these medial temporal lobe structures, patients in the preclinical stage of AD are able to effectively recruit enough compensatory brain resources (e.g., frontal and temporal cortical regions important for executive functions and semantic memory) to halt or slow further memory decline for a period of time. A similar compensatory response in cholinergic activity may also attenuate memory changes for a time, as DeKosky and colleagues (DeKosky et al., 2002) recently found higher than normal levels of cholinergic activity in the hippocampal region of patients with MCI. As the disease progresses, however, all of these additional resources become compromised and the patient exhibits a period of rapid decline in episodic memory abilities.
The present results must be interpreted cautiously for a number of reasons. First, the relatively small samples of APOE-ε4-positive and ε4-negative preclinical AD patients may not provide enough power to detect subtle effects of APOE genotype on the rate of memory decline in this cohort. Thus, this generally negative finding must be considered tentative until it is replicated with larger numbers of prospectively studied preclinical AD patients. Second, the findings obtained with this restricted sample of preclinical AD patients may not generalize to other samples that differ in age, education, or distribution of APOE genotypes (e.g., those with an APOE-ε2 alelle or those homozygous for the APOE-ε4 alelle). Third, the 1-year follow-up interval may be too short to provide a highly reliable evaluation of possible APOE genotype effects on memory decline in normal older adults (see Zelinski et al., 1997). Fourth, the rigorous tests of memory used in the present study may be so difficult for patients with AD that floor effects could attenuate differences in the rate of decline associated with the ε4-positive and ε4-negative APOE genotypes once the disease becomes overt. It should be noted, however, that several of the memory measures used in the present study (e.g., CVLT recognition discriminability) did not appear to be highly susceptible to floor effects and provided the same pattern of results as the more difficult measures.
Despite these caveats, the results of the study provide important information about the changes in episodic memory that occur immediately prior to the development of dementia, and about the potential effects of APOE genotype on those changes. The demonstration of a decline in episodic memory in the period immediately preceding the development of the dementia syndrome is consistent with previous findings (Chen et al., 2001) and extends them by showing that there is little effect of APOE genotype on the course of this decline. In addition, these results compliment previous studies that have documented the stability of memory in the earlier preclinical period (Bäckman et al., 2001) and help to clarify the course of memory decline during the preclinical and overt stages of AD.
ACKNOWLEDGMENTS
Portions of this study were presented at the 1998 Annual Convention of the American Psychological Association in San Francisco, CA. This study was supported in part by grants from the Medical Research Service of the Department of Veterans Affairs (MWB), the National Institute on Aging (AG05131 to LJT, AG12674 to MWB), and by funds from the State of California (ARCC to DG).
REFERENCES
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Bäckman L, Small BJ. Influences of cognitive support on episodic remembering: Tracing the loss from normal aging to Alzheimer's disease. Psychology and Aging. 1998;13:267–276. doi: 10.1037//0882-7974.13.2.267. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- Basun H, Grut M, Winblad B, Lannfelt L. Apolipoprotein ε4 allele and disease progression in patients with late-onset Alzheimer's disease. Neuroscience Letters. 1995;183:32–34. doi: 10.1016/0304-3940(94)11107-t. [DOI] [PubMed] [Google Scholar]
- Becker JT, Mintum MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Galasko D, Butters N, Salmon DP, Delis DC. Preclinical cognitive markers of dementia of the Alzheimer type. Neuropsychology. 1994;8:374–384. [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. New England Journal of Medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Bratzke H. Evolution of Alzheimer's disease related cortical lesions. Journal of Neural Transmission. 1998;54(Suppl):97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY. Specificity of brain activation patterns in people at genetic risk for Alzheimer's disease. American Journal of Geriatric Psychiatry. 2002;10:44–51. [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: A prospective community study. Archives of General Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neuroscience and Biobehavioral Reviews. 2000;24:365–374. doi: 10.1016/s0149-7634(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Rimmler JB, Locke PA, Conneally PM, Schmader KE, Tanzi RE, Gusella JF, Small GW, Roses AD, Pericak-Vance MA, Haines JL. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology. 1995;45:1323–1328. doi: 10.1212/wnl.45.7.1323. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk ofAlzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Craft S, Teri L, Edland SD, Kukull WA, Schellenberg G, McCormick WC, Bowen JD, Larsen EB. Accelerated decline in apolipoprotein E-ε4 homozygotes with Alzheimer's disease. Neurology. 1998;51:149–153. doi: 10.1212/wnl.51.1.149. [DOI] [PubMed] [Google Scholar]
- Dal Forno G, Rasmusson X, Brandt J, Carson KA, Brookmeyer R, Troncoso J, Kawas CH. Apolipoprotein E genotype and rate of decline in probable Alzheimer's disease. Archives of Neurology. 1996;53:345–350. doi: 10.1001/archneur.1996.00550040085017. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ferrell R, Kamboh MI, Becker JT. Natural history of definite AD as a function of APOE genotypes. Neurology. 1995;5(Suppl 4):A373. [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Annals of Neurology. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. The Psychological Corporation; New York: 1987. [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, Kramer JH, Cermak L. Profiles of demented and amnesic patients on the CVLT: Implications for the assessment of memory disorders. Psychological Assessment. 1991;3:19–26. [Google Scholar]
- Desgranges B, Baron JC, Eustache F. The functional neuroanatomy of episodic memory: The role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Bouter LM, Geerlings MI, van Kamp GJ, Deeg DJH. APOE-ε4 is associated with memory decline in cognitively impaired elderly. Neurology. 2000;54:1492–1497. doi: 10.1212/wnl.54.7.1492. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein S, McHugh PR. Mini-Mental State: A practical method of grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Govoni S, Geroldi C, Bianchetti A, Calabresi L, Franceschini G, Trabucchi M. Gene dose of the ε4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer's disease. Annals of Neurology. 1995;37:596–604. doi: 10.1002/ana.410370509. [DOI] [PubMed] [Google Scholar]
- Fuld PA, Masur DM, Blau AD, Crystal H, Aronson MK. Object-memory evaluation for prospective detection of dementia in normal functioning elderly: Predictive and normative data. Journal of Clinical and Experimental Neuropsychology. 1990;12:520–528. doi: 10.1080/01688639008400998. [DOI] [PubMed] [Google Scholar]
- Galasko D, Kwo-on-Yuen PF, Klauber MR, Thal LJ. Neurological findings in Alzheimer's disease and normal aging. Archives of Neurology. 1990;47:625–627. doi: 10.1001/archneur.1990.00530060033012. [DOI] [PubMed] [Google Scholar]
- Geroldi C, Laasko MP, DeCarli C, Beltramello A, Bianchetti A, Soininen H, Trabucchi M, Frisoni GB. Apolipoprotein E genotype and hippocampal asymmetry in Alzheimer's disease: A volumetric MRI study. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;68:93–96. doi: 10.1136/jnnp.68.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, Perls TT, Lipsitz LA, Hyman BT. Clinical and pathological correlates of apolipoprotein E ε4 in Alzheimer's disease. Annals of Neurology. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer's disease. Psychology and Aging. 1997;12:183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- Growdon JH, Locascio JJ, Corkin S, Gomez-Isla T, Hyman BT. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- Howieson DB, Dame A, Camicioli R, Sexton G, Payami H, Kaye JA. Cognitive markers preceding Alzheimer's dementia in the healthy oldest old. Journal of the American Geriatrics Society. 1997;45:584–589. doi: 10.1111/j.1532-5415.1997.tb03091.x. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Gomez-Isla T, Briggs M, Chung H, Nichols S, Kohout F, Wallace R. Apolipoprotein E and cognitive change in an elderly population. Annals of Neurology. 1996;40:55–66. doi: 10.1002/ana.410400111. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Gomez-Isla T, Rebeck GW, Briggs M, Chung H, West HL, Greenberg S, Mui S, Nichols S, Wallace R, Growdon JH. Epidemiological, clinical, and neuropathological study of apolipoprotein E genotype in Alzheimer's disease. Annals of the New York Academy of Sciences. 1996;802:1–5. doi: 10.1111/j.1749-6632.1996.tb32592.x. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio A, Barnes CL. Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Sano M, Dooneief G, Marder K, Bell KL, Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurology. 1995;45:957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Albert MS, Sandor T, Tieman J, Joleaz F. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Archives of Neurology. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- Katzman R. Alzheimer's disease. New England Journal of Medicine. 1986;314:964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Kurz A, Egensperger R, Haupt M, Lautenschlager N, Romero B, Graeber MB, Muller U. Apolipoprotein E ε4 allele, cognitive decline, and deterioration of everyday performance in Alzheimer's disease. Neurology. 1996;47:440–443. doi: 10.1212/wnl.47.2.440. [DOI] [PubMed] [Google Scholar]
- Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D'Agostino RB. The ’preclinical phase’ of probable Alzheimer's disease. Archives of Neurology. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional manual. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- Mayeux R, Small SA, Tang M-X, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer's disease: Effects of time and apolipoprotein-E. Neurobiology of Aging. 2001;22:683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCSD-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Hindley NJ, Braak H, Braak E, Yilmazer-Hanke DM, Schultz C, Barnetson L, King EM, Jobst KA, Smith AD. The progression of Alzheimer's disease from limbic to the neocortex: Clinical, radiological and pathological relationships. Dementia: Geriatric and Cognitive Disorders. 1999;10:115–120. doi: 10.1159/000017111. [DOI] [PubMed] [Google Scholar]
- Normann J, Brookes AJ, Yates C, St. Clair D. Apolipoprotein E genotype and its effect on duration and severity of early and late onset Alzheimer's disease. British Journal of Psychiatry. 1995;167:533–536. doi: 10.1192/bjp.167.4.533. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Kirca M, Bohl J, Scharnagl H, Grob W, Marz W. Apolipoprotein E polymorphism influences not only cerebral senile plaque load but alsoAlzheimer-type neurofibrillary tangle formation. Neuroscience. 1995;66:583–587. doi: 10.1016/0306-4522(94)00596-w. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kokmen E, Waring SC, Kurland LT. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. Journal of the American Medical Association. 1995;273:1274–1278. [PubMed] [Google Scholar]
- Pfeffer R, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, Berg L. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychology of Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 39–56. [Google Scholar]
- Salmon DP, Butters NM. Neuropsychologic assessment of dementia in the elderly. In: Katzman R, Rowe JW, editors. Principles of geriatric neurology. F.A. Davis; New York: 1992. pp. 144–163. [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, St. George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proceedings of the National Academy of Sciences (USA) 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Basun H, Bäckman L. Three-year changes in cognitive performance as a function of apolipoprotein E genotype: Evidence from very old adults without dementia. Psychology and Aging. 1998;13:80–87. doi: 10.1037//0882-7974.13.1.80. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Bäckman L. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Archives of Neurology. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Soininen H, Partanen K, Pitkanen A, Hallikainen M, Hanninen T, Helisalmi S, Mannermaa A, Ryynanen M, Koivisto K, Riekkinen P., Sr. Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E epsilon 4 allele. Neurology. 1995;45:391–392. doi: 10.1212/wnl.45.2.391. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Riekkinen PJ. Apolipoprotein E, memory and Alzheimer's disease. Trends in Neurosciences. 1996;19:224–228. doi: 10.1016/0166-2236(96)10027-8. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences (USA) 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Goedert M, Saunders AM, Huang D, Corder EH, Dong L-M, Jakes R, Alberts MJ, Gilbert JR, Han S-H, Hulette C, Einstein G, Schmechel DE, Pericak-Vance MA, Roses AD. Hypothesis: Microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Experimental Neurology. 1994;125:163–171. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Szalai JP, Snow WG, Fisher RH, Nores A, Nadon G, Dunn E, St George-Hyslop PH. Prediction of probable Alzheimer's disease in memory-impaired patients: A prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale–Revised. The Psychological Corporation; New York: 1987. [Google Scholar]
- Zelinski EM, Burnight KP. Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychology and Aging. 1997;12:503–513. doi: 10.1037//0882-7974.12.3.503. [DOI] [PubMed] [Google Scholar]