Abstract
The profiles of neuropsychological deficits associated with Alzheimer's disease (AD) in Young-Old (M age < 70) and Very-Old (M age > 80) patients were compared, along with possible modifying effects of apolipoprotein E (APOE) genotype on these profiles. A comprehensive battery of neuropsychological tests was administered to the two AD patient groups (Young-Old: n = 33; Very-Old: n = 48) and their respective age-matched normal control (NC) groups who remained free of dementia on follow-up examinations over a 1 to 10 year period (Young-Old: n = 43; Very-Old: n = 36). AD and NC groups did not differ in education levels or gender distributions. Young-Old AD and Very-Old AD groups were comparable in education, gender, dementia severity, and disease duration. Results showed that both AD groups achieved comparable raw scores on all the neuropsychological measures. However, when scores were standardized on the basis of performance of their respective NC groups (i.e., age-corrected z scores), Very-Old AD patients significantly outperformed Young-Old AD patients on tests of executive functions, visuospatial skills, and delayed memory. Furthermore, the relationship between age and memory and executive function deficits in AD was modified by APOE genotype. These data suggest that the profile of neuropsychological deficits associated with AD in the Very-Old lacks the disproportionate saliency of episodic memory and executive function deficits typical of the Young-Old.
Keywords: Aging, Alzheimer's disease, Neuropsychology, Apolipoprotein E, Very-old
INTRODUCTION
Community (population) studies in many different countries have confirmed that the prevalence of AD rises in an approximately exponential fashion between the ages of 65 and 85 (see Kawas & Katzman, 1999, for review). The prevalence of AD in the population over the age of 85 is less clear, but emerging epidemiologic evidence suggests that prevalence rates may continue to rise in this advanced age group (Evans et al., 1989; Fichter et al., 1996; McDowell et al., 1998; Rocca et al., 1998). It is now estimated that between 25% and 50% of individuals 85 years old and older will develop AD. Because individuals over the age of 80 represent the fastest growing segment of our population (U.S. Bureau of the Census, 1992), the development of AD in the so-called “Very-Old” (i.e., age 80 and above) is a public health problem of increasing magnitude.
The clinical detection of AD in the Very-Old poses a number of unique challenges. The boundaries between normal age-related cognitive changes and the very earliest signs ofAD may be particularly difficult to delineate in the Very-Old, primarily because many of the early structural and functional changes of AD overlap with changes observed either in normal aging or in the context of other disease processes. A number of studies have shown that normal aging is associated with mild brain atrophy on structural magnetic resonance (MR) imaging (Jack et al., 1998a, 1999; Jernigan et al., 2001; Pfefferbaum et al., 1994), decreased hemodynamic response on functional MR imaging (D'Esposito et al., 1999), reduced synaptic density (Masliah et al., 1993), increased white matter abnormalities (Guttman et al., 1998; Jernigan et al., 2001; Salat et al., 1999), and a subclinical accumulation of neuritic plaques and neurofibrillary tangles in medial temporal lobe brain regions (Green et al., 2000; Hulette et al., 1998). These brain changes are accompanied by age-related declines in information processing speed, executive functions, and efficiency of learning and recall (Corey-Bloom et al., 1996; Desgranges et al., 1998; Grady et al., 1995; Gunning-Dixon & Raz, 2000; Hulette et al., 1998; Mittenberg et al., 1989; Schacter et al., 1996; Ylikoski et al., 1993). The structural and functional decline that occurs in the Very-Old has led some investigators to suggest that less AD pathology may be needed to produce pathologic cognitive decline in the Very-Old compared to the Young-Old (see Terry et al., 1999). However, dementia may be more difficult to detect against the background of lower and more variable cognitive test scores in the appropriate age-matched normal reference group.
An additional challenge for detecting dementia in the Very-Old is that the typical profile of neuropsychological deficits associated with AD in the Young-Old may be less salient in the Very-Old patient. Numerous studies over the last two decades have shown that the dementia syndrome of AD is initially characterized by a prominent amnesia with rapid forgetting of information over time, marked executive dysfunction most evident as a deficit in shifting cognitive set, and additional deficits in certain aspects of language, visuospatial abilities, and attention (for review, see Salmon & Bondi, 1999). Because many of these abilities are those that are also detrimentally affected by normal aging (e.g., executive functions, memory processes), the prominence of specific deficits related to AD may be much less evident in the Very-Old than in the Young-Old, especially after performance is standardized to the age-appropriate normal cohort. This would result in a less distinct and somewhat atypical cognitive deficit profile associated with AD in the Very-Old compared to that of the Young-Old.
Another factor that could alter the cognitive deficit profile of AD in the Very-Old is a possible age-related change in the influence of the ε4 allele variant of the apolipoprotein E (APOE) gene. The APOE ε4 allele has been identified as a major risk factor for late-onset AD (Corder et al., 1993; Saunders et al., 1993; Strittmatter et al., 1993, 1994), but there is evidence that the associated risk wanes with advancing age (Corder et al., 1994, 1995, 1996; Rebeck et al., 1994; but see Gebner et al., 1997; Payami et al., 1997). This change in risk suggests that the phenotypic expression of the APOE ε4 allele may be age dependent. If this is the case, previously observed differences in the clinical and neuropsychological presentation of Young-Old AD patients with or without the ε4 allele (Lange et al., 2002; Smith et al., 1998) may be absent or changed in Very-Old AD patients. Several studies that have compared cognitive deficits in predominantly Young-Old AD patients with or without the ε4 allele suggest that those with the ε4 allele may have generally more severe memory impairment (Smith et al., 1998) or less ability to use strategic processes or semantic abilities in support of memory (Lange et al., 2002) than those without the ε4 allele, even when duration of illness and severity of global dementia is comparable. A decrease in the influence of the APOE ε4 allele on the manifestation of AD in the Very-Old may attenuate these differences and modify the profile of cognitive deficits that characterizes the disease.
Despite these several factors that could alter the clinical presentation of AD in the Very-Old, there are few detailed neuropsychological studies comparing the profile of early cognitive deficits associated with AD in Very-Old and Young-Old cohorts. Furthermore, there are no studies that compare the impact of APOE genotype on the early neuropsychological manifestation ofAD in these two age groups. To address these issues, the present study compared the performances of mildly demented AD patients whose mean age was over 80 (i.e., Very-Old) and under 70 (i.e., Young-Old) on a battery of neuropsychological tests known to be sensitive to the cognitive deficits that typify AD. Cognitive test performances of the Very-Old and Young-Old AD patients were normalized to their respective age-matched healthy control participants' performance prior to the comparison in order to reduce the impact of normal aging on their deficit profile. APOE genotype was determined so that a possible interaction between age and ε4 allele status on the cognitive deficit profile engendered by AD could be examined.
Thus, the present study was designed to (1) identify the most salient cognitive markers of early AD in the Very-Old, (2) compare the profiles of cognitive deficits in Young-Old and Very-Old patients with AD on both raw and standardized scores, and (3) determine whether APOE genotype differentially affects neuropsychological deficit patterns in these two cohorts. Specifically, we predicted that Very-Old AD patients would exhibit apparently less severe cognitive impairment than Young-Old patients with a similar estimated disease duration, primarily because their performance would be referenced to lower and more variable test scores in their age-matched normal control group. We further predicted that the profile of cognitive deficits that characterizes early AD in the Young-Old would be less salient in the Very-Old, since many of the most prominent deficits are those that are also affected in normal aging. Finally, given the rather specific effect of APOE ε4 genotype on memory and executive functions (Albert et al., 2001; Bondi et al., 1999; see also Collie and Maruff, 2000, for review), we expected that any interactive effect of age and APOE genotype on cognitive performance would be limited to those abilities.
METHODS
Research Participants
One-hundred sixty individuals participated in this study: 43 Young-Old NC participants, 33 Young-Old AD patients, 36 Very-Old NC participants, and 48 Very-Old AD patients. All participants were part of larger cohorts participating in either the University of California, San Diego (UCSD) Alzheimer's Disease Research Center, or a UCSD/San Diego VA Healthcare longitudinal study of normal aging. Participants were selected without regard to ethnicity or race. Written informed consent was obtained from all participants (or their conservators) after the protocol of the study had been fully explained. The diagnosis of AD was made by two senior staff neurologists according to the criteria developed by the NINCDS-ADRDA (McKhann et al., 1984). Historically, diagnostic accuracy rates (i.e., histopathologic confirmation at autopsy of individuals clinically diagnosed with probable or possible AD) at our center have been 90% or higher (Galasko et al., 1994). The NC participants were either spouses of the patients or were volunteers obtained through newspaper advertisements or community lectures. Volunteers with a history of alcoholism, drug abuse, learning disability, neurologic or severe psychiatric illness were excluded.
Subjects were divided into two groups on the basis of their age at testing: (1) a Young-Old group that was comprised of individuals aged 70 years or younger (range: ages 56–70), and (2) a Very-Old group that was comprised of individuals aged 75 years or greater (range: ages 75–90). In addition, following this initial step, we selected all those who had finished a complete neuropsychological evaluation that included the California Verbal Learning Test (CVLT; Delis et al., 1987). This method of selection and group assignment, with a five year age gap (i.e., subjects were not enrolled if they fell between the ages of 71 and 75), resulted in an approximately 15 year age difference between the Young-Old and Very-Old groups (see Table 1).
Table 1.
Summary of demographic variables, APOE genotypes, and global cognitive status of young-old and very-old normal control and Alzheimer disease groups
| Normal control groups |
Alzheimer disease groups |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Young-old (n = 43) |
Very-old (n = 36) |
Young-old (n = 33) |
Very-old (n = 48) |
||||||
| Variables | M | SD | M | SD | M | SD | M | SD | p-valuesd |
| Demographics and Global Cognition | |||||||||
| Age | 66.72 | (2.85) | 79.17 | (2.90) | 65.12 | (3.97) | 80.44 | (2.80) | <.001a |
| Education | 14.67 | (2.26) | 14.53 | (2.43) | 14.09 | (2.44) | 14.38 | (3.65) | .83a |
| Gender (women/men) | 19/24 | 18/18 | 17/16 | 21/28 | .86b | ||||
| Disease duration | – | – | 4.21 | (2.16) | 3.54 | (2.45) | .21 c | ||
| Mattis Dementia Rating Scale | 139.70 | (3.20) | 138.47 | (3.24) | 112.85 | (11.65) | 114.21 | (10.07) | <.001a |
| APOE Genotypes (ε4/non-ε4) | 19/24 | 10/26 | 21/12 | 23/25 | .03b | ||||
| Years of Follow-up Evaluations (in which NCs remained non-demented) | 3.27 | (2.02) | 3.50 | (2.63) | – | – | .67c | ||
P-value associated with 4 group (Young-Old NC; Young-Old AD; Very-Old NC; Very-Old AD) one-way ANOVA.
P-value associated with 4 group (Young-Old NC; Young-Old AD; Very-Old NC; Very-Old AD) chi-square.
P-value associated with an independent samples t test (Young-Old AD vs. Very-Old AD or Young-Old NC vs. Very-Old NC).
Age, education, gender
Consistent with the design of the study, a one-way ANOVA confirmed a highly significant difference in age among the four groups [F(3,156) = 267.53, p < .001]; however, posthoc comparisons using Tukey's HSD statistic revealed that neither the Young-Old NC and Young-Old AD groups (p = .12), nor the Very-Old NC and Very-Old AD groups (p = .25) differed from one another in age. All other age comparisons between Young-Old and Very-Old groups (e.g., Young-Old AD vs. Very-Old AD; Young-Old AD vs. Very-Old NC, etc.) were significant (all Tukey HSDs: p < .001; see Table 1). The four groups did not differ in years of education completed [one-way ANOVA: F(3,156) = 0.29, p = .83] or in gender distribution [χ2 (3, N = 160) = 0.74, p = .86].
APOE genotype
The distribution of APOE genotype polymorphisms differed significantly across the four groups [χ2 (3, N = 160)= 9.08, p = .03; see Table 1], but did not differ significantly within each diagnostic group [Young-Old vs. Very-Old NC: χ2(1, N = 79) = 2.27, p = .13; Young-Old vs. Very-Old AD: χ2(1, N = 81) = 1.95, p = .16]. The Very-Old NC participants had the lowest ε4 allelic frequency (28%), followed by the Young-Old NC (44%), Very-Old AD (48%), and Young-Old AD (64%) groups.
Dementia severity, functional status, and disease duration
As expected, AD patients scored significantly lower than NC participants on the Mattis Dementia Rating Scale (Mattis, 1988; DRS) [one-way ANOVA F(3,156)= 136.72, p < .001]; however, the DRS scores did not differ significantly between the two AD groups [Tukey HSD: p = .88] or the two NC groups [Tukey HSD: p = .90]. The two AD groups did not differ from one another in the estimated years of disease duration [t(79) = 1.27, p = .21]. Furthermore, a subset of participants with Pfeffer Outpatient Disability ratings (Young-Old NC: n = 23; Young-Old AD: n = 25; Very-Old NC: n = 25; Very-Old AD: n = 44) demonstrated that AD patients scored significantly worse on ratings of functional status [one-way ANOVA F(3,113) = 79.35, p < .001]; however, Pfeffer scores did not differ significantly between the twoAD groups [Tukey HSD: p = .54] or the two NC groups [Tukey HSD: p = .93].
Follow-up intervals
In order to minimize the possibility that the NC groups were contaminated by individuals with preclinical AD, all but 4 of the 43 Young-Old NC participants and 3 of the 36 Very-Old NC participants had 1 to 11 years of annually-administered follow-up testing to ensure that their non-demented status was maintained beyond the time of their initial assessment (i.e., the data used in the present study). The NC groups averaged more than three years of follow-up testing and did not differ significantly in this regard [t(77) = 0.43, p = .67; see Table 1]. Participants were not included in the NC groups if any of the follow-up evaluations resulted in a change in diagnostic status from normal to demented, “preclinical” dementia,Mild Cognitive Impairment (MCI), or other classification indicative of significant cognitive decline.
Materials and Procedure
All participants were administered a comprehensive battery of neuropsychological tests that included measures of confrontation naming, letter and category fluency, vocabulary, visuospatial ability, psychomotor speed, visuomotor sequencing, set-shifting skills, novel problem solving, and learning and memory. The specific tests used in the present study (see Table 2) have been described previously (Salmon & Butters, 1992). Each participant was tested individually by a trained psychometrist in a quiet, well-lit room.
Table 2.
Mean (and SD) raw scores of the neuropsychological tests for young-old and very-old normal control and Alzheimer disease groups
| Normal control groups |
Alzheimer disease groups |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young-old (n = 43) |
Very-old (n = 36) |
Young-old (n = 33) |
Very-old (n = 48) |
|||||||
| Variables | M | SD | M | SD | p-valuesa | M | SD | M | SD | p-valuesb |
| Language | ||||||||||
| Boston Naming Test (30-item) | 28.09 | (1.39) | 27.19 | (2.27) | .03 | 21.27 | (6.35) | 20.58 | (5.44) | .60 |
| Letter Fluency (FAS Total) | 39.05 | (10.29) | 39.31 | (12.01) | .92 | 23.00 | (11.38) | 26.73 | (12.33) | .17 |
| Category Fluency (AFV Total) | 48.49 | (10.74) | 42.11 | (8.98) | .006 | 21.82 | (8.09) | 24.42 | (9.13) | .19 |
| WAIS–R Vocabulary | 55.05 | (8.33) | 58.53 | (6.15) | .04 | 43.06 | (13.44) | 44.73 | (12.49) | .57 |
| Visuoconstructional/Psychomotor Skills | ||||||||||
| WAIS–R Digit Symbol | 48.07 | (10.53) | 40.11 | (9.99) | .001 | 17.19 | (13.20) | 22.04 | (12.12) | .10 |
| WISC Block Design | 43.91 | (10.48) | 38.72 | (9.72) | .03 | 16.82 | (12.76) | 21.13 | (11.99) | .13 |
| Trail Making Test (Part A; s) | 41.02 | (14.99) | 47.72 | (14.90) | .05 | 97.82 | (43.91) | 86.54 | (37.06) | .22 |
| Sequencing/Executive Function | ||||||||||
| Trail Making Test (Part B; s) | 88.79 | (30.36) | 102.81 | (33.77) | .06 | 266.18 | (60.72) | 249.44 | (67.00) | .26 |
| Modified Wisconsin Card Sort | ||||||||||
| Categories | 5.63 | (0.82) | 4.94 | (1.67) | .01 | 1.91 | (1.71) | 2.47 | (1.80) | .17 |
| Perseverative errors | 0.40 | (1.22) | 2.08 | (3.99) | .02 | 7.88 | (9.86) | 12.51 | (12.37) | .08 |
| Learning and Memory | ||||||||||
| WMS–R Logical Memory | ||||||||||
| Immediate Recall | 25.70 | (6.37) | 22.94 | (7.18) | .08 | 7.09 | (4.88) | 8.04 | (5.44) | .42 |
| Delayed Recall | 21.19 | (6.48) | 16.17 | (6.68) | .001 | 1.64 | (2.18) | 2.00 | (3.11) | .56 |
| Percent Delay Recall Savings | 81.93 | (12.23) | 69.13 | (18.75) | .001 | 20.97 | (23.57) | 19.98 | (27.94) | .87 |
| California Verbal Learning Test | ||||||||||
| Total List A Immediate Recall | 48.07 | (10.81) | 40.89 | (7.72) | .001 | 16.91 | (7.46) | 18.67 | (7.37) | .30 |
| List A Trial 5 Recall | 11.74 | (2.80) | 10.03 | (2.32) | .004 | 4.06 | (1.94) | 4.33 | (1.62) | .49 |
| Short-Delay Free Recall | 9.79 | (3.61) | 7.72 | (2.69) | .006 | 1.39 | (1.80) | 1.02 | (1.56) | .33 |
| Short-Delay Cued Recall | 10.79 | (3.32) | 9.14 | (2.28) | .01 | 2.82 | (2.07) | 2.77 | (2.20) | .92 |
| Long-Delay Free Recall | 9.60 | (3.98) | 8.31 | (2.48) | .09 | 0.94 | (1.77) | 0.48 | (1.24) | .17 |
| Long-Delay Cued Recall | 10.81 | (3.45) | 9.08 | (2.80) | .02 | 2.55 | (2.04) | 2.00 | (1.99) | .23 |
| Percent Long-Delay Savings | 79.57 | (24.07) | 84.02 | (22.65) | .40 | 20.56 | (33.90) | 9.24 | (22.12) | .07 |
P-value associated with an independent samples t test comparing Young-Old NC to Very-Old NC groups.
P-value associated with an independent samples t test comparing Young-Old AD to Very-Old AD groups.
RESULTS
Profile Analysis
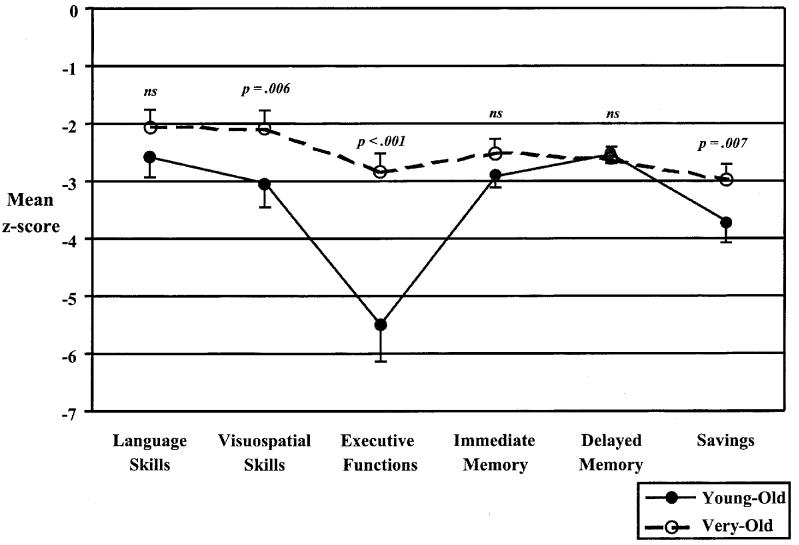
Mean (and SD) raw scores from each of the four groups are presented in Table 2. Before inspecting individual neuropsychological test performances, each of the test scores of the two AD groups were z-transformed relative to their respective NC group and submitted to a profile analysis using multivariate analysis of variance (MANOVA). Prior to analysis, z scores were modified to ensure that negative scores represented poorer performance and then averaged into the following neuropsychological domains based largely on prior factor analytic groupings demonstrated by Bondi et al. (2002): (1) Language: Boston Naming Test, Letter and Category Fluency, and WAIS–R Vocabulary; (2) Executive Functions: modified WCST Categories and Perseverative Errors, Trailmaking Test Part B; (3) Visuoconstructive and Psychomotor Skills: WISC–R Block Design, WAIS–R Digit Symbol, Trailmaking Test Part A; (4) Immediate Recall: CVLT Trials 1–5 Total Recall, WMS–R Logical Memory Immediate Recall; (5) Delayed Recall: CVLT Long-Delay Freeand Cued-Recall, WMS–R Logical Memory Delayed Recall, and (6) Recall Savings: CVLT Percent Long-Delay Savings, WMS–R Percent Delayed Recall Savings. The resulting mean levels of performance in each of the six neuropsychological domains for the Young-Old and Very-Old AD groups are shown in Figure 1.
Fig. 1.
Mean levels of performance indicated in z-score units of young-old (dotted lines) and Very-Old (solid lines) Alzheimer's disease groups on each of six neuropsychological domains. Error bars denote standard error of the mean.
The six neuropsychological domain composites, representing a within-subjects factor, were then submitted along with two between-subjects factors [age group (Young-Old AD vs. Very-Old AD) and APOE genotype (ε4 vs. non-ε4)] to a mixed-model MANOVA. Results of the MANOVA revealed a significant main effect of age group [F(1,72) = 21.07, p < .001, η2 = .23] but not APOE genotype [F(1,72) = 0.32, p = .57, η2 = .01], a significant within-subjects domain effect [Multivariate F(5,68) = 21.42, p < .001, η2 = .61], a significant age group by domain interaction [Multivariate F(5,68) = 11.41, p < .001, η2 = .46] but not APOE × Domain interaction [Multivariate F(5,68) = 1.68, p = .15, η2 = .11], and a significant three-way interaction of Age Group × APOE Genotype × Neuropsychological Domain [Multivariate F(5,68) = 3.90, p = .004, η2 = .22].
As shown in Figure 1, pairwise comparisons (with a Bonferroni-corrected significance level: .05/6 = .008) revealed that the age group by domain interaction was the result of significantly worse z scores for the Young-Old AD group than for the Very-Old AD group on tests of executive functions [t(77) = 5.02, p < .001, η2 = .25], visuoconstructive and psychomotor skills [t(76) = 2.85, p = .006, η2 = .10], and delayed recall savings [t(79) = 2.78, p = .007, η2 = .09]. A borderline non-significant difference was observed for immediate recall [t(79) = 2.73, p = .008, η2 = .09], and no significant group differences were noted for language skills [t(79) = 1.74, p = .09, η2 = .04] or delayed recall [t(79) = 0.75, p = .46, η2 = .01].
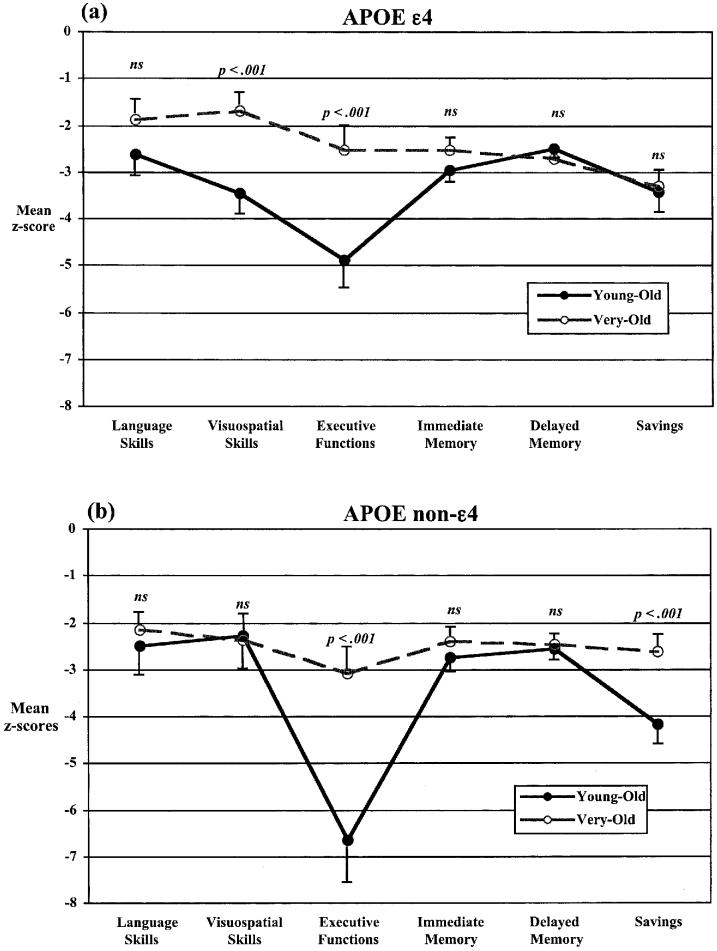
As shown in Figure 2, pairwise comparisons (with a Bonferroni-corrected significance level: .05/12 = .004) revealed that the three-way interaction appeared to be due to a more deleterious effect of the APOE ε4 allele for the Very-Old AD group on recall savings [Young-Old AD vs. Very-Old AD with the ε4 allele: t(42) = 0.34, p = .74, η2 = .003; see Figure 2a] compared to its non-ε4 counterparts who significantly outperformed its Young-Old AD group on recall savings [Young-Old AD vs. Very-Old AD without a copy of the ε4 allele: t(35) = 4.02, p < .001, η2 = .32; see Figure 2b]. An opposite pattern of greater deficits on tests of visuospatial functions for the Young-Old AD group with an ε4 allele compared to its Very-Old AD counterparts was demonstrated [Young-Old AD vs. Very-Old AD with the ε4 allele: t(41) = 4.29, p < .001, η2 = .31; see Figure 2a], whereas the two non-ε4 AD age groups were comparably impaired on visuospatial functions [Young-Old AD vs. Very-Old AD without a copy of the ε4 allele: t(33) = 0.18, p = .86, η2 = .001; see Figure 2b]. On tests of executive functions, the Young-Old AD group performed significantly worse than the Very-Old AD group, whether or not they were ε4 carriers [ε4: t(41) = 4.65, p < .001, η2 = .35; non-ε4: t(34) = 3.48, p = .001, η2 = .26].
Fig. 2.
Mean levels of performance indicated in z-score units of young-old AD (solid lines) and Very-Old AD (dotted lines) groups either with an APOE ε4 allele (a) or without an ε4 allele (b) on each of the six neuropsychological domains. Error bars denote standard error of the mean.
Univariate Analyses
Raw score comparisons of individual test performances
As shown in Table 2, the Very-Old NC group produced lower mean raw scores than the Young-Old NC group on most of the 20 neuropsychological test measures (ps < .05 on 15 of the 20 independent samples t-test comparisons), although variability in scores for the two age groups were comparable on the majority of measures (ps > .05 associated with Levene's test for equality of variances on 14 of the 20 measures). In contrast, independent samples t-tests revealed that the Young-Old and Very-Old AD groups scored comparably on all of the raw neuropsychological test score comparisons (all p-values > .05; also, ps > .05 associated with Levene's test for equality of variances on 17 of the 20 measures). It should also be highlighted that the results were equivalent, whether or not equal variance assumptions were met.
Standardized score comparisons of individual test performances
This pattern of discrepant raw score results between the NC andAD groups would be expected to result in Very-Old AD patients appearing less cognitively impaired than the Young-Old AD patients once their performances are viewed against the backdrop of normal age-related cognitive decline. To directly examine this notion, and as discussed above, scores achieved by the Young-Old and Very-Old AD patients on each test measure were converted to z scores based upon the means and standard deviations of their respective NC groups (see Table 3).Aseries of 2 (Young-Old AD vs. Very-Old AD) × 2 (APOE ε4 vs. non-ε4) ANOVAs using z-transformed test scores were performed to examine the effects of age and APOE genotype on the degree of impairment in AD groups on each of the individual test measures.
Table 3.
Means (and SDs) of age-corrected z-scores of the young-old (M age < 70) and very-old (M age > 80) Alzheimer disease groups
| Alzheimer disease group Age-corrected z scores |
|||||
|---|---|---|---|---|---|
| Young-old (n = 33) |
Very-old (n = 48) |
||||
| Variables | M | SD | M | SD | p-valuesa |
| Language | |||||
| Boston Naming Test (30-item) | −4.89 | (4.56) | −2.92 | (2.40) | .013 |
| Letter Fluency (FAS Total) | −1.56 | (1.11) | −1.05 | (1.03) | .035 |
| Category Fluency (AFV Total) | −2.48 | (0.75) | −1.97 | (1.02) | .016 |
| WAIS–R Vocabulary | −1.44 | (1.61) | −2.24 | (2.03) | .061 |
| Visuoconstructive/Psychomotor Skills | |||||
| WAIS–R Digit Symbol | −2.93 | (1.25) | −1.81 | (1.21) | <.001 |
| WISC Block Design | −2.58 | (1.22) | −1.81 | (1.23) | .007 |
| Trail Making Test (Part A; sec) | 3.79 | (2.93) | 2.61 | (2.49) | .054* |
| Sequencing/Executive Function | |||||
| Trail Making Test (Part B; s) | 5.84 | (2.00) | 4.34 | (1.98) | .001* |
| Modified Wisconsin Card Sort | |||||
| Categories | −4.55 | (2.09) | −1.48 | (1.08) | <.001 |
| Perseverative Errors | 6.14 | (8.10) | 2.61 | (3.10) | .008* |
| Learning and Memory | |||||
| WMS–R Logical Memory | |||||
| Immediate Recall | −2.92 | (0.77) | −2.07 | (0.76) | <.001 |
| Delayed Recall | −3.02 | (0.34) | −2.12 | (0.47) | <.001* |
| Percent Delay Recall Savings | −4.98 | (1.93) | −2.62 | (1.49) | <.001* |
| Learning and Memory | |||||
| California Verbal Learning Test | |||||
| Total List A Immediate Recall | −2.88 | (0.69) | −2.88 | (0.95) | .991 |
| List A Trial 5 Recall | −2.75 | (0.69) | −2.45 | (0.70) | .061 |
| Short-Delay Free Recall | −2.33 | (0.50) | −2.49 | (0.58) | .188 |
| Short-Delay Cued Recall | −2.40 | (0.62) | −2.79 | (0.97) | .045 |
| Long-Delay Free Recall | −2.18 | (0.44) | −3.15 | (0.50) | <.001 |
| Long-Delay Cued Recall | −2.40 | (0.59) | −2.53 | (0.71) | .397 |
| Percent Long-Delay Savings | −2.45 | (1.41) | −3.30 | (0.98) | .002 |
P-value associated with main effect for age group (young-old AD; very-old AD).
Significant Age × APOE genotype interactions indicated by an asterisk (*) at p < .025.
Age effects
Main effects for age (with a Bonferroni-corrected significance level: .05/20 = .002) using the age-normalized z scores revealed significantly poorer performance in the Young-Old AD group compared to the Very-Old AD group on tests of executive functions [modified WCST perseverative errors: F(1,75) = 14.65, p < .001, η2 = .16; modified WCST categories: F(1,77) = 64.96, p < .001, η2 = .46], psychomotor skills [WAIS–R Digit Symbol: F(1,75) = 12.66, p = .001, η2 = .14], and learning and memory [WMS–R Logical Memory Immediate Recall: F(1,77) = 22.12, p < .001, η2 = .22; Delayed Recall: F(1,77) = 99.30, p < .001, η2 = .56; Delayed Recall Savings: F(1,77) = 44.52, p < .001, η2 = .37]. The Young-Old AD group performed significantly better than the Very-Old AD group on only one test measure [CVLT Long-Delay Free Recall: F(1,77) = 78.17, p < .001, η2 = .50], although this latter score had a pronounced floor effect (i.e., both AD groups' means averaged less than one item). Thus, in all but one case (CVLT Long-Delay Free Recall), Very-Old AD patients demonstrated better scores than Young-Old AD patients.
APOE genotype polymorphism effects
There was only one significant main effect of APOE genotype, with APOE ε4 non-carriers [mean z = −7.05] performing below that of ε4 carriers [mean z = −2.86] in terms of perseverative errors on the modified WCST [F(1,75) = 11.48, p = .001, η2 = .13].
Age × APOE Genotype interactions
Significant Age × APOE Genotype interaction effects were obtained for five of the neuropsychological test measures. The presence of an APOE ε4 allele had a more deleterious effect on performance in the Very-Old AD group than in the Young-Old AD group on two measures of story recall [WMS–R Logical Memory Delayed Recall: F(1,77) = 8.38, p = .005, η2 = .10; WMS–R Logical Memory Delayed Recall Savings: F(1,77) = 8.34, p = .005, η2 = .10]. In contrast, the APOE ε4 allele had a more deleterious effect on performance in the Young-Old AD group than in the Very-Old AD group on measures of visuomotor sequencing [Trails A: F(1,77) = 10.86, p < .001, η2 = .12; Trails B: F(1,77) = 5.35, p = .02, η2 = .07] and in terms of perseverative errors on the modified WCST [F(1,75) = 7.35, p = .008, η2 = .09; see Table 3].
DISCUSSION
The results of the present study suggest that, when AD patients are compared to their age-appropriate control groups, the profile and severity of neuropsychological dysfunction typified in the Young-Old is no longer observed in the Very-Old. That is, the profile of neuropsychological deficits associated with AD in the Very-Old is less severe from that in the Young-Old when patients are compared using standardized scores. Despite being matched on education, gender, frequency of the APOE ε4 allele, disease duration, global dementia severity (as measured by the Mattis DRS), and degree of functional impairment, Very-Old AD patients exhibited a more mild degree of deficit on age-corrected z scores than did their Young-Old AD counterparts. This was evident in the significant group effect in the profile analysis (see Figure 1) which confirmed that the composite z score collapsed across the six cognitive domains showed less overall impairment for the Very-Old AD group (overall mean z = −2.51) than for the Young-Old AD group (overall mean z= −3.35). Furthermore, cognitive domains most affected following age-corrections in the Very-Old AD patients included those that are usually the most severely affected early in the disease in the Young-Old patients, namely, retention of episodic memories (i.e., savings scores) and executive functions. Because of these age-related differences, the profile of cognitive deficits associated with AD in the Very-Old lacks the disproportionate saliency of episodic memory and executive function deficits typical of the disease in the Young-Old. Thus, clinicians may be likely to commit false negative diagnostic errors in the Very-Old if they expect the level of severity and pattern of impairment (relative to age-appropriate normative data) to be the same as in the Young-Old patient.
Our results also demonstrate dramatic differences in the rawscore versus standardized score profiles of neuropsychological impairment between Young-Old and Very-Old AD patients. When inspecting raw scores, both AD groups showed comparable impairments across all of the neuropsychological variables examined. Given that the rawscoreswere comparable between AD groups, the distinct profiles of cognitive impairment associated withAD in the Very-Old and Young-Old arose primarily from differences in performance exhibited by the respective age-matched normative reference cohorts. The Very-Old control participants performed significantly worse than the Young-Old control participants on nearly all of the cognitive tests, with the largest differences apparent on tests of memory, executive functions, and category fluency. These findings are consistent with previous reports of the adverse effects of normal aging on these cognitive abilities (Corey-Bloom et al., 1996; Gunning-Dixon & Raz, 2000; Hulette et al., 1998; Mittenberg et al., 1989). It is important to note that this decline in cognitive ability was evident in the Very-Old NC group despite largely excluding individuals who may have been in a preclinical stage of AD. In addition, although the mean scores of the Very-Old NC group tended to be significantly lower than those of the Young-Old NC group, the variance associated with the different measures was similar for the two groups. In fact, the standard deviations were nominally larger in the Young-Old than in the Very-Old NC group for 11 of the 20 cognitive measures (see Table 2). This similarity in the degree of test score variability between the two groups makes it unlikely that the “better” z scores of the Very-Old AD patients compared to the Young-Old AD patients is an artifact of increased variability with aging in the control group; rather, the current results suggest that it is the result of lower mean scores in the Very-Old normal control group.
As such, the present results underscore the importance of a normative reference group that is as free as possible of individuals who may be in a preclinical stage of AD. Numerous studies have now shown that subtle cognitive changes can precede the diagnosis of AD by a few years or more (Bäckman et al., 2001; Bondi et al., 1994, 1995, 1999; Chen et al., 2001; Lange et al., 2002; Linn et al., 1995; Masur et al., 1994; Rubin et al., 1998; Small et al., 1998, 2000; Smith et al., 1998). As Sliwinski and colleagues (1996) have shown, the inclusion of such individuals with preclinical AD in a normative sample leads to an underestimate of the mean, an overestimate of the variance, and an overestimate of the effect of age on a given cognitive measure, all of which reduces the sensitivity of the measure for detecting mild impairment. Thus, future studies of the effects of normal aging on cognition might consider risk factors for the development of dementia in their samples and longitudinally follow individuals to document that no obvious signs of dementia develop in the years soon after the collection of normative data (La Rue et al., 1992; but see Bäckman et al., 2002).
Our results also suggest that using highly screened samples of normal older adults helped to limit variability in test performances among the Very-Old more than it helped to buttress mean scores. However, national standardization samples from which many standardized scores are derived tend to demonstrate both lower mean values and greater variability in test performances with advancing age (Heaton et al., 1990, 1996). Less stringent inclusion and exclusion criteria, greater percentages of racial and ethnic subgroups, socioeconomic substrata, lower education levels, and greater numbers of sites and examiners involved in data collection, would presumably increase the variance associated with test scores. In all likelihood, the net effect would result in even less sensitive standardized measures to detect normal from deficient test performances in the Very-Old than was demonstrated in the current study with our highly screened NC samples.
One might argue that an approach using highly screened samples of older adults might be setting standards too high and that the normative reference groups would be comprised of only those “optimally” aging individuals and, thus, would not be representative of “normal” aging. However, the inclusion of NC participants with a variety of medical and systemic conditions in this—and other—studies is contrary to this notion, as long as those conditions are not thought to adversely affect cognition. Indeed, the strategy used by the Mayo clinic's older American normative studies (Ivnik et al., 1992; Malec et al., 1992) have included in their samples individuals with chronic illnesses such as hypertension and diabetes, but whose cognitive capacity and daily functioning were not considered to be adversely affected by their illness.
Our findings also imply that similar decrements in sensitivity for AD may be observed both in the classification of Mild Cognitive Impairment (MCI; Petersen et al., 1995, 1999) as well as in other, non-cognitive measures such as volumetric assessments on structural MR imaging (e.g., Jack et al., 1998a, 1998b). For example, in contrast to the obvious cognitive impairment of Young-Old AD patients (i.e., scores −3 to −6 SDs below normal), the cognitive impairment of Very-Old AD patients was less apparent (i.e., scores −2 to −3 SDs below normal). Thus, the suggested use of −1.5 SDs or below on memory testing for the identification of MCI may need adjustment upward if it is to retain sensitivity for the detection of MCI in the Very-Old. With respect to MR imaging, there may be less volumetric integrity (and more variability) in medial temporal lobe structures in the Very-Old. Consequently, imaging approaches that measure change in these structures as a diagnostic sign of AD may also be rendered less useful in this cohort because of the greater backdrop of hippocampal atrophy and variability with age (see Jernigan et al., 2001).
Consistent with previous studies (Basun et al., 1995; Bondi et al., 1999; Corder et al., 1995; Normann et al., 1995; Dal Forno et al., 1996; DeKosky et al., 1995; Gomez-Isla et al., 1996; Growdon et al., 1996; Kurz et al., 1996; Small et al., 1998; Smith et al., 1998), there was little overall effect of APOE genotype on the cognitive performance of the NC participants or AD patients in the Very-Old or Young-Old cohorts (e.g., no main effect of APOE genotype in the multivariate analysis and in all but one of the ANOVAs). However, there were interactions between age and the presence of the APOE ε4 allele on the severity of impairment exhibited by patients with AD on some measures of memory, visuomotor sequencing, and perseverative responding, but these interaction effects were not consistent across measures. The ε4 allele was associated with worse performance in AD on story recall measures in the Very-Old, but not Young-Old, whereas it was associated with better performance on the Trail-Making Test (Parts A and B) and perseverative responses on the Wisconsin Card Sorting Task in the Very-Old, but not Young-Old. These findings, though preliminary, are consistent with prior studies demonstrating APOE-related effects to be limited to tests of episodic memory and executive functions (see Bondi et al., 1999; Smith et al., 1998).
Finally, there appeared to be some differences with respect to the sensitivity of the two types of memory tests. It appeared that the Very-Old AD patients were better able than the Young-Old AD patients to exploit the additional semantic and contextual support provided by the story-learning format of the WMS–R Logical Memory test relative to the list-learning format of the CVLT. Also, APOE genotype appeared to further modify this relationship since the Very-Old AD patients with the APOE ε4 allele derived less benefit from this additional support than did the non-carriers. These findings must be interpreted cautiously, however, in light of their raw scores on these measures, because both groups were performing near floor levels on the memory tests. For example, both groups of AD patients were retaining only 10 to 20 of the material after a delay period.
The presence of these floor effects demonstrates that inspection of the raw scores in such cases may clarify—rather than cloud—interpretation of episodic memory performance in the Very-Old. For example, a number of studies in the literature demonstrate that healthy older adults typically produce retention rates on the WMS–R at or above 60% for delayed recall of the story material (Butters et al., 1988; Cullum et al., 1990; Incalzi et al., 1995; Tröster et al., 1993). Thus, any individual for whom the retention rate falls below 30 or 40% should raise the possibility of significant episodic memory disturbance. Because the raw score comparisons of the two AD groups failed to reveal any significant differences on the test measures, examining raw scores might help obviate some of the diminution in standard score profiles observed in the present study. At a minimum, our results demonstrate that mild decrements on standardized neuropsychological test scores in the Very-Old likely represent large and clinically significant deficits, particularly for tests of episodic memory, executive functions, and visuospatial skills.
Taken together, our results clearly argue against the simple application of our understanding of neuropsychological changes in early AD in the Young-Old to the detection of the disease in the Very-Old. Because of normal age-related changes in cognitive performance, and possible age-related changes in the influence of the APOE ε4 allele on cognition, a multi-faceted approach that integrates neuropsychological assessment, APOE genotyping, and emerging neuroimaging technologies, may be needed to characterize the early and preclinical stages of AD in this fastest growing and most vulnerable segment of our population. The development and refinement of methods for the early and accurate detection of AD in the Very-Old is an important goal of neuropsychological research given that preventative and neuroprotective agents designed to impede the progression of the disease are under development (see Thal, 1999).
ACKNOWLEDGMENTS
This work was supported in part by Department of Veterans Affairs Merit Review grants (M. Bondi and D. Delis) and National Institute on Aging Grants AG12674 (M. Bondi) and AG05131 (UCSD Alzheimer's Disease Research Center). The authors would like to thank Natalie Marchant for her assistance in the preparation of the manuscript as well as the staff and participants of the Alzheimer's Disease Research Center at the University of California, San Diego.
REFERENCES
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jonsson Laukka E, Wahlin Å, Small BJ, Fratiglioni L. Influences of preclinical dementia and impending death on the magnitude of age-related cognitive decline. Psychology and Aging. 2002;17:435–442. doi: 10.1037//0882-7974.17.3.435. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- Basun H, Grut M, Winblad B, Lannfelt L. Apolipoprotein ε4 allele and disease progression in patients with late-onset Alzheimer's disease. Neuroscience Letters. 1995;183:32–34. doi: 10.1016/0304-3940(94)11107-t. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Galasko D, Butters N, Salmon DP, Delis DC. Preclinical cognitive markers of dementia of the Alzheimer type. Neuropsychology. 1994;8:374–384. [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T. Episodic memory changes are associated with the ApoE-ε4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Serody AB, Chan AS, Eberson-Shumate S, Delis DC, Hansen LA, Salmon DP. Cognitive and neuropathologic correlates of Stroop color-word test performance in Alzheimer's disease. Neuropsychology. 2002;16:335–343. doi: 10.1037//0894-4105.16.3.335. [DOI] [PubMed] [Google Scholar]
- Butters N, Salmon DP, Cullum MC, Cairns P, Tröster AI, Jacobs D, Moss M, Cermak LS. Differentiation of amnesic and demented patients with the Wechsler Memory Scale–Revised. Clinical Neuropsychologist. 1988;2:133–148. [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: A prospective community study. Archives of General Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neuroscience and Biobehavioral Reviews. 2000;24:365–374. doi: 10.1016/s0149-7634(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Corder EH, Lannfelt L, Viitanen M, Corder LS, Manton KG, Winblad B, Basun H. Apolipoprotein E genotype determines survival in the oldest old (85 years or older) who have good cognition. Archives of Neurology. 1996;53:418–422. doi: 10.1001/archneur.1996.00550050048022. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr., Rimmler JB, Locke PA, Conneally PM, Schmader KA, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature Genetics. 1994;7:180–183. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Rimmler JB, Locke PA, Conneally PM, Schmader KE, Tanzi RE, Gusella JF, Small GW, Roses AD, Pericak-Vance MA, Haines JL. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology. 1995;45:1323–1328. doi: 10.1212/wnl.45.7.1323. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines GL, Perick-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Wiederholt WC, Edelstein S, Salmon DP, Cahn D, Barrett-Connor E. Cognitive and functional status of the oldest old. Journal of the American Geriatrics Society. 1996;44:671–674. doi: 10.1111/j.1532-5415.1996.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Butters N, Tröster AI, Salmon DP. Normal aging and forgetting rates on the Wechsler Memory Scale–Revised. Archives of Clinical Neuropsychology. 1990;5:23–30. doi: 10.1016/0887-6177(90)90004-9. [DOI] [PubMed] [Google Scholar]
- Dal Forno G, Rasmusson X, Brandt J, Carson KA, Brookmeyer R, Troncoso J, Kawas CH. Apolipoprotein E genotype and rate of decline in probable Alzheimer's disease. Archives of Neurology. 1996;53:345–350. doi: 10.1001/archneur.1996.00550040085017. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ferrell R, Kamboh MI, Becker JT. Natural history of definite AD as a function of APOE genotypes. Neurology. 1995;45(Suppl 4):A373. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. Psychological Corporation; New York: 1987. [Google Scholar]
- Desgranges B, Baron JC, Eustache F. The functional neuroanatomy of episodic memory: The role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. NeuroImage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein H, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer's disease in a community population of older persons. Journal of the American Medical Association. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Fichter MM, Schröppel H, Meller I. Incidence of dementia in a Munich community sample of the oldest old. European Archives of Psychiatry and Clinical Neurosciences. 1996;246:320–328. doi: 10.1007/BF02189026. [DOI] [PubMed] [Google Scholar]
- Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, Hill LR, Lessin P, Thal LJ. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Archives of Neurology. 1994;51:888–895. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- Gebner R, Reischies FM, Kage A, Geiselmann B, Borchelt M, Steinhagen-Thiessen E, Köttgen E. In an epidemiological sample the apolipoprotein E4 allele is associated to dementia and loss of memory function only in the very old. Neuroscience Letters. 1997;222:29–32. doi: 10.1016/s0304-3940(97)13334-1. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locasio JJ, Perls TT, Lipsitz LA, Hyman BT. Clinical and pathological correlates of apolipoprotein E ε4 in Alzheimer's disease. Annals of Neurology. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Grady CL, Mcintosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Green MS, Kaye JA, Ball MJ. The Oregon Brain Aging Study: Neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54:105–113. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- Growdon JH, Locascio JJ, Corkin S, Gomez-Isla T, Hyman BT. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Guttman CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Mathews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Psychological Assessment Resources, Inc.; Odessa, FL: 1990. [Google Scholar]
- Heaton RK, Ryan L, Grant I, Matthews CG. Demographic influences on neuropsychological test performance. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric disorders. 2nd ed. Oxford University Press; New York: 1996. pp. 141–163. [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. Journal of Neuropathology and Experimental Neurology. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Incalzi RA, Capparella O, Gemma A, Marra C, Carbonin PU. Effects of aging and of Alzheimer's Disease on verbal memory. Journal of Clinical and Experimental Neuropsychology. 1995;17:580–589. doi: 10.1080/01688639508405147. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo's older americans normative studies: WMS–R norms for ages 56 to 94. Clinical Neuropsychologist. 1992;6(suppl):49–82. [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O'Brien P, Waring S, Tangalos E, Smith G, Ivnik R, Thibodeau S, Kokmen E. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer's disease. Annals of Neurology. 1998b;43:303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TJ, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kawas C, Katzman R. Epidemiology of dementia and Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. Raven Press; New York: 1999. pp. 95–116. [Google Scholar]
- Kurz A, Egensperger R, Haupt M, Lautenschlager N, Romero B, Graeber MB, Muller U. Apolipoprotein E ε4 allele, cognitive decline, and deterioration of everyday performance in Alzheimer's disease. Neurology. 1996;47:440–443. doi: 10.1212/wnl.47.2.440. [DOI] [PubMed] [Google Scholar]
- Lange KL, Bondi MW, Salmon DP, Galasko D, Delis DC, Thomas RG, Thal LJ. Decline in verbal memory during preclinical Alzheimer's disease: Examination of the effect ofAPOE genotype. Journal of the International Neuropsychological Society. 2002;8:943–955. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue A, Matsuyama SS, McPherson S, Sherman J, Jarvik LF. Cognitive performance in relatives of patients with probable Alzheimer disease: An age at onset effect? Journal of Clinical and Experimental Neuropsychology. 1992;14:533–538. doi: 10.1080/01688639208402842. [DOI] [PubMed] [Google Scholar]
- Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D'Agostino RB. The ‘preclinical phase’ of probable Alzheimer's disease. Archives of Neurology. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- Malec JF, Ivnik RJ, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo's older americans normative studies: Utility of corrections for age and education for the WAIS–R. The Clinical Neuropsychologist. 1992;6(suppl):31–47. [Google Scholar]
- Masliah E, Mallory M, Hansen LA, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- McDowell I, Hill GB, Lindsay JP, Helliwell B. The Canadian study of health and aging: Incidence of dementia; Paper presented at the Sixth International Conference on Alzheimer's Disease and Related Disorders; Amsterdam, The Netherlands. Jul, 1998. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mittenberg W, Seidenberg M, O'Leary DS, DiGiulio DV. Changes in cerebral functioning associated with normal aging. Journal of Clinical and Experimental Neuropsychology. 1989;11:918–932. doi: 10.1080/01688638908400945. [DOI] [PubMed] [Google Scholar]
- Normann J, Brookes AJ, Yates C, St. Clair D. Apolipoprotein E genotype and its effect on duration and severity of early and late onset Alzheimer's disease. British Journal of Psychiatry. 1995;167:533–536. doi: 10.1192/bjp.167.4.533. [DOI] [PubMed] [Google Scholar]
- Payami H, Grimslid H, Oken B, Camicioli R, Sexton G, Dame A, Howieson D, Kaye J. A prospective study of cognitive health in the elderly (Oregon Brain Aging Study): Effects of family history and apolipoprotein E genotype. American Journal of Human Genetics. 1997;60:948–956. [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith G, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kokmen E, Waring SC, Kurland LT. APOE status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. Journal of the American Medical Association. 1995;273:1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E . Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Perls TT, West HL, Sodhi P, Lipsitz LA, Hyman BT. Reduced apolipoprotein ε4 allele frequency in the oldest old Alzheimer's patients and cognitively normal individuals. Neurology. 1994;44:1513–1516. doi: 10.1212/wnl.44.8.1513. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer's disease: A reanalysis of data from Rochester,Minnesota, 1975–1984. American Journal of Epidemiology. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, Berg L. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Archives of Neurology. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychology ofAlzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 39–56. [Google Scholar]
- Salmon DP, Butters N. Neuropsychological assessment of dementia in the elderly. In: Katzman R, Rowe JW, editors. Principles of geriatric neurology. F. A. Davis; Philadelphia: 1992. pp. 144–163. [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, St. George-Hyslop PH, Perick-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory changes: A PET study. NeuroReport. 1996;7:1165–1169. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- Sliwinski M, Lipton RB, Buschke H, Stewart WF. The effect of preclinical dementia on estimates of normal cognitive function in aging. Journal of Gerontology: Psychological Sciences. 1996;51B:P217–P225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- Small BJ, Basun H, Bäckman L. Three-year changes in cognitive performance as a function of apolipoprotein E genotype: Evidence from very old adults without dementia. Psychology and Aging. 1998;13:80–87. doi: 10.1037//0882-7974.13.1.80. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Bäckman L. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Archives of Neurology. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schemchel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences (USA) 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Goedert M, Saunders AM, Huang D, Corder EH, Dong L-M, Jakes R, Alberts MJ, Gilbert JR, Han S-H, Hulette C, Einstein G, Schmechel DE, Pericak-Vance MA, Roses AD. Hypothesis: Microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Experimental Neurology. 1994;125:163–171. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Hansen LA. The neuropathology of Alzheimer disease and the structural basis of its cognitive alterations. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 187–206. [Google Scholar]
- Thal LJ. Clinical trials in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 423–440. [Google Scholar]
- Tröster AI, Butters N, Salmon DP, Cullum CM, Jacobs D, Brandt J, White RF. The diagnostic utility of savings scores: differentiating Alzheimer's and Huntingtons diseases with the logical memory and visual reproduction tests. Journal of Clinical and Experimental Neuropsychology. 1993;15:773–788. doi: 10.1080/01688639308402595. [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of the Census . Profiles in Americas elderly, No. 2. U.S. Government Printing Office; Washington, DC: 1992. Growth of America's Very-Old population. [Google Scholar]
- Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Archives of Neurology. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]