Abstract
Alzheimer's disease (AD) is a common, devastating form of dementia. With the advent of promising symptomatic treatment, the importance of recognizing AD at its very earliest stages has increased. We review the extant neuropsychological and neuroimaging literature on preclinical AD, focusing on longitudinal studies of initially nondemented individuals and cross-sectional investigations comparing at-risk with normal individuals. We systematically reviewed 91 studies of neuropsychological functioning, structural neuroimaging, or functional neuroimaging in preclinical AD. The neuropsychological studies indicated that preclinical AD might be characterized by subtle deficits in a broad range of neuropsychological domains, particularly in attention, learning and memory, executive functioning, processing speed, and language. Recent findings from neuroimaging research suggest that volume loss and cerebral blood flow or metabolic changes, particularly in the temporal lobe, may be detected before the onset of dementia. There exist several markers of a preclinical period of AD, in which specific cognitive and biochemical changes precede the clinical manifestations. The preclinical indicators of AD reflect early compromise of generalized brain integrity and temporal lobe functioning in particular.
Keywords: Dementia, Neuropsychology, Magnetic resonance imaging, Positron emission tomography, Cognition disorders, Memory disorders
INTRODUCTION
The neuropathologic changes of Alzheimer's disease (AD) begin well before the disease becomes clinically apparent. The brain may compensate for such changes until cognitive decline becomes obvious and disrupts daily functioning, and the clinical diagnosis of AD can be made. However, cognitive signs and brain changes are subtly present prior to clinical diagnosis, thus denoting a “preclinical” stage of AD in which affected individuals exhibit only very mild changes in cognition despite an ongoing disease process. This stage gradually progresses to mild cognitive impairment (MCI; Petersen et al., 2001), characterized by mild but detectable cognitive impairment, and then to the more severe and clinically apparent stages of AD.
Much research into new AD treatments has focused on slowing or halting the disease process, rather than reversing its neuropathology. Many patients with MCI already exhibit substantial neuropathologic signs of AD (Price & Morris, 1999), illustrating the need to identify the preclinical stages of AD so that treatment can begin promptly. Identifying preclinical disease markers would improve characterization of predisposing factors for AD, promote our understanding of disease course, and improve our ability to assess treatment response without waiting for evidence of significant cognitive or functional declines.
Better recognition of preclinical markers of AD could have profound social and financial effects. Early detection could help reduce the cost of Alzheimer's care ($100 billion per year in the United States; DeKosky & Orgogozo, 2001); an intervention delaying AD onset by two years would result in nearly two million fewer cases 50 years from now (Brookmeyer et al., 1998). Additionally, early and accurate diagnosis will aid patients and families in healthcare decision-making.
We reviewed the current literature on neuropsychological and neuroimaging changes that may reflect preclinical AD. In doing so, we distinguished between “preclinical AD” and “MCI,” typically defined as mild memory impairment without other cognitive or functional deficits (Petersen et al., 2001). In contrast, preclinical AD is conceptualized as a stage featuring subtle cognitive declines (not necessarily impairments, however, and not necessarily in the domain of memory) and no perceptible declines in daily functioning. Another distinction exists on autopsy, at which those with MCI may exhibit substantial AD pathology, whereas preclinical AD patients may exhibit only minor changes (Price & Morris, 1999). Detecting AD in its preclinical stage, before the development of MCI, is crucial, particularly given that the conversion rate from MCI to AD within two years is up to 30% (Petersen et al., 2001). In a recent meta-analysis, Bäckman et al. (2005) found that neuropsychological tests of global cognition, delayed recall, perceptual speed, and executive functioning best discriminated subjects who developed AD from those who remained well. However, of the 47 studies included in their meta-analysis, 27 investigated cognition in MCI, cognitively impaired, or memory clinic samples, thereby making memory measures the most likely to emerge as predictors of future AD. The current review excluded studies of subjects with MCI or memory complaints in favor of a more pure focus on preclinical AD. We present a model of accelerated, nonlinear cognitive decline in the preclinical period and provide evidence that helps reconcile the heterogeneity in AD presentation.
As a backdrop to our focus on preclinical AD, we first review briefly the neuropathology of AD and the diagnostic criteria for the disease. Study designs and the rationale for using at-risk samples in this research are outlined. A comprehensive review of the current neuropsychological and neuroimaging research follows. Finally, the empirical evidence is put into a clinical context, gaps in our understanding are presented, and the potential impact of research on preclinical AD is discussed.
Neuropathologic and Diagnostic Criteria for AD
AD is a progressive degenerative disease characterized by neocortical atrophy, neuron and synapse loss, neuritic plaques, and neurofibrillary tangles (Alzheimer, 1907; Terry et al., 1991). These neuropathologic changes occur primarily in the hippocampus and entorhinal cortex, with later changes occurring in frontal, temporal, and parietal association cortices (Braak & Braak, 1991; Hyman et al., 1984). Eventually, the limbic regions and neocortex are affected (Bobinski et al., 1999; Xu et al., 2000). Additionally, subcortical neuron loss in the nucleus basalis of Meynert and locus coeruleus results in decreased levels of cholinergic and noradrenergic markers, respectively (Bondareff et al., 1982).
Clinically, AD is characterized by profound global dementia, with severe amnesia and additional deficits in other cognitive domains, such as language, executive functions, attention, and visuospatial/constructional abilities (American Psychiatric Association, 1994; Salmon & Bondi, 1999). The current DSM-IV criteria (American Psychiatric Association, 1994) include: (a) Memory impairment and at least one additional cognitive impairment; (b) Impaired functioning and functional decline; (c) Gradual onset and continuing cognitive decline; (d) Cognitive deficits not due to other causes. The criteria for AD suggest that cognitive deficits, structural volume loss, or functional brain changes appear gradually and thus might be identified before the neural degeneration produces clinically diagnosable dementia.
Study Designs
The prototypic longitudinal case-control research design used to identify potential preclinical markers of AD involves a baseline assessment of a large cohort of normal older adults and periodic follow-up evaluations (Collie & Maruff, 2000). Then, individuals who later develop AD are compared with those who remain nondemented on the presence and magnitude of cognitive declines, structural changes, and/or functional imaging changes. Retrospective studies of nondemented individuals who came to autopsy have also been published, comparing neuropsychological profiles of individuals with and without AD pathology.
Studying individuals at high risk for AD has been a common approach to research on preclinical indicators of the disease. Early studies recruited individuals at risk for AD due to family history (e.g., Hom et al., 1994), whereas more recent studies have focused on older adults with genetic susceptibilities such as the presence of an apolipoprotein E (APOE) ε4 allele (Corder et al., 1993). Such studies have used both cross-sectional designs and longitudinal evaluations to examine cognitive decline or neuroimaging changes. To provide a backdrop for our review of longitudinal and cross-sectional studies of preclinical AD, we briefly discuss the established risk factors for AD next.
Risk Factors for AD
Advancing age is the single most important risk factor for AD (Kawas & Katzman, 1999), with exponential increases in AD prevalence in individuals between 65 and 85. Epidemiologic studies of people over age 85, the fastest growing segment of our population (U.S. Census Bureau, 2004), show that the incidence of AD does not plateau, as was previously speculated, but continues to rise in this advanced age group, with 25–50% of this cohort developing the disease (Kawas & Katzman, 1999; Rocca et al., 1998).
In addition to age, certain genetic mutations have been implicated in the pathogenesis of AD. Early-onset AD is associated with defects on chromosome 21, particularly a mutation of the amyloid precursor protein (APP) gene, chromosome 1, and chromosome 14, both due to mutations in the presenilin-2 and presenilin-1 genes (Kawas & Katzman, 1999). Together, these known genetic mutations account for only about 2% of all cases of early-onset AD (Pericak-Vance et al., 2000).
The vast majority of people who develop AD do so after age 65, and the APOE gene on chromosome 19 has been identified as a susceptibility gene for both familial and sporadic late-onset AD (Corder et al., 1993). APOE is a protein influencing cholesterol transport in the blood and appears to be related to the deposition of amyloid and0or the formation of neurofibrillary tangles in the brain. The three different alleles (ε2, ε3, and ε4) yield different disease risks, with the ε4 allele conferring the greatest risk. APOE ε4 is present in up to 50% to 60% of AD patients, compared to only 16% of nondemented individuals. The risk of developing AD is three to four times higher for individuals with one copy of the ε4 allele, and approximately ten-fold in those with two copies of ε4 (Corder et al., 1993). Although APOE genotyping cannot be used alone as a diagnostic test for AD, it significantly improves diagnostic specificity when used in combination with clinical criteria (Mayeux et al., 1998). In a recent meta-analysis, Small et al. (2004) found significant differences between nondemented APOE ε4 carriers and noncarriers in global cognition, episodic memory, and executive functioning.
Numerous environmental, medical, and social factors have also been suggested as putative risk factors for AD, including head injury, hypertension, hypotension, toxin exposure, and low education. Some of these risk factors may interact with each other, and some may contribute to an individual's “cognitive reserve” or brain reserve capacity that buffers one against the cognitive effects of neurodegeneration (Kawas & Katzman, 1999).
METHOD
The literature providing a basis for this review was obtained by searching the Medline and PsycInfo databases for English-language articles containing the key terms “preclinical” and “Alzheimer.” Other terms entered into the search included “neuropsychologic,” “neuropsychological,” and “neuroimaging.” All articles up to April 2005 were included. Relevant papers from the reference lists of identified articles were also reviewed. Only studies that promoted knowledge on how to screen and diagnose AD using noninvasive, in vivo methods were included. Given our focus on ante mortem indicators, we excluded retrospective autopsy studies aimed at identifying biomarkers of AD after death. Family pedigree studies were only included if subjects were compared to normal control groups or genetically unaffected relatives. Given our specific focus on identifying AD before the manifestation of any clinical symptoms, we included only cross-sectional or prospective studies of nondemented, cognitively normal individuals; whereas studies of age-associated memory impairment (AAMI), MCI, questionable AD, or memory clinic populations were excluded. We also excluded investigations that focused solely on evoked potentials, event-related potentials, or olfactory testing, due to the relative paucity of research in these areas. Seventy-three neuropsychological studies, eleven structural neuroimaging studies, and seven functional neuroimaging studies were included in the present review. Comprehensive tables of relevant research were constructed (see the Appendices). When articles presented both neuropsychological and neuroimaging results, they were listed in both tables; cross-sectional and longitudinal results from the same study were listed in those respective sections of the tables. We then summarized the results across studies in Table 1.
Table 1.
Summary of neuropsychological and neuroimaging results across studies
| (A) Neuropsychological studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All comparisons (n = 79) |
Longitudinal AD+/AD− (n = 30) |
Longitudinal decline ε4+/ε4− (n = 16) |
Neuropsychological ε4+/ε4− (n = 26) |
Autopsy AD+/AD− (n = 3) |
Neuropsychological FH+/FH− (n = 4) |
||||||
| Percent of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | |
| General cognitive | 38% | 23 | 14 (61%) | 12 | 5 (42%) | 19 | 3 (16%) | 3 | 0 (0%) | 4 | 1 (25%) |
| Attention | 71% | 3 | 3 (100%) | 0 | 0 (0%) | 3 | 1 (33%) | 0 | 0 (0%) | 1 | 1 (100%) |
| Processing speed | 43% | 8 | 6 (75%) | 4 | 2 (50%) | 11 | 3 (27%) | 3 | 0 (0%) | 2 | 1 (50%) |
| Verbal learning | 57% | 21 | 19 (90%) | 10 | 4 (40%) | 18 | 5 (28%) | 3 | 1 (33%) | 4 | 3 (75%) |
| Verbal memory | 50% | 15 | 13 (87%) | 11 | 5 (45%) | 17 | 5 (29%) | 3 | 1 (33%) | 4 | 1 (25%) |
| Visual learning | 29% | 13 | 8 (62%) | 4 | 0 (0%) | 11 | 2 (18%) | 3 | 0 (0%) | 3 | 0 (0%) |
| Visual memory | 28% | 6 | 1 (17%) | 6 | 3 (50%) | 11 | 3 (27%) | 0 | 0 (0%) | 2 | 0 (0%) |
| Working memory | 12% | 9 | 0 (0%) | 3 | 1 (33%) | 11 | 2 (18%) | 1 | 0 (0%) | 2 | 0 (0%) |
| Language | 33% | 16 | 13 (81%) | 9 | 2 (22%) | 13 | 0 (0%) | 3 | 0 (0%) | 4 | 0 (0%) |
| Motor speed | 17% | 3 | 0 (0%) | 0 | 0 (0%) | 2 | 1 (50%) | 0 | 0 (0%) | 1 | 0 (0%) |
| Executive functioning | 44% | 13 | 9 (69%) | 7 | 3 (43%) | 11 | 4 (36%) | 2 | 0 (0%) | 3 | 0 (0%) |
| Visuospatial | 26% | 12 | 5 (42%) | 7 | 2 (29%) | 8 | 1 (13%) | 2 | 0 (0%) | 2 | 0 (0%) |
| Praxis | 17% | 3 | 1 (33%) | 1 | 0 (0%) | 1 | 0 (0%) | 1 | 0 (0%) | 0 | 0 (0%) |
| Olfactory | 100% | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 1 | 1 (100%) |
| (B) Neuroimaging studies | |||||||||||
| All comparisons (n = 18) |
Longitudinal MRI AD+/AD− (n = 3) |
Longitudinal MRI E4+/E4− (n = 1) |
Longitudinal PET E4+/E4− (n = 1) |
Cross-sectional MRI E4+/E4− (n = 7) |
Cross-sectional fMRI/PET E4+/E4− or FH+/FH− (n = 6) |
||||||
| Percent of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | Number of studies assessing | Number of studies significant | |
| Whole brain | 27% | 2 | 1 (50%) | 0 | 0 (0%) | 1 | 0 (0%) | 2 | 1 (50%) | 6 | 1 (17%) |
| Frontal lobe | 40% | 1 | 0 (0%) | 0 | 0 (0%) | 1 | 1 (100%) | 2 | 0 (0%) | 6 | 3 (50%) |
| Temporal lobe | 64% | 2 | 2 (100%) | 0 | 0 (0%) | 1 | 1 (100%) | 2 | 0 (0%) | 6 | 4 (67%) |
| Parietal lobe | 45% | 2 | 1 (50%) | 0 | 0 (0%) | 1 | 0 (0%) | 2 | 0 (0%) | 6 | 4 (67%) |
| Hippocampus | 39% | 3 | 2 (67%) | 1 | 1 (100%) | 1 | 0 (0%) | 7 | 3 (43%) | 6 | 1 (17%) |
| Amygdala | 9% | 1 | 0 (0%) | 0 | 0 (0%) | 1 | 0 (0%) | 3 | 1 (33%) | 6 | 0 (0%) |
| Parahippocampal g. | 9% | 2 | 0 (0%) | 0 | 0 (0%) | 1 | 1 (100%) | 2 | 0 (0%) | 6 | 0 (0%) |
| Posterior cingulate | 36% | 2 | 1 (50%) | 0 | 0 (0%) | 1 | 1 (100%) | 2 | 0 (0%) | 6 | 2 (33%) |
| Thalamus | 0% | 1 | 0 (0%) | 0 | 0 (0%) | 1 | 0 (0%) | 2 | 0 (0%) | 6 | 0 (0%) |
| Basal forebrain | 10% | 1 | 0 (0%) | 0 | 0 (0%) | 1 | 1 (100%) | 2 | 0 (0%) | 6 | 0 (0%) |
RESULTS
Neuropsychological Changes in Preclinical AD
Compared with older adults who remain nondemented, those who later develop AD perform more poorly across a broad range of neuropsychological measures. Appendix 1, presents summaries of 73 studies of neuropsychological changes in the preclinical period. There were 30 longitudinal case-control studies (Appendix 1, part A); 16 longitudinal studies examining decline in APOE ε4+ and ε4− subjects (Appendix 1, part B); 26 cross-sectional studies comparing neuropsychological performance in subjects with and without the APOE ε4 allele (Appendix 1, part C); 3 retrospective studies using autopsy data (Appendix 1, part D); and 4 studies comparing neuropsychological performance in subjects with and without a family history of AD (Appendix 1, part E).
The domains most consistently associated with preclinical AD were attention (71% of studies in which it was assessed), verbal learning and memory (57% and 50%, respectively), executive functioning (44%), processing speed (43%), and language (33%), with studies showing either early declines in these abilities or significant differences between at-risk subjects and control subjects (see Table 1). Global measures of cognition (e.g., the Mini-Mental State Examination) were less consistently associated with preclinical AD (38% of studies). Although not the focus of the current review, there was one study (Schiffman et al., 2002) that included olfactory processing as part of a comprehensive neuropsychological battery; this and other studies (e.g., Murphy et al., 1998) have shown that olfactory impairment may be an important preclinical indicator.
The current review revealed that attention, although not as commonly assessed as learning and memory in studies of preclinical AD, is even more consistently associated with the later development of AD. Only 10% of the longitudinal case-control studies measured attention, but of those, 100% found that attention performance discriminated cases from controls. Furthermore, verbal learning was a somewhat more consistent indicator of preclinical AD than was verbal delayed recall; these findings suggest that the deficits in verbal delayed recall in preclinical AD may partly reflect poor attention and encoding.
Episodic memory decline is one of the earliest and most prominent features of preclinical AD (Bondi et al., 1995, 1999; Linn et al., 1995). Subtle declines in episodic memory often occur several years before the emergence of the obvious cognitive and behavioral changes required for a clinical diagnosis of AD (Albert et al., 2001; Bäckman et al., 2001; Bondi et al., 1994, 1999; Chen et al., 2000, 2001; Fox et al., 1998; Grober & Kawas, 1997; Howieson et al., 1997; Jacobs et al., 1995; Lange et al., 2002; Masur et al., 1994). It is thought that episodic memory tasks are strong predictors of future AD, because the brain structures subserving episodic memory, such as the medial temporal lobes and the hippocampal formation (Squire, 1992), are among the first affected.
Some studies suggest that, compared to those who remain nondemented, individuals who later develop AD exhibit lower baseline levels of cognitive functioning. Fox and colleagues (1998), for example, tested asymptomatic at-risk members of early-onset familial AD pedigrees over a six-year period. Those who developed AD had demonstrated normal, but significantly lower verbal memory and performance IQ scores at their initial assessment (when they were ostensibly unaffected) compared to the group that remained nondemented. Similarly, Elias and colleagues (2000), in a 22-year prospective study of the Framingham cohort, found that only abstract reasoning (Similarities) and verbal retention performance predicted AD in subjects who were dementia-free for at least ten years after baseline neuropsychological assessment.
Lower baseline functioning among APOE ε4 carriers could represent either a preclinical stage of AD or simply a genetic phenotype for poorer cognition. One study suggested that the ε4 allele is associated with low normal cognitive performance (Berr et al., 1996). However, a later study found that ε4 and non-ε4 subjects' CVLT profiles did not differ once incident AD cases were excluded from the analyses (Bondi et al., 1999). Thus, it seems unlikely that allelic group differences represent evidence for a cognitive phenotype of the APOE gene (Reed et al., 1994). Instead, it appears that more ε4 individuals were demonstrating subtle memory decrements indicative of preclinical AD (see also Small et al., 1998 and Smith et al., 1998).
Although few studies have systematically examined the course of episodic memory changes during the preclinical phase (Bäckman et al., 2001; Chen et al., 2001; Lange et al., 2002; Rubin et al., 1998), summarizing and combining their findings may have important implications for detecting AD preclinically and projecting the course of decline. In two studies that have examined memory changes over time, Small et al. (2000a) and Bäckman et al. (2001) measured changes in episodic memory in individuals who eventually developed probable AD. Both studies found that the subjects who had mild episodic memory declines six years prior to diagnosis showed little memory change in the following three years, but exhibited precipitous declines just prior to the development of AD. Similarly, Chen et al. (2001) found a significant decline in episodic memory and executive functioning in individuals with preclinical AD during the 3.5 to 1.5 years before diagnosis, and Lange et al. (2002) found abrupt declines in episodic memory one to two years before AD onset.
The APOE ε4 genotype may hasten the decline in episodic memory that occurs prior to the emergence of diagnosable AD (Bondi et al., 1995, 1999; Reed et al., 1994). In the studies we reviewed, those comparing APOE genotype groups found that the ε4 allele is associated with both verbal and visual learning and memory. Caselli and colleagues (1999) compared performances of ε4 homozygotes, ε4 heterozygotes (all ε3/ε4), and non-ε4 carriers on various neuropsychological measures and found that older subjects in the ε4 homozygote group performed worse on immediate and delayed recall tasks. Baxter et al. (2003) also found that verbal learning ability declined over two years in a group of cognitively normal individuals who had the ε4 allele, but only in those who were 60 years of age or older. Thus, age-related memory decline seems to occur earlier in cognitively normal ε4 homozygotes than in ε4 heterozygotes and noncarriers, and precedes clinically detectable AD.
Neuroimaging Changes in Preclinical AD
Although no routine diagnostic test confirms the presence of AD, imaging techniques are an adjunctive screening measure for undetected pathology (Knopman et al., 2001) and represent an important expanding field in biological neuropsychiatry. Structural imaging techniques can detect early volumetric changes predictive of dementia, and functional imaging can detect preclinical changes in cerebral blood flow, metabolic activity, and neurotransmitter and receptor function. Appendix 2 presents summaries of 18 studies of neuroimaging changes in the preclinical period. There were three longitudinal case-control studies (Appendix 2, part A); one longitudinal magnetic resonance imaging (MRI) study that examined decline in APOE ε4+ and ε4− subjects (Appendix 2, part B); one longitudinal PET study that examined decline in APOE ε4+ and ε4− subjects (Appendix 2, part C); seven cross-sectional studies comparing structural MRI data in subjects with and without the APOE ε4 allele (Appendix 2, part D); and six studies comparing functional imaging data in at-risk versus not-at-risk subjects (Appendix 2, part E).
Of the eleven structural imaging studies and seven functional imaging studies we reviewed (see Appendix 2), most hypothesized that, given the histopathology of AD and the cognitive hallmark of AD (rapid forgetting), changes in temporal and hippocampal functioning would be among the earliest indicators of AD. Indeed, temporal lobe changes were the most common finding in preclinical AD, with 64% of studies measuring temporal lobe changes finding significant differences; changes in the parietal lobe (45% of studies), frontal lobe (40% of studies), hippocampus (39% of studies), and posterior cingulate (36% of studies) were also identified (see Table 1). Global brain changes were not as consistently associated with preclinical AD (27% of studies). We review these studies further in the two following sections on structural and functional brain imaging.
Structural Brain Imaging
Autopsy studies reveal that neurofibrillary changes in incident AD cases occur initially in the transentorhinal and entorhinal cortex, then in the hippocampal formation, and later in the neocortical structures (Braak & Braak, 1995). Amyloid plaque deposition may occur early in other cortical structures (e.g., anterior cortical regions; Morris et al., 1996). MRI can measure volumes of specific brain structures and can thereby distinguish normally aging subjects from potential AD patients even in the earliest stages of the disease. MRI studies reveal that portions of the medial temporal lobe (MTL), particularly the entorhinal cortex and hippocampus, are initially affected in AD.
Studies of the relationship between morphological and cognitive measures of AD have demonstrated that atrophy of the MTL region, especially the hippocampus, correlates with episodic memory impairment in AD (de Leon et al., 1997). Other cognitive abilities are affected as the neuropathologic changes of AD spread from limbic structures to neocortical association areas (Braak et al., 1998). The longitudinal MRI studies reviewed in Appendix 2, part A, uniformly found that MTL volume loss was detectable in preclinical AD cases and that the rate of volume loss also predicted future AD. A similar pattern implicating hippocampal volume loss emerged when APOE status was investigated longitudinally (Cohen et al., 2001).
Controversy exists as to whether greater MTL volume reduction among APOE ε4 carriers reflects preclinical AD or a structural phenotype. Some studies have shown an ε4 effect on MTL volumes (den Heijer et al., 2002; Plassman et al., 1997; Tohgi et al., 1997), but others have not (Jernigan et al., 2001; Schmidt et al., 1996). Cohen et al. (2001), however, showed that ε4 carriers demonstrated greater hippocampal volume loss over time than did noncarriers, suggesting that the cross-sectional differences between groups may reflect a preclinical AD state.
Functional Brain Imaging
Positron emission tomography
Positron emission tomography (PET) studies of AD patients reveal hypometabolism in the neocortical structures, mainly the parietal, frontal, and posterior temporal association cortices, the same areas where neuronal and synaptic degeneration appear most severe in post-mortem AD brains. Later, more advanced stages of AD are denoted by functional brain changes across the neocortex, with relative preservation of the sensorimotor and visual cortices.
Many of the PET studies in this area have compared APOE ε4 carriers with noncarriers. In the first PET study of this kind, Kennedy and colleagues (1995) found that ε4 carriers had lower global and temporoparietal glucose metabolism than did noncarriers. Reiman and colleagues (1996), who compared cognitively normal, middle-aged ε4 homozygotes and matched non-ε4 control participants, found that the ε4 homozygotes had reduced glucose metabolism in the posterior cingulate, parietal, temporal, and prefrontal regions, all of which were regions demonstrating specific metabolic reductions in mild AD. In a later study, Reiman et al. (2004) found that these same abnormalities existed in even younger (20–39 years old) ε4 carriers.
Functional magnetic resonance imaging
Functional MRI (fMRI) studies have generally shown decreased hippocampal activation in elderly subjects with memory decline relative to normal control groups. In one of the first studies to use fMRI to explore preclinical brain changes, Smith and colleagues (1999) examined cortical activation in two groups of cognitively normal middle-aged women who differed only in terms of their AD risk (i.e., family history of AD and APOE status). The groups performed equally well on visual naming and letter fluency tasks during scanning, but their activation patterns during both tasks differed, with the high-risk group demonstrating lower activations in the bilateral mid- and posterior inferotemporal regions.
Other researchers, however, have found increased activation associated with incipient AD. Bookheimer and colleagues (2000) found that during a word recall task, nondemented ε4 carriers had greater activation than did noncarriers in the left prefrontal region and bilateral orbitofrontal, superior temporal, and inferior and superior parietal regions. These abnormal patterns of activation may represent a compensatory functional response, that is, the use of additional brain resources to perform the task. The increased activations in the ε4 carriers were specific to episodic encoding, and were not seen in attentional tasks studied by the same group (Burggren et al., 2002).
Such studies suggest that compensatory mechanisms in brain activity exist in preclinical AD, before the manifestation of frank cognitive and functional impairments (Becker et al., 1996). Several functional neuroimaging studies have shown that the brain activity associated with performance on memory tasks is more diffuse in patients with early AD than in normal older individuals, probably because of the need to recruit additional brain resources to maintain performance. It may be that after an initial decline in memory following damage to MTL structures, patients in the preclinical stage of AD are able to recruit enough compensatory brain resources to slow further memory decline for a period of time. As the disease progresses, however, these additional resources become compromised and rapid episodic memory decline ensues (Lange et al., 2002).
It could be argued that functional neuroimaging methods will be more sensitive to early MTL dysfunction than will structural MRI (Haxby et al., 1986). However, PET studies of at-risk older adults or patients with early AD have not typically demonstrated MTL metabolic changes (Reiman et al., 1996; Small et al., 1995), findings that differ from those derived from structural neuroimaging and neuropathologic studies that demonstrate that the earliest changes in AD occur in MTL regions (Braak & Braak, 1991). Furthermore, studies of brain changes using PET have not unequivocally demonstrated the characteristic temporal-parietal hypoperfusion or hypometabolism in all cases of early AD (Azari et al., 1993).
DISCUSSION
This literature review reveals that a preclinical phase of detectable cognitive decline and structural and functional brain changes precedes the clinical diagnosis of AD by several years or more. Declines in attention, episodic memory, atrophy in medial temporal lobe structures, and/or hypoperfusion in temporoparietal areas appear to be the most common markers of preclinical AD. In contrast to other reviews focusing solely or partially on MCI subjects (Bäckman et al., 2005; Wolf et al., 2003), we found relatively greater evidence for deficits in attention and more evidence for early differences in parietal and posterior cingulate volumes. Bäckman and colleagues' (2005) review found the largest effect for global cognitive measures [e.g., the Mini-Mental State Examination (MMSE) or the Dementia Rating Scale (DRS)], also reported that the effect size confidence intervals overlapped for the domains of episodic memory, executive functioning, and perceptual speed, and found moderate effect sizes for attention tests. Also consistent with our review, Greenwood et al. (2005a, 2005b) demonstrated deleterious effects of the APOE ε4 allele on experimental tests of visuospatial attention, and Jacobson and colleagues (2005a) have shown that performance discrepancies between auditory and spatial attention are associated with presence of the APOE ε4 allele in older adults. Together with this emerging literature, our review suggests that episodic memory is certainly not the only marker of preclinical AD. Using novel measures from cognitive neuroscience or comparing standardized neuropsychological measures in novel ways (e.g., cognitive asymmetry calculations) will be important new directions (Houston et al., 2005; Jacobson et al., 2005b).
Neuroimaging studies have predominantly focused on the temporal lobe, but newer methods examining, for example, the whole brain (e.g., voxel-based morphometry), amyloid imaging (Wu et al., 2005), or diffusion imaging (Medina et al., 2005), are suggesting other areas of interest in preclinical AD. Although neurofibrillary tangle pathology spreads from medial temporal to association cortices (Braak & Braak, 1991), the amyloid plaque burden is more widely dispersed (Arnold et al., 1991), variable in progression, and may also mediate the association of genetic risk to cognition (Bennett et al., 2005). Consistent with this early heterogeneity, AD may initially present with cognitive (Jacobson et al., 2002) or metabolic (Haxby et al., 1985) asymmetries. Recent MR morphometric studies also show that changes sensitive to progression to AD appear asynchronous across brain regions and are more pronounced with global indices, such as whole brain atrophy and ventricular enlargement rates, than with MTL structures (Gunter et al., 2003; Jack et al., 2005; Kaye et al., 2005). Thus, it may be that many possible brain regions are susceptible, and earlier than previously thought, in preclinical AD. The neuropsychological and neuroimaging studies to date argue for comprehensive measurement of cognitive domains and brain regions in future studies.
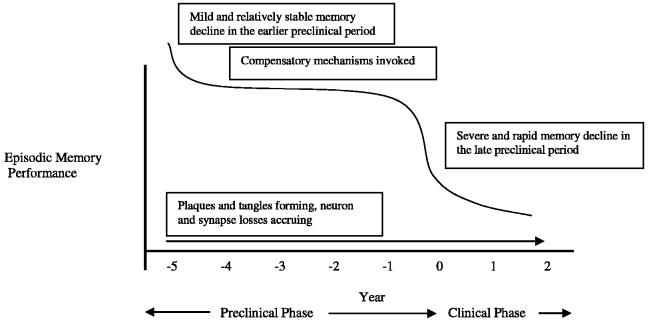
The cognitive and neuroimaging changes of incipient AD appear to remain relatively stable until a few years before clinical diagnosis, when there is a more notable decline. The mild course of decline in the early preclinical period may reflect the initial invocation of compensatory brain mechanisms, whereas the more rapid decline of the clinical period may reflect the inability of these brain resources to overcome the accrual of plaques, tangles, and neuron and synapse losses. A growing body of fMRI evidence among at-risk persons supports this notion (Bookheimer et al., 2000; Bondi et al., 2005; Dickerson et al., 2005; Han et al., 2006; Johnson et al., 2004), and a similar compensatory response in brain-derived neurotrophic factors (Durany et al., 2000; Egan et al., 2003) or cholinergic activity (DeKosky et al., 2002) may also occur. Given these converging lines of evidence for brain compensation, we propose a nonlinear model of episodic memory decline and neuroimaging changes to characterize this preclinical period of accruing AD pathology (see Figure 1). Consistent with this model, Martins et al. (2005) have demonstrated that APOE ε4 possession is associated with earlier and faster cognitive decline in patients with AD, whereas the ε2 allele is related to slower decline, and that a nonlinear model best predicts these differential rates of decline.
Fig. 1.
Proposed model of nonlinear pattern of episodic memory decline during the preclinical period of Alzheimer's disease (based on data of Bäckman et al., 2001; Bunce et al., 2004; Chen et al., 2001; Lange et al., 2002).
We believe this review contributes to the field in four major ways. First, it provides the only comprehensive review of both neuropsychological and neuroimaging indicators of preclinical AD. Second, it addresses a significant flaw of previous studies or review articles by excluding studies of subjects with MCI, AAMI, or memory complaints. In doing so, it concludes that if one is looking for preclinical AD, one needs to look beyond memory and the temporal lobe. Third, from the extant literature we present a model of cognitive decline showing that there appears to be an accelerated and nonlinear decline in the period immediately preceding AD diagnosis. Fourth, we provide both evidence for the often conflicting presentation of AD changes in brain and cognition during the preclinical period, as well as possible explanations for these wide-ranging findings across the literature.
Our methodology for the present review has limitations. Although memory deficits are the hallmark of AD, we did not assume that memory declines would be the foremost marker of preclinical AD, and wanted to avoid “stacking the deck” in favor of memory. Thus, we excluded articles on MCI, AAMI, and memory clinic populations to focus specifically on normal older adults or younger people at risk for AD. However, some studies recruited participants via advertisements rather than population sampling, and some of the participants in the studies we reviewed may have met criteria for MCI or AAMI or may have had memory complaints. A second and related limitation is that by excluding research on memory-impaired populations, the present review may over-represent certain groups (e.g., APOE ε4 carriers) whose cognitive profiles may not characterize those of the general population. For this reason, we separated the APOE studies from the others in our review. We focused on neuropsychological and neuroimaging tests, excluding other potentially promising methodologies without substantial published literature (e.g., olfactory testing, Murphy et al., 1998). Finally, although a formal meta-analysis that would have weighted each study based on the number of participants, we chose to use a “box score” approach to examining the findings of the included studies, due to considerable heterogeneity in study methodologies and measures.
Although there have been many exciting developments in this growing area of research, conclusions about the nature and course of preclinical AD remain limited by contradictory findings in at-risk groups and/or studies that rely on retrospective or cross-sectional designs or small datasets. To improve detection of preclinical markers, the neuropsychological functioning, brain structure, and brain functioning of at-risk individuals who develop AD should ultimately be tested against that of persons who remain dementia-free over the same follow-up period. Furthermore, they might be compared to individuals with other conditions (e.g., depression, other dementias) and validated by autopsy-confirmed diagnosis.
In addition to documenting the cognitive deficits in preclinical AD, the longitudinal course of these deficits is also important. There has been a lack of consensus regarding when the preclinical period begins and how early preclinical changes may be detected. Most investigations have used relatively short test-retest intervals (e.g., two to three years after initial assessment), but other studies have used longer intervals (decades or more). With longer test-retest intervals, cognitive changes are more likely to be detected, but it is more difficult to determine when decline began. With some groups finding differences between AD cases and controls five to six decades before the onset of AD (see Snow-don et al., 1996 and Whalley et al., 2000), future research should address the question of the age range in which it is possible to detect a preclinical AD state and whether such states can be distinguished from lower levels of cognitive reserve.
With respect to fMRI studies of at-risk groups, contradictory findings across studies [e.g., decreased vs. increased blood oxygen level dependent (BOLD) responses] may reflect different mechanisms linking the hemodynamic response to its underlying neuroanatomy and neurophysiology. One concern is that differences in the “resting state” will influence the amplitude of the BOLD response (Cohen et al., 2002). If the AD brain is in a lower resting state, as suggested by Reiman et al. (1996), it should show an increased hemodynamic response compared with a non-AD brain in the context of equal stimulation. The BOLD signal must be calibrated to the resting state to prevent over-interpretation of greater BOLD responses as signifying heightened compensatory responses. Recent research using APP23 transgenic mice has demonstrated that amyloid plaques have a direct effect on the hemodynamic response, due partly to compromised cerebrovascular reactivity (Mueggler et al., 2002). Human studies also demonstrate that the hemodynamic response itself changes with age (D'Esposito et al., 1999). Thus, future efforts should incorporate other MR-based techniques such as perfusion imaging and structural morphometry to help delineate the contributions of the neuroanatomic and neurophysiologic underpinnings of the BOLD signal.
It remains that there is still no single marker of AD. Combined cognitive, imaging, and genetic assessments may ultimately be needed to achieve accurate and reliable identification of preclinical AD (Albert, 1996; Small et al., 1996). Detecting preclinical AD will probably be best accomplished by examining decline longitudinally with sensitive cognitive tests and structural or functional markers of brain integrity. Early detection may be enhanced if risk factors such as advancing age or the presence of the APOE ε4 allele (Mayeux et al., 1998) are also considered.
The ability to detect AD in its earliest, preclinical phase will continue to be an important topic of neuropsychological and neuroimaging research. The rapid development of neuroprotective agents designed to impede the progression of the disease is a testament to the recognized importance of early identification and treatment of AD (DeLaGarza, 2003). Moreover, preserving cognitive status and functional independence with more effective interventions will have far-reaching implications for maintaining patients' quality of life and decreasing caregivers' financial and emotional burden (Brookmeyer et al., 1998).
ACKNOWLEDGMENTS
This work was supported by NIMH grants K23 MH066011, T32 MH019934, and NIA grants R01 AG012674 and P50 AG005131.
APPENDIX 1
Neuropsychological Studies
| Part A. Prospective longitudinal studies of initially nondemented subjects, comparing cases (subjects who developed AD) and controls (subjects who remained nondemented) | ||||
|---|---|---|---|---|
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Tests Used | Results |
| Bäckman and Small (1998) | 24 cases 134 controls 3-year study |
Cases A = 84 G = 4% male E = 8 Controls A = 83 G = 18% male E = 9 |
MMSE Immediate memory tasks involving: 1. Free recall of slowly and rapidly presented unrelated words 2. Free and cued recall of related words |
At baseline, cases performed worse on: MMSE Free and cued recall (regardless of speed of presentation or relatedness of words) |
| Bäckman et al. (2001) | 15 cases 105 controls 6-year study |
Cases A = 84 G = 7% male E = 9 Controls A = 82 G = 20% male E = 10 |
MMSE Free recall and recognition of slowly presented unrelated words WAIS-R Digit Span |
At baseline and 3-year follow-up, cases performed worse on: MMSE Free recall and recognition These results held after controlling for age, education, and gender Cases and controls had similar slopes of decline |
| Bondi et al. (1994) | 5 cases 51 controls 28/56 subjects were FH+ 3-year study Subjects also were compared to 25 patients with probable AD at baseline Cross-sectional results from baseline testing reported below |
FH+: A = 70 G = 36% male E = 16 FH−: A = 71 G = 36% male E = 15 AD at follow-up: A = 74 G = 40% male E = 15 AD at baseline: A = 71 G = 56% male E = 14 |
CVLT MMSE DRS Number Information Test (general knowledge) Letter and category fluency BNT WAIS-R (Digit Span, Vocabulary, Arithmetic, Similarities, Digit Symbol) WISC-R Block Design Trail Making Test, Parts A and B Clock Drawing Test Clock Setting Test Grooved Pegboard Test Modified WCST WMS-R (Visual Memory Span, Logical Memory) SRT |
Cases performed worse than controls but better than AD patients on: CVLT learning and delayed recall measures MMSE DRS Total Category Fluency WAIS-R Digit Symbol Logical Memory I SRT Total Cases performed worse than controls and comparably to AD patients on: CVLT learning and delayed recall measures DRS Conceptualization Letter Fluency WAIS-R Similarities Logical Memory II SRT Recall |
| Bondi et al. (1999) | 7 cases 79 controls 23 ε4+ 63 ε4− Mean length of follow-up wa 3 years Sample included 52 subjects from Bondi et al. (1995) Cross-sectional results from baseline testing reported below |
ε4+ A = 68 G = 47% male E = 15 ε4− A = 70 G = 43% male E = 15 |
MMSE DRS CVLT WMS Visual Reproduction WAIS-R (Digit Span, Digit Symbol, Vocabulary) WISC Block Design BNT Letter fluency Trail Making Test, Parts A and B Modified WCST |
Cases performed worse on: CVLT List A Total Analyses controlled for age and education |
| Bunce et al. (2004) | 162 cases 373 controls 161 ε4+ 374 ε4− 6-year study |
ε4+ A = 80 G = 16% male E = 8 ε4− A = 80 G = 25% male E = 9 |
MMSE | Cases at 3-year follow-up and at 6-year follow-up exhibited greater declines on MMSE Decline was most marked in the 3 years prior to diagnosis E4 status did not predict rate of decline or AD diagnosis over time |
| Chen et al. (2000) | 120 cases 483 controls 10-year study |
Cases A = 78 G = 43% male E = 50% less than high school Controls A = 75 G = 38% male E = 32% less than high school |
CERAD word list learning and memory test WMS Logical Memory Trail Making Test, Parts A and B Letter and category fluency CERAD version of BNT CERAD Praxis Clock Drawing MMSE |
Cases performed worse on: Word List Delayed Recall Word List learning measures Trail Making Test, Part B MMSE |
| Chen et al. (2001) | 68 cases 483 controls 10-year study Same sample as above, examined change between assessments 3.5 and 1.5 years before AD diagnosis |
Cases A = 77 G = 43% male E = 47% less than high school Controls A = 73 G = 38% male E = 32% less than high school |
CERAD word list learning and memory test WMS Logical Memory Trail Making Test, Parts A and B Letter and category fluency CERAD version of BNT CERAD Praxis Clock Drawing MMSE Orientation |
Cases declined most on: Trail Making Test, Part A Trail Making Test, Part B Word List Delayed Recall and Recognition measures Word List learning measures CERAD Praxis Clock Drawing CERAD Boston Naming Test MMSE Orientation |
| Dartigues et al. (1997) | 59 cases 2667 controls 3-year study |
A = 75 G = 40% male E = mostly grade school |
MMSE BVRT Isaaks Set Test (category fluency) |
Cases performed worse on: MMSE BVRT Isaaks Set Test All three tests were independent predictors of conversion to AD even after adjusting for age and education |
| Elias et al. (2000) | 109 cases 967 controls 22-year study controlling for age, education, occupation level, and gender |
Cases G = 21% male Controls G = 39% male A: 65–94 E: Majority of subjects had ≥HS |
WMS (Logical Memory, Paired Associate Learning, Visual Reproduction) COWAT WAIS (Similarities, Digit Span) MMSE |
Cases performed worse on: Logical Memory (% retained) Similarities Paired Associate Learning WMS Learning and Immediate Recall Among an older (age 75–94), but not a younger (age 65–74) cohort, lower COWAT scores were associated with later AD diagnosis |
| Fabrigoule et al. (1998) | 16 cases 1143 controls 2-year study |
A = 73 G = 44% male E = 56% grade school educated, remainder secondary or university education |
MMSE BVRT WMS Paired Associates Test Isaacs Set Test (category fluency) Zazzo's Cancellation Task (processing speed) WAIS (Digit Symbol, Similarities) |
Cases performed worse on: BVRT WAIS Digit Symbol Isaacs Set Test Zazzo's Cancellation Task Wechsler Paired Associates Test WAIS Similarities MMSE |
| Fox et al. (1998) | 10 cases 53 controls All subjects at risk for autosomal dominant familial AD 6-year study |
A = 45 G = 42% male E = NR |
MMSE WAIS-R (Vocabulary, Arithmetic, Digit Span, Similarities, Block Design, Picture Completion, Picture Arrangement) Recognition Memory Test (words and faces) Graded Naming Test Visual Object and Spatial Perception Test Psychomotor Speed Tests Graded Difficulty Arithmetic Test Graded Difficulty Spelling Test NART |
Cases performed worse on: Recognition Memory Test (words) WAIS-R Block Design, Picture Completion, and Picture Arrangement |
| Grober and Kawas (1997) | 20 cases 60 controls 3-year study |
Cases A = 79 G = 45% male E = 17 Controls A = 79 G = 48% male E = 17 |
SRT | Cases performed worse on: SRT learning measures |
| Hall et al. (2000) | 35 cases 293 controls 15-year study |
Cases A = 80 G = NR E = NR Controls A = 80 G = NR E = NR |
SRT learning | Cases performed worse on: SRT learning |
| Howieson et al. (1997) | 16 cases 31 controls Mean length of follow-up was 2.8 years |
Cases A = 90 G = 44% male E = 15 Controls A = 85 G = 42% male E = 14 |
CERAD version of BNT WAIS-R (Vocabulary, Picture Completion, Block Design) CERAD Word List Memory Test WMS-R Logical Memory and Visual Reproduction |
Cases performed worse on: Boston Naming Test Logical Memory I and II Block Design CERAD Word List Delayed Recall Picture Completion Visual Reproduction I |
| Jacobs et al. (1995) | 41 cases 402 controls 4-year study |
Cases A = 79 G = 22% male E = 8 Controls A = 73 G = 27% male E = 11 |
SRT BVRT MMSE Orientation items WAIS-R Similarities subtest DRS Identities and Oddities BNT (15 item version) Letter and category fluency BDAE Complex Ideational Material Rosen Drawing Test (visuoconstruction) Visual matching test (visuoperception) |
Cases performed worse on: Boston Naming Test SRT Immediate Recall WAIS-R Similarities Analyses controlled for age, education, sex, and language of test administration |
| Katzman et al. (1989) | 32 cases 402 controls 5-year study |
Cases A = 81 G = 16% male E = modal 7–9 Entire sample A = 79 G = 36% male E = modal 7–9 |
Blessed Information-Memory- Concentration Test |
Cases performed worse on: Blessed Information-Memory- Concentration Test Those with 0–2 errors developed AD at a rate less than 0.6% per year, whereas those with 5–8 errors developed AD at a rate of over 12% per year |
| Klages et al. (2003) | 27 cases 182 controls 5-year study |
A = 77 G = 38% male E = 10 |
SRT | Cases performed worse on: SRT (delayed free recall) |
| Laukka et al. (2004) | 43 cases 149 controls 6-year study |
A = 84 G = 16% male E = 9 |
WAIS-R Digit Span Episodic Memory (Random Recall, Organized Recall, Word Recognition, Face Recognition) Visuospatial Ability (WAIS-R Block Design, Clock Reading, Clock Setting) Letter and category fluency |
Cases performed worse on: All episodic memory measures Fluency measures WAIS-R Block Design Clock Setting |
| Lindeboom et al. (2002) | 24 cases 204 controls 3-year study |
Cases A = 79 G = 25% male E = completed primary education Controls A = 73 G = 45% male E = completed primary education |
MMSE | Cases performed worse on: MMSE |
| Lindsay et al. (2002) | 194 cases 3894 controls 5-year study |
Cases A = 81 G = 32% male E = 10 Controls A = 73 G = 42% male E = 11 |
3MS Examination | Cases performed worse on: 3MS |
| Linn et al. (1995) | 55 cases 990 controls 13-year study |
Cases A = 76 G = 29% male Controls A = 72 G = 38% male E = Majority of subjects had ≥HS |
WMS (Logical Memory, Visual Reproduction, Paired Associate Learning, Digit Span) COWAT WAIS Similarities |
Cases performed worse on: Logical Memory I and II and percent retained Visual Reproduction I Paired Associate Learning Controlled Oral Word Association Similarities Analyses controlled for age and education |
| Masur et al. (1994) | 64 cases 253 controls Minimum length of follow-up was 4 years |
Cases A = 80 G = 41% male E = modal 7–9 Controls A = 79 G = 39% male E = modal 7–x9 |
WAIS (Information, Vocabulary, Similarities, Digit Span, Block Design, Object Assembly, Digit Symbol) Fuld Object Memory Evaluation SRT Category fluency Raven's Colored Matrices Purdue Pegboard Test |
Cases performed worse on: SRT delayed recall Fuld Object Memory recall Category Fluency WAIS Digit Symbol |
| Nielsen et al. (1999) | 102 cases 2350 controls 2-year study |
A = 65–84 G = NR E = NR |
CAMCOG: Orientation Comprehension Naming Category fluency Definitions Memory Recognized pictures General knowledge Attention Copying Ideomotor praxis Abstraction Visual perception |
Cases performed worse on: Category fluency Memory General knowledge Attention |
| Rapp and Reischies (2005) | 15 cases 172 controls 4-year study |
A = 80 G = 50% male E = 11 |
MMSE Trail Making Test, Part B Digit Letter Test (processing speed) WAIS Digit Symbol WMS Paired Associate Learning Identical Pictures (attention) Memory for Text (learning) Activity Recall (recall of tests given) |
Cases performed worse on: Trail Making Test, Part B Digit Letter Test Digit Symbol Substitution Test Identical Pictures Paired Associates Test Memory for Text Activity Recall After controlling for age differences between groups, cases performed worse on: Identical Pictures Trail Making Test, Part B |
| Saxton et al. (2004) | 72 cases 621 controls Study combined data from 3 separate prospective studies with 8 years of follow-up |
A = 73 G = 44% male E = 13 |
WMS-R Orientation WMS-R Immediate Memory (Verbal, Visual, General) WMS-R Delayed Memory Speed/Attention (WMS-R Attention/ Concentration, WAIS-R Digit Symbol, Trail Making Test, Parts A and B) Verbal Productivity/Vocabulary (WAIS-R Vocabulary, Letter and category fluency) BNT WAIS-R Block Design |
For 1.5–3.4 year follow-up, cases performed worse on: WMS-R Verbal WMS-R Visual WMS-R General WMS-R Delayed WMS-R Attention/Concentration Trail Making Test, Parts A and B Category Fluency Boston Naming Test For 3.5–5 year follow-up, cases performed worse on: WMS-R General WMS-R Delayed Trail Making Test, Part B Category Fluency For 5.1–8 year follow-up, cases performed worse on: WMS-R Verbal WMS-R Delayed |
| Small et al. (1997b) | 32 cases 189 controls 3-year study |
A = 84 G = 19% male E = 9 |
MMSE | Cases performed worse on: MMSE (particularly delayed recall and orientation to time) |
| Small et al. (1997a) | 26 cases 179 controls 3-year study |
Cases A = 85 G = 12% male E = 9 Controls A = 83 G = 21% male E = 9 |
MMSE Face Recognition Task Free Recall and Recognition of Random and Organizable Words Letter and category fluency Poppelreuter's Figures (visual perception) Clock Test WAIS-R (Block Design, Digit Span) |
Cases performed worse on: MMSE Face Recognition Task Free Recall and Recognition of Random and Organizable Words Letter and Category Fluency Poppelreuter's Figures Clock Test WAIS-R Block Design |
| Small et al. (2000a) | 73 cases 459 controls 6-year study |
Cases A = 82 G = 21% male E = 8 |
MMSE | Cases performed worse on: MMSE delayed memory item |
| Cases were those diagnosed with AD at 6-year, but not 3-year, follow-up |
Controls A = 79 G = 23% male E = 9 |
|||
| Yoshitake et al. (1995) | 42 cases 784 controls 7-year study |
A = 74 G = 40% male E = 26% had ε6 |
Hasegawa Dementia Scale (11-item mental status exam including orientation, memory, common knowledge, and calculation) |
Cases performed worse on: Hasegawa Dementia Scale |
| Zonderman et al. (1995) | 7 cases 364 controls Longitudinal study since 1960 with testing every 6–8 years |
A = 72 G = 68% male E = mostly HS or college educated |
BVRT | Cases performed worse on: BVRT learning Cases declined more rapidly on the BVRT in the 6 years before diagnosis of AD |
| Part B. Prospective longitudinal studies examining differential cognitive decline in ε4+ and ε4− subjects | ||||
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Tests Used | Results |
| Baxter et al. (2003) | 54 ε4+ 59 ε4− 2-year study |
ε4+ A = 58 G = 33% male E = 16 |
RAVLT COWAT |
ε4+ subjects over age 60 declined in their novel word learning ability |
| ε4− A = 59 G = 37% male E = 16 |
||||
| Bretsky et al. (2003) | 227 ε4+ 738 ε4− 7-year study |
A = 74 G = 44% male E = NR |
Overall cognitive performance: 4 items from WAIS-R Similarities Delayed spatial recognition BNT (18-item version) Spatial ability Memory: Delayed verbal and visual memory Story recall |
At 3-year follow-up, ε4+ subjects declined in naming and spatial ability At 7-year follow-up, ε4+ subjects declined in overall cognitive performance, naming, spatial ability, abstraction, and verbal and visual memory |
| Cohen et al. (2001) | 16 ε4+ 9 ε4− 2-year study Groups also compared on structural MRI (see findings reported below) |
ε4+ A = 55 G = 0% male E = NR ε4− A = 61 G = 0% male E = NR |
SRT free recall Letter and category fluency Rey CFT WMS-R WAIS-R Block Design |
No significant group differences |
| Dik et al. (2000) | 213 ε4+ 653 ε4− Mean length of follow-up was 3.1 years |
A = 72 G = 51% male E = 9 |
MMSE (baseline only) Alternate forms of an abbreviated (3-trial) RAVLT |
At baseline, ε4+ and ε4− subjects did not differ on the MMSE At follow-up, ε4+ subjects over 75 per- formed better on RAVLT immediate recall |
| Ercoli et al. (2003) | 23 ε4+ 31 ε4− 2-year study |
ε4+ A = 65 G = 39% male E = 15 ε4− A = 66 G = 42% male E = 15 |
MMSE MMSE subset items (delayed 3-item recall; serial 7s; intersecting pentagons; time and place orientation) |
No significant group differences For ε4+ subjects, lower baseline scores on the MMSE subset items predicted decline in visual construction and naming |
| Helkala et al. (1996) | 192 ε4+ (including ε2/ε4) 378 ε3/ε3 62 ε2+ 3-year study |
A = 73 G = 35% male E = 7 |
MMSE SRT (total score) Visual Reproduction test (immediate, delay, copy) Letter and category fluency Trail Making Test, Parts A and B |
At follow-up, ε2+ subjects performed better than ε4+ and ε3/ε3 subjects on: SRT Letter fluency |
| Hofer et al. (2002) | 94 ε4+ 340 ε4− 7-year study |
A = 76 G = 49% male E = 12 |
Verbal ability (Vocabulary, Similarities, NART items) Memory (word recognition test, 3 word recall with 2 minute delay, address recall with 2 minute delay) Speed (symbol-letter modalities test) |
ε4+ subjects declined on: Memory |
| Jonker et al. (1998) | 25 ε4+ 292 ε4− 3-year study |
A = 75 G = 43% male E = 8 |
CAMCOG (total score, memory and nonmemory subscales) |
ε4+ subjects declined on: CAMCOG total score memory subscale nonmemory subscale |
| Mayeux et al. (2001) | 80 ε4+ 483 ε4− 7-year study |
A = 76 G = 31% male E = 10 |
Visuospatial/Cognitive factor (Rosen Drawing Test, BVRT, DRS Identities and Oddities) Language factor (BNT, COWAT, WAIS-R Similarities) Memory factor (seven subtests of the SRT) |
ε4+ subjects declined faster on: Memory |
| Pendleton et al. (2002) | 201 ε4+ 566 ε4− 15-year study |
A = modal 60–69 G = 30% male E = NR |
Heim AH4 part 1 (fluid general intelligence) |
No significant group differences |
| Reiman et al. (2001) | 10 ε4+ 15 ε4− All subjects had a family history of AD 2-year study Groups also compared on PET (see findings reported below) |
ε4+ A = 56 G = 30% male E = 15 ε4− A = 57 G = 33% male E = 16 |
MMSE RAVLT CFT BNT WAIS-R (Information, Digit Span, Block Design, Arithmetic, Similarities) COWAT |
No significant group differences |
| Riley et al. (2000) | 34 ε4+ 207 ε4− All subjects were nuns 4-year study |
A = 81 G = 0% male E = 17 |
CERAD: MMSE Delayed word recall Verbal fluency BNT Constructional praxis |
ε4+ subjects declined on: MMSE Delayed word recall |
| Small et al. (1998) | 20 ε4+ 54 ε4− 3-year study Cross-sectional results from baseline testing reported below |
ε4+ A = 80 G = 25% male E = 10 E4− A = 82 G = 30% male E = 10 |
MMSE Face Recognition Task Free Recall and Recognition of Random and Organizable Words Letter and category fluency Poppelreuter 's Figures Clock Test WAIS-R (Block Design, Digit Span) |
ε4+ subjects declined on: Recognition memory for faces and words |
| Wilson et al. (2002) | 161 ε4+ 450 ε4− 6-year follow-up Cross-sectional results from baseline testing reported below |
A = 76 G = 38% male E = 18 |
Episodic memory (CERAD Word List Memory, Recall, and Recognition, Immediate and Delayed Story Memory, WMS-R Logical Memory Story A) Semantic memory (BNT, Verbal Fluency, Extended Range Vocabulary, NART) Working memory (WMS-R Digit Span, Digit Ordering, Alpha Span) Perceptual speed (Symbol Digit Modalities Test, Number Comparison) Visuospatial ability (Judgment of Line Orientation, Raven's Standard Progressive Matrices) |
ε4+ subjects declined on all cognitive domains, with the most decline on episodic memory |
| Winnock et al. (2002) | 130 ε4+ 470 ε4− 7-year study Cross-sectional results from baseline testing reported below |
A = 74 G = 76% male E = mostly primary education or above |
MMSE | No significant group differences in decline |
| Yaffe et al. (before 1997) | 271 ε4+ 1479 ε4− 6-year study Cross-sectional results from baseline testing reported below |
A = 71 G = 0% male E = 12 |
MMSE Trail Making Test, Part B WAIS-R Digit Symbol |
ε4+ subjects declined on all cognitive tests |
| Part C. Cross-sectional studies comparing neuropsychological profiles of healthy, nondemented ε4+ and ε4− subjects | ||||
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Tests Used | Results |
| Albert et al. (1995) | 60 ε4+ 158 ε4− |
A = 74 G = 26% male E = 8 |
SRT (total recall and delayed recall) | No significant group differences |
| Berr et al. (1996) | 270 ε4+ 904 ε4− |
A = 65 G = 42% male E = 11 |
MMSE Trail Making Test, Part B RAVLT Benton Face Recognition Test WAIS-R Digit Symbol Finger Tapping Test BVRT Raven's Progressive Matrices PASAT Word Fluency Test |
After adjusting for age, education, and gender, the number of ε4 alleles was associated with worse performance on: MMSE Trail Making Test, Part B Finger Tapping Test |
| Bondi et al. (1999) | 43 ε4+ 90 ε4− Sample included 52 subjects from Bondi et al. (1995) Longitudinal results reported above |
ε4+ A = 68 G = 47% male E = 15 ε4− A = 70 G = 43% male E = 15 |
MMSE DRS CVLT WMS Visual Reproduction WAIS-R (Digit Span, Digit Symbol, Vocabulary) WISC Block Design BNT Letter fluency Trail Making Test, Parts A and B Modified WCST |
ε4+ subjects performed worse on: WCST nonperseverative errors CVLT learning and memory measures |
| Caselli et al. (1999) | 25 ε4/ε4 25 ε4 heterozygotes 50 ε4− All subjects were first-degree relatives of AD patients |
ε4/ε4 A = 56 G = 28% male E = 16 ε4 heterozygotes A = 56 G = 28% male E = 15 |
COWAT WAIS-R (Arithmetic, Digit Span, Similarities, Block Design, Information) WMS-R Orientation RAVLT BNT CFT BVRT |
No significant group differences, whether all three groups were compared or whether ε4+ subjects were compared with ε4− subjects |
| ε4− A = 57 G = 28% male E = 15 |
||||
| Caselli et al. (2001) | 20 ε4/ε4 20 ε4 heterozygotes 40 ε4− All subjects were first-degree relatives of AD patients |
ε4/ε4 A = 57 G = 40% male E = 16 ε4 heterozygotes A = 56 G = 40% male E = 16 |
COWAT WAIS-R (Arithmetic, Digit Span, Similarities, Block Design, Information) WMS-R Orientation RAVLT BNT CFT BVRT |
ε4 heterozygotes performed worse than did ε4− subjects on: Complex Figure Test Recall |
| ε4− A = 56 G = 40% male E = 16 |
||||
| Caselli et al. (2002) | 42 ε4/ε4 38 ε4− ε4/ε4 subjects were matched to ε4− subjects on age, gender, education, family history of dementia |
A = 55 G = 31% male E = 16 |
MMSE RAVLT BVRT Rey-Osterrieth CFT WAIS-R (Information, Digit Span, Arithmetic, Similarities, Block Design) Letter fluency BNT |
ε4/ε4 subjects performed worse than did ε4− subjects on: RAVLT learning Similarities |
| Chen et al. (2002) | 72 ε4+ 81 ε4− |
A = 67 G = 50% male E = 16 |
MMSE CVLT |
No significant group differences |
| Deary et al. (2004) | 120 ε4+ 342 ε4− All subjects born in 1921 |
A = 79 G = 41% male E = NR |
MMSE WMS-R Logical Memory Raven's Progressive Matrices Letter fluency |
ε4+ subjects performed worse on: Logical Memory I and II |
| Fillenbaum et al. (2001) | 548 ε4+ 1343 ε4− Longitudinal study with applicable cross-sectional data at baseline |
A = 77 G = 33% male E = 10 |
Short Portable Mental Status Questionnaire |
ε4+ subjects performed worse on: Short Portable Mental Status Questionnaire |
| Flory et al. (2000) | 61 ε4+ 159 ε4− |
A = 46 G = 51% male E = 16 |
Verbal associative learning Verbal associative learning delayed recall Verbal associative learning recognition CFT delayed recall WAIS-R (Digit Symbol immediate recall, Digit Span) |
ε4+ subjects performed worse on: CFT delayed recall Verbal associative learning |
| Greenwood et al., (2000) | 38 ε4+ 48 ε3/ε3 11 ε2+ |
A = 59 G = 39% male E = 17 |
Cued letter discrimination task Cued visual search task Vigilance task |
No significant group differences in accuracy or reaction time on any task ε4+ subjects had slower reaction times to invalid cues than did other groups on the cued letter discrimina- tion task and had reduced spatial scaling of attention on the cued visual search task |
| Greenwood et al. (2005) | 64 ε4+ 113 ε4− Cross-sectional study examining ε4 “dose” |
A = 59 G = 42% male E = 17 |
Spatial cued letter discrimination task Spatial working memory task |
ε4 dose was associated with impair- ment in: Redirecting visuospatial attention to unexpected locations Retaining locations in working memory Using attentional scaling to enhance spatial working memory |
| Kim et al. (2002) | 74 ε4+ 392 ε4− |
A = 70 G = 27% E = 6 |
Korean version of CERAD: MMSE Verbal fluency test Modified BNT Word list memory test Word list recall test Word list recognition test Constructional praxis test Constructional recall test |
No significant group differences |
| Levy et al. (2004) | 61 ε4+ 115 ε4− |
A = 59 G = 36% male E = 17 |
Prose Recall (WMS-R Logical Memory I and II, percent retained) Word Recall (SRT, WMS-R Verbal Paired Associates I and II) Design Recall (WMS-R Visual Reproduction I and II, percent retained, CFT 3 minute recall) Visuospatial Ability (CFT copy, WAIS-R Block Design, Digit Symbol) Language (Letter and category fluency, BNT) |
ε4+ subjects performed worse on: Logical Memory II Logical Memory percent retained |
| Reed et al. (1994) | 40 dizygotic twins (20 pairs) discordant for presence of the ε4 allele Cross-sectional study comparing education- adjusted scores on neuropsychological tests in twins discordant for ε4 |
A = 63 G = 100% male E = NR |
BVRT COWAT MMSE WAIS-R Digit Symbol Modified version of the Telephone Interview for Cognitive Status |
ε4+ twins performed worse than their ε4− co-twins on: BVRT |
| Rosen et al. (2002) | 21 ε4+ 21 ε4− |
A = 62 G = 43% male E = 17 |
Operation Span Task (working memory) SRT |
ε4+ subjects performed worse on: Operation Span Task |
| Salo et al. (2001) | 12 ε4+ 34 ε4− |
A = 89 G = 48% male E = 4 |
MMSE Fuld Object Memory Evaluation Letter and category fluency WAIS-R Similarities |
No significant group differences |
| Schmidt et al. (1996) | 39 ε4+ 175 ε4− Groups also compared on structural MRI (see findings reported below) |
ε4+ A = 59 G = about 50% male E = 11 ε4− A = 61 G = about 50% male E = 12 |
German verbal and visual learning and memory test German cancellation (attention and speed) test German complex reaction time test WCST Trail Making Test, Part B WAIS-R Digit Span Purdue Pegboard Test |
ε4+ subjects performed worse on: Verbal and visual memory |
| Small et al. (1998) | 20 ε4+ 54 ε4− Longitudinal results reported above |
ε4+ A = 80 G = 25% male E = 10 ε4− A = 82 G = 30% male E = 10 |
MMSE Face Recognition Task Free Recall and Recognition of Random and Organizable Words Letter and category fluency Poppelreuter's Figures Clock Test WAIS-R (Block Design, Digit Span) |
No significant group differences |
| Small et al. (2000b) | 91 ε4+ 322 ε4− |
A = 73 G = 49% male E = 14 |
3MS Spot-the-Word (premorbid IQ test) Hopkins Verbal Learning Test Word stem Completion Task (implicit memory) Trail Making Test, Parts A and B Stroop Test |
No significant group differences |
| Smith et al. (1998) | 90 ε4+ 251 ε4− |
ε4+ A = 80 G = 30% male E = 14 ε4− A = 80 G = 31% male E = 13 |
Verbal Comprehension (WAIS-R Vocabulary, Information) Perceptual Organization (WAIS-R Block Design, Picture Arrangement, Picture Completion; WMS-R Visual Reproduction I) Attention/Concentration (WMS-R Digit Span, Mental Control; WAIS-R Arithmetic) Learning (RAVLT acquisition; WMS-R Verbal and Visual Paired Associates I) Retention (percent retention for RAVLT, WMS-R Logical Memory, and WMS-R Visual Reproduction) BNT RAVLT delayed recall |
No significant group differences |
| Staehelin et al (1999) | 72 ε4+ 198 ε3/ε3 62 ε2+ (including 11 ε2/ε4) |
A = 76 G = 68% male E = NR |
Reaction Time Delayed free recall WAIS-R Vocabulary (German version) |
ε3/ε3 and ε4+ subjects performed worse than did ε2 subjects on: Reaction Time ε4+ subjects performed worse than did ε3/ε3 on: WAIS-R Vocabulary |
| Tohgi et al. (1997) | 14 ε4+ 40 ε4− Groups also compared on structural MRI (see findings reported below) |
A = 59 G = 52% male E = 12 |
MMSE | No significant group differences |
| Wilson et al. (2002) | 161 ε4+ 450 ε4− Longitudinal results reported above |
A = 76 G = 38% male E = 18 |
Episodic memory (CERAD Word List Memory, Recall, and Recognition, Immediate and Delayed Story Memory, WMS-R Logical Memory Story A) Semantic memory (BNT, Verbal Fluency, Extended Range Vocabulary, NART) Working memory (WMS-R Digit Span, Digit Ordering, Alpha Span) Perceptual speed (Symbol Digit Modalities Test, Number Comparison) Visuospatial ability (Judgment of Line Orientation, Raven's Standard Progressive Matrices) |
ε4+ subjects performed worse on: Episodic memory Visuospatial ability |
| Winnock et al. (2002) | 130 ε4+ 470 ε4− Longitudinal results reported above |
A = 74 G = 76% male E = mostly primary education or above |
MMSE | ε4+ subjects performed worse on: MMSE (but not after controlling for education) |
| Yaffe et al. (1997) | 271 ε4+ 1479 ε4− Longitudinal results reported above |
A = 71 G = 0% male E = 12 |
MMSE Trail Making Test, Part B WAIS-R Digit Symbol |
ε4/ε4 subjects performed worse on: Trail Making Test, Part B |
| Part D. Cross-sectional studies comparing neuropsychological profiles of nondemented subjects with AD-like and normal brains at autopsy | ||||
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Tests Used | Results |
| Goldman et al. (2001) | 5 preclinical AD brains with neuritic and diffuse plaques 11 normal brains |
Preclinical AD brain A = 80 G = 40% male E = 14 Normal brain A = 83 G = 78% male E = 14 |
WMS (Mental Control, Logical Memory, Digit Span, Paired Associate Learning) BVRT WAIS (Information, Block Design, Digit Symbol) Trail Making Test, Part A Crossing-off (processing speed) BNT Letter fluency |
No significant group differences |
| Hulette et al. (1998) | 4 “possible AD” brains per CERAD autopsy criteria 8 normal brains |
Possible AD brain A = 83 G = 50% male E = 15 Normal brain A = 82 G = 50% male E = 17 |
MMSE Letter and category fluency Naming test Constructional Praxis Symbol-Digit Modalities Test Trail Making Test, Parts A and B CERAD Word List Memory WMS Logical Memory BVRT |
Subjects with possible AD at autopsy performed worse on: Memory percent retained Trail Making Test, Parts A and B Category fluency (trends only; sample size too small to permit inferential tests) |
| Schmitt et al. (2000) | 7AD-like brains 52 normal brains |
A = 84 G = 46% male E = 16 |
MMSE Blessed Information-Memory-Concentration Test Temporal Orientation Test WMS (Mental Control, Logical Memory) BVRT Alzheimer's Disease Assessment Scale (word list learning/recognition and design reproduction) COWAT Animal naming BNT WAIS-R (Vocabulary, Digit Symbol) Trail Making Test, Parts A and B |
Subjects with AD-like brains performed worse at the evaluation before death on: Logical Memory I Word list delayed recall |
| Part E. Cross-sectional studies comparing neuropsychological profiles of healthy, nondemented, FH+ and FH− subjects | ||||
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Tests Used | Results |
| Bondi et al. (1994) | 5 cases 51 controls 28/56 subjects were FH+ Longitudinal results reported above |
FH+: A = 70 G = 36% male E = 16 FH−: A = 71 G = 36% male E = 15 |
CVLT MMSE DRS Number Information Test (general knowledge) Letter and category fluency BNT WAIS-R (Digit Span, Vocabulary, Arithmetic, Similarities, Digit Symbol) WISC-R Block Design Trail Making Test, Parts A and B Clock Drawing Test Clock Setting Test Grooved Pegboard Test Modified WCST WMS-R (Visual Memory Span, Logical Memory) SRT |
FH+ subjects performed worse on: CVLT learning and delayed recall measures |
| Diaz-Olavarrieta et al. (1997) | 14 FH+ (from FAD families) 14 FH− Cognitive test scores were averaged over two evaluations in a one-year period |
FH+ A = 39 G = 30% male E = 11 FH− A = 38 G = 71% male E = 11 |
MMSE Digit Span Corsi cubes WMS (Orientation, Mental Control) Immediate verbal memory Paired word association Verbal learning curve Rey-Osterreith CFT (immediate and delayed recall) Block Design BNT Letter and category fluency |
No significant group differences |
| Hom et al. (1994) | 20 FH+ (first-degree relatives of AD probands) 20 FH− |
FH+ A = 55 G = 15% male E = 14 FH− A = 56 G = 15% male E = 12 |
WAIS WMS-R (Logical Memory, Visual Reproduction) Halstead Category Test Tactual Performance Test Seashore Rhythm Test Speech-Sounds Perception Test Reitan-Indiana Aphasia Screening Examination |
FH+ subjects performed worse on: WAIS Verbal IQ Seashore Rhythm Test Logical Memory I WAIS Information Halstead Impairment Index |
| Schiffman et al. (2002) | 33 FH+ 32 FH− |
A = 61 G = 54% male E = 15 |
MMSE CERAD battery WMS-R Logical Memory BVRT COWAT Trail Making Test, Parts A and B Symbol Digit Modalities Test Taste Detection Threshold, Memory, Recognition, Identification Smell Detection Threshold, Memory, Recognition, Identification |
FH+ subjects performed worse on: Logical Memory I Trail Making Test Part A Smell Detection Threshold Smell Memory Taste Memory |
Abbreviations Used:
3MS = Modified Mini-Mental State Exam
AD = Alzheimer's disease
BDAE = Benton Diagnostic Aphasia Examination
BNT = Boston Naming Test
BVRT = Benton Visual Retention Test
CAMCOG = Cambridge Mental Disorders of the Elderly Examination—Cognitive Section
CERAD = Consortium to Establish a Registry for Alzheimer's Disease
CFT = Complex Figure Test
COWAT = Controlled Oral Word Association Test
CVLT = California Verbal Learning Test
DRS = Dementia Rating Scale
ε4 = APOE ε4 allele
ε3 = APOE ε3 allele
ε2 = APOE ε2 allele
FAD = Familial Alzheimer's disease
FH = Family history of AD
HS = High School
MMSE = Mini-Mental State Examination
MRI = Magnetic resonance imaging
NART = National Adult Reading Test
NR = Not reported
PASAT = Paced Auditory Serial Addition Test
PET = Positron emission tomography
RAVLT = Rey Auditory Verbal Learning Test
SRT = Selective Reminding Test
WAIS = Wechsler Adult Intelligence Scale
WAIS-R = Wechsler Adult Intelligence Scale-Revised
WCST = Wisconsin Card Sorting Test
WISC = Wechsler Intelligence Scale for Children
WISC-R = Wechsler Intelligence Scale for Children–Revised
WMS = Wechsler Memory Scale
WMS-R = Wechsler Memory Scale-Revised
APPENDIX 2
Neuroimaging Studies of Preclinical Alzheimer's Disease
| Part A. Longitudinal structural MRI studies examining development of Alzheimer's disease | ||||
|---|---|---|---|---|
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Regions Examined | Results |
| Fox et al. (1996) | 3FH+ cases 4FH+ controls (All 7 from a familial AD pedigree) 38 normal controls 3-year study |
FH+ A = 45 G = 86% male E = NR Normal controls A = 48 G = 50% male E = NR |
Hippocampal formation volume | In the 3 subjects who developed AD, significant, asymmetric hippocampal atrophy was detectable before the development of symptoms The 4 FH+ subjects who remained well did not differ from NCs on hippocampal volume |
| Fox et al. (2001) | 4 FH+ cases (All 4 from a familial AD pedigree) 20 normal controls 8-year study |
FH+ A = median 43 G = NR E = NR Normal controls A = median 51 G = NR E = NR |
Whole brain | FH+ subjects had higher annual rates of global volume loss, particularly in the medial temporal lobe, inferolateral temporal lobe, parietal lobe, and posterior cingulate |
| Kaye et al. (1997) | 12 cases 18 controls Mean length of follow-up was 3.5 years |
Cases A = 90 G = 42% male E = 15 Controls A = 87 G = 44% male E = 14 |
Supratentorial intracranial cavity volume; Temporal lobe tissue volume; Parahippocampal gyrus volume; Hippocampal volume |
Subjects who developed AD had smaller hippo- campal and temporal lobe tissue volumes at baseline and showed greater temporal lobe atrophy over time. Hippocampal and temporal lobe tissue volumes at baseline and rate of temporal lobe volume loss were significant predictors of group status. |
| Part B. Longitudinal structural MRI study comparing nondemented ε4+ and ε4− subjects | ||||
| Cohen et al. (2001) | 16 ε4+ 9 ε4− 2-year study Groups also compared on neuropsychological performance (see find- ings reported above) |
ε4+ A = 55 G = 0% male E = NR ε4− A = 61 G = 0% male E = NR |
Hippocampal volume | Compared with ε4− subjects, ε4+ subjects demonstrated greater hippocampal volume loss |
| Part C. Longitudinal functional imaging study comparing nondemand ε4+ ε4− subjects | ||||
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Tasks Used | Results |
| Reiman et al. (2001) | 10 ε4+ 15 ε4− All subjects were FH+ 2-year study Groups also compared on neuropsychological performance (see find- ings reported above) |
ε4+ A = 56 G = 30% male E = 15 ε4− A = 57 G = 33% male E = 16 |
Resting PET examining regional rates of glucose metabolism |
ε4+ subjects had significantly greater glucose metabolism declines over 2 years in temporal, posterior cingulate, prefrontal cortex, basal forebrain, parahippocampal gyrus, and thalamus |
| Part D. Cross-sectional structural MRI studies comparing nondemented ε4+ and ε4− subjects | ||||
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Regions Examined | Results |
| den Heijer et al. (2002) | 117 ε4+ 259 ε3/ε3 52 ε2+ |
A = 72 G = 48% male E = NR |
Hippocampal and amygdalar volume |
ε4+ subjects had smaller hippocampal and amygdalar volumes bilaterally No significant differences between ε2+ subjects and ε3/ε3 subjects |
| Jack et al. (1998) | 30 ε4+ 95 ε4− |
ε4+ A = 80 E = 13 ε4− A = 79 E = 13 G = 26% male |
Hippocampal volume | Trend toward smaller hippocampi in the ε4+ group |
| Jernigan et al. (2001) | 21 ε4+ 22 ε4− |
A = NR G = NR E = NR |
Whole brain | ε4+ subjects had lower subcortical gray matter volumes than did ε4− subjects; the difference was due to lower lenticular nucleus volumes in the ε4+ subjects |
| Plassman et al. (1997) | 3 ε4+ twin pairs 7ε4− twin pairs |
ε4+ A = 65 G = 33% male E = 15 ε4− A = 62 G = 29% male E = 13 |
Hippocampal volume | Controlling for education, the ε4+ group had smaller hippocampi than did the ε4− group; there were no group differences in hippocampal volume asymmetry. |
| Reiman et al. (1998) | 11 ε4/ε4 22 ε4− All subjects were FH+ |
ε4+ A = 55 G = 27% male E = 17 ε4− A = 56 G = 27% male E = 16 |
Hippocampal volume | ε4 homozygotes had 8% smaller left and right hippocampal volumes, but the difference was not statistically significant |
| Schmidt et al. (1996) | 39 ε4+ 175 ε4− Groups also compared on neuropsychological performance (see find- ings reported above) |
ε4+ A = 59 G = about 50% male E = 11 ε4− A = 61 G = about 50% male E = 12 |
Whole brain | No differences in presence of infarcts, white matter hyperintensities, sulcal widening, ventricular enlargement, or hippocampal/ parahippocampal volumes |
| Tohgi et al. (1997) | 14 ε4+ 40 ε4− Groups also compared on neuropsychological performance (see find- ings reported above) |
A = 59 G = 52% male E = 12 |
Hippocampal volume | Compared to ε4− subjects, ε4+ subjects had smaller right hippocampal volumes |
| Part E. Cross-sectional functional imaging studies comparing at-risk subjects (ε4+ or FH+) to non-at-risk subjects (ε4− or FH−) | ||||
| Authors | Number and Type of Subjects; Methodological Comments |
Age (A = mean years unless otherwise indicated) Gender (G = percent male) Education Level (E = mean years) |
Tasks Used | Results |
| Bookheimer et al. (2000) | 16 ε4+ 14 ε4− fMRI |
ε4+ A = 63 G = 44% male E = 15 ε4− A = 62 G = 50% male E = 15 |
Word pair learning and recall task compared with resting condition |
ε4+ subjects exhibited greater magnitude and extent of activation in left hippocampal, parietal, and prefrontal regions than did ε4− subjects |
| Burggren et al. (2002) | 13 ε4+ 12 ε4− fMRI |
ε4+ A = 65 G = 38% male E = 16 ε4− A = 66 G = 42% male E = 16 |
Digit span forward task (1–8 digits) | No significant group differences in activation, even as task difficulty increased |
| Kennedy et al. (1995) | 24 FH+ subjects from a familial AD pedigree 16 age-matched control subjects PET |
FH+ A = 45 G = NR E = NR Controls: A = 50 G = NR E = NR |
Resting PET examining global and regional rates of glucose metabolism |
Compared to controls, the at-risk subjects had lower global and temporoparietal glucose metabolism |
| Reiman et al. (1996) | 11 ε4/ε4 22 ε4− All subjects were FH+ PET |
ε4+ A = 55 G = 27% male E = 17 ε4− A = 56 G = 27% male E = 16 |
Resting PET examining regional rates of glucose metabolism |
ε4 homozygotes had significantly lower glucose metabolism in posterior cingulate and bilateral parietal, temporal, and prefrontal regions |
| Reiman et al. (2004) | 12 ε4+ (all heterozygotes) 15 ε4− PET |
ε4+ A = 31 G = 25% male E = 16 ε4− A = 31 G = 20% male E = 16 |
Resting PET examining regional rates of glucose metabolism |
ε4+ subjects had significantly lower glucose metabolism bilaterally in posterior cingulate, parietal, temporal, and prefrontal regions |
| Smith et al. (1999) | 14 ε4+, FH+ 12 ε4−, FH− fMRI |
ε4+, FH+ A = 52 G = 0% male E = 15 ε4−, FH− A = 53 G = 0% male E = 15 |
Visual naming Letter fluency |
ε4+, FH+ subjects had lower activation in the bilateral mid- and posterior inferotemporal regions during both tasks |
Abbreviations Used:
AD = Alzheimer's disease
ε4 = APOE ε4 allele
ε3 = APOE ε3 allele
ε2 = APOE ε2 allele
fMRI = Functional magnetic resonance imaging
FH = Family history of AD
MMSE = Mini-Mental State Examination
MRI = Magnetic resonance imaging
NC = Normal control
NR = Not reported
PET = Positron emission tomography
REFERENCES
- Albert MS. Cognitive and neurobiologic markers of early Alzheimer's disease. Proceedings of the National Academy of Sciences. 1996;93:13,547–13,551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Albert SM, Gurland B, Maestre G, Jacobs DM, Stern Y, Mayeux R. APOE genotype influences functional status among elderly without dementia. American Journal of Medical Genetics. 1995;60:583–587. doi: 10.1002/ajmg.1320600621. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift für Psychiatrie und Psychisch-Gerichtliche Medizin. 1907;64:146–148. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association Press; Washington, DC: 1994. [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cerebral Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Azari NP, Pettigrew KD, Schapiro MR, Haxby JV, Grady CL, Pietrini P, Salerno JA, Heston LL, Rapoport SI, Horwitz B. Early detection of Alzheimer disease: A statistical approach using positron emission tomographic data. Journal of Cerebral Blood Flow and Metabolism. 1993;13:438–447. doi: 10.1038/jcbfm.1993.58. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger A, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: A meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ. Influences of cognitive support on episodic remembering: Tracing the process of loss from normal aging to Alzheimer's disease. Psychology of Aging. 1998;13:267–276. doi: 10.1037//0882-7974.13.2.267. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- Baxter LC, Caselli RJ, Johnson SC, Reiman E, Osborne D. Apolipoprotein E ε4 affects new learning in cognitively normal individuals at risk for Alzheimer's disease. Neurobiology of Aging. 2003;24:947–952. doi: 10.1016/s0197-4580(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Becker JT, Mintum MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E ε4 allele to cognitive function in older people. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berr C, Dufouil C, Brousseau T, Richard F, Amouyel P, Marceteau E, Alperovitch A. Early effect of ApoE-ε4 allele on cognitive results in a group of highly performing subjects: The EVA study. Neuroscience Letters. 1996;218:9–12. doi: 10.1016/0304-3940(96)13059-7. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Convit A, De Saniti S, Wegiel J, Tarschish CY, Saint Louis LA, Wisniewski HM. MRI of entorhinal cortex in mild Alzheimer's disease. Lancet. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164–168. doi: 10.1212/wnl.32.2.164. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. FMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer's disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Galasko D, Butters N, Salmon DP, Delis DC. Preclinical cognitive markers of dementia of the Alzheimer type. Neuropsychology. 1994;8:374–384. [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and Apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T. Episodic memory changes are associated with the ApoE-ε4 allele in non-demented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. New England Journal of Medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Neuropathologica. 1991;82:259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiology of Aging. 1995;16:271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Bratzke H. Evolution of Alzheimer's disease related cortical lesions. Journal of Neural Transmission. 1998;54:97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-ε4 in longitudinal cognitive decline: MacArthur studies of successful aging. Neurology. 2003;60:1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D, Fratiglioni L, Small BJ, Winblad B, Bäckman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63:816–821. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY. Specificity of brain activation patterns in people at genetic risk for Alzheimer's disease. American Journal of Geriatric Psychiatry. 2002;10:44–51. [PubMed] [Google Scholar]
- Caselli RJ, Graff-Radford NR, Reiman EM, Weaver A, Osborn D, Lucas J, Uecker A, Thiobodeau SN. Preclinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology. 1999;53:201–213. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Osbourne D, Reiman EM, Hentz JG, Barbieri CJ, Saunders AM, Hardy J, Graff-Radford NR, Hall GR, Alexander GE. Preclinical cognitive decline in late middle-aged asymptomatic apolipoprotein E-ε404 homozygotes: A replication study. Journal of the Neurological Sciences. 2001;189:93–98. doi: 10.1016/s0022-510x(01)00577-9. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE, Boeve BF. A distinctive interaction between memory and chronic daytime somnolence in asymptomatic APOE ε4 homozygotes. Sleep. 2002;25:447–453. [PubMed] [Google Scholar]
- Chen JG, Edwards CL, Vidyarthi S, Pitchumoni S, Tabrizi S, Barboriak D, Charles HC, Doraiswamy PM. Learning and recall in subjects at genetic risk for Alzheimer's disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:58–63. doi: 10.1176/jnp.14.1.58. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55:1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: A prospective community study. Archives of General Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. Journal of Cerebral Blood Flow & Metabolism. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neuroscience and Biobehavioral Reviews. 2000;24:365–374. doi: 10.1016/s0149-7634(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines HL, Pericak-Vance MA. Gene dose of apolipoprotein E ε4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dartigues JF, Commenges D, Letenneur L, Barberger-Gateau P, Gilleron V, Fabrigoule C, Mazaux JM, Orgogozo JM, Salamon R. Cognitive predictors of dementia in elderly community residents. Neuroepidemiology. 1997;16:29–39. doi: 10.1159/000109668. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Visscher PM, Tynan MC, Whalley LJ. Apolipoprotein E gene variability and cognitive functions at age 79: A follow-up of the Scottish Mental Survey of 1932. Psychology and Aging. 2004;19:367–371. doi: 10.1037/0882-7974.19.2.367. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Annals of Neurology. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Orgogozo JM. Alzheimer disease: Diagnosis, costs, and dimensions of treatment. Alzheimer's Disease and Associated Disorders. 2001;15:S3–S7. [PubMed] [Google Scholar]
- DeLaGarza VW. Pharmacologic treatment of Alzheimer's disease: An update. American Family Physician. 2003;68:1365–1372. [PubMed] [Google Scholar]
- de Leon MJ, George AE, Golomb J, Tarshish CY, McRae T, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisiniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiology of Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Dujin CM, Hofman A, Breteler MMB. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Diaz-Olavarrieta C, Ostrosky-Solis F, de al Cadena CG, Rodriguez Y, Alonso E. Neuropsychological changes in subjects at risk of inheriting Alzheimer's disease. Neuroreport. An International Journal for the Rapid Communication of Research in Neuroscience. 1997;8:2449–2453. doi: 10.1097/00001756-199707280-00008. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;5:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Bouter LM, Geerlings MI, van Kamp GJ, Deeg DJ. APOE-epsilon4 is associated with memory decline in cognitively impaired elderly. Neurology. 2000;54:1492–1497. doi: 10.1212/wnl.54.7.1492. [DOI] [PubMed] [Google Scholar]
- Durany N, Michel T, Kurt J, Cruz-Sanchez FF, Crevos-Navarro J, Riederer P. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer's disease brains. International Journal of Developmental Neuroscience. 2000;18:807–813. [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino EZ, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Elias M, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer's disease: A 22-year prospective study of the Framingham cohort. Archives of Neurology. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Ercoli LM, Siddarth P, Dunkin JJ, Bramen J, Small GW. MMSE items predict cognitive decline in persons with genetic risk for Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology. 2003;16:67–73. doi: 10.1177/0891988703016002001. [DOI] [PubMed] [Google Scholar]
- Fabrigoule C, Rouch I, Taberly A, Letenneur L, Commenges D, Mazaux JM, Orgogozo JM, Dartigues JF. Cognitive process in preclinical phase of dementia. Brain. 1998;121:135–141. doi: 10.1093/brain/121.1.135. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Landerman LR, Blazer DG, Saunders AM, Harris TB, Launer LJ. The relationship of APOE genotype to cognitive functioning in older African-American and Caucasian community residents. Journal of the American Geriatrics Society. 2001;49:1148–1155. doi: 10.1046/j.1532-5415.2001.49230.x. [DOI] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Ryan CM, Muldoon MF. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:707–711. doi: 10.1002/1096-8628(20001204)96:6<707::aid-ajmg1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN. Presymptomatic hippocampal atrophy in Alzheimer's disease: A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Seiffer AK, Agnew SK, Rossor MN. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer's disease: A longitudinal prospective study. Brain. 1998;121:1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- Goldman WP, Price JL, Storandt M, Grant EA, McKeel DW, Rubin EH, Morris JC. Absence of cognitive impairment or decline in preclinical Alzheimer's disease. Neurology. 2001;56:361–367. doi: 10.1212/wnl.56.3.361. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Putnam K, Levy J, Parasuraman R. Scaling of visuospatial attention undergoes differential longitudinal change as a function of APOE genotype prior to old age: Results from the NIMH BIOCARD study. Neuropsychology. 2005a;19:830–842. doi: 10.1037/0894-4105.19.6.830. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of the apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005b;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the ε4 allele of the apolipoprotein E gene. Proceedings of the National Academy of Sciences. 2000;97:11,661–11,666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer's disease. Psychology and Aging. 1997;12:183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- Gunter JL, Shiung MM, Manduca A, Jack CR. Methodological considerations for measuring rates of brain atrophy. Journal of Magnetic Resonance Imaging. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. Statistics in Medicine. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Eyler LT, Brown GG, Salmon DP, Fleisher AS, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemisphere compensatory response. Neurobiology of Aging. 2006 doi: 10.1016/j.neurobiolaging.2005.12.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Duara R, Grady CL, Cutler NR, Rapoport SI. Relations between neuropsychological and cerebral metabolic asymmetries in early Alzheimer's disease. Journal of Cerebral Blood Flow and Metabolism. 1985;5:193–200. doi: 10.1038/jcbfm.1985.25. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Duara R, Schlageter N, Berg G, Rapoport SI. Neocortical metabolic abnormalities precede nonmemory cognitive defects in early Alzheimer's-type dementia. Archives of Neurology. 1986;43:882–885. doi: 10.1001/archneur.1986.00520090022010. [DOI] [PubMed] [Google Scholar]
- Helkala EL, Koivisto K, Hänninen T, Vanhanen M, Kervinen K, Kuusisto J, Mykkänen L, Kesäniemi YA, Laakso M, Riekkinen P. Memory functions in human subjects with different apolipoprotein E phenotypes during a 3-year population-based follow-up study. Neuroscience Letters. 1996;204:177–180. doi: 10.1016/0304-3940(96)12348-x. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Easteal S. Change in cognitive functioning associated with ApoE genotype in a community sample of older adults. Psychology and Aging. 2002;17:194–208. [PubMed] [Google Scholar]
- Hom J, Turner MB, Risser R, Bonte FJ, Tintner R. Cognitive deficits in asymptomatic first-degree relatives of Alzheimer's disease patients. Experimental Neuropsychology. 1994;16:568–576. doi: 10.1080/01688639408402668. [DOI] [PubMed] [Google Scholar]
- Houston WS, Delis DC, Lansing A, Cobell C, Jacobson M, Salmon DP, Bondi MW. Executive function asymmetry in older adults genetically at risk for Alzheimer's disease: Verbal versus design fluency. Journal of the International Neuropsychological Society. 2005;11:863–870. doi: 10.1017/s1355617705051015. [DOI] [PubMed] [Google Scholar]
- Howieson DB, Dame A, Camicioli R, Sexton G, Payami H, Kaye JA. Cognitive markers preceding Alzheimer's dementia in the healthy oldest old. Journal of the American Geriatric Society. 1997;45:584–589. doi: 10.1111/j.1532-5415.1997.tb03091.x. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. Journal of Neuropathology and Experimental Neurology. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O'Brien P, Waring S, Tangalos E, Smith G, Ivnik R, Thibodeau S, Kokmen E. Hippocampal atrophy and ApoE genotype are independently associated with Alzheimer's disease. Annals of Neurology. 1998;43:303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DM, Sano M, Dooneief G, Marder K, Bell KL, Stern Y. Neuropsychological detection and characterization of preclinicalAlzheimer's disease. Neurology. 1995;45:957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Delis DC, Bondi MW, Salmon DP. Asymmetry in auditory and spatial attention span in normal elderly genetically at risk for Alzheimer's disease. Journal of Clinical and Experimental Neuropsychology. 2005a;27:240–253. doi: 10.1080/13803390490515441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Lansing A, Houston WS, Olsen R, Wetter S, Bondi MW, Salmon DP. Asymmetries in global-local processing abilities in elderly with the Apolipoprotein-E ε4 allele. Neuropsychology. 2005b;19:822–829. doi: 10.1037/0894-4105.19.6.822. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Delis DC, Bondi MW, Salmon DP. Do neuropsychological tests detect preclinical Alzheimer's disease: Individual-test versus cognitive-discrepancy score analysis. Neuropsychology. 2002;16:132–139. doi: 10.1037//0894-4105.16.2.132. [DOI] [PubMed] [Google Scholar]
- Jernigan TJ, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42:980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Jonker C, Schmand B, Lindeboom J, Havekes LM, Launer LJ. Association between apolipoprotein E ε4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementia. Archives of Neurology. 1998;55:1065–1069. doi: 10.1001/archneur.55.8.1065. [DOI] [PubMed] [Google Scholar]
- Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL. Development of dementing illness in an 80-year-old volunteer cohort. Annals of Neurology. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- Kawas C, Katzman R. Epidemiology of dementia and Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. Raven Press; New York: 1999. pp. 95–116. [Google Scholar]
- Kaye JA, Moore MM, Dame A, Quinn J, Camicioloi R, Howieson D, Corbridge E, Care B, Nesbit G, Sexton G. Journal of Alzheimer's Disease. 2005;8:51–56. doi: 10.3233/jad-2005-8106. [DOI] [PubMed] [Google Scholar]
- Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B, Sexton G. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- Kennedy AM, Frackowiak RS, Newman SK, Bloomfield PM, Seaward J, Roques P, Lewington G, Cunningham VJ, Rossor MN. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer's disease. Neuroscience Letters. 1995;186:17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- Kim KW, Youn JC, Jhoo JH, Lee DY, Lee KU, Lee JH, Woo JI. Apolipoprotein E ε4 allele is not associated with the cognitive impairment in community-dwelling normal elderly individuals. International Journal of Geriatric Psychiatry. 2002;17:635–640. doi: 10.1002/gps.664. [DOI] [PubMed] [Google Scholar]
- Klages JD, Fisk JD, Rockwood K. APOE genotype, memory test performance, and the risk of Alzheimer's disease in the Canadian Study of Health and Aging. Dementia and Geriatric Cognitive Disorders. 2003;15:1–5. doi: 10.1159/000066670. [DOI] [PubMed] [Google Scholar]
- Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC. Practice parameter: Diagnosis of dementia (an evidence-based review) Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- Lange KL, Bondi MW, Galasko DG, Delis DC, Salmon DP, Thal LJ. Decline in verbal memory during preclinical Alzheimer's disease: Examination of the effect of Apolipoprotein E genotype. Journal of the International Neuropsychological Society. 2002;8:943–955. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukka EJ, Jones S, Small BJ, Fratiglioni L, Bäckman L. Similar patterns of cognitive deficits in the preclinical phases of vascular dementia and Alzheimer's disease. Journal of the International Neuropsychological Society. 2004;10:382–391. doi: 10.1017/S1355617704103068. [DOI] [PubMed] [Google Scholar]
- Levy JA, Bergeson J, Putnam K, Rosen V, Cohen R, Lalonde F, Mirza N, Linker G, Sunderland T. Context-specific memory and apolipoprotein E (ApoE) ε4: Cognitive evidence from the NIMH prospective study of risk for Alzheimer's disease. Journal of the International Neuropsychological Society. 2004;10:362–370. doi: 10.1017/S1355617704103044. [DOI] [PubMed] [Google Scholar]
- Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:126–133. doi: 10.1136/jnnp.73.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: A prospective analysis from the Canadian Study of Health and Aging. American Journal of Epidemiology. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D'Agostino RB. The ‘preclinical phase’ of probable Alzheimer's disease: A 13-year prospective study of the Framingham cohort. Archives of Neurology. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- Martins CAR, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: A nonlinear model. Neurology. 2005;65:1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, Hyman BT, Crain B, Tang M-X, Phelps CH. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer's disease. The New England Journal of Medicine. 1998;338:506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Small SA, Tang M-X, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer's disease: Effects of time and apolipoprotein-E. Neurobiology of Aging. 2001;22:683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Medina D, Detoledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiology of Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Mueggler T, Sturchler-Pierrat C, Baumann D, Rausch M, Staufenbiel M, Rudin M. Compromised hemodynamic response in amyloid precursor protein transgenic mice. The Journal of Neurosciences. 2002;22:7218–7224. doi: 10.1523/JNEUROSCI.22-16-07218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Annals of the New York Academy of Science. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Lolk A, Andersen K, Andersen J, Kragh-Sørenson P. Characteristics of elderly who develop Alzheimer's disease during the next two years: A neuropsychological study using CAMCOG. The Odense study. International Journal of Geriatric Psychiatry. 1999;14:957–963. doi: 10.1002/(sici)1099-1166(199911)14:11<957::aid-gps43>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Pendleton N, Payton A, van den Boogerd EH, Holland F, Diggle P, Rabbitt PMA, Horan MA, Worthington J, Ollier WER. Apolipoprotein E genotype does not predict decline in intelligence in healthy older adults. Neuroscience Letters. 2002;324:74–76. doi: 10.1016/s0304-3940(02)00135-0. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance MA, Grubber J, Bailey LR, Hedges D, West S, Santoro L, Kenmerer B, Hall JL, Saunders AM, Roses AD, Small GW, Scott WK, Conneally PM, Vance JM, Haines JL. Identification of novel genes in lateonset Alzheimer's disease. Experimental Gerontology. 2000;35:1343–1352. doi: 10.1016/s0531-5565(00)00196-0. [DOI] [PubMed] [Google Scholar]
- Petersen RD, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter. Early detection of dementia: Mild cognitive impairment (an evidencebased review) Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Saunders AM, Breitner JC. Apolipoprotein E ε4 allele and hippocampal volume in twin with normal cognition. Neurology. 1997;48:985–989. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in non-demented aging and “preclinical” Alzheimer's disease. Annals of Neurology. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life. American Journal of Geriatric Psychiatry. 2005;13:134–141. doi: 10.1176/appi.ajgp.13.2.134. [DOI] [PubMed] [Google Scholar]
- Reed T, Carmelli D, Swan GE, Breitner JC, Welsh KA, Jarvik GP, Deeb S, Auwerx J. Lower cognitive performance in normal older adult male twins carrying the apolipoprotein E ε4 allele. Archives of Neurology. 1994;51:1189–1192. doi: 10.1001/archneur.1994.00540240033012. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E ε4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proceedings of the National Academy of Sciences. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in person's homozygous for the ε4 allele for apolipoprotein E. New England Journal of Medicine. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli R, Brandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proceedings of the National Academy of the Sciences. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli R, Lewis S, Brandy D, de Leon M, De Santi S, Convit A, Osborne D, Weaver A, Thibodeau SN. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer's disease. Annals of Neurology. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Riley KP, Snowdon DA, Saunders AM, Roses AD, Mortimer JA, Nanayakkara N. Cognitive function and apolipoprotein E in very old adults: Findings from the nun study. Journal of Gerontology. 2000;55B:S69–S75. doi: 10.1093/geronb/55.2.s69. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer's disease: A reanalysis of data from Rochester, Minnesota, 1975–1984. American Journal of Epidemiology. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Bergeson JL, Putman K, Harwell A, Sunderland T. Working memory and apolipoprotein E: What's the connection? Neuropsychologia. 2002;40:2226–2233. doi: 10.1016/s0028-3932(02)00132-x. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller P, Kinscherf DA, Grant EA, Morris JC, Berg L. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychology of Alzheimer disease. In: Salmon DP, Bondi MW, editors. Alzheimer disease. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 39–56. [Google Scholar]
- Salo A, Ylikoski R, Verkkoniemi A, Polvikoski T, Juva K, Rastas S, Kontula K, Kainulainen K, Niinistö L, Notkola I-L, Sulkava R. Does apolipoprotein E influence learning and memory in the nondemented oldest old? International Psychogeriatrics. 2001;13:451–459. doi: 10.1017/s1041610201007864. [DOI] [PubMed] [Google Scholar]
- Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L. Preclinical Alzheimer disease: Neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG, Sattely-Miller EA, Zervakis J, Welsh-Bohmer K. Taste, smell and neuropsychological performance of individuals at familial risk for Alzheimer's disease. Neurobiology of Aging. 2002;23:397–404. doi: 10.1016/s0197-4580(01)00337-2. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Schmidt R, Fazekas F, Semmler J, Kapeller P, Reinhart B, Kostner GM. Apolipoprotein E ε4 allele in the normal elderly: Neuropsychological and brain MRI correlates. Clinical Genetics. 1996;50:293–299. doi: 10.1111/j.1399-0004.1996.tb02377.x. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: Neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Small BJ, Basun H, Bäckman L. Three-year changes in cognitive performance as a function of Apolipoprotein E genotype: Evidence from very old adults without dementia. Psychology and Aging. 1998;13:80–87. doi: 10.1037//0882-7974.13.1.80. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Archives of Neurology. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Small BJ, Graves AB, McEvoy CL, Crawford FD, Mullan M, Mortimer JA. Is APOE-ε4 a risk factor for cognitive impairment in normal aging? Neurology. 2000;54:2082–2088. doi: 10.1212/wnl.54.11.2082. [DOI] [PubMed] [Google Scholar]
- Small BJ, Herlitz A, Fratiglioni L, Almkvist O, Bäckman L. Cognitive predictors of incidentAlzheimer's disease: Aprospective longitudinal study. Neuropsychology. 1997;11:413–420. doi: 10.1037//0894-4105.11.3.413. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Small BJ, Viitanen M, Bäckman L. Mini-Mental State Examination item scores as predictors of Alzheimer's disease: Incidence data from the Kungsholmen Project, Stockholm. Journal of Gerontology. 1997;52A:M299–M304. doi: 10.1093/gerona/52a.5.m299. [DOI] [PubMed] [Google Scholar]
- Small GW, Komo S, La Rue A, Saxena S, Phelps ME, Massiotta JC, Saunders AM, Haines JL, Pericak-Vance MA, Roses AD. Early detection of Alzheimer's disease by combining apolipoprotein E and neuroimaging. Annals of the New York Academy of Sciences. 1996;802:70–78. doi: 10.1111/j.1749-6632.1996.tb32600.x. [DOI] [PubMed] [Google Scholar]
- Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, Kaplan A, La Rue A, Adamson CF, Chang L, Guza BH, Corder EH, Saunders AM, Haines JL, Pericak-Vance MA, Roses AD. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. Journal of the American Medical Association. 1995;273:942–947. [PubMed] [Google Scholar]
- Smith CD, Anderson AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ. Altered brain activation in cognitively intact individuals at high risk for Alzheimer's disease. Neurology. 1999;53:1391–1396. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. Findings from the nun study. Journal of the American Medical Association. 1996;275:528–532. [PubMed] [Google Scholar]
- Squire LR. Memory and brain. 2nd ed. Oxford University Press; New York: 1992. [Google Scholar]
- Staehelin HB, Perrig-Chiello P, Mitrache C, Miserez AR, Perrig WJ. Apolipoprotein E genotypes and cognitive functions in healthy elderly persons. Acta Neurologica Scandinavica. 1999;100:53–60. doi: 10.1111/j.1600-0404.1999.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss in the major correlate of cognitive impairment. Annals of Neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Takahashi S, Kato E, Homma A, Niina R, Sasaki K, Yonezawa H, Sasaki M. Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E ε4 allele. Neuroscience Letters. 1997;236:21–24. doi: 10.1016/s0304-3940(97)00743-x. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau U.S. interim projections by age, sex, race, and Hispanic origin. 2004 http://www.census.gov/ipc/www/usinterimproj/
- Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55:1428–1429. [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E ε4 allele and decline in different cognitive systems during a 6-year period. Archives of Neurology. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, Dartigues JF. Longitudinal analysis of the effect of the apolipoprotein E ε4 and education on cognitive performance in elderly subjects: The PAQUID study. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:794–797. doi: 10.1136/jnnp.72.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Jelic V, Gertz H-J, Nordberg A, Julin P, Wahlund L-O. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurologica Scandinavica. 2003;107:52–76. doi: 10.1034/j.1600-0404.107.s179.10.x. [DOI] [PubMed] [Google Scholar]
- Wu C, Pike VW, Wang Y. Amyloid imaging: From benchtop to bedside. Current Topics in Developmental Biology. 2005;70:171–213. doi: 10.1016/S0070-2153(05)70008-9. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jack CR, O'Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Archives of Neurology. 1997;54:1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawaon H, Ueda K, Sueishi K, Tsuneyosh M, Fujishima M. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: The Hisayama study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- Zonderman AB, Giambra LM, Arenberg D, Resnick SM, Costa PT, Kawas CH. Changes in immediate visual memory predict cognitive impairment. Archives of Clinical Neuropsychology. 1995;10:111–123. [PubMed] [Google Scholar]