Abstract
Background
Celecoxib inhibits PGE2 production in cancerous tissue. We previously reported that PGE2 levels in nipple aspirate fluid (NAF) and plasma were not decreased in women at increased breast cancer risk who received celecoxib 200 mg twice daily (bid). The endpoints of the current study were to determine if a short course of celecoxib 400 mg bid would decrease PGE2 levels in women 1) at increased breast cancer risk, and 2) with established breast cancer.
Methods
NAF and plasma samples were collected before, 2 weeks after taking celecoxib 400 mg bid, and two weeks after washout from 26 women who were at increased breast cancer risk. From 13 women with newly diagnosed breast cancer, NAF from the incident breast and plasma were collected before and on average 2 weeks after taking celecoxib. Additionally, in nine of the 13 women with breast cancer, NAF was collected from the contralateral breast.
Results
No consistent change in NAF or plasma PGE2 levels was noted in high risk premenopausal women. NAF PGE2 levels decreased after celecoxib administration in postmenopausal high risk women (p = 0.02), and in both the NAF (p = 0.02) and plasma (p = 0.03) of women with breast cancer.
Conclusion
Celecoxib 400 mg bid taken on average for 2 weeks significantly decreased NAF, but not plasma, PGE2 levels in postmenopausal high risk women, and decreased both NAF and plasma PGE2 levels in women with newly diagnosed breast cancer. PGE2 levels may predict celecoxib breast cancer prevention and treatment efficacy. Our observations are preliminary, and larger studies to confirm and extend these findings are warranted.
Background
Women at increased breast cancer risk include those with a strong family history of breast cancer, those with a history of ductal carcinoma in situ (DCIS) or invasive breast cancer (IBC), and those with precancerous changes in their breasts. Eligibility for enrollment in the first National Surgical Adjuvant Breast and Bowel Project (NSABP) breast cancer prevention trial required that a participant have a > 1.66% projected 5 year probability of developing IBC [1]. The only accepted treatment for these women is tamoxifen, which has side effects of hot flashes, deep vein thrombosis, and uterine cancer. An effective alternative treatment is desirable.
Cyclooxygenase (COX)-1 and COX-2 may be present in breast tumors to catalyze the conversion of arachidonic acid to prostaglandins, prostacyclins, or thromboxanes. While COX-1 expression is constitutive, COX-2 is inducible [2] and is upregulated in a variety of tumors, including breast cancers [3]. Prostaglandin (PG)E2 is produced from arachidonic acid by either COX-1 or -2. PGE2 has tumor and cell growth promoting activity [4]. Women with breast cancer with PGE2 levels above 15 ng/g appear to have a significantly worse survival rate than those with levels ≤ 15 ng/g [5]. Malignant breast tumors produce more PGE2 than benign breast tumors or normal breast tissue [3]. A retrospective analysis of women with and without breast cancer seen at a single hospital for a one year period found that the women without breast cancer were more likely to have taken celecoxib than women who had developed breast cancer [6].
Nonsteroidal antiinflammatory drugs (NSAIDs), including aspirin, indomethacin and ibuprofen, inhibit both COX-1 and COX-2. Inhibition of COX-1 leads to a number of adverse effects, including gastrointestinal ulcers and renal toxicity [7]. Recent efforts have, therefore, focused on pharmacologic agents such as celecoxib, a clinically available medication which selectively inhibits COX-2. Preclinical studies suggest that celecoxib is effective both in preventing and in treating breast cancer in a dose dependant manner [8,9].
We are currently able to collect breast nipple aspirate fluid (NAF) from 95% of nonlactating adult females ages 18 to 80 with the use of a modified breast pump [10]. Using our NAF collection technique, we performed a preliminary study to determine if 200 mg bid of the COX-2 inhibitor celecoxib administered for two weeks to women at increased breast cancer risk would significantly decrease PGE2 levels in the breast, as measured both in NAF and in plasma, providing both organ specific and systemic information [11]. We observed that PGE2 is concentrated in NAF compared to matched plasma, but that celecoxib 200 mg bid administered for two weeks did not significantly decrease PGE2 concentrations in NAF or plasma.
Subjects with familial adenomatous polyposis (FAP) who received celecoxib for six months at 400 mg bid, but not those receiving 100 mg bid, demonstrated a significant reduction, compared to placebo, in mean polyp number [12]. Consequently, we sought to determine if a short two week course of celecoxib at a dose of 400 mg bid would alter NAF and/or plasma PGE2 levels in women at high risk for, or with newly diagnosed breast cancer. We observed that celecoxib 400 mg bid lowered PGE2 levels in the NAF and plasma of women with breast cancer, and in the NAF of high risk postmenopausal women.
Methods
Subject recruitment
Women were provided an Institutional Review Board approved protocol and required to give written informed consent in order to enroll in the study. Subjects evaluated had to be ≥ 18 years old and be at increased breast cancer risk, based on the subject having either a Gail model risk of developing IBC in a 5 year period of > 1.66%, or previously treated DCIS or IBC (now finished with treatment and free of disease).
Pregnant and lactating women were not eligible. Women could not have been currently on NSAIDs, aspirin, a COX-2 inhibitor, warfarin, or have taken such a medication within two weeks of enrollment. Subjects could not have a significant history of peptic ulcer disease, upper gastrointestinal bleeding, asthma, or be allergic to sulfonamides or NSAIDs. A complete blood count, serum electrolytes and liver panel had to be within normal limits. Subjects were recruited from the Breast Evaluation Clinics at the University of Missouri-Columbia.
Intervention
Celecoxib was taken daily for 14 days by women at increased risk, and for 10 to 24 (median 14) days by women with recently diagnosed breast cancer. Compliance was assessed through the count of returned pills. All subjects were required to have taken at least 80% of the prescribed medication. While the goal was to have women at increased risk and women with cancer take celecoxib for the same time period, variability in the operative date required some flexibility in the latter group.
Specimen collection
For all women, both those with cancer and those at increased risk, NAF was analyzed from only one breast. If one breast contained cancer and the other did not, only NAF from the breast with cancer was analyzed. For serum collected from women with cancer, PGE2 values were assigned to the cancer group. For all subjects, NAF was collected from the same breast for each of the three visits. A detailed account of the NAF collection technique has been reported [10,13].
Baseline NAF and blood collection were performed prior to the ingestion of celecoxib. For women at increased risk, washout NAF and blood samples were collected 14 days after stopping celecoxib. Thus, subjects at increased risk were asked to provide three NAF and three plasma samples (baseline, after celecoxib, and after washout), whereas subjects with recently diagnosed cancer provided two NAF and two plasma samples. Each subject granted permission for us to contact them yearly to determine if they had developed new or recurrent breast cancer.
NAF samples were collected into capillary tubes and stored at -80°C until analysis. Eight mL of blood were also collected from the subject in a tube containing heparin, the blood spun for 10 min at 1600 rpm, and the plasma fraction decanted and stored at -80°C until analysis. All women had NAF and plasma collected within 12 hrs of their last dose of celecoxib. The half life of the medication is 11.5 hrs.
PGE2 analysis
The biomarker chosen for analysis was PGE2, due to its established link to cancer growth. NAF and plasma samples were analyzed by immunoassay for their PGE2 content. NAF and plasma samples were analyzed as per the manufacturer's instructions (R&D Systems, Minneapolis, MN). The kit uses a monoclonal antibody to PGE2 to competitively bind the PGE2 in the standard or sample. Briefly, samples were diluted in 100 μL assay buffer supplied by the manufacturer, pipetted into appropriate wells, incubated for 18–24 hrs at 4°C, washed, substrate solution added, followed by 1 hr incubation, and absorbance measured at 405 nm.
For NAF and plasma analyses, a standard curve was prepared using serial dilutions of PGE2. A linear regression equation was created from standards of known PGE2 concentration, and PGE2 concentrations of unknown samples fit to the standard curve regression equation, corrected for aliquot volume and expressed as nanograms of PGE2/mL of original sample. The goodness of fit of the standard curve, R2, for NAF samples was 0.999. The goodness of fit was similar for the plasma samples.
Statistical analysis
Median values of continuous variables were computed for the various groups of subjects. Due to the potential non-normality of the data, ranking procedures were used for all analyses with continuous variables. The Wilcoxon Rank Sum Test was used to compare independent groups. Examples of these comparisons include comparing pre- and postmenopausal women, comparing the cancer to the high risk group, etc. The Wilcoxon Signed Ranks Test was used to make within group comparisons such as comparing pretreatment to posttreatment, pretreatment to washout, etc.
Results
Subjects
Subjects were enrolled from May 2003 to December 2004. Complete recruitment data were available for all subjects screened in 2004, during which the majority of the subjects analyzed in the study were recruited. In 2004, 158 women were screened, of which 27 were eligible, enrolled and were evaluable. Reasons for ineligibility included use of nonallowed medication, medical history, and blood screen abnormalities. All eligible subjects were evaluable, which required successful collection of NAF or plasma and detection of PGE2 before and after treatment in the samples.
NAF and plasma samples were collected before, 2 weeks after taking celecoxib 400 mg bid, and two weeks after washout from 26 women who were at high risk for developing breast cancer. NAF was successfully collected from the same breast at for 95% (113/120) of subject visits. From 13 women with newly diagnosed cancer, NAF from the incident breast and plasma were collected before and on average 2 weeks after taking celecoxib 400 mg bid. Additionally, in nine of the 13 women with breast cancer, NAF was collected from the contralateral breast. Thus, we collected 26 NAF and 26 plasma samples from the 26 women at increased risk of breast cancer, plus 9 NAF samples from breasts contralateral to a breast with cancer, for a total of 35 NAF samples from breasts at increased cancer risk. We collected NAF from 12/13 subjects from the breast containing cancer, as well as plasma in the 13 subjects with cancer (Table 1). In total, 47 NAF and 39 blood samples were collected at baseline from 39 subjects.
Table 1.
| Demographics | ||
| High Risk | Cancer | |
| NAF(plasma) | NAF(plasma) | |
| Samples | 35(26)1 | 12(13)2 |
| Age (years) | ||
| Median | 53(50.5) | 59(58) |
| Range | 30–81(30–81) | 35–77(35–77) |
| Premenopausal | 14(11) | 3(3) |
| Race | ||
| White | 34(25) | 12(13) |
| Black | 0 | 0 |
| Asian | 0 | 0 |
| American Indian | 1(1) | 0 |
| Primary risk factors for enrollment | ||
| Family history | 17(17) | 0 |
| Atypia | 6(6) | 0 |
| History of breast cancer | 3(3) | 0 |
| New contralateral breast cancer | 9(0) | 0 |
| New ipsilateral breast cancer | 0 | 12(13) |
| Concomitant medications | ||
| Vitamins | 14(12) | 3(3) |
| Alternative therapies | 7(4) | 1(2) |
1: NAF was collected from both breasts of nine women who had cancer diagnosed on one side. NAF from the incident breast was placed in the cancer group and from the contralateral breast in the high risk group. Plasma from these women are included only in the cancer group.
2: We were unable to collect NAF from one cancer subject at baseline.
Fewer than half of subjects were premenopausal in both the high risk and cancer groups. Median age was lower in high risk subjects than in subjects with breast cancer. All but one subject recruited was Caucasian. The median number of celecoxib pills taken was over 95% by subjects in both the high risk and cancer groups, and all subjects in both groups took over 80% of the pills that they were given.
PGE2 concentration decreases in both NAF and plasma of women with breast cancer after celecoxib treatment
We evaluated all NAF samples for PGE2 (Table 2). PGE2 levels were not significantly different when comparing subjects at increased risk to women with cancer. When evaluating the effect of celecoxib on PGE2, levels did not significantly change in high risk women, but decreased significantly among women with newly diagnosed breast cancer (p = 0.02). Changes in individual subjects after treatment are illustrated in Figure 1A.
Table 2.
Comparison of Median PGE2 Concentrations in ng/mL in NAF Based on Disease Status1
| Before (N) | After (N) | Washout (N) | P value | |||
|
Before v. After (N) |
After v. Washout (N) |
Before v. Washout (N) |
||||
| Population 2 | ||||||
| High risk | 11.43 (26) | 13.22 (22) | 14.30 (22) | 0.84 (22) | 0.91 (21) | 0.67 (22) |
| Cancer | 10.70 (12) | 8.11 (11) | NA | 0.02 (11) | ||
| P value | 0.37 | 0.11 | ||||
1: N: sample size; NAF: nipple aspirate fluid; NA: not applicable.
2: Although NAF was collected from both breasts of women with cancer, only NAF from the breast with cancer is included in this Table. In the high risk group, after taking celecoxib NAF collection was unsuccessful in two subjects, and in one the tube broke. In the cancer group, one subject refused NAF collection, and in a second subject the after treatment aspiration was not attempted due to the presence of a needle localization wire.
Figure 1.
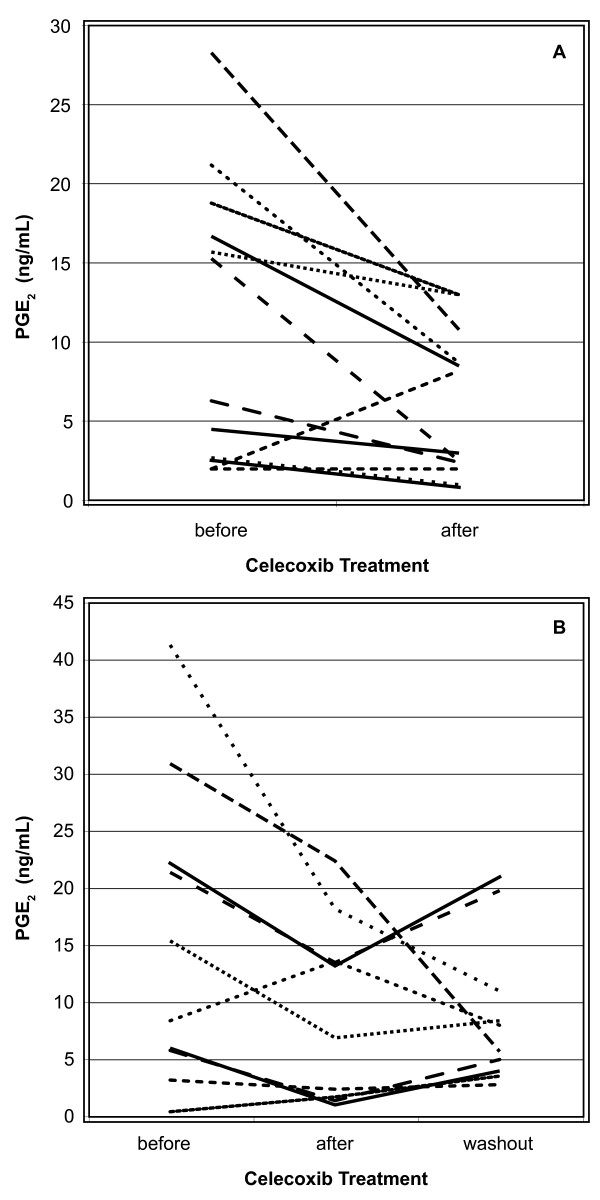
PGE2 values in NAF from A) women with breast cancer, and B) high risk postmenopausal women. Only subjects with before and after treatment values (cancer subjects) or with before, after and washout values (high risk subjects) are shown.
PGE2 concentrations in plasma were determined in all samples (Table 3). Similar to findings in NAF, PGE2 levels were not significantly different when comparing samples from subjects at increased risk to women with cancer. When evaluating the changes in PGE2 before vs. after treatment, levels did not significantly change in high risk women, but decreased significantly in samples from women with newly diagnosed breast cancer (p = 0.03). Changes in individual subjects after treatment are illustrated in Figure 2.
Table 3.
Comparison of Median PGE2 Concentrations in ng/mL in Plasma Based on Celecoxib Dose and Disease Status1
| Before (N) | After (N) | Washout (N) | P value | |||
|
Before v. After (N) |
After v. Washout (N) |
Before v. Washout (N) |
||||
| Population 2 | ||||||
| High risk | 0.29 (26) | 0.21 (26) | 0.25 (23) | 0.72 (26) | 0.50 (23) | 0.70 (23) |
| Cancer | 0.37 (13) | 0.30 (12) | NA | 0.03 (12) | ||
| P value | 0.16 | 0.99 | ||||
1: N: sample size; NA: not applicable.
2: Reasons why blood was not collected: In the high risk group at washout, three subjects refused. In the cancer group, one subject refused blood collection after treatment.
Figure 2.
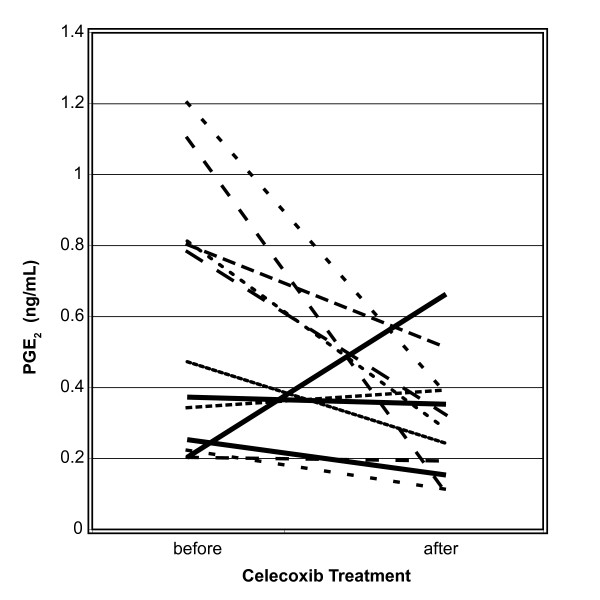
PGE2 values in plasma from women with breast cancer. Only subjects with before and after treatment values are shown.
Celecoxib decreases PGE2 levels in the NAF and plasma of postmenopausal women
We next determined if menopausal status influenced the effect of celecoxib on PGE2 levels (Tables 4 and 5). Celecoxib significantly decreased PGE2 levels in the NAF (p = 0.02) but not the plasma of postmenopausal high risk women. Changes in individual subjects after treatment and after washout are illustrated in Figure 1B. Celecoxib did not significantly alter PGE2 levels in premenopausal women.
Table 4.
Effect of Menopausal Status on Median PGE2 Concentrations in ng/mL in NAF1
| Before (N) | After (N) | Washout (N) | P value | |||
|
Before v. After (N) |
After v. Washout (N) |
Before v. Washout (N) |
||||
| Menopausal Population | ||||||
| High risk | ||||||
| Pre- | 15.72 (11) | 36.06 (11) | 27.64 (11) | 0.17 (11) | 0.76 (11) | 0.04 (11) |
| Post- | 5.88 (15)2 | 6.81 (11) | 5.57 (11) | 0.03 (11) | 0.77 (10) | 0.03 (11) |
| P value | 0.18 | 0.04 | 0.003 | |||
| Cancer | ||||||
| Pre- | 15.2 (3) | 2.40 (3) | NA | 0.25 (3) | ||
| Post- | 10.9 (8) | 8.27 (8) | NA | 0.11 (8) | ||
| P value | 1.00 | 0.61 | ||||
1: Abbreviations: NAF: nipple aspirate fluid; N: sample size; NA: not applicable.
2: Before treatment median PGE2 for the 11 subjects with an after treatment comparison value was 8.33, which was significantly higher than the after treatment group.
Table 5.
Effect of Menopausal Status on Median PGE2 Concentrations in ng/mL in Plasma1
| Before (N) | After (N) | Washout (N) | P value | |||
|
Before v. After (N) |
After v. Washout (N) |
Before v. Washout (N) |
||||
| Menopausal Population | ||||||
| High risk | ||||||
| Pre- | 0.37 (11) | 0.37 (11) | 0.45 (10) | 0.77 (11) | 0.61 (10) | 0.77 (10) |
| Post- | 0.28 (15) | 0.19 (15) | 0.18 (13) | 0.64 (15) | 0.59 (13) | 0.45 (13) |
| P value | 0.53 | 0.47 | 0.04 | |||
| Cancer | ||||||
| Pre- | 0.25 (3) | 0.35 (3) | NA | 0.99 (3) | ||
| Post- | 0.78 (9) | 0.28 (9) | NA | 0.01 (9) | ||
| P value | 0.27 | 0.58 | ||||
1: Abbreviations: N: sample size; NA: not applicable
PGE2 levels are higher in pre- than in postmenopausal high risk women
Both after treatment (p = 0.04) and after washout (p = 0.003), NAF PGE2 levels were significantly higher in pre- than in postmenopausal high risk women (Table 4). PGE2 levels in NAF were not different in women with newly diagnosed breast cancer. After washout, plasma PGE2 levels were higher (p = 0.04) in pre- than in postmenpausal women (Table 5). No differences in plasma PGE2 levels were found, either before or after treatment, in women with newly diagnosed breast cancer. Moreover, NAF and plasma PGE2 levels were not correlated in either the cancer or high risk groups, whether or not they were subdivided by menopausal status.
PGE2 is concentrated in NAF compared to matched plasma
When both NAF and plasma were available from the same subject, relative concentrations of PGE2 were compared before and after treatment with celecoxib. In the high risk (cancer) groups, median concentrations of PGE2 in NAF were 27.9 (16.4), 63.7 (13.9), and 48.1 (not applicable) times higher than in corresponding plasma before celecoxib, after celecoxib, and after washout. The differences in the ratios of NAF to serum PGE2 levels before vs. after treatment, before vs. after washout, and after treatment vs. after washout were significant in neither the high risk nor the cancer group.
Toxicity
In the high risk group, there were 11 subjects who experienced side effects from celecoxib, four of whom dropped out. In the other 7 subjects, the side effects resolved spontaneously. In the four who dropped out, the side effects (edema in two, diarrhea in one, and heart palpitations in one) all resolved shortly after stopping celecoxib. Of the 13 subjects with recently diagnosed cancer who received celecoxib, one had insomnia(this subject is also included in the at risk group, since NAF was collected from the non-involved breast) which resolved spontaneously. There were no dropouts in the cancer group.
Discussion
We carried out experiments to investigate whether the COX-2 inhibitor celecoxib at a dose of 400 mg bid could significantly affect endogenous levels of PGE2 in NAF or plasma of women at risk for breast cancer or in women with known cancer. This study builds on our initial study, which found that celecoxib 200 mg bid did not alter PGE2 levels in NAF or plasma under normal, nonstressed conditions.
In the current study, we observed two important findings. First, PGE2 levels significantly decreased in the NAF, but not in the plasma, of postmenopausal women at increased risk of breast cancer. Second, PGE2 levels significantly decreased in both the NAF and plasma of celecoxib-treated women with newly diagnosed breast cancer.
It is unclear why PGE2 levels significantly decreased in post- but not premenopausal high risk women. We did not routinely record whether premenopausal subjects started celecoxib in the first or second half of their menstrual cycle. We have this information for 10 women in the high risk group and one woman for the cancer group. We did not find a significant difference in PGE2 response to celecoxib in either the high risk or cancer group based on when the subject started medication in the first or second half of her cycle, although sample size limits the reliability of these findings.
In order to assess if PGE2 was concentrated in NAF, we determined its relative concentration in NAF vs. plasma in both high risk women and in women with newly diagnosed breast cancer. In both groups, PGE2 was concentrated in NAF relative to plasma. The NAF/plasma relative concentration was numerically, although not significantly, lower in women with cancer than in those at increased risk.
The relative concentration of PGE2 in NAF vs. plasma differed in the current study compared to our earlier study [11]. There are a number of possible reasons for this. First, relative concentrations were lower in the cancer group, which was not present in the earlier study. Second, we used different kits in the two studies. The kit used in the current study was chosen because it has been shown capable of measuring PGE2 in human bodily fluids by other investigators [14]. Third, we used a higher dose of celecoxib (400 mg bid vs. 200 mg bid) in the current study.
COX-2 expression has been evaluated in preclinical models and in clinical breast specimens. In a dimethylbenzanthracene (DMBA) rat model of breast cancer, celecoxib was found to dramatically reduce the incidence, multiplicity, and volume of breast tumors relative to control [15]. While COX-2 expression appears to be a good marker of breast cancer in tissue, the lack of reliable commercial immunoassays for bodily fluids at the time that the study was conducted limited our ability to measure this marker in NAF and plasma.
Conclusion
PGE2 is measurable and is concentrated in NAF compared to plasma. Plasma concentrations of PGE2 in women with a predisposition to breast cancer are within the normally accepted basal range (less than 1 nanogram/mL). Celecoxib 400 mg bid significantly decreased PGE2 levels in women with newly diagnosed breast cancer in both NAF and plasma, and in the NAF of high risk postmenopausal women, consistent with clinical studies evaluating its effects on colon and duodenal polyps. We acknowledge that additional studies, with a placebo control, are required to determine whether monitoring NAF eicosanoid concentrations will be of value in describing the development and progression of breast cancer, or in monitoring the effect of candidate chemopreventive agents such as celecoxib.
Abbreviations
ADH: atypical ductal hyperplasia; DCIS: ductal carcinoma in situ; DMBA: dimethylbenzanthracene; FAP: Familial Adenomatous Polyposis; IBC: invasive breast cancer; NAF-nipple aspirate fluid; NSABP: National Surgical Adjuvant Breast and Bowel Project; NSAIDs: nonsteroidal anti-inflammatory drugs; PG: prostaglandin
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
ERS designed the study, enrolled subjects, and performed the majority of manuscript preparation. WQ conducted all PGE2 analyses. JTF assisted with manuscript preparation and critique. LS enrolled subjects and entered data. JEH performed the statistical analyses.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Funding to conduct this study was provided by Department of Defense grant DAMD17-01-1-0427.
Contributor Information
Edward R Sauter, Email: sautere@health.missouri.edu.
Wenyi Qin, Email: qunw@health.missouri.edu.
Lisa Schlatter, Email: schlatterl@health.missouri.edu.
John E Hewett, Email: hewettj@health.missouri.edu.
John T Flynn, Email: john.flynn@jefferson.edu.
References
- Fisher B. Highlights from recent National Surgical Adjuvant Breast and Bowel Project studies in the treatment and prevention of breast cancer. CA Cancer J Clin. 1999;49:159–177. doi: 10.3322/canjclin.49.3.159. [DOI] [PubMed] [Google Scholar]
- Whittle BJ. Gastrointestinal effects of nonsteroidal anti-inflammatory drugs. Fundam Clin Pharmacol. 2003;17:301–313. doi: 10.1046/j.1472-8206.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II) J Natl Cancer Inst. 1998;90:1609–1620. doi: 10.1093/jnci/90.21.1609. [DOI] [PubMed] [Google Scholar]
- Fulton AM, Gimotty P, Alonsozana E, Dorsey R, Kundu N. Elevated prostaglandin E2 (PGE2) levels in human breast cancer are associated with poor long-term survival. Proc Am Assn Cancer Res. 2000;41:3660A. [Google Scholar]
- Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. doi: 10.1186/1471-2407-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher EA. Balancing gastroprotection and cardioprotection with selective cyclo-oxygenase-2 inhibitors: clinical implications. Drug Saf. 2003;26:913–924. doi: 10.2165/00002018-200326130-00001. [DOI] [PubMed] [Google Scholar]
- Lanza-Jacoby S, Miller S, Flynn J, Gallatig K, Daskalakis C, Masferrer JL, Zweifel BS, Sembhi H, Russo IH. The cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu mice. Cancer Epidemiol Biomarkers Prev. 2003;12:1486–1491. [PubMed] [Google Scholar]
- Alshafie G, Abou-Issa HM, Seibert K, Harris RE. Chemotherapeutic evaluation of celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, in a rat mammary tumor model. Proc Am Assn Cancer Res. 2000;41:3144A. doi: 10.3892/or.7.6.1377. [DOI] [PubMed] [Google Scholar]
- Sauter ER, Ross E, Daly M, Klein-Szanto A, Engstrom PF, Sorling A, Malick J, Ehya H. Nipple aspirate fluid: a promising non-invasive method to identify cellular markers of breast cancer risk. Br J Cancer. 1997;76:494–501. doi: 10.1038/bjc.1997.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter ER, Schlatter L, Hewett J, Koivunen D, Flynn JT. Lack of effect of celecoxib on prostaglandin E2 concentrations in nipple aspirate fluid from women at increased risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1745–1750. [PubMed] [Google Scholar]
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- Sauter ER, Daly M, Linahan K, Ehya H, Engstrom PF, Bonney G, Ross EA, Yu H, Diamandis E. Prostate-specific antigen levels in nipple aspirate fluid correlate with breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:967–970. [PubMed] [Google Scholar]
- Hidalgo GE, Zhong L, Doherty DE, Hirschowitz EA. Plasma PGE-2 levels and altered cytokine profiles in adherent peripheral blood mononuclear cells in non-small cell lung cancer (NSCLC) Mol Cancer. 2002;1:5. doi: 10.1186/1476-4598-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–2103. [PubMed] [Google Scholar]


