Abstract
Background
Inflammation and inflammatory biomarkers play an important role in atherosclerosis and cardiovascular disease. Little information is available, however, on time course of serum markers of inflammation after stroke.
Methods
First ischemic stroke patients ≥40 years old had levels of high-sensitivity C-reactive protein (hsCRP), serum amyloid A (SAA), and fibrinogen measured in plasma samples drawn at 1, 2, 3, 7, 14, 21 and 28 days after stroke. Levels were log-transformed as needed, and parametric and non-parametric statistical tests were used to test for evidence of a trend in levels over time. Levels of hsCRP and SAA were also compared with levels in a comparable population of stroke-free participants.
Results
Mean age of participants with repeated measures (n = 21) was 65.6 ± 11.6 years, and 13 (61.9%) were men, and 15 (71.4%) were Hispanic. Approximately 75% of patients (n = 15) had mild strokes (NIH Stroke Scale score 0–5). There was no evidence of a time trend in levels of hsCRP, SAA, or fibrinogen for any of the markers during the 28 days of follow-up. Mean log(hsCRP) was 1.67 ± 1.07 mg/L (median hsCRP 6.48 mg/L) among stroke participants and 1.00 ± 1.18 mg/L (median 2.82 mg/L) in a group of 1176 randomly selected stroke-free participants from the same community (p = 0.0252).
Conclusion
Levels of hsCRP are higher in stroke patients than in stroke-free subjects. Levels of inflammatory biomarkers associated with atherosclerosis, including hsCRP, appear to be stable for at least 28 days after first ischemic stroke.
Background
Basic and clinical studies provide evidence that inflammation plays a crucial role in atherosclerosis and cardiovascular disease [1]. Despite a growing literature on the role of acute phase proteins, particularly high-sensitivity C-reactive protein (hsCRP), and other inflammatory markers in risk stratification and prediction of outcomes among patients with cardiovascular disease [2-6], very little is known about the role of these markers in predicting outcome in patients with cerebrovascular disease. Some investigators have suggested that levels of acute phase reactants predict prognosis after stroke [7-13], although a recent review concluded that there was insufficient data available to recommend testing for these markers in stroke patients at present [14]. Little information is available, however, on the time course of serum markers of inflammation and related proteins after stroke. This consensus statement [14] also concluded that further studies were needed to determine the time course of levels of these markers after stroke.
Our objective was to determine the stability of levels of inflammatory biomarkers after ischemic stroke. We measured plasma levels of acute phase proteins at seven pre-specified time points up to 28 days in patients with ischemic stroke and assessed for trends in these values. We also compared these levels to those in a comparable stroke-free population.
Methods
The Northern Manhattan Study (NOMAS) includes a population-based incident ischemic stroke follow-up study in a multi-ethnic, urban population. The methods of patient identification and enrollment have been described in previous publications [15-17]. Briefly, stroke patients were enrolled if they: (1) were diagnosed with a first stroke; (2) were over age 40, and (3) resided in Northern Manhattan for ≥3 months in a household with a telephone. For this analysis, a subsample (n = 21) of hospitalized ischemic stroke patients who had blood collected at seven pre-specified time intervals after stroke were included [17]. The study was approved by the CUMC Institutional Review Board. All participants gave consent directly or through a surrogate when appropriate.
Data were collected through interviews by trained research assistants, and physical and neurological examinations were conducted by study neurologists, as previously described [15-17]. When possible, data were obtained directly from subjects using standardized data collection instruments. When the subject was unable to provide answers, a proxy knowledgeable about the subject's history was interviewed. Race-ethnicity was based upon self-identification. Medical history was determined using standardized questions adapted from the Behavioral Risk Factor Surveillance System from the Centers for Disease Control and Prevention [18]. Hypertension was defined as a history of hypertension or use of anti-hypertensive medications, and diabetes mellitus was defined by a fasting blood glucose level ≥126 mg/dl, the subject's self-report of such a history, or insulin or oral hypoglycemic use.
Stroke diagnostic evaluation included computerized tomography and/or magnetic resonance imaging of the brain, ultrasound evaluation of the extracranial and intracranial cerebral vessels, and transthoracic or transesophageal echocardiogram as appropriate. Assessment of stroke subtype using modified TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria was determined by a consensus of stroke neurologists, using all available information, as described in a previous publication [19]. Baseline stroke severity was assessed using a derived National Institutes of Health Stroke Scale (NIHSS), and categorized as mild (0–5), moderate (6–13) or severe (≥14) [16].
Measurement of acute phase proteins
Both serum and plasma (in EDTA) samples were collected in the morning at days 1, 2, 3, 7, 14, 21, and 28 after stroke onset. The mean number of hospital days for the patient sample was 11.7 ± 18.7 days and 74 samples were drawn in the hospital and the remainder was collected after discharge. All blood tubes were centrifuged at 3000 g for 10 minutes. Serum and plasma were immediately separated, aliquoted and stored at -70°C until specimens could be assayed. High sensitivity C-reactive protein (hsCRP), serum amyloid A (SAA) and fibrinogen were measured using plasma samples in the Columbia University Medical Center laboratory using the BNII system (Dade-Behring, Deerfield, IL). The intra-assay and inter-assay coefficients of variation were, respectively, as follows: for hsCRP, 1.8% and 4.5%; for SAA, 7.2% and 6.1%; and for fibrinogen, 1.6% and 5.7%.
Statistical analyses
Statistical analyses were conducted using SAS Version 8.2 (SAS Institute, Cary, NC). Means and their standard deviations of hsCRP, SAA, and fibrinogen were calculated. Levels of all markers were log-transformed before analysis to stabilize the variance. Mean log-transformed values for each marker were compared across groups defined by demographic characteristics, stroke characteristics (NIHSS and diagnostic subtype), and risk factors. Tests for time trends were performed using both parametric and non-parametric methods. Random effects models were used to calculate the adjusted differences between mean values at each time point while taking the correlation among the repeated measurements into account. The best model for all outcomes was the model with linear, quadratic, and cubic time trends. Friedman's test was also used to test for statistical significance of differences between the markers at each time point without assuming multivariate normality. The Welch modified two-sample t-test was used to test for differences between the marker levels in the sample of stroke patients and a sample of stroke-free participants. Throughout, Type I error of the test was set as 0.05.
Results
Table 1 lists the baseline demographic characteristics and vascular disease risk factor profile of the 21 participants. The mean age of the subjects was 65.6 ± 11.6 years, and 61.9% (n = 13) were male. The prevalence of vascular risk factors was high. Previous history of cardiovascular disease was also prevalent (38.1% had a history of coronary artery disease). Most had mild strokes (71.4%, n = 15), 23.8% (n = 5) had moderate strokes, and only 1 had a severe stroke.
Table 1.
Baseline characteristics of study population
| Number of participants | 21 |
| Age, mean ± SD, y | 65.6 ± 11.6 |
| Male, N (%) | 13 (61.9) |
| Race-ethnicity, N (%) | |
| Non-Hispanic White, | 4 (19.1) |
| Non-Hispanic Black | 2 (9.5) |
| Hispanic | 15 (71.4) |
| Risk factors, N (%) | |
| History of coronary artery disease | 8 (38.1) |
| Current smoking | 4 (19.1) |
| Diabetes mellitus | 3 (14.3) |
| Hypertension | 14 (66.7) |
| Hypercholesterolemia | 7 (33.3) |
| NIH Stroke Scale score, N (%) | |
| 0–5 | 15 (71.4) |
| 6–13 | 5 (23.8) |
| ≥14 | 1 (4.8) |
| Stroke etiologic subtype, N (%) | |
| Atherosclerotic | 2 (9.5) |
| Lacunar | 7 (33.3) |
| Embolic | 1 (4.8) |
| Cryptogenic | 11 (52.4) |
There was no evidence of a time trend in levels of hsCRP, SAA, or fibrinogen for any of the markers during the 28 days of follow-up (Table 2, Figure). Results were similar using non-parametric tests. Although mean hsCRP increased from day 1 (8.08 ± 6.54 mg/L) to day 2 (16.42 ± 24.71 mg/L) and remained elevated throughout the period of follow-up (day 28: 20.89 ± 35.40 mg/L), this was not statistically significant.
Table 2.
Mean levels of log-transformed measures during 28 days of follow-up
| Day | N | log(CRP) mg/L | log (SAA) mg/L | log (fibrinogen) mg/dL |
| 1 | 16 | 0.72 ± 0.46 | 0.77 ± 0.51 | 2.64 ± 0.09 |
| 2 | 15 | 0.91 ± 0.53 | 0.97 ± 0.67 | 2.65 ± 0.11 |
| 3 | 20 | 0.99 ± 0.51 | 1.05 ± 0.71 | 2.70 ± 0.06 |
| 7 | 20 | 0.87 ± 0.56 | 0.93 ± 0.65 | 2.69 ± 0.08 |
| 14 | 19 | 0.90 ± 0.60 | 0.88 ± 0.60 | 2.60 ± 0.37 |
| 21 | 18 | 0.76 ± 0.61 | 0.85 ± 0.65 | 2.66 ± 0.12 |
| 28 | 17 | 0.91 ± 0.58 | 0.93 ± 0.64 | 2.66 ± 0.09 |
| P* | 0.7005 | 0.4764 | 0.6382 |
* p for trend over time using random effect models.
In a random effect model adjusted for measurement day, age, sex, race-ethnicity, history of hypertension, diabetes mellitus, current smoking, coronary artery disease, stroke subtype, and stroke severity, log(hsCRP) was associated with current smoking (beta = 2.46 ± 1.22, p = 0.0469) and stroke severity (beta = 0.15 ± 0.08, p = 0.0539), but not with other risk factors. On day 1, mean log(hsCRP) was 1.54 ± 1.18 mg/L among current smokers versus 2.04 ± 0.58 mg/L among non-smokers. Mean log(hsCRP) was 1.54 ± 1.11 mg/L among those with mild stroke (NIH stroke scale <6) versus 2.04 ± 0.98 among those with moderate to severe stroke (NIH stroke scale ≥6).
In a random effect model SAA was associated with sex (p = 0.0017) and history of diabetes mellitus (p = 0.0349), but not with stroke severity, smoking, or other risk factors. Mean values of log(SAA) were 3.16 ± 0.55 mg/L for those with diabetes mellitus compared with 1.59 ± 1.14 mg/L for those without diabetes. Fibrinogen levels were not associated with patient characteristics.
In a group of 1176 stroke-free subjects (mean age 68.9 ± 10.0 years) drawn from the same underlying population as part of an ongoing prospective cohort study among stroke patients, mean levels of hsCRP were lower than the day 1 values in this population of stroke patients. The mean log(hsCRP) was 1.00 ± 1.18 (median hsCRP 2.82 mg/L) among stroke-free participants, and 1.67 ± 1.07 (median 6.48 mg/L) among stroke patients (p = 0.0252). Mean log(SAA) was not higher among stroke patients (1.78 ± 1.20 mg/L) than 1122 stroke-free participants (1.52 ± 0.80 mg/L; p = 0.4031). Median SAA among stroke patients was 5.70 versus 4.60 mg/L among stroke-free participants.
Discussion
We found that levels of the acute phase proteins hsCRP, SAA, and fibrinogen are stable after stroke for at least one month in patients with their first mild to moderate ischemic strokes. There is no significant time trend in levels over this period of time. Although we did not have available levels of these proteins in these patients prior to their stroke, the levels of hsCRP in a comparable population of stroke-free participants was significantly lower, suggesting that hsCRP levels are higher in those with recent stroke. We think this is most likely because hsCRP levels increase at the time of stroke, although we cannot exclude the possibility that hsCRP is elevated in those stroke-free individuals who subsequently go on to develop stroke [2,14]. It thus appears that if levels of hsCRP increase at the time of stroke, they remain elevated for at least one month. Whether levels decrease after that time is not addressed by these data.
Several epidemiological studies provide evidence that inflammatory markers, particularly hsCRP, predict incident ischemic stroke in stroke-free populations [14]. Some investigators have provided evidence that inflammatory markers measured at the time of ischemic stroke predict future recurrent ischemic events [14], including stroke [13]. The prognostic significance of inflammatory markers after a vascular event has already occurred has been assessed in relatively few studies, however, particularly with regard to recurrent stroke as an outcome. Among stroke patients, IL-6 and IL-1 receptor antagonist, but not TNFα, measured at baseline were independent predictors of worsening in the first 24 hours after stroke [7,8]. Others found that hsCRP levels above 10.1 when measured within 72 hours of stroke predicted mortality over 4 years [10]. Others found that the measurement of CRP at 24 or 48 hours, but not at admission, also predicted outcome [12]. In one study, hsCRP levels ≥15 mg/L at discharge were associated with occurrence of a new vascular event or death at 1 year [11]. HsCRP levels in the highest quintile measured at least 3 months after a first ischemic stroke or TIA were associated with an increased risk of subsequent stroke or MI in another study [9]. In a nested case-control analysis of a large clinical trial population, elevated levels of both fibrinogen and hsCRP independently predicted recurrent ischemic stroke [13].
The time course of inflammatory markers and acute phase reactant proteins after ischemic events has been investigated in relatively few studies, particularly with regard to stroke. In one study [20], levels of fibrinogen and leukocyte count, but not hsCRP, were elevated up to one year after acute stroke compared to healthy control subjects. These data were limited to blood samples drawn at stroke onset, 6 weeks, 6 months, and 1 year after enrollment. In another study [21], levels of hsCRP measured at hospital discharge were more strongly associated with prognosis than measurements made at admission or between 48 and 72 hours. There was evidence of a change in hsCRP levels during hospitalization, but with some patients showing an increase and others a decrease in levels. Our data suggest that, on average, levels measured within the first month after stroke may not vary significantly, and that timing of measurement may not be crucial if levels within one month are used for prognostication. Currently, however, while several studies have indicated hsCRP may be used for purposes of prognostication [10-12], there is no consensus that this is appropriate [14].
We also found an association of post-stroke levels of hsCRP with smoking and stroke severity. Other studies have found an association between hsCRP levels and smoking in disease-free individuals [22-24], but few studies have evaluated this association in patients after stroke. In those studies in which this was assessed, no association was found [9-12]. We may have had greater power to detect associations, however, because of the greater number of measurements made at multiple time points. Many other inflammatory markers are similarly elevated in smokers [2], moreover, and this may provide a mechanism through which smoking increases the risk of vascular disease. Similar to some [10,11], but not all [9], other studies, we found an association of post-stroke hsCRP to stroke severity. Serum amyloid A has not been systematically studied in stroke patient populations.
Diabetes mellitus has also been associated with levels of hsCRP in cross-sectional analyses of stroke-free individuals [25,26], and the increased risk associated with elevated levels of hsCRP may be attenuated or absent in study participants with a history of diabetes [26,27]. Previous studies in stroke patients, however, have not demonstrated differences in hsCRP levels according to history of diabetes mellitus [9-12,21]. In studies that have found an effect of elevated hsCRP on risk of recurrent stroke, moreover, analyses were not adjusted for diabetes mellitus [13]. Further studies will be needed to determine whether the prognostic value of hsCRP after stroke is present in patients with diabetes mellitus.
Levels of inflammatory markers may remain elevated for at least a month after stroke for several reasons. First, patients with ischemic stroke may suffer ongoing complications, such as infections and deep venous thrombosis, which lead to persistent elevations in these markers. The patients in our study, however, were largely free of such complications and had milder strokes than would be expected among patients with these complications. Second, levels of acute phase reactants could be markers of an increased inflammatory state that first predisposed the patient to stroke, and we cannot exclude this possibility on the basis of our data. We found few correlations between levels of acute phase proteins and other vascular risk factors, however. Third, the cerebral or vascular injury caused by stroke and the subsequent recovery may lead to an upregulation in the production of these markers, and this process may persist for a month or longer [20]. Further studies in larger populations of patients, with correlation with stroke severity and markers of cerebral injury, are needed to resolve these questions.
A strength of our study is the availability of levels of several markers at multiple time points after ischemic stroke. Most studies have relied on a relatively small number of measurements. We also focused on patients with milder strokes, which limits the likelihood of confounding due to major medical complications.
There are also limitations to our study. Because of the requirement for frequent phlebotomy, we studied a relatively small number of participants, which limited our power to detect changes over time and between patient subgroups. We also do not have available measurements beyond 28 days. Future studies should assess these markers at longer time intervals after stroke.
Conclusion
Levels of hsCRP are higher in stroke patients than in stroke-free subjects. Levels of inflammatory biomarkers associated with atherosclerosis, including hsCRP, appear to be stable for at least 28 days after first ischemic stroke. This information may be useful in planning future studies of the effect of inflammatory biomarkers on prognosis after stroke.
Abbreviations
hsCRP = high-sensitivity C-reactive protein
MI = myocardial infarction
NIHSS = National Institutes of Health Stroke Scale
SAA = serum amyloid A
TIA = transient ischemic attack
TOAST = Trial of Org 10172 in Acute Stroke Treatment
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
MSVE conceived of the study, participated in its design and coordination, and drafted the manuscript. KC supervised the immunoassays. WT performed the statistical analysis. MCP participated in the study design and coordination, and supervised the statistical analysis, providing important intellectual input. BBA participated in the study design and coordination. RLS conceived of the study, and supervised its design and coordination. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Figure 1.
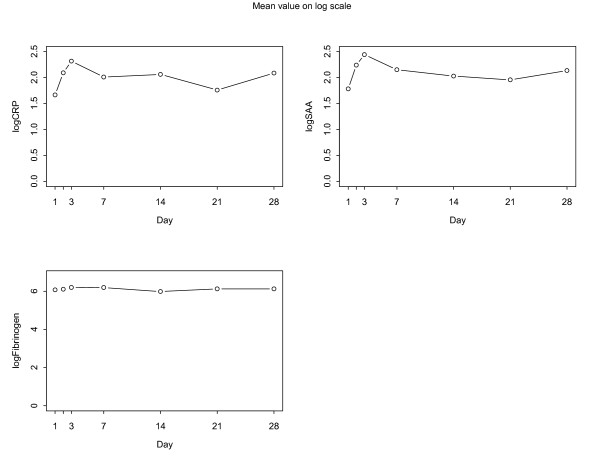
Mean values of log-transformed levels of acute phase proteins over time.
Acknowledgments
Acknowledgements
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (K23 NS42912, R01 NS48134, MSVE; R01 29993, RLS) the General Clinical Research Center (2 M01 RR00645), and the American Heart Association (Kathleen Scott Research Fellowship, MSVE).
Contributor Information
Mitchell SV Elkind, Email: mse13@columbia.edu.
Kristen Coates, Email: kc509@columbia.edu.
Wanling Tai, Email: wt2109@columbia.edu.
Myunghee C Paik, Email: mcp@biostat.columbia.edu.
Bernadette Boden-Albala, Email: bb87@columbia.edu.
Ralph L Sacco, Email: rls1@columbia.edu.
References
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease. Application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- Biasucci LN, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–84. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- Biasucci LM, Liuzzo G, Grillo RL, Caligiuri G, Rebuzzi AG, Buffon A, Summaria F, Ginnetti F, Fadda G, Maseri A. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation. 1999;99:855–60. doi: 10.1161/01.cir.99.7.855. [DOI] [PubMed] [Google Scholar]
- Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–9. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800–5. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Montaner J, Rovira A, Ibarra B, Quintana M. C-reactive protein predicts further ischemic events in first-ever transient ischemic attack or stroke patients with intracranial large-artery occlusive disease. Stroke. 2003;34:2463–70. doi: 10.1161/01.STR.0000089920.93927.A7. [DOI] [PubMed] [Google Scholar]
- Muir KW, Weir CJ, Alwan W, Squire IB, Lees KR. C-reactive protein and outcome after ischemic stroke. Stroke. 1999;30:981–5. doi: 10.1161/01.str.30.5.981. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32:133–8. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- Winbeck K, Poppert H, Etgen T, Conrad B, Sander D. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke. 2002;33:2459–64. doi: 10.1161/01.STR.0000029828.51413.82. [DOI] [PubMed] [Google Scholar]
- Woodward M, Lowe GDO, Campbell DJ, Colman S, Rumley A, Chalmers J, Neal BC, Patel A, Jenkins AJ, Kemp BE, MacMahon SW. Associations of inflammatory and hemostatic variables with the risk of recurrent stroke. Stroke. 2005;36:2143–2147. doi: 10.1161/01.STR.0000181754.38408.4c. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, Emsley HC, Forconi S, Hopkins SJ, Masotti L, Muir KW, Paciucci A, Papa F, Roncacci S, Sander D, Sander K, Smith CJ, Stefanini A, Weber D. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–29. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Elkind MS, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- Elkind MSV, Cheng J, Rundek T, Boden-Albala B, Sacco RL. Leukocyte count predicts outcome after ischemic stroke: The Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Kargman DE, Tuck C, Berglund L, Lin IF, Mukherjee RS, Thompson EV, Jones J, Boden-Albala B, Paik MC, Sacco RL. Lipid and lipoprotein levels remain stable in acute ischemic stroke: the Northern Manhattan Stroke Study. Atherosclerosis. 1998;139:391–9. doi: 10.1016/S0021-9150(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Gentry EM, Kalsbeek WD, Hegelin G, Jones JT, Gaines KL, Forman MR, Marks JS, Trowbridge FL. The Behavioral Risk Factor Surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed] [Google Scholar]
- Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: The Northern Manhattan Stroke Study experience. Neurology. 1997;48:1204–11. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- Beamer NB, Coull BM, Clark WM, Briley DP, Wynn M, Sexton G. Persistent inflammatory response in stroke survivors. Neurology. 1998;50:1722–1728. doi: 10.1212/wnl.50.6.1722. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F, Bocola V. C-Reactive Protein in ischemic stroke. An independent prognostic factor. Stroke. 2001;32:917–924. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- Yamada S, Gotoh T, Nakashima Y, Kayaba K, Ishikawa S, Nago N, Nakamura Y, Itoh Y, Kajii E. Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population. Am J Epidemiol. 2001;153:1183–90. doi: 10.1093/aje/153.12.1183. [DOI] [PubMed] [Google Scholar]
- Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89:1117–9. doi: 10.1016/S0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, Kuller LH. Lifetime smoking exposure affects the association between of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17:2167–76. doi: 10.1161/01.atv.17.10.2167. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Best LG, Zhang Y, Lee ET, Yeh JL, Cowan L, Palmieri V, Roman M, Devereux RB, Fabsitz RR, Tracy RP, Robbins D, Davidson M, Ahmed A, Howard BV. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation. 2005;112:1289–1295. doi: 10.1161/CIRCULATIONAHA.104.489260. [DOI] [PubMed] [Google Scholar]
- Curb JD, Abbott RD, Rodriguez BL, Sakkinen P, Popper JS, Yano K, Tracy RP. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003;107:2016–20. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]


