Abstract
Photolyases and cryptochrome blue-light photoreceptors are evolutionarily related flavoproteins that perform distinct functions. Photolyases repair UV-damaged DNA in many species from bacteria to plants and animals. Cryptochromes regulate growth and development in plants and the circadian clock in animals. Recently, a new branch of the photolyase/cryptochrome family was identified. Members of this branch exhibited no or trace levels of DNA repair activity in vivo and in vitro and, therefore, were considered to be cryptochromes, and they were named cryptochrome-DASH. Here, we show that Cry-DASH proteins from bacterial, plant, and animal sources actually are photolyases with high degree of specificity for cyclobutane pyrimidine dimers in ssDNA.
Keywords: circadian clock, DNA repair, photoreception
Cryptochromes are defined as proteins with a high degree of sequence homology to DNA photolyase but with no DNA repair activity (1–3). Cryptochromes first were discovered in plants and shown to mediate blue-light-dependent growth and development (4, 5). Subsequently, cryptochromes were found in animals, where they regulate the circadian clock by light-dependent and light-independent mechanisms (6–8). Most recently, members of the photolyase/cryptochrome family were found in the cyanobacterium Synechocystis sp. PCC6803 (9, 10), and in Vibrio cholerae (11), organisms which both also possess a bona fide photolyase, which repairs cyclobutane pyrimidine dimers in DNA in a light-dependent reaction. Genetic and biochemical analyses revealed that the proteins encoded by these photolyase/cryptochrome family genes had no or very marginal repair activities and hence these proteins were considered to be bacterial cryptochromes (9–11). Subsequent work revealed that these bacterial cryptochromes have sequence homologs in Arabidopsis thaliana (12), in Xenopus laevis, and in several other species (13). The entire branch was named Cryptochrome-Drosophila, Arabidopsis, Synechocystis, Human (Cry-DASH) to indicate that members of the branch that included Arabidopsis cryptochrome 3 (AtCry3) and Synechocystis sp. PCC6803 cryptochrome had higher sequence homology to Drosophila and Human cryptochromes than bacterial photolyases (13). Most recently, it was reported that some Cry-DASH proteins may have residual photolyase activity (14), and this was considered to be an evolutionary relic.
Results and Discussion
Vibrio cholerae contains three members of the photolyase/cryptochrome family (15). Both genetic and biochemical analyses indicate that one of these, named VcPhr, is a bona fide cyclobutane pyrimidine dimer photolyase; the other two had no demonstrable photolyase activity and, thus, were designated cryptochromes, VcCry1 and VcCry2 (11). We were interested in VcCry1 for two reasons. First, it is a member of the Cry-DASH branch which was reported to have transcriptional regulatory activity and residual photolyase activity (9, 13). Second, VcCry1 is unique among the photolyases and cryptochromes characterized to date in that it appears to have high affinity for RNA (11). We reasoned that VcCry1 might be an RNA-specific photolyase with some residual activity toward DNA, in contrast to conventional photolyase, which, in addition to its primary DNA repair activity, exhibits very weak RNA repair capacity (16). To test for RNA repair activity we used UV-irradiated poly (rU) as substrate and UV-irradiated poly (dU) as a negative control. Unexpectedly, with these substrates, VcCry1 demonstrated essentially the same activity as the photolyase VcPhr (16), repairing photodimers in the deoxyribonucleotide homopolymers poly dU (U<>U, uracil cyclobutane dimer) (Fig. 1) and poly dT(T<>T) (data not shown) much more efficiently than the dimers in the ribopolynucleotide homopolymer (Fig. 1).
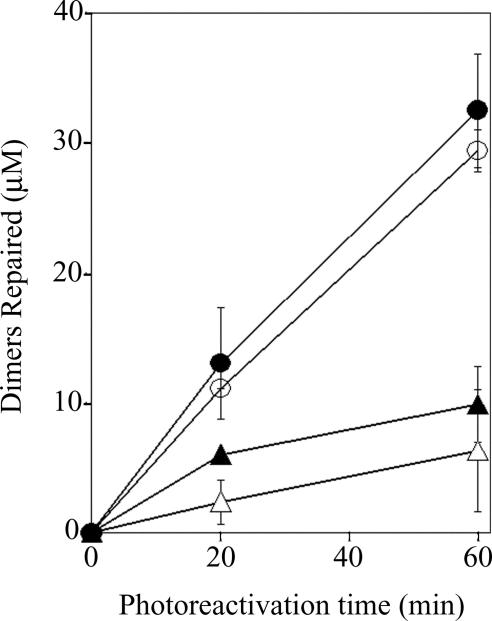
Fig. 1.
Repair of cyclobutane pyrimidine dimers in poly dU(U<>U) and poly rU(U<>U). VcPhr or VcCry1 at a 100 nM concentration were mixed with a 100 μM concentration of rU<>rU or dU<>dU in the corresponding nucleotide polymers and exposed to photoreactivating light for the indicated time periods. The level of repair was determined from the increase in absorbance at 260 nm (16). ○, VcPhr with poly dU(U<>U) substrate; ●, VcCry1 with poly dU(U<>U) substrate. ▵, VcPhr with poly rU(U<>U) substrate; ▴, VcCry1 with poly rU(U<>U) substrate. Error bars denote SDs where they are larger than the symbols.
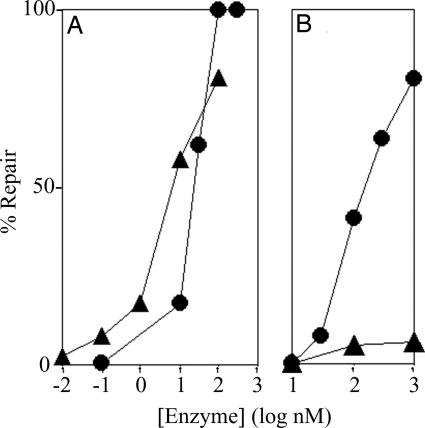
Previously we failed to detect photolyase activity in VcCry1 by using as substrate an oligonucleotide duplex containing a single cyclobutane thymine dimer (T<>T) (11). We reasoned that the repair activity of VcCry1 that we detect here with pyrimidine cyclobutane dimer (Pyr<>Pyr)-containing poly (dU) and poly (dT) was due to unique features of the homopolymers. We noted that the homopolymers, which are repaired robustly, are single-stranded. Weak repair of uniquely modified oligonucleotide duplexes by Cry-DASH enzymes has been reported (14), and could arise from repair of contaminating single-stranded substrate introduced in the preparation or storage of the duplexes. Hence, we tested both the single- and double-stranded forms of a 48-mer oligonucleotide containing a single T<>T as substrates for VcPhr and VcCry1. The results in Fig. 2 show that whereas photolyase demonstrates some preference for single-stranded over double-stranded substrates, in agreement with previous reports (17, 18), VcCry1 unequivocally repairs the dimer but only in ssDNA. Furthermore, the other Cry-DASH proteins from plant (AtCry3) and animal (XlCry-DASH) sources also demonstrated photoreactivation specifically toward ssDNA. In contrast, AtCry1 and BsUvrC had no detectable photorepair activity under any condition. AtCry1 is known to be a blue-light photoreceptor involved in the growth and development of Arabidopsis, and exhibits remarkable structural similarity to photolyase and the so called Cry-DASH branch of the photolyase/cryptochrome family (19). BsUvrC, a negative control, was selected for testing as a generic MBP fusion protein based upon availability.
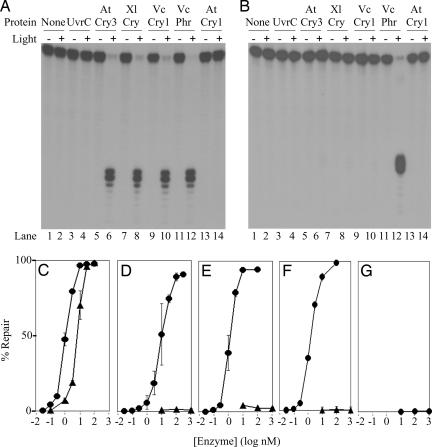
Fig. 2.
Repair of a cyclobutane thymine dimer (T<>T) in ss- and dsDNAs by photolyase and Cry-DASH family enzymes. (A and B) A 48-nt-long radiolabeled oligomer with a T<>T in the TTAA sequence (MseI recognition site) in the center, in single-stranded (A) or double-stranded (B) form, was mixed with the appropriate enzyme and exposed to photoreactivating light as indicated. The level of repair was determined by the susceptibility of the DNA to cleavage by MseI endonuclease. Single-stranded repair products were annealed with the complementary strand before digestion with MseI. The reaction products were separated on 5% polyacrylamide gels. Shown are autoradiograms of representative gels. Photolyase from Vibrio cholerae (VcPhr) was used as a positive control, and UvrC from Bacillus subtilis (UvrC) was used as a negative control. AtCry3, A. thaliana Cry-DASH; XlCry, Xenopus laevis Cry-DASH; VcCry1, V. cholerae Cry-DASH; AtCry1, A. thaliana cryptochrome blue-light photoreceptor. The substrate concentration was 0.27 nM, and the enzymes were at a concentration of 100 nM. Photoreactivation was for 2 h. (C–G) Repair of cyclobutane thymine dimer as a function of enzyme concentration for VcPhr (C), VcCry1 (D), XlCry (E), AtCry3 (F), and AtCry1 (G). Substrate at 0.27 nM was incubated with the indicated concentrations of enzymes and subjected to photoreactivating light for 2 h. ●, ssDNA; ▴, dsDNA. Error bars denote standard deviations where they are larger than the symbols.
Electrophoretic mobility shift assays were conducted to examine the binding of Cry-DASH enzymes to the T<>T in ss- and dsDNA. The results, in Fig. 3, show that dimer-specific binding parallels repair. Thus, the Cry-DASH enzymes fail to appreciably bind to or repair the dimer in dsDNA, but robustly bind to and repair the same dimer in ssDNA
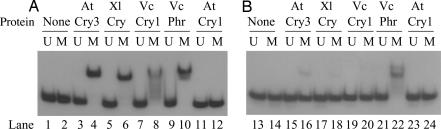
Fig. 3.
Binding of VcPhr and Cry-DASH enzymes to a cyclobutane thymine dimer. A radiolabeled 48-mer, either unmodified (U) or modified (M) to contain a cyclobutane thymine dimer, was prepared in a single-stranded (A) or double-stranded (B) form. Substrates were incubated with the enzymes shown, and then the bound and free DNAs were separated with 5% polyacrylamide gels. Note that weak, apparent binding of AtCry3 and Xl-DASH to the dimer in dsDNA likely results from binding to contaminating ssDNA substrate as indicated by the loss of free ssDNA in lanes 16 and 18.
Next, we examined whether VcCry1 in particular and Cry-DASH enzymes in general contribute to photoreactivation in vivo. In fact, we previously reported that null mutation of VcPhr abolished photoreactivation in V. cholerae under the experimental conditions used in our study (11). Having found that VcCry1 does have a potent photolyase activity toward ssDNA, we reasoned that by using a more sensitive assay we might be able to detect the contribution of VcCry1 to biological photoreactivation. To this end, E. coli strain UNC523 (uvrA−phr−), transformed with plasmids expressing MBP, MBPVcCry1, or MBPVcPhr at high levels, was exposed first to 254 nm light and then to photoreactivating light for various times. The photoresponses shown in Fig. 4 indicate that VcCry1 mediates photoreactivation in vivo at a rate that is <0.3% the rate seen with VcPhr. The in vivo photorepair mediated by VcCry1 may result from the actual repair of dimers in dsDNA, or from repair in regions of the supercoiled genomic DNA which transiently assume single-stranded character in the vicinity of the damage. In any event, the observed photorepair reinforces the notion that VcCry1 contributes very little, if any, to repair of genomic DNA.
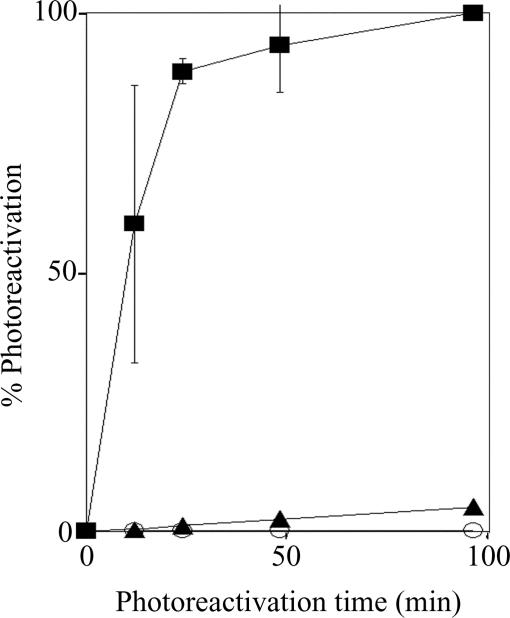
Fig. 4.
In vivo assay of photoreactivation by VcPhr and VcCry1 (Cry-DASH) enzymes. E. coli UNC523 (phr−uvrA−) transformed with vectors expressing maltose binding protein (○), MBP-VcCry1 (▴), or MBP-VcPhr (■) was irradiated with 8 J·m−2 of 254 nm light to ≈2 × 10−5 survival. Cultures then were exposed to photoreactivating light (366 nm) at a fluence rate of 24 J·m−2·sec−1 for the indicated times and plated. Percent photoreactivation is plotted as a function of photoreactivation time. Error bars denote standard deviations where they are larger than the symbols.
Next, we tested VcCry1 for its ability to restore the biological activity of single- and double-stranded plasmid DNAs that were inactivated with UV. Plasmids were irradiated with 254 nm to produce five to six photoproducts per molecule, 70–90% of which are cyclobutane pyrimidine dimers and hence are substrates for photolyase. The irradiated plasmids were mixed with VcPhr or VcCry1, exposed to photoreactivating light, and then transformed into a nucleotide excision repair deficient strain of E. coli. From the transformation efficiency, the level of repair at various enzyme concentrations was calculated (20). As seen in Fig. 5, the conventional photolyase, VcPhr, repaired ssDNA and dsDNA with comparable efficiencies. VcCry1, as expected, was very inefficient in repairing the supercoiled dsDNA, repairing it with ≈0.1% the efficiency of VcPhr. However, VcCry1 was essentially as active on ssDNA as VcPhr. We suggest that this activity is of biological relevance: lateral gene transfer has played an important role in evolution and some of this transfer may be in the form of ssDNA viruses or free ssDNA. The ssDNA photolyase family may contribute to this process by repairing the ssDNA that has been damaged by UV in transit.
Fig. 5.
Repair of UV-irradiated single- and double-stranded plasmids by VcPhr (A) and VcCry1 (Cry-DASH) (B) proteins. The plasmids were irradiated with 254 nm to generate approximately five photoproducts per plasmid as determined by the transformation assay (20). The irradiated plasmids were incubated with VcPhr or VcCry1 at the indicated concentrations, exposed to photoreactivating light for 2 h, and then used to transform E. coli strain CA1 (uvrA−). From the number of transformants before and after light exposure, the percent dimers repaired by the photoenzyme was calculated (20). ●, single-stranded plasmid; ▴, double-stranded plasmid. Data points are from a representative experiment. Note that some subtle differences exist between the repair of single- and double-stranded plasmid DNA in relation to the T<>T in the single-stranded and double-stranded oligomers (Fig. 1). We presume that these differences reflect the heterogeneity of the photodimers in the plasmid substrates and the nature of the assays used to measure repair of the plasmids and the oligonucleotides.
In conclusion, we define a new class of photolyases with specificity for cyclobutane pyrimidine dimers in ssDNA. These enzymes, which are found in bacteria, plants, and animals, and were previously designated Cry-DASH, because of the lack of significant photorepair activity on dsDNA, should be reclassified as ssDNA photolyases. It should be noted, however, that this classification does not necessarily exclude an additional nonrepair function for ssDNA photolyases, as indeed even some conventional photolyases have both repair and transcriptional regulatory functions (21). With regard to single-strand specificity of this class of enzymes, it must be noted that both biochemical (17) and crystallographic (22, 23) data indicate that conventional photolyases make most of their contacts with the damaged strand and the contacts with the undamaged strand are mostly van der Waals contacts of secondary importance. It appears that this preference for the damaged strand is amplified in this new family of photolyases by the presence of amino acid residues in the binding groove that clash with the phosphodiester backbone or the bases of the complementary strand. The accompanying paper by Deisenhoffer and coworkers (24) presents the structural basis for the single-strand preference for these enzymes.
Materials and Methods
Strains, Plasmids, and Phage.
E. coli K-12 strain UNC523 (uvrA::Tn10 phr::kan) was used (25). E. coli C strain CA1 (uvrA::Tn10) was constructed by transduction with phage P1 by using UNC523 as the donor. Plasmids include the vector pMal-c2 (New England Biolabs, Beverly, MA) and plasmids encoding MBP-VcPhr (pUNC2001) and MBP-VcCry1 (pUNC2002) described in ref. 11. We also used plasmids encoding the X. laevis Cry-DASH (pMalXL), the A. thaliana Cry3 (pMalAtC3), and B. subtilis UvrC (pMalUvrC) as MBP fusion proteins.
Proteins.
AtCry1 purified from insect cells and active in autophosphorylation assays was provided by S. Özgür (26, 27). It possesses a polyhistidine tag and was purified from insect cells by nickel affinity chromatography. MBP fusion proteins were purified according to the manufacturer's recommendations (New England Biolabs). Specifically, transformed UNC523 was grown in 6 × 1-liter batches at room temperature. IPTG was added to 0.3 mM at an OD600 of 0.6, and growth continued overnight. Cell pellets were washed and resuspended in 6 × 20 ml of column buffer (20 mM Tris, pH 7.4/200 mM NaCl/1 mM EDTA/10 mM 2-mercaptoethanol) and then frozen. Thawed suspensions were sonicated and pelleted. Supernatants were applied to columns containing 20 ml of amylose resin (New England Biolabs). Columns were washed with 10 volumes of column buffer and then eluted with 10 mM maltose in column buffer. Fractions were analyzed on polyacrylamide gels, pooled, and dialyzed against 50 mM Tris, pH 7.5/100 mM NaCl/1 mM EDTA/5 mM DTT/50% glycerol. Purified proteins were denatured by incubation at 60°C, and then absorbance at 450 nm was used to determine flavin concentration. The concentrations of flavoenzymes given in this work represent the concentration of flavin-containing protein. The UvrC concentration is based on comparison with known quantities of BSA that were separated alongside UvrC on SDS-polyacrylamide gels stained with Coomassie blue.
Assays.
Photoreactivation of homopolymeric substrates.
Substrate materials included poly (rU) from Sigma, poly (dU) from Midland Certified Reagent Co. (Midland, TX), and poly (dT) from GE Healthcare (Piscataway, NJ). Acetone-sensitized generation and quantitation of poly rU(U<>U), poly dU(U<>U), and poly dT(T<>T) were as described (16).
Photoreactivation buffer was 50 mM Tris (pH 7.5), 100 mM NaCl, 1 mM EDTA, and 10 mM DTT. Proteins at 100 nM each were incubated with a 100 μM concentration of pyrimidine dimer for 30 min and then exposed to 366 nm photoreactivating light, 2.4 mW/cm2, from two black lights (F15T8-BLB; General Electric), filtered through one plate of glass. Binding and photoreactivation were done on ice. After photoreactivation, samples were diluted 1:10 in water, and the level of repair was measured by absorbance change at 260 nm (16).
Photoreactivation of oligonucleotide with a single cyclobutane thymine dimer (T<>T).
The DNA substrate was synthesized from a dimer-containing 12-mer (GCGAAT<>TAAGCG; Phoenix BioTechnologies, Huntsville, AL), two arm oligomers of 18-nucleotides each, and a complementary 48-mer (GACGCAGATCTACGAATTCGCTTAATTCGCTGCACCGGATCCCGCTAG; Lineberger Nucleic Acids Core Facility, University of North Carolina), as described (6). Briefly, the oligonucleotides were phosphorylated, and the 12-mer was labeled with 32P. After annealing, ligation, and incubation with MseI (to eliminate the small fraction of substrate free of dimer), the 48-mer radiolabeled product was purified through a denaturing polyacrylamide gel.
Care was taken in preparing substrates for these experiments to minimize contamination of double-stranded labeled substrate with single-stranded labeled substrate. Toward this end, to make double-stranded substrate, the gel-purified radiolabeled strand at 5.3 nM was mixed with the unlabeled 48-mer complementary strand at 530 nM before annealing. To anneal, the DNAs were heated to 95°C and then cooled slowly. To make single-stranded substrate, the gel-purified material was heated to 95°C for 5 minutes, cooled on ice, and then the 100-fold excess of unlabeled 48-mer complementary strand was added so as to have identical DNA concentrations in both the ssDNA and dsDNA substrates. Photoreactivation buffer was as described for experiments with the homopolymers, except BSA was added to 100 μg/ml. Reactions contained radiolabeled substrate at 0.28 nM and the indicated enzyme concentrations. Binding and photoreactivation conditions were as described for the homopolymers. Reaction products were extracted with phenol, precipitated with ethanol, and resuspended in 10 mM Tris, pH 7.9/50 mM NaCl/10 mM MgCl2/1 mM DTT, with the labeled DNA strand at 0.14 nM. The complementary 48-mer was added to 175 nM. The DNA was annealed and then digested with MseI (10 units per reaction) after adding BSA to 100 μg/ml. Products were analyzed on 8% polyacrylamide sequencing gels.
Eletrophoretic mobility shift assay.
The gel-purified, radiolabeled 48-mer was also used for gel shift assays. To make double-stranded substrate, the gel-purified 48-mer was mixed with an equimolar amount of the complementary strand 48-mer before annealing. To make single-strand substrate, the gel-purified, radiolabeled 48-mer was heated at 95°C for 5 minutes, cooled on ice, and then an equimolar amount of complementary strand 48-mer was added. Binding reactions included 15 mM Tris (pH 7.5), 20 mM NaCl, 5 mM DTT, 50 μg/ml BSA, and 2 nM radiolabeled substrate. Proteins were at 100 nM except VcCry1 which was at 3 μM. Enzymes and substrates were mixed and incubated for 30 min on ice, and then protein-DNA binding was analyzed by separating products with nondenaturing 5% polyacrylamide gels in a buffer of 25 mM Tris, 25 mM borate, and 0.6 mM EDTA, pH 8.3. Gels were run in the dark at 4°C.
Photoreactivation in vivo.
Survival of E. coli UNC523 (phr−uvrA−) expressing MBP, MBPVcPhr, or MBPVcCry1 was measured by using overnight cultures, which express these proteins at a level of ≈10,000 molecules per cell under these conditions, compared with ≈10 molecules of native photolyase per wild-type E. coli. Cells were collected by centrifugation, resuspended in PBS, and then diluted from ≈109 cells/ml to 108 cells/ml for irradiation. UV damage was produced with germicidal light, 8 J/m2, delivered at a rate of 0.21 to 0.23 J·m−2·sec−1. Cultures were then exposed, with mixing, to blacklight, ≈5 J·m−2·sec−1, and samples were withdrawn at various time points. Samples of irradiated and unirradiated cultures were diluted and plated on LB agar and incubated in the dark at 37°C to allow surviving cells to grow into colonies. Survival was calculated as (colonies + UV)/(colonies − UV). UV produced ≈2 × 10−5 survival. Photoreactivation was calculated as (survival + photoreactivation)/(survival − photoreactivation). In each experiment, the maximum level of photoreactivation observed in the culture expressing VcPhr was given a value of 100%, and all of the other values within the experiment were divided by this value to give percent photoreactivation.
Repair of ssDNA and dsDNA Plasmids by VcPhr and VcCry1.
These assays were carried out essentially as described elsewhere (20). We used pPU192 (28) isolated in single-stranded form after superinfection with M13 helper phage, and the supercoiled, double-stranded pGL3 promoter vector (Promega, Madison, WI). Plasmids were irradiated with 400 J·m−2 (single-stranded) or 300 J·m−2 (double-stranded) 254 nm light. VcPhr or VcCry1 at the indicated concentrations were incubated with plasmid at 150 ng/μl in 50 mM Tris, pH 7.5/10 mM NaCl/1 mM EDTA/10 mM DTT. Binding and photoreactivation were as described for experiments with homopolymeric substrates, with photoreactivation lasting 2 h. E. coli CA1 competent cells were then transformed, and appropriate dilutions of transformed cells were plated on media containing 200 μg·ml−1 ampicillin. Transformant colonies were counted, and quantitation was as described (20).
Acknowledgments
We thank I. H. Kavakli (University of North Carolina) for providing the MBPXL and MBPAtCry3 constructs, S. Özgür (University of North Carolina) for providing the AtCry1 protein, and Carla McDowell-Buchanan for initiating the comparative analysis of the activities of VcPhr and VcCry1 with homopolymeric substrates and for her discovery of RNA photolyase activity in VcCry1. This work was supported by National Institutes of Health Grant GM31082.
Abbreviations
- Cry-DASH
Cryptochrome-Drosophila, Arabidopsis, Synechocystis, Human
- Pyr<>Pyr
pyrimidine cyclobutane dimer
- T<>T
thymine cyclobutane dimer
- U<>U
uracil cyclobutane dimer.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cashmore AR. Cell. 2003;122:537–543. [PubMed] [Google Scholar]
- 2.Lin C, Shalitin D. Annu Rev Plant Biol. 2003;54:469–496. doi: 10.1146/annurev.arplant.54.110901.160901. [DOI] [PubMed] [Google Scholar]
- 3.Sancar A. Chem Rev. 2003;103:2203–2237. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad M, Cashmore AR. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 5.Guo H, Yang H, Mockler TC, Lin C. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- 6.Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang R-P, Todo T, Wei Y-F, Sancar A. Biochemistry. 1996;35:13871–13877. doi: 10.1021/bi962209o. [DOI] [PubMed] [Google Scholar]
- 7.Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, Sancar A. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- 8.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 9.Hitomi K, Okamoto K, Daiyasu H, Miyashita H, Iwai S, Toh H, Ishiura M, Todo T. Nucleic Acids Res. 2000;28:2353–2362. doi: 10.1093/nar/28.12.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng WO, Pakrasi HB. Mol Gen Genet. 2001;264:924–930. doi: 10.1007/s004380000383. [DOI] [PubMed] [Google Scholar]
- 11.Worthington EN, Kavakli H, Berrocal-Tito G, Bondo BE, Sancar A. J Biol Chem. 2003;278:39143–39154. doi: 10.1074/jbc.M305792200. [DOI] [PubMed] [Google Scholar]
- 12.Kleine T, Lockhart P, Batschauer A. Plant J. 2003;35:93–103. doi: 10.1046/j.1365-313x.2003.01787.x. [DOI] [PubMed] [Google Scholar]
- 13.Brudler R, Hitomi K, Daiyasu H, Toh H, Kucho K, Ishiura M, Kanehisa M, Roberts VA, Todo T, Tainer JA, et al. Mol Cell. 2003;11:59–67. doi: 10.1016/s1097-2765(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 14.Daiyasu H, Ishikawa T, Kuma K, Iwai S, Todo T, Toh H. Genes Cells. 2004;9:479–495. doi: 10.1111/j.1356-9597.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- 15.Heidelberg J. F., JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L, et al. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S-T, Sancar A. Biochemistry. 1991;30:8623–8630. doi: 10.1021/bi00099a019. [DOI] [PubMed] [Google Scholar]
- 17.Husain I, Sancar GB, Holbrook SR, Sancar A. J Biol Chem. 1987;262:13188–13197. [PubMed] [Google Scholar]
- 18.Zhao X, Liu J, Hsu DS, Zhao S, Taylor J-S, Sancar A. J Biol Chem. 1997;272:32580–32590. doi: 10.1074/jbc.272.51.32580. [DOI] [PubMed] [Google Scholar]
- 19.Brautigam CA, Smith BS, Ma Z, Palnitkar M, Tomchick DR, Machius M, Deisenhofer J. Proc Natl Acac Sci USA. 2004;101:12142–12147. doi: 10.1073/pnas.0404851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sancar A, Smith FW, Sancar GB. J Biol Chem. 1984;259:6028–6032. [PubMed] [Google Scholar]
- 21.Berrocal-Tito GB, Rosales-Saavedra T, Herrera-Estrella A, Horwitz BA. Photochem Photobiol. 2000;71:662–668. doi: 10.1562/0031-8655(2000)071<0662:coblad>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Park H, Kim S, Sancar A, Deisenhofer J. Science. 1995;268:1866–1872. doi: 10.1126/science.7604260. [DOI] [PubMed] [Google Scholar]
- 23.Mees M, Klar T, Gnau P, Hennecke U, Eker AP, Carell T, Essen LO. Science. 2004;306:1789–1793. doi: 10.1126/science.1101598. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Baxter R, Smith BS, Partch CL, Colbert CL, Deisenhofer J. Proc Natl Acad Sci USA. 2006 doi: 10.1073/pnas.0608554103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra K, Kim ST, Batschauer A, Dawut L, Sancar A. Biochemistry. 1995;34:6892–6899. doi: 10.1021/bi00020a037. [DOI] [PubMed] [Google Scholar]
- 26.Partch CL, Clarkson MW, Ozgur S, Lee AL, Sancar A. Biochemistry. 2005;44:3795–3805. doi: 10.1021/bi047545g. [DOI] [PubMed] [Google Scholar]
- 27.Özgür S. PhD thesis. Chapel Hill: University of North Carolina; 2005. [Google Scholar]
- 28.Selby CP, Drapkin R, Reinberg D, Sancar A. Nucleic Acids Res. 1997;25:787–793. doi: 10.1093/nar/25.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]