Abstract
GR231118 (also known as 1229U91 and GW1229), a purported Y1 antagonist and Y4 agonist was radiolabelled using the chloramine T method.
[125I]-GR231118 binding reached equilibrium within 10 min at room temperature and remained stable for at least 4 h.
Saturation binding experiments showed that [125I]-GR231118 binds with very high affinity (Kd of 0.09–0.24 nM) in transfected HEK293 cells with the rat Y1 and Y4 receptor cDNA and in rat brain membrane homogenates. No specific binding sites could be detected in HEK293 cells transfected with the rat Y2 or Y5 receptor cDNA demonstrating the absence of significant affinity of GR231118 for these two receptor classes.
Competition binding experiments revealed that specific [125I]-GR231118 binding in rat brain homogenates is most similar to that observed in HEK293 cells transfected with the rat Y1, but not rat Y4, receptor cDNA.
Autoradiographic studies demonstrated that [125I]-GR231118 binding sites were fully inhibited by the Y1 antagonist BIBO3304 in most areas of the rat brain. Interestingly, high percentage of [125I]-GR231118/BIBO3304-insensitive binding sites were detected in few areas. These [125I]-GR231118/BIBO3304-insensitive binding sites likely represent labelling to the Y4 receptor subtype.
In summary, [125I]-GR231118 is a new radiolabelled probe to investigate the Y1 and Y4 receptors; its major advantage being its high affinity. Using highly selective Y1 antagonists such as BIBO3304 or BIBP3226 it is possible to block the binding of [125I]-GR231118 to the Y1 receptor allowing for the characterization and visualization of the purported Y4 subtype.
Keywords: Neuropeptide Y, receptor subtype, receptor distribution, radioligand
Introduction
Neuropeptide Y (NPY) is a 36 amino acid peptide which shares high sequence homology with peptide YY (PYY) and the pancreatic polypeptides (PP) (Tatemoto et al., 1992). NPY is one of the most abundant peptides found in the mammalian brain (de Quidt & Emson, 1986a,1986b). Several biological effects have been observed following intracerebroventricular injections of NPY and its congeners including increased feeding, decreased anxiety related-behaviours, modulation of LH-RH and ACTH secretions, and the regulation of various neuronal, activities (for reviews see Colmers & Bleakman, 1994; Dumont et al., 1992; Gehlert, 1998; Inui, 1999; Kalra & Crowley, 1992; Vezzani et al., 1999; Wahlestedt & Reis, 1993). These various effects of NPY are mediated by the activation of at least five receptor subtypes expressed in mammalian brain (Dumont et al., 1996a; 1997; 1998a,1998b; Gehlert & Gackenheimer, 1997; Gehlert et al., 1997; Jacques et al., 1997; Statnick et al., 1997; Trinh et al., 1996; Whitcomb et al., 1997).
Several studies using [125I]-NPY or [125I]-PYY as radioligands have demonstrated that the rat brain contained high amounts of NPY receptors distributed in various brain structures including the cortex, the hippocampus, multiple thalamic, hypothalamic and brainstem nuclei, and the cerebellum and the spinal cord (Dumont et al., 1992; Lynch et al., 1989; Martel et al., 1990; Kar & Quirion, 1992; Quirion & Martel, 1992). Subsequent autoradiograph studies using [125I]-PYY in the presence of [Pro34]-PYY or C-terminal NPY/PYY fragments revealed the existence of at least two classes (Y1-like and Y2-like) of receptors in the rat brain (Aicher et al., 1991; Dumont et al., 1990; 1993; Gehlert et al., 1992; Larsen et al., 1993). The differential distribution of these receptor subtypes was confirmed using more selective radioligands such as [125I]-Leu31,Pro34]-PYY (Y1-like) and [125I]-PYY3–36 (Y2-like) (Dumont et al., 1995; 1996a; Gehlert & Gackenheimer, 1997). However, further competition binding experiments revealed that both radioligands also recognized additional receptor sites suggesting further NPY receptor heterogeneity in the CNS (Dumont et al., 1995; 1998a; Statnick et al., 1997).
The cloning of additional NPY receptors confirmed this hypothesis. In addition to the well established Y1 and Y2 subtypes proposed by Wahlestedt et al. (1986) on the basis of the differential potencies of C-terminal fragments in various bioassays, three additional NPY receptors have been cloned namely by Y4 (Bard et al., 1995; Gregor et al., 1996a; Lundell et al., 1995; 1996), Y5 (Gerald et al., 1996; Hu et al., 1996) and y6 (Gregor et al., 1996b; Matsumoto et al., 1996; Weinberg et al., 1996) subtypes. They are all expressed in various mammalian species except for the y6 subtype which has not been found in the rat (Burkhoff et al., 1998) while in human and primates, its cDNA contains a single base deletion resulting in the expression of a non-functional receptor protein (Gregor et al., 1996b; Matsumoto et al., 1996). Structure-activity studies revealed that each receptor subtype has a unique pharmacological profile (Michel et al., 1998). However, none of the synthetic agonists used thus far demonstrated high selectivity for a single receptor subtype. In fact, [125I]-[Leu31,Pro34]-PYY, first developed as a Y1 agonist radioligand (Dumont et al., 1995), was later demonstrated to bind with high affinity to the Y4 (Gehlert et al., 1996a,1996b; 1997) and Y5 (Dumont et al., 1998a; Gerald et al., 1996) receptors. Similarly, [125I]-PYY3–36 originally proposed as a Y2 receptor probe (Dumont et al., 1995) was found to also bind to the Y5 (Gerald et al., 1996) and possibly y6 (Matsumoto et al., 1996) subtypes. Additionally, [125I]-human (h) PP and [125I]-rat (r) PP (Trinh et al., 1996) as well as [125I]-bovine (b) PP (Gehlert et al., 1997; Whitcomb et al., 1997) used to characterize the Y4 subtype, also recognize the Y5 (Dumont et al., 1998a; Gerald et al., 1996) and likely y6 (Gregor et al., 1996b) receptor subtypes.
Few antagonists have also been used as radioligands to study NPY receptors. Among them, (R-N2-(Diphenylacetyl)-N-(4-hydroxyphenyl)-methyl argininamide), known as BIBP3226, a non-peptide Y1 receptor antagonist (Rudolf et al., 1994) which demonstrated no activity at the Y2, Y4 and Y5 subtypes (Gerald et al., 1996; Gehlert et al., 1996b,1996c; Jacques et al., 1995; Rudolf et al., 1994) was used as radioligand (Entzeroth et al., 1995). However, autoradiographic studies revealed that [3H]-BIBP3226 lacked adequate affinity leading to high non-specific labelling and low resolution compared to [125I]-[Leu31,Pro34]-PYY (Dumont et al., 1996b). More recently, the same group of investigators reported the development of a second non-peptide Y1 receptor antagonist ((R)-N-[[4-(aminocarbonylaminomethyl) - phenyl]methyl] - N2 - (diphenylacetyl)-argininamide trifluoroacetate) or BIBO3304 having a 10 fold higher affinity than BIBP3226 for the Y1 receptor with a similar selectivity profile (Dumont et al., 1999; Wieland et al., 1998). However, BIBO3304 is not available in radiolabelled form. In that context, the peptidergic Y1 antagonist, homodimeric Ile-Glu-Pro-Dpr-Tyr-Arg-Leu-Arg-Tyr-CONH2 known as GR231118 (or 1229U91, Daniels et al., 1995; and GW1229, Bitran et al., 1997) could prove most useful taking into account its very high affinity for the Y1 (Daniels et al., 1995; Dumont et al., 1998a) and Y4 (Parker et al., 1998; Schober et al., 1998) receptor subtypes. GR231118, first reported as a potent Y1 antagonist (Daniels et al., 1995) was recently shown to be a potent agonist at the Y4 (Parker et al., 1998; Schober et al., 1998) and possibly y6 (Parker et al., 1998) receptors; the later being absent in rat and primate tissues (Burkhoff et al., 1998; Gregor et al., 1996b; Matsumoto et al., 1996). We report here on the development of [125I]-GR231118 as a new radioligand having very high affinity for the Y1 (Kanatani et al., 1996) and Y4 receptors expressed in HEK293 cells and endogenously in rat brain tissues.
Methods
Materials
Male Sprague Dawley CD rats (200–250 g) obtained from Charles River Canada (St-Constant, Québec, Canada) were kept on a 12 h light-dark cycle (light on at 07.00 h) in temperature and humidity controlled rooms. Animals were fed with standard laboratory chow and had access to tap water ad libitum. Animal care was according to protocols and guidelines approved by McGill University and the Canadian Council of Animal Care.
Analogues and fragments of hPYY, porcine (p) NPY and hPP were synthesized as previously described (Forest et al., 1990) while rPP was purchased from Bachem California (Torrance, CA, U.S.A.). R-N2-(Diphenylacetyl)-N-(4-hydroxyphenyl)-methyl argininamide, known as BIBP3226 and ((R)-N-[[4-(aminocarbonylaminomethyl) - phenyl]methyl] - N2 - (diphenylacetyl)-argininamide trifluoracetate), code name BIBO3304 were generously provided by Boehringer Ingelheim (Germany) while homodimeric Ile-Glu-Pro-Dpr-Tyr-Arg-Leu-Arg-Tyr-CONH2, (firstly known as 1229U91, GW1229 and now GR231118) was a gift from Glaxo Wellcome (Research Triangle Park NC, U.S.A.). Human embryonic kidney cells (HEK293) were obtained from Drs S.H. Shen and Y. Tong, Biotechnology Research institute (Montréal, QC, Canada). Bovine serum albumin (BSA) and Iodine-125 were obtained from ICN Pharm. Canada Ltd. (Montréal, QC, Canada) and bacitracin was purchased from Sigma Chemical (St-Louis, MI, U.S.A.). Schleicher and Schuell #32 glass filters were obtained from Xymotech (Montréal, QC, Canada). [3H]-Hyperfilms and 125I-microscale standards were purchased from Amersham (Mississauga, ON, Canada). All tissue culture media, antibiotics and reagents were obtained from Gibco-BRL (Burnington, ON, Canada). The expressing vector, pcDNA3, was purchased from Invitrogen (San Diego, CA, U.S.A.). All other chemicals were of analytical grade and obtained from Fisher Scientific (Montréal, QC, Canada) or Sigma Chemical (St-Louis, MI, U.S.A.).
Iodine-125 was incorporated into the tyrosine residue of GR231118 using the chloramine T method as previously described (Dumont et al., 1995) and the specific activity was assumed to be of the theoretical value (2000 Cimmol−1).
Membrane preparations
Membranes were prepared as previously described (Dumont et al., 1995). Briefly, rats were killed by decapitation and their brains rapidly removed and homogenized in a Krebs Ringer phosphate (KRP) buffer at pH 7.4 of the following composition (mM): NaCl 120, KCl 4.7, CaCl2 2.2, KH2PO4 1.2, MgSO4 1.2, dextrose 5.5 and NaHCO3 25, using a Brinkman polytron (at setting 6 for 15–20 s). Homogenates were centrifuged at 49,000 × g for 20 min, supernatants discarded and pellets washed, resuspended, and recentrifuged twice. Protein concentration was determined with BSA as the standard (Bradford et al., 1976).
Transfected cells
HEK 293 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% foetal calf serum and antibiotics (penicillin G sodium, streptomycin sulphate and amphotericin B). Cultured cells were transfected with either of the rat Y1, Y2, Y4 or Y5 receptor cDNA using a calcium phosphate method (Tong et al., 1995). Briefly, 125 μl of 2.5 M calcium phosphate was added to 1.125 ml water containing 50 μg of either rat Y1, Y2, Y4 or Y5 receptor cDNA which was previously inserted in expressing pcDNA3 vectors and was slowly mixed with 1.25 ml 2× HEPES buffer at pH 7.05 and left at room temperature for 20 min. The mixture was added to a 150 mm dish containing HEK293 cells at 30% confluent and returned to the incubator. The medium was changed the next morning. Forty-eight h later, cells were washed with KRP buffer pH 7.4 and scratched. Detached cells were then centrifuged at 400×g for 10 min and the pellet washed with KRP buffer (pH 7.4), recentrifuged twice, and resuspended in 8 ml of KRP buffer pH 7.4 and used for receptor binding assay.
Binding assays
All binding assays were initiated by adding 100 μl of membrane preparations in a final volume of 500 μl of KRP containing 0.1% (w v−1) BSA, 0.05% (w v−1) bacitracin, [125I]-GR231118 and unlabelled peptide or competitor as needed. Time dependency was established using 25 pM [125I]-GR231118 at both 4°C and room temperature. Isotherm saturations and competition binding assays were performed at room temperature. Saturation experiments were performed in the presence of increasing concentrations of [125I]-GR231118 whereas competition binding experiments were performed in the presence of 25–30 pM [125I]-GR231118 and various competitors (pNPY, hPYY, [Leu31,Pro34]-pNPY, [Leu31,Pro34]-pPYY, pNPY2–36, pNPY13–36, hPYY3–36, hPYY13–36, rPP, hPP, GR231118, BIBO3304 and BIBP3226) at concentrations ranging from 10−13 M to 10−6 M. Non-specific binding was determined in the presence of 1 μM GR231118. After a 1 h incubation, the binding reaction was terminated by rapid filtration through Schleicher and Schuell #32 glass filters (previously soaked in 1.0% polyethyleneimine) using a cell harvester filtering apparatus (Brandel Instruments, Gaithersburg, MD, U.S.A.). Filters were rinsed three times with 3 ml cold KRP and the radioactivity remaining on filters was quantified using a gamma counter with 85% efficiency (Camberra Packard Instruments, Meriden CT, U.S.A.).
All binding experiments were repeated three to six times (each in triplicate), and results (mean±s.e.mean) expressed as percentage of specific binding or fmol mg−1 protein. All data obtained from the saturation isotherm experiments were subtracted for [125I]-GR231118 values found on filters in absence of membrane homogenates. Kd, Bmax and half time association values were calculated from data using the GraphPad Prism (GraphPad Software Inc. San Diego, CA, U.S.A.). IC50 values (i.e. concentration of unlabelled competitor required to compete for 50% of specific binding of the radioligand) for the various competitors were calculated using the GraphPad Prism.
Quantitative receptor autoradiography
Receptor autoradiography was performed as described in details elsewhere (Dumont et al., 1996a; 1998a). Briefly, rats were sacrificed by decapitation, and their brains rapidly removed from the skull, frozen in 2-methylbutane at −40°C for 15 s, and then kept at −80°C until needed. Sections (20 μM) were obtained using a cryomicrotome at −17°C, mounted on gelatin-chrome-alum-coated slides, dried overnight in a desiccator at 4°C, and then kept at −80°C until use.
On the days of the experiments, adjacent coronal sections were preincubated for 60 min at room temperature in KRP buffer at pH 7.4 and then incubated for 60 min in a fresh preparation of KRP buffer containing 0.1% BSA, 0.05% bacitracin, 25 pM [125I]-GR231118 in the presence and absence of 100 nM BIBO3304 (Y1 antagonist), hPP(Y4/Y5) or GR231118. Following a 1 h incubation, sections were washed four times, 2 min each in ice-cold KRP buffer then dipped in deionized water to remove salts and rapidly dried. Non-specific binding was determined using 100 nM GR231118. Incubated sections were apposed against 3H-Hyperfilms for 4 days alongside radioactive standards. Films were developed and quantified as described in details elsewhere (Dumont et al., 1996a; 1998a).
Results
GR231118, a Y1 receptor antagonist/Y4 agonist was iodinated using the chloramine T method (Hunter & Greenwood, 1962) and purified by HPLC. All binding experiments were performed with enriched fractions of the iodinated peptide. We tested first if [125I]-GR231118 could bind to the glass fibre filters used to terminate incubation. Various concentrations (5–2000 pM) of [125I]-GR231118 were incubated in 0.5 ml of KRP at room temperature for 1 h in the presence or absence of 1 μM GR231118 but without membrane homogenates. [125I]-GR231118 binding increased linearly with increasing concentrations of radioligands and no difference was observed between [125I]-GR231118 bound to filters in the presence or absence of 1 μM GR231118 (not shown) indicating that [125]-GR231118 did not bind specifically to filters. Amounts of [125I]-GR231118 found on filters represented less than 1% of the total radioactivity of the incubation buffer.
As shown in Figure 1, [125I]-GR231118 binding reached equilibrium in a time- and temperature-dependent manner in rat brain membrane homogenates. Specific [125I]-GR231118 binding reached equilibrium after 45 min at 4°C and remained stable for at least 3 h (Figure 1). Similarly, specific [125I]-GR231118 binding reached equilibrium within 10 min at room temperature and remained stable for up to 4 h (Figure 1). Half time association (t½) was 2.4 min at room temperature and 9.8 min at 4°C. All subsequent experiments were performed at room temperature using 60 min incubation periods.
Figure 1.
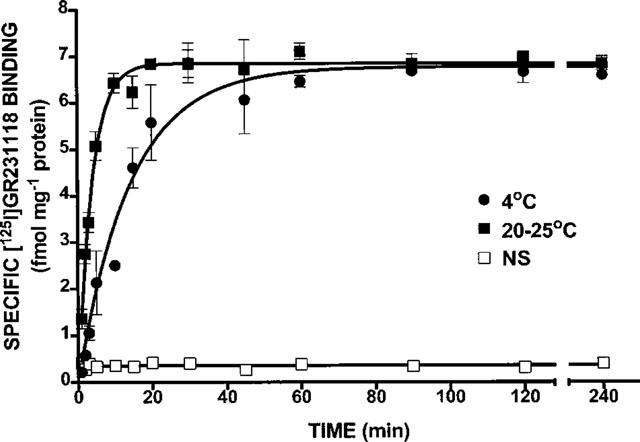
Typical profiles of the time course association of [125I]-GR231118 binding in rat brain membrane homogenates at 4°C and room temperature. Data represent the mean±s.e.mean of a prototypical experiment performed in triplicate. This experiment was repeated three times with similar results.
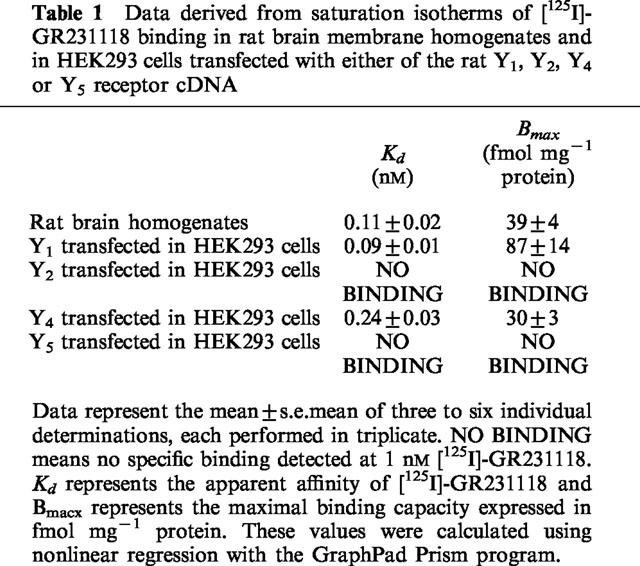
Saturation parameters of [125I]-GR231118 binding in rat brain membrane homogenates and HEK293 cells transfected with either of the rat Y1, Y2, Y4 or Y5 receptor cDNA were established next. In rat brain membrane preparations, specific [125I]-GR231118 binding (25 pM) represented 95% of totally bound ligand (Figure 2). Binding parameters derived from saturation isotherms (Figure 2) demonstrated that [125I]-GR231118 binds with very high affinity (Kd of 0.11±0.02 nM) to an apparent single class of saturable sites (Bmax of 39±4 fmol mg−1 protein) in rat brain homogenates (Table 1). The nature and specificity of [125I]-GR231118 binding was demonstrated further using HEK293 cells transfected with either of the rat Y1, Y2, Y4 or Y5 receptor cDNA. Using concentrations up to 1 nM, we could not detect significant amounts of specific [125I]-GR231118 binding sites in HEK293 cells expressing the rat Y2 and Y5 receptors. In contrast, saturation isotherms demonstrated that [125I]-GR231118 bound with very high affinity (Kd of 0.09±0.01 nM for Y1 and 0.24±0.03 nM for Y4) to saturable population of sites in HEK293 cells transfected with the rat Y1 or Y4 receptor cDNA (Table 1).
Figure 2.
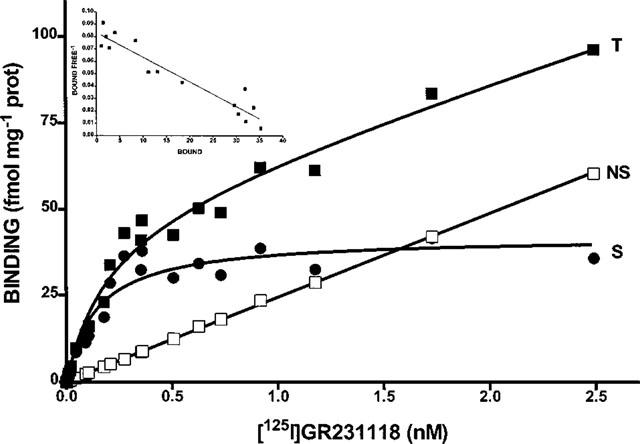
Typical profiles of saturation binding isotherms of [125I]-GR231118 binding in rat brain membrane homogenates. Membranes were incubated with increasing concentrations of [125I]-GR231118 (T; total binding) in the presence of 1 μM GR231118 (NS; non-specific binding). Specific (S) binding represents the difference between total and non-specific binding. Insert is a Scatchard transformation of the isotherm saturation binding experiment. Data represent the mean±s.e.mean of a prototypical experiment performed in triplicate.
Table 1.
Data derived from saturation isotherms of [125I]-GR231118 binding in rat brain membrane homogenates and in HEK293 cells transfected with either of the rat Y1, Y2, Y4 or Y5 receptor cDNA
The ligand binding profile of sites targeted by [125I]-GR231118 was investigated next in HEK293 cells transfected with the rat Y1 or Y4 receptor cDNA, as well as in rat brain homogenates. In HEK293 cells expressing the rat Y1 receptor cDNA, the observed competition binding profile was as follows: BIBO3304 (Y1 antagonist)=GR231118> BIBP3226 (Y1 antagonist)=pNPY, [Leu31,Pro34]-pNPY>hPYY, [Leu31,Pro34]-pPYY>>pNPY2–36, hPYY3–36>pNPY13-36, hPYY 13–36, hPP and rPP (Table 2). As expected, a completely different profile was obtained in HEK293 cells transfected with the rat Y4 receptor. While hPP and rPP were basically unable to compete for [125I]-GR231118 binding sites in Y1-transfected HEK293 cells, both peptides potently inhibited binding in HEK293 cells transfected with the rat Y4 receptor cDNA (Table 2). In contrast, BIBP3226 and BIBO3304 were inactive on Y4 transfected cells (Table 2). In rat brain homogenates, competition binding experiments revealed that GR231118, BIBO3304 and BIBP3226 competed with high affinities for specific [125I]-GR231118 sites (Figure 3; Table 2). Interestingly, while GR231118 was able to fully inhibit specific [125I]-GR231118 binding, the non-peptide Y1 receptor antagonists, BIBP3226 and BIBO3304 competed for approximately 95% of specific [125I]-GR321118 binding sites. These data may indicate that [125I]-GR321118 is labelling at least two population of sites in the rat brain, the major one being the Y1 subtype.
Table 2.
Competition binding parameters of various agonists and antagonists of the NPY family against [125I]-GR231118 binding in rat brain homogenates and HEK 293 cells transfected with either of the rat Y1 or Y4 receptor cDNA
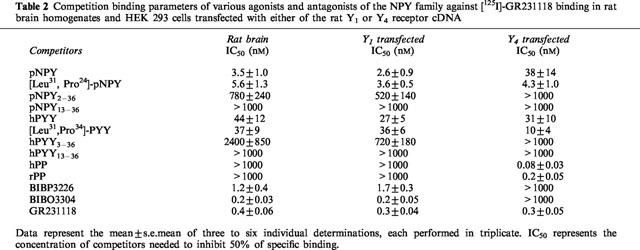
Figure 3.
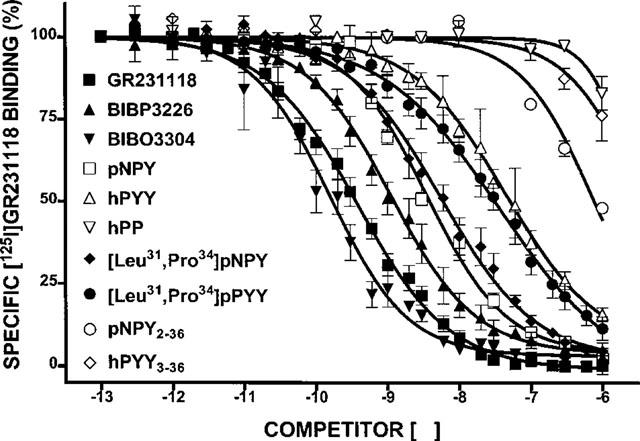
Competition binding profiles of various agonists and antagonists of the NPY family against specific [125I]-GR231118 binding in brain membrane homogenates. Data represent the mean±s.e.mean of four to six determinations, each performed in triplicate.
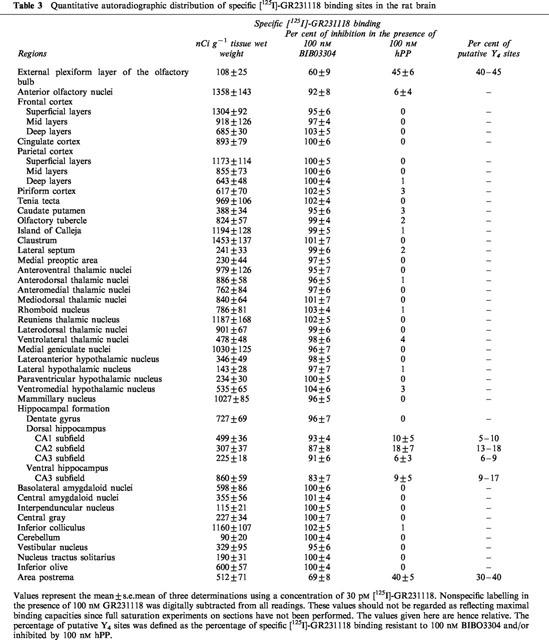
Further evidence for the existence of [125]-GR231118 binding sites which are resistant to BIBO3304 was provided using adjacent coronal rat brain sections incubated with [125I]-GR231118 (25–30 pM) in the presence of either 100 nM BIBO3304 (to occlude Y1 sites), hPP (to block Y4 sites) or GR231118 (total population of labelled sites). The concentration of competitors used in autoradiographic studies was chosen on the basis of data obtained in membrane binding assays which demonstrated that 100 nM of GR231118 and BIBO3304 generated the maximal inhibition of [125I]-GR231118 binding obtainable by each molecule (Figure 3). As shown in Figure 4, specific [125I]-GR231118 binding sites are fully competed by the Y1 antagonist BIBO3304 in the anterior olfactory nuclei, tenia tecta, claustrum, olfactory tubercle, islands of Calleja, lateral septum, various cortical areas, the dentate gyrus of the hippocampus, various thalamic, hypothalamic and brainstem nuclei, the nucleus tractus solitarius and the cerebellum, revealing the Y1 nature of specific [125I]-GR231118 labelling in these structures. Interestingly, relatively high levels of [125I]-GR231118/BIBO3304-insensitive binding sites were detected in the area postrema (Figure 4). Quantitative autoradiographic data confirmed that most, if not all, specific [125I]-GR231118 binding sites seen in most areas of the rat brain are fully competed by 100 nM BIBO3304 (Table 3). However, significant amounts of specific [125I]-GR231118/BIBO3304-insensitive sites were observed in the area postrema (Table 3). Additionally, few other areas such as the external plexiform layer of the olfactory bulb and the CA1, CA2 and CA3 subfields of the hippocampus contained low but still significant amounts of [125I]-GR231118 sites that were resistant to BIBO3304 (Table 3). These sites may belong to the Y4 subtype since hPP was able to compete for a fraction of specific [125I]-GR231118 labelling in these regions (Table 3).
Figure 4.
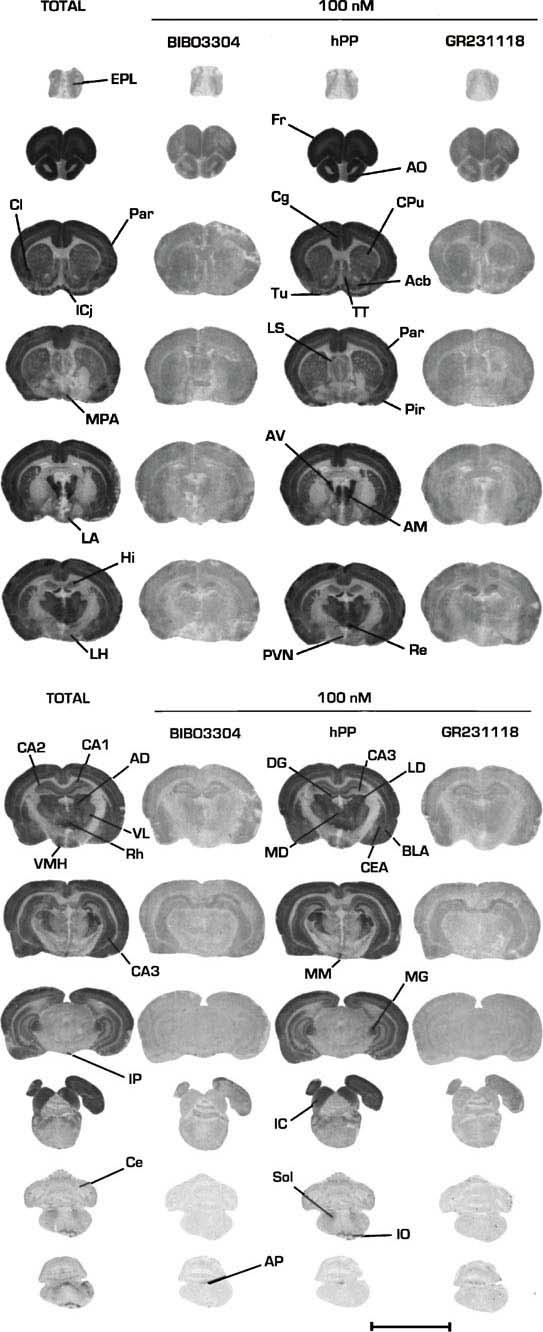
Photomicrographs of the autoradiographic distribution of [125I]-GR231118 binding sites in the rat brain. Adjacent coronal rat brain sections were incubated in the presence of 25 pM [125I]-GR231118 (total binding, Total) and either 100 nM BIBO3304 (to block Y1 sites), 100 nM hPP (to block Y4 sites), 100 nM hPP (to block Y4 sites) or 100 nM GR231118 (non-specific binding). Abbreviations: Acb, Accumbens; AD, anterodorsal thalamic nuclei; AM, anteromedial thalamic nuclei; AO, anterior olfactory nuclei; AP, area postrema; AV, anteroventral thalamic nuclei; BLA, basolateral amygdaloid nuclei; CA1, CA1 subfield of the hippocampus; CA2, CA2 subfield of the hippocampus; CA3, CA3 subfield of the hippocampus; Ce, cerebellum; CEA, Central amygdaloid nucleus; Cg, cingulate cortex; Cl, claustrum; CPu, caudate putamen; DG, dentate gurus; EPL, external plexiform layer of the olfactory bulb; Fr, frontal cortex; Hi, hippocampus; IC, inferior colliculus; ICj, Island of Calleja; IO, inferior olive; IP, interpeduncular nucleus; LA, lateroanterior hypothalamic nucleus; LD, laterodorsal thalamic nuclei; LH, lateral hypothalamic nucleus; LS, lateral septum; MD, mediodorsal thalamic nuclei, MG, medial geniculate nuclei, MM, mamilary nucleus; MPA, medial preoptic area; Par, parietal cortex; Pir, piriform cortex; PVN, paraventricular hypothalamic nucleus; Re, reuniens thalamic nucleus; Rh, Rhomboid thalamic nucleus; Sol, nucleus tractus solitarius; TT, tenia tecta; Tu, olfactory tubercle; VL, ventrolateral thalamic nuclei; VMH, ventromedial hypothalamic nucleus. Scale bar represents 10 mm.
Table 3.
Quantitative autoradiographic distribution of specific [125I]-GR231118 binding sites in the rat brain
Discussion
Our results demonstrated that [125I]-GR231118 has very high affinity (Kd in the sub-nM range) for the Y1 and Y4 receptor subtypes while being mostly inactive on the Y2 and Y5 receptors. In fact, [125I]-GR231118 is one of the highest affinity radioligand developed thus far to target the Y1 receptor subtype including [125I]-[Leu31,Pro34]-PYY (Dumont et al., 1995) and [3H]-BIBP3226 (Entzeroth et al., 1995). These two radioligands have at least a 10 fold lower affinity for the Y1 receptor compared to [125I]-GR231118. This new radioligand also has very low non-specific binding, this being a major advantage for both homogenate binding assays and receptor autoradiography. Moreover, despite its relatively poor selectivity for the Y1 vs Y4 receptor subtypes, [125I]-GR231118 in the presence of a saturating concentration of a highly selective Y1 antagonist such as BIBO3304, allows for the investigation of the Y4 receptor without interference from the Y5 subtype, in contrast to PP-related radioligands (Gerald et al., 1996; Hu et al., 1996). Hence, [125I]-GR231118 should become a most useful probe to investigate both the Y1 (as a peptide antagonist ligand) and Y4 (as a peptide agonist ligand) receptors in mammalian tissues.
Receptor binding assays (Dumont et al., 1995; 1998a), autoradiographic studies (Dumont et al., 1996a, 1996b; 1998a, 1998b; Gehlert & Gackenheimer, 1997; Gehlert et al., 1997; Trinh et al., 1996; Whitcomb et al., 1997) and in vivo assays (Colmers & Bleakman, 1994; Dumont et al., 1992; Inui, 1999; Kalra & Crowley, 1992; Quirion et al., 1990; Vezzani et al., 1999) have demonstrated the existence of heterogeneous populations of NPY receptors in the rat brain. Similarly, mouse, guinea-pig, monkey and human brains are also enriched with multiple NPY receptors (Caberlotto et al., 1997; Dumont et al., 1997; 1998b; Gehlert & Gackenheimer, 1997; Jacques et al., 1997; Statnick et al., 1997; Widdowson, 1993). Furthermore, in situ hybridization studies demonstrated that Y1, Y2, Y4 and Y5 mRNA are expressed in various structures of the rat (Gerald et al., 1996; Gustafson et al., 1997; Larsen et al., 1993; Larsen & Kristensen, 1997; 1998; Parker & Herzog, 1998; 1999; Tong et al., 1997) and human (Caberlotto et al., 1997; 1998; Jacques et al., 1996; 1998; Statnick et al., 1998) brains. Accordingly, the development of optimal radioreceptor assay conditions to investigate each NPY receptor subtype is still a most significant objective.
Using [125I]-[Leu31,Pro34]-PYY in the presence of selective non-peptide Y1 receptor antagonists, we were able to demonstrate that [125I]-[Leu31,Pro34]-PYY/BIBP3226-insensitive sites have a ligand binding profile similar to the Y5 receptor subtype in the rat CNS (Dumont et al., 1998a). However, under these conditions, the possible labelling of putative Y4 receptor could not be fully excluded as [125I]-[Leu31,Pro34]-PYY also possesses some affinity for this subtype (Gehlert et al., 1996a). Interestingly, the present study demonstrated that [125I]-GR231118 binds with very high affinity to the Y1 and Y4 receptors, but not to the Y2 and Y5 subtypes, transfected in HEK293 cells. Moreover, competition binding experiments using various analogues of NPY, PYY and the PPs as well as selective Y1 antagonists such as BIBP3226 (Rudolf et al., 1994) and BIBO3304 (Wieland et al., 1998) demonstrated that [125I]-GR231118 binds to the Y1 and Y4 receptors with a different ligand binding profile depending upon the receptor subtype expressed in transfected HEK293 cells. The apparent affinity of various agonists and antagonists to compete against [125I]-GR231118 binding in Y1 or Y4 receptor transfected in HEK 293 cells is rather similar to those previously reported for these two receptors using other radioligands and/or preparations (Bard et al., 1995; Gerald et al., 1996; Gehlert et al., 1996a, 1996b; Gregor et al., 1996a).
In rat brain membrane homogenates, various agonists and antagonists of the Y family competed for [125I]-GR231118 binding with a ligand selectivity profile similar to that observed in HEK293 cells transfected with the rat Y1 but not with the Y4 receptor cDNA. These data suggest that most of the sites targeted by [125I]-GR231118 in the rat brain are of the Y1 subtype. This hypothesis is supported further by the high affinities of BIBP3226 and BIBO3304 to compete for specific [125I]-GR231118 binding sites in the rat CNS. Interestingly however, and in contrast to non-radioactive GR231118, BIBP3226 and BIBO3304 competed for up to a maximum of 95% of specific [125I]-GR231118 binding in the rat brain suggesting that in addition to the Y1 receptor subtype, another population of sites (possibly the Y4 receptor) is expressed and recognized by [125I]-GR231118 in this tissue.
Autoradiographic studies revealed that the distribution of [125I]-GR231118 labelling is largely similar to those seen using [125I]-[Leu31,Pro34]-PYY as radioligand (Dumont et al., 1996a; 1998a, 1998b; Gehlert & Gackenheimer, 1997). Additionally, most specific [125I]-GR231118 binding is competed by 100 nM BIBO3304 (and not by 100 nM hPP) in various brain structures supporting further the recognition of the Y1 receptor subtype. However, adjacent coronal rat brain sections incubated with 25 pM [125I]-GR231118 in the presence of a saturating concentration (100 nM) of BIBO3304 (to block Y1 sites) revealed the existence of [125I]-GR231118/BIBO3304-insensitive sites. These specific binding sites are mainly found in the area postrema. Additionally, lower but still significant amounts of [125]-GR231118/BIBO3304-insensitive sites are expressed in the external plexiform layer of the olfactory bulb and in CA1, CA2 and CA3 subfields of the hippocampal formation. Specific [125I]-GR231118 binding sites seen in these regions were also partly sensitive to hPP (100 nM). Considering that [125I]-GR231118 failed to recognize the Y2 and Y5 receptors, it may be taken as an indication that [125I]-GR231118/BIBO3304-insensitive sites represent a Y4 subtype. This is in accordance with data reported here in Y4-transfected HEK293 cells that demonstrated the high affinity (Kd=0.24 nM) of [125I]-GR231118 for this receptor. Moreover, other purported Y4 ligands including [125I]-hPP, [125I]-rPP and [125I]-oPP (Gehlert et al., 1997; Trinh et al., 1996; Whitcomb et al., 1990; 1997) and Y4 receptor mRNA studies (Larsen & Kristensen, 1997) highlighted the area postrema as a targeted structure. However, moderate to very high levels of specific [125I]-PP binding sites were also found in the medial preoptic area, paraventricular nucleus of the hypothalamus and interpeduncular nucleus (Dumont et al., 1998b; Gehlert et al., 1997; Trinh et al., 1996; Whitcomb et al., 1997). These structures contained much lower amounts of specific [125I]-Leu31,Pro34]-PYY (Dumont et al., 1996a; 1998a, 1998b; Gehlert et al., 1997) and [125I]-GR231118 (this study) binding sites even if these two radioligands possess high affinities for the Y4 receptor subtype (Gehlert et al., 1996a; this study). This may be taken as evidence for the existence in these regions of yet another receptor that is preferentially recognized by PP-related molecules, in addition to the Y4 and Y5 subtypes (Gehlert et al., 1996a, 1996b; Gerald et al., 1996). Naturally, molecular information is currently lacking to support this hypothesis. On the other hand, as reported by Walker et al. (1997) for [125I]-PYY, it could be that [125I]-[Leu31,Pro34]-PYY and [125I]-GR231118 have a different sensitivity for guanine nucleotides as compared to [125I]-rPP or [125I]-hPP at the Y4 receptor subtype. This could explain differences in labelling intensity observed between these radioligands. Further studies will be required to clarify this issue.
In summary, our results demonstrated that [125I]-GR231118 binds with very high affinity to rat brain homogenates, rapidly reaching equilibrium at room temperature. Isotherm saturation experiments revealed that [125I]-GR231118 binds with very high affinity to the Y1 and Y4 receptors while it is basically inactive at the Y2 and Y5 subtypes transfected and expressed in HEK293 cells. Additionally, in the presence of a selective Y1 antagonist, it is possible to discriminate between the Y1 and Y4 subtypes in tissues expressing both receptors such as the rat brain. Hence, [125I]-GR231118 should prove most useful to investigate in detail the respective characteristics of these two NPY receptor subtypes in a variety of tissues.
Acknowledgments
This study was supported by the Medical Research Council of Canada. R. Quirion is ‘Chercheur-Boursier' of the ‘Fonds de la recherche en Santé du Québec'.
Abbreviations
- BIBO3304
(R)-N-[[4-(aminocarbonylaminomethyl)-pheyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate
- BIBP3226
R-N2-(Diphenylacetyl)-N-(4-hydroxyphenyl)-methyl argininamide
- GR231118
homodimeric Ile-Glu-Pro-Dpr-Tyr-Arg-Leu-Arg-Tyr-CONH2
- h
human
- HEK293
human embryonic kidney cells
- KRP
Krebs Ringer phosphate buffer
- NPY
neuropeptide Y
- p
porcine
- PP
pancreatic polypeptide
- PYY
peptide YY
- r
rat
References
- AICHER S.A., SPRINGSTON M., BERGER S.B., REIS D.J., WAHLESTEDT C. Receptor-selective analogs demonstrate NPY/PYY receptor heterogeneity in rat brain. Neurosci. Lett. 1991;130:32–36. doi: 10.1016/0304-3940(91)90220-n. [DOI] [PubMed] [Google Scholar]
- BARD J.A., WALKER M.W., BRANCHEK T.A., WEINSHANK R.L. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J. Biol. Chem. 1995;270:26762–26765. doi: 10.1074/jbc.270.45.26762. [DOI] [PubMed] [Google Scholar]
- BITRAN M., DANIELS A.J., BORIC M.P. GW1229, a novel neuropeptide Y Y1 receptor antagonist, inhibits the vasoconstrictor effect on neuropeptide Y in the hamster microcirculation. Eur. J. Pharmacol. 1997;319:43–47. doi: 10.1016/s0014-2999(96)00832-1. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BURKHOFF A., LINEMEYER D.L., SALON J.A. Distribution of a novel hypothalamic neuropeptide Y receptor gene and its absence in rat. Mol. Brain Res. 1998;53:311–316. doi: 10.1016/s0169-328x(97)00302-1. [DOI] [PubMed] [Google Scholar]
- CABERLOTTO L., FUXE K., RIMLAND J.M., SEDVALL G., HURD Y.L. Regional distribution of neuropeptide Y Y2 receptor messenger RNA in the human post mortem brain. Neuroscience. 1998;86:167–178. doi: 10.1016/s0306-4522(98)00039-6. [DOI] [PubMed] [Google Scholar]
- CABERLOTTO L., FUXE K., SEDVALL G., HURD Y.L. Localization of neuropeptide Y Y1 mRNA in the human brain: abundant expression in cerebral cortex and striatum. Eur. J. Neurosci. 1997;9:1212–1225. doi: 10.1111/j.1460-9568.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- COLMERS W.F., BLEAKMAN D. Effects of neuropeptide Y on the electrical properties of neurons. Trends Neurosci. 1994;17:373–379. doi: 10.1016/0166-2236(94)90046-9. [DOI] [PubMed] [Google Scholar]
- DANIELS A.J., MATTHEWS J.E., SLEPETIS R.J., JANSEN M., VIVEROS O.H., TADEPALLI A., HARRINGTON W., HEYER D., LANDAVAZO A., LEBAN J.J. High-affinity neuropeptide Y receptor antagonists. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9067–9071. doi: 10.1073/pnas.92.20.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE QUIDT M.E., EMSON P.C. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system–I. Radioimmunoassay and chromatographic characterisation. Neuroscience. 1986a;18:527–543. doi: 10.1016/0306-4522(86)90056-4. [DOI] [PubMed] [Google Scholar]
- DE QUIDT M.E., EMSON P.C. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system–II. Immunohistochemical analysis. Neuroscience. 1986b;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H.N., FOURNIER A., QUIRION R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral nervous systems: BIBO304 (Y1) and CGP71683A (Y5 Can. J. Physiol. Pharmacol. 1999. In Press [PubMed]
- DUMONT Y., FOURNIER A., QUIRION R. Expression and characterization of the neuropeptide Y Y5 receptor subtype in the rat brain. J. Neurosci. 1998a;18:5565–5574. doi: 10.1523/JNEUROSCI.18-15-05565.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., ST-PIERRE S., QUIRION R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J. Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., ST-PIERRE S., QUIRION R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31,Pro34]peptide YY and [125I]peptide YY3–36 as selective Y1 and Y2 radioligands. J. Pharmacol. Exp. Ther. 1995;272:673–680. [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., ST-PIERRE S., QUIRION R. Autoradiographic distribution of [125I][Leu31,Pro34]PYY and [125I]PYY3–36 binding sites in the rat brain evaluated with two newly developed Y1 and Y2 receptor radioligands. Synapse. 1996a;22:139–158. doi: 10.1002/(SICI)1098-2396(199602)22:2<139::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., ST-PIERRE S., SCHWARTZ T.W., QUIRION R. Differential distribution of neuropeptide Y1 and Y2 receptors in the rat brain. Eur. J. Pharmacol. 1990;191:501–503. doi: 10.1016/0014-2999(90)94189-5. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., JACQUES D., BOUCHARD P., QUIRION R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea-pig, and primates brains. J. Comp. Neurol. 1998b;402:372–384. [PubMed] [Google Scholar]
- DUMONT Y., JACQUES D., ST-PIERRE J.A., QUIRION R. Neuropeptide Y receptor types in the mammalian brain: species differences and status in the human central nervous system Neuropeptide Y and Drug Development 1997Academic Press: London; 57–86.Grundemar, L. & Bloom, S.R. (eds) [Google Scholar]
- DUMONT Y., MARTEL J.C., FOURNIER A., ST-PIERRE S., QUIRION R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog. Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., ST-PIERRE J.A., QUIRION R. Comparative autoradiographic distribution of neuropeptide Y Y1 receptors visualized with the Y1 receptor agonist [125I][Leu31,Pro34]PYY and the non-peptide antagonist [3H]BIBP3226. Neuroreport. 1996b;7:901–904. doi: 10.1097/00001756-199603220-00013. [DOI] [PubMed] [Google Scholar]
- ENTZEROTH M., BRAUNGER H., EBERLEIN W., ENGEL W., RUDOLF K., WIENEN W., WIELAND H.A., WILLIM K.D., DOODS H.N. Labeling of neuropeptide Y receptors in SK-N-MC cells using the novel, nonpeptide Y1 receptor-selective antagonist [3H]BIBP3226. Eur. J. Pharmacol. 1995;278:239–242. doi: 10.1016/0014-2999(95)00161-d. [DOI] [PubMed] [Google Scholar]
- FOREST M., MARTEL J.C., ST-PIERRE S., QUIRION R., FOURNIER A. Structural study of the N-terminal segment of neuropeptide tyrosine. J. Med. Chem. 1990;33:1615–1619. doi: 10.1021/jm00168a014. [DOI] [PubMed] [Google Scholar]
- GEHLERT D.R. Multiple receptors for the pancreatic polypeptide (PP-fold) family: physiological implications. Proc. Soc. Exp. Biol. Med. 1998;218:7–22. doi: 10.3181/00379727-218-44263. [DOI] [PubMed] [Google Scholar]
- GEHLERT D.R., BEAVERS L.S., JOHNSON D., GACKENHEIMER S.L., SCHOBER D.A., GADSKI R.A. Expression cloning of a human brain neuropeptide Y Y2 receptor. Mol. Pharmacol. 1996c;49:224–228. [PubMed] [Google Scholar]
- GEHLERT D.R., GACKENHEIMER S.L. Differential distribution of neuropeptide Y Y1 and Y2 receptors in rat and guinea-pig brains. Neuroscience. 1997;76:215–224. doi: 10.1016/s0306-4522(96)00340-5. [DOI] [PubMed] [Google Scholar]
- GEHLERT D.R., GACKENHEIMER S.L., SCHOBER D.A. [Leu31-Pro34] neuropeptide Y identifies a subtype of 125I-labeled peptide YY binding sites in the rat brain. Neurochem. Int. 1992;21:45–67. doi: 10.1016/0197-0186(92)90067-2. [DOI] [PubMed] [Google Scholar]
- GEHLERT D.R., GACKENHEIMER S.L., SCHOBER D.A., BEAVERS L., GADSKI R., BURNETT J.P., MAYNE N., LUNDELL I., LARHAMMAR D. The neuropeptide Y Y1 receptor selective radioligand, [125I][Leu31,Pro34]peptide YY, is also a high affinity radioligand for human pancreatic polypeptide 1 receptors. Eur. J. Pharmacol. 1996a;318:485–490. doi: 10.1016/s0014-2999(96)00797-2. [DOI] [PubMed] [Google Scholar]
- GEHLERT D.R., SCHOBER D.A., BEAVERS L., GADSKI R., HOFFMAN J.A., SMILEY D.L., CHANCE R.E., LUNDELL I., LARHAMMAR D. Characterization of the peptide binding requirements for the cloned human pancreatic polypeptide-preferring receptor. Mol. Pharmacol. 1996b;50:112–118. [PubMed] [Google Scholar]
- GEHLERT D.R., SCHOBER D.A., GACKENHEIMER S.L., BEAVERS L., GADSKI R., LUNDELL I., LARHAMMAR D. [125I]Leu31,Pro34-PYY is a high affinity radioligand for rat PP1/Y4 and Y1 receptors: evidence for heterogeneity in pancreatic polypeptide receptors. Peptides. 1997;18:397–401. doi: 10.1016/s0196-9781(96)00346-4. [DOI] [PubMed] [Google Scholar]
- GERALD C., WALKER M.W., CRISCIONE L., GUSTAFSON E.L., BATZL-HARTMANN C., SMITH K.E., VAYSSE P., DURKIN M.M., LAZ T.M., LINEMEYER D.L., SCHAFFHAUSER A.O., WHITEBREAD S., HOFBAUER K.G., TABER R.I., BRANCHEK T.A., WEINSHANK R.L. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- GREGOR P., FENG Y., DECARR L.B., CORNFIELD L.J., MCCALEB M.L. Molecular characterization of a second mouse pancreatic polypeptide receptor and its inactivated human homologue. J. Biol. Chem. 1996b;271:27776–27781. doi: 10.1074/jbc.271.44.27776. [DOI] [PubMed] [Google Scholar]
- GREGOR P., MILLHAM M.L., FENG Y., DECARR L.B., MCCALEB M.L., CORNFIELD L.J. Cloning and characterization of a novel receptor to pancreatic polypeptide, a member of the neuropeptide Y receptor family. FEBS Lett. 1996a;381:58–62. doi: 10.1016/0014-5793(96)00067-1. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON E.L., SMITH K.E., DURKIN M.M., WALKER M.W., GERALD C., WEINSHANK R., BRANCHEK T.A. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol. Brain Res. 1997;46:223–235. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- HU Y., BLOOMQUIST B.T., CORNFIELD L.J., DECARR L.B., FLORES-RIVEROS J.R., FRIEDMAN L., JIANG P., LEWIS-HIGGINS L., SADLOWSKI Y., SCHAEFER J., VELAZQUEZ N., MCCALEB M.L. Identification of a novel hypothalamic neuropeptide Y receptor associated with feeding behavior. J. Biol. Chem. 1996;271:26315–26319. [PubMed] [Google Scholar]
- HUNTER W.M., GREENWOOD F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature (Lond.) 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- INUI A. Neuropeptide Y feeding receptors: are multiple subtypes involved. Trends Pharmacol. Sci. 1999;20:43–46. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- JACQUES D., CADIEUX A., DUMONT Y., QUIRION R. Apparent affinity and potency of BIBP3226, a non-peptide neuropeptide Y receptor antagonist, on purported neuropeptide Y Y1, Y2 and Y3 receptors. Eur. J. Pharmacol. 1995;278:R3–R5. doi: 10.1016/0014-2999(95)00179-o. [DOI] [PubMed] [Google Scholar]
- JACQUES D., DUMONT Y., FOURNIER A., QUIRION R. Characterization of neuropeptide Y receptor subtypes in the normal human brain, including the hypothalamus. Neuroscience. 1997;79:129–148. doi: 10.1016/s0306-4522(96)00639-2. [DOI] [PubMed] [Google Scholar]
- JACQUES D., TONG Y., DUMONT Y., SHEN S.H., QUIRION R. Expression of the neuropeptide Y Y1 receptor mRNA in the human brain: an in situ hybridization study. Neuroreport. 1996;7:1053–1056. doi: 10.1097/00001756-199604100-00020. [DOI] [PubMed] [Google Scholar]
- JACQUES D., TONG Y., SHEN S.H., QUIRION R. Discrete distribution of the neuropeptide Y Y5 receptor gene in the human brain: an in situ hybridization study. Mol. Brain Res. 1998;61:100–107. doi: 10.1016/s0169-328x(98)00208-3. [DOI] [PubMed] [Google Scholar]
- KALRA S.P., CROWLEY W.R. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- KANATANI A., ISHIHARA A., ASAHI S., TANAKA T., OZAKI S., IHARA M. Potent neuropeptide Y Y1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 1996;137:3177–3182. doi: 10.1210/endo.137.8.8754736. [DOI] [PubMed] [Google Scholar]
- KAR S., QUIRION R. Quantitative autoradiographic localization of [125I]neuropeptide Y receptor binding sites in rat spinal cord and the effects of neonatal capsaicin, dorsal rhizotomy and peripheral axotomy. Brain Res. 1992;574:333–337. doi: 10.1016/0006-8993(92)90836-x. [DOI] [PubMed] [Google Scholar]
- LARSEN P.J., KRISTENSEN P. The neuropeptide Y (Y4) receptor is highly expressed in neurones of the rat dorsal vagal complex. Mol. Brain Res. 1997;48:1–6. doi: 10.1016/s0169-328x(97)00069-7. [DOI] [PubMed] [Google Scholar]
- LARSEN P.J., KRISTENSEN P. Distribution of neuropeptide Y receptor expression in the rat suprachiasmatic nucleus. Mol. Brain. Res. 1998;60:69–76. doi: 10.1016/s0169-328x(98)00168-5. [DOI] [PubMed] [Google Scholar]
- LARSEN P.J., SHEIKH S.P., JAKOBSEN C.R., SCHWARTZ T.W., MIKKELSEN J.D. Regional distribution of putative NPY Y1 receptors and neurons expressing Y1 mRNA in forebrain areas of the rat central nervous system. Eur. J. Neurosci. 1993;5:1622–1637. doi: 10.1111/j.1460-9568.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- LUNDELL I., BLOMQVIST A.G., BERGLUND M.M., SCHOBER D.A., JOHNSON D., STATNICK MA., GADSKI R.A., GEHLERT D.R., LARHAMMAR D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J. Biol. Chem. 1995;270:29123–29128. doi: 10.1074/jbc.270.49.29123. [DOI] [PubMed] [Google Scholar]
- LUNDELL I., STATNICK M.A., JOHNSON D., SCHOBER D.A., STARBACK P., GEHLERT D.R., LARHAMMAR D. The cloned rat pancreatic polypeptide receptor exhibits profound differences to the orthologous receptor. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5111–5115. doi: 10.1073/pnas.93.10.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNCH D.R., WALKER M.W., MILLER R.J., SNYDER S.H. Neuropeptide Y receptor binding sites in rat brain: differential localization with [125I]peptide YY and [125I]neuropeptide Y imply receptor heterogeneity. J. Neurosci. 1989;9:2607–2619. doi: 10.1523/JNEUROSCI.09-08-02607.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTEL J.C., FOURNIER A., ST PIERRE S., QUIRION R. Quantitative autoradiographic distribution of [125I]Bolton-Hunter neuropeptide Y receptor binding sites in rat brain. Comparison with [125I]peptide YY receptor sites. Neuroscience. 1990;36:255–283. doi: 10.1016/0306-4522(90)90367-d. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO M., NOMURA T., MOMOSE K., IKEDA Y., KONDOU Y., AKIHO H., TOGAMI J., KIMURA Y., OKADA M., YAMAGUCHI T. Inactivation of a novel neuropeptide Y/peptide YY receptor gene in primate species. J. Biol. Chem. 1996;271:27217–27220. doi: 10.1074/jbc.271.44.27217. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T., WESTFALL T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- PARKER E.M., BABIJ C.K., BALASUBRAMANIAM A., BURRIER R.E., GUZZI M., HAMUD F., MUKHOPADHYAY G., RUDINSKI M.S., TAO Z., TICE M., XIA L., MULLINS D.E., SALISBURY B.G. GR231118 (1229U91) and other analogues of the C-terminus of neuropeptide Y are potent neuropeptide Y Y1 receptor antagonists and neuropeptide Y Y4 receptor agonists. Eur. J. Pharmacol. 1998;349:97–105. doi: 10.1016/s0014-2999(98)00171-x. [DOI] [PubMed] [Google Scholar]
- PARKER R.M., HERZOG H. Comparison of Y-receptor subtype expression in the rat hippocampus. Regul. Pept. 1998;7576:109–115. doi: 10.1016/s0167-0115(98)00059-7. [DOI] [PubMed] [Google Scholar]
- PARKER R.M., HERZOG H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- QUIRION R., MARTEL J.C. Neuropeptide Y receptors in mammalian brains Peptide Receptor Handbook of Chemical Neuroanatomy 1992Vol 11Elsevier: Amsterdam; 247–287.In: Bjorklund, A., Hokfelt, T. & Kuhar, M.J. (eds.) [Google Scholar]
- QUIRION R., MARTEL J.C., DUMONT Y., CADIEUX A., JOLICOEUR F., ST-PIERRE S., FOURNIER A. Neuropeptide Y receptors: autoradiographic distribution in the brain and structure-activity relationships. Ann. N.Y. Acad. Sci. 1990;611:58–72. doi: 10.1111/j.1749-6632.1990.tb48922.x. [DOI] [PubMed] [Google Scholar]
- RUDOLF K., EBERLEIN W., ENGEL W., WIELAND H.A., WILLIM K.D., ENTZEROTH M., WIENEN W., BECK-SICKINGER A.G., DOODS H.N. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur. J. Pharmacol. 1994;271:R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- SCHOBER D.A., VAN ABBEMA A.M., SMILEY D.L., BRUNS R.F., GEHLERT D.R. The neuropeptide Y Y1 antagonist, 1229U91, a potent agonist for the human pancreatic polypeptide-preferring (NPY Y4) receptor. Peptides. 1998;19:537–542. doi: 10.1016/s0196-9781(97)00455-5. [DOI] [PubMed] [Google Scholar]
- STATNICK M.A., SCHOBER D.A., GACKENHEIMER S., JOHNSON D., BEAVERS L., MAYNE N.G., BURNETT J.P., GADSKI R., GEHLERT D.R. Characterization of the neuropeptide Y5 receptor in the human hypothalamus: a lack of correlation between Y5 mRNA levels and binding sites. Brain Res. 1998;810:16–26. doi: 10.1016/s0006-8993(98)00855-5. [DOI] [PubMed] [Google Scholar]
- STATNICK M.A., SCHOBER D.A., GEHLERT D.R. Identification of multiple neuropeptide Y receptor subtypes in the human frontal cortex. Eur. J. Pharmacol. 1997;332:299–305. doi: 10.1016/s0014-2999(97)01031-5. [DOI] [PubMed] [Google Scholar]
- TATEMOTO K., MANN M.J., SHIMIZU M. Synthesis of receptor antagonists of neuropeptide Y. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1174–1178. doi: 10.1073/pnas.89.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG Y., DUMONT Y., SHEN S.H., HERZOG H., SHINE J., QUIRION R. Expression of the neuropeptide Y Y1 receptor in human embryonic kidney 293 cells: ligand binding characteristics, in situ hybridization and receptor autoradiography. Mol. Brain Res. 1995;34:303–308. doi: 10.1016/0169-328x(95)00176-s. [DOI] [PubMed] [Google Scholar]
- TONG Y., DUMONT Y., SHEN S.H., QUIRION R. Comparative developmental profile of the neuropeptide Y Y1 receptor gene and protein in the rat brain. Mol. Brain Res. 1997;48:323–332. doi: 10.1016/s0169-328x(97)00107-1. [DOI] [PubMed] [Google Scholar]
- TRINH T., DUMONT Y., QUIRION R. High levels of specific neuropeptide Y/pancreatic polypeptide receptors in the rat hypothalamus and brainstem. Eur. J. Pharmacol. 1996;318:R1–R3. doi: 10.1016/s0014-2999(96)00863-1. [DOI] [PubMed] [Google Scholar]
- VEZZANI A., SPERK G., COLMERS W.F. Neuropeptide Y: emerging evidence for a functional role of seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- WAHLESTEDT C., REIS D.J. Neuropeptide Y-related peptides and their receptors–are the receptors potential therapeutic drug targets. Annu. Rev. Pharmacol. Toxicol. 1993;33:309–352. doi: 10.1146/annurev.pa.33.040193.001521. [DOI] [PubMed] [Google Scholar]
- WAHLESTEDT C., YANAIHARA N., HAKANSON R. Evidence for different pre- and post-junctional receptors for neuropeptide Y and related peptides. Regul. Pept. 1986;13:307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]
- WALKER M.W., SMITH K.E., BARD J., VAYSSE P.J., GERALD C., DAOUTI S., WEINSHANK R.L., BRANCHEK T.A. A structure-activity analysis of the cloned rat and human Y4 receptors for pancreatic polypeptide. Peptides. 1997;18:609–612. doi: 10.1016/s0196-9781(97)00070-3. [DOI] [PubMed] [Google Scholar]
- WEINBERG D.H., SIRINATHSINGHJI D.J., TAN C.P., SHIAO L.L., MORIN N., RIGBY M.R., HEAVENS R.H., RAPOPORT D.R., BAYNE M.L., CASCIERI M.A., STRADER C.D., LINEMEYER D.L., MACNEIL D.J. Cloning and expression of a novel neuropeptide Y receptor. J. Biol. Chem. 1996;271:16435–16438. doi: 10.1074/jbc.271.28.16435. [DOI] [PubMed] [Google Scholar]
- WHITCOMB D.C., PUCCIO A.M., VIGNA S.R., TAYLOR I.L., HOFFMAN G.E. Distribution of pancreatic polypeptide receptors in the rat brain. Brain Res. 1997;760:137–149. doi: 10.1016/s0006-8993(97)00295-3. [DOI] [PubMed] [Google Scholar]
- WHITCOMB D.C., TAYLOR I.L., VIGNA S.R. Characterization of saturable binding sites for circulating pancreatic polypeptide in rat brain. Am. J. Physiol. 1990;259:G687–G691. doi: 10.1152/ajpgi.1990.259.4.G687. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON P.S. Quantitative receptor autoradiography demonstrates a differential distribution of neuropeptide Y Y1 and Y2 receptor subtypes in human and rat brain. Brain Res. 1993;631:27–38. doi: 10.1016/0006-8993(93)91182-r. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., ENGEL W., EBERLEIN W., RUDOLF K., DOODS H.N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br. J. Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]


