Abstract
In order to elucidate further the role of nitric oxide (NO) as an endogenous antiangiogenic mediator, mRNA expression of inducible nitric oxide synthase (iNOS), enzyme activity and production of NO were determined in the chick chorioallantoic membrane (CAM), an in vivo model of angiogenesis. In this model, maximum angiogenesis is reached between days 9–12 of chick embryo development. After that period, vascular density remains constant.
Inducible NO synthase (iNOS) mRNA expression, determined by reverse transcriptase polymerase chain reaction (RT–PCR), increased from the 8th day reaching a maximum (70% increase) at days 10–11.
NO synthase activity, determined as citrulline formation in the presence of calcium, also increased from day 8 reaching a maximum around day 10 (100% increase). Similar results were obtained in the absence of calcium suggesting that the NOS determined was the inducible form.
Nitric oxide production, determined as nitrites, increased from day 8 reaching a maximum around day 10 (64% increase) and remaining stable at day 13.
Finally, the bacterial lipopolysaccharide LPS (which activates transcriptionally iNOS), inhibited dose dependently angiogenesis in the CAM. These results in connection with previous findings from this laboratory, showing that NO inhibits angiogenesis in the CAM, suggest that increases in iNOS expression, enzyme activity and NO production closely parallel the progression of angiogenesis in the CAM, thus providing an endogenous brake to control this process.
Keywords: Nitric oxide synthase, nitric oxide, angiogenesis, chick chorioallantoic membrane
Introduction
Nitric oxide (NO) in addition to its multiple roles in pathophysiology, has recently been added to the list of endogenous mediators of angiogenesis (Pipili-Synetos et al., 1994). Angiogenesis is a highly regulated process implicated in many pathological circumstances including tumour growth and involving a great number of mediators such as growth factors, thrombin, thrombospondin and angiostatin (Maragoudakis, 1993; O'Reilly et al., 1994). NO however, unlike most of the above substances, has been shown to possess both pro- and anti-angiogenic properties. For instance, it has been shown that in the rabbit cornea, NO mediates stimulated angiogenesis (Gallo et al., 1998; Ziche et al., 1994; 1997). In addition, NO promotes proliferation of the coronary endothelium in culture (Parenti et al., 1998; Ziche et al., 1993; 1997; Morbidelli et al., 1996) and appears to mediate vascular endothelial growth factor (VEGF)-induced proliferation in human endothelial cells (Papapetropoulos et al., 1997; Hood & Granger, 1998). All the above effects involve NO produced through the constitutive NO synthase (cNOS) and are cyclic GMP-dependent.
There is, on the other hand, a considerable body of evidence supporting the antiangiogenic role of NO. Work from this laboratory has shown that, in the chick chorioallantoic membrane (CAM), NO donors inhibit basal and stimulated angiogenesis (Pipili-Synetos et al., 1993; 1994; 1995; Sakkoula et al., 1997). Moreover, we and a number of laboratories have shown that NO either has no effect or inhibits proliferation of a variety of endothelial cell types (Pipili-Synetos et al., 1994; Babaei et al., 1998; RayChaudhury et al., 1996; Yang et al., 1994). The type of NO involved in some of these effects appears to be that produced through activation of the inducible NO synthase (iNOS) (Sakkoula et al., 1997; RayChaudhury et al., 1996). It is therefore possible that the production of high amounts of NO via iNOS might inhibit angiogenesis, while small amounts produced via cNOS might be proangiogenic. If this were the case, iNOS should be the main type involved in the antiangiogenic effects of NO in the CAM.
In order to test this hypothesis, the expression of iNOS mRNA, the type and the activity of the translation product (NOS) and the enzyme product (NO) were determined in the CAM from day 8 to day 13 of embryo development. This time frame was chosen because in this model, maximum angiogenesis is reached between days 9 and 12 of chick embryo development. After that period, vascular density remains constant (Maragoudakis et al., 1988).
Methods
RNA isolation
Total cellular RNA isolation was performed by the acid guanidinium isothiocyanate- phenol-chloroform method (Chomczynski & Sacchi, 1987). For each sample, 4-5 CAMs from days 8–13 were taken, washed with ice-cold PBS, drained to remove excess fluid and homogenized in solution D (4 M guanidinium chloride, 25 mM sodium citrate, 0.5% sarcosyl, 0.2 M mercaptoethanol) by passing several times through a 18-gauge syringe needle. The homogenates were kept at −80°C. Total cellular RNA was extracted from collected frozen homogenates and the integrity of RNA was tested by running an aliquot on a 2.2 M formaldehyde 1% agarose gel.
RT–PCR
A 530 bp fragment of chick iNOS cDNA was amplified by RT–PCR in a thermal cycler PTC-200, Peltier (MJ Research). Primers were designed according to the published cDNA sequence of chick iNOS (Lin et al., 1996) and were : 5′-CCAGAGAGATTCATCTGACCG-3′ (sense) and 5′-GGTCCCTACAACGAGTCTGAA-3′ (antisense). The reporter gene was the chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the primers : 5′-ACGGATTTGGCCGTATTGGC-3′ (sense) and 5′-GCAGGATGCGAAACTGAGCG-3′ (antisense) were designed according to the published sequence (Stone et al., 1985) to yield a fragment of 1223 bp. The RT–PCR reactions were performed in a single step using the Access RT–PCR system (Promega) according to the following conditions: The Reverse Transcriptase reaction was performed by AMV-RT for 1 h at 48°C. After an initial denaturation step for 2 min at 94°C, 30 cycles of amplification (94°C for 1 min, 57°C for 40 s and 68°C for 1.5 min) were performed and ended with a final annealing step at 57°C for 1 min, followed by a final DNA synthesis step at 68°C for 7 min. DNA contamination was excluded by performing PCR reactions in the absence of the Reverse Transcription step. To further establish that the 530 bp PCR fragment represents iNOS cDNA, we performed a nested PCR reaction using the initial antisense primer (5′-GGTCCCTACAACGAGTCTGAA-3′) and a new sense primer (5′-GATATTACTGTTTGGCTGCCG-3′), internal to the 530 bp fragment. This reaction yielded the expected 253 bp fragment, thus confirming that the 530 bp PCR product corresponds to the iNOS cDNA (Figure 1).
Figure 1.

Reverse transcriptase-polymerase chain (RT–PCR) reactions for chicken GAPDH and iNOS mRNA. Lane 1: Φ×174 marker. Lane 2: The product of RT–PCR for GAPDH mRNA that showed the expected band of 1223 bp and a double non-specific band of 200 bp. Lane 3: The product of RT–PCR for the mRNA of chicken iNOS that yielded the expected band of 530 bp. Lane 4: The product of nested PCR for iNOS cDNA that yielded a 253 bp band from the 530 bp band that represents chicken iNOS.
The RT–PCR products were subjected to electrophoresis on 1.5% agarose gels containing ethidium bromide and photographed. The resulting photographs were scanned and the PCR bands were measured (width and intensity) using ImagePC. The ratio of iNOS/GAPDH electrophoretic band values represents the expression of iNOS gene for the days 8–13 of chicken embryo development.
The chick chorioallantoic membrane (CAM)
The in vivo CAM angiogenesis model, initially described by Folkman (Folkman, 1985) and modified as previously reported (Maragoudakis et al., 1988), was used. Biochemical evaluation of angiogenesis was performed by determining the extent of collagenous protein biosynthesis in the CAM lying directly under the disc applied at day 9 of chick embryo development. After 48 h, the tissue containing radioactivity was subjected to collagenase digestion. The resulting radiolabelled tripeptides, corresponding to basement membrane collagen and other collagenous material synthesized by the CAM from (U-14C)-proline, were counted and expressed as c.p.m. mg−1 protein. For each egg, collagenous protein biosynthesis under the disc containing the test material was then expressed as % of that under the control disc in the same egg (n signifies the number of eggs for each treatment).
Determination of NOS activity
NOS was determined in tissue homogenates as the conversion of [14C]-L-arginine to [14C]-L-citrulline, as described elsewhere (Rachel et al., 1998). Briefly, 4–5 CAMs from day 8–13 were dissected and washed three times with PBS pH 7.3 and were then homogenized (1 : 5 w v−1 in 20 mM Tris-HCl buffer, pH 7.4 containing 2 mM EDTA). The tissue homogenates were centrifuged at 10 000×g for 10 min and the supernatants stored at −80°C until assayed for NOS activity.
Combined NOS activity was determined in incubations (15 min at 37°C; total volume 100 μl) containing supernatants from CAMs (82 μl), [14C]-L-arginine (0.2 μCi, 670 nM), NADPH (0.5 mM), CaCl2 (0.75 mM), tetra-hydro-biopterin (BH4, 0.03 mM) and L-arginine (0.01 mM), in the presence or absence of NG-nitro-L-arginine methyl ester (L-NAME) (100 mM). Specific NOS activity was calculated as the activity in the absence minus the activity in the presence of L-NAME. To determine iNOS activity, CaCl2 was omitted and replaced by homogenization buffer (P. Moore, personal communication). The reaction was terminated by addition of 3 ml HEPES buffer (20 mM pH 5.5) containing 2 mM EDTA and reaction mixtures were applied to 1.0 ml columns of Dowex AG50WX-8 (Na+) followed by 0.5 ml distilled water. [14C]-L-citrulline was quantified by liquid scintillation spectroscopy (Beckman 1801 LS) of 0.6 ml of the combined flow-through. Protein concentration of the tissue homogenates was determined using the Bradford reagent (Bradford, 1976). Results are expressed as pmol citrulline min−1 mg−1 protein.
Determination of nitrites
For the determination of the nitrites formed by the CAM, 4–5 CAMs from day 8–13, were dissected, washed three times with PBS, pH 7.3, and cut into small pieces. They were then placed in 24-well plates so that each well contained approximately 0.5–1.0 mg protein and incubated at 37°C for 6 h in Krebs salt solution. At the end of the incubation period, the samples were centrifuged at 420×g for 4 min in an Eppendorf microfuge. Nitrites were subsequently measured in the supernatant with the use of the Griess reagent as previously described by Szabo et al. (1994) and protein was measured in the precipitate as described above. Results are expressed as nmoles mg−1 protein.
Materials
Fertilized eggs were obtained locally (Ioannina, Greece). PCR primers were obtained from Minotech Inc., Crete, Greece. U-14C-proline and 14C-L-arginine were from ICN. Guanidinium chloride, sodium citrate, sarcosyl, mercaptoethanol, Tris, EDTA, NADPH, L-NAME and HEPES were from Sigma. BH4 was a generous gift from Dr J. Catravas, University of Atlanta, Georgia, U.S.A.
Results
The expression of iNOS in the CAM was studied from day 8 to day 13 of chicken embryo development. RT–PCR reactions were performed for three different series of chicken embryos. For each series, the reactions were repeated twice.
RT–PCR for the mRNA of chicken iNOS yielded the expected band of 530 bp (Figure 1). This band, representing chicken iNOS, showed an increasing intensity from day 8 to day 12 and fell to a lower level at day 13 (Figure 2). In addition, the reference gene (GAPDH) produced the expected band of 1223 bp (Figure 1) and a double non-specific band of 200 bp. This band was of comparable intensity between lanes 2–7 (Figure 2a). The calculated ratios of iNOS/GAPDH (Figure 2b) similarly to the results of the representative experiment in Figure 2a, showed that iNOS expression increased significantly from 0.58±0.038, n=6, at day 8 to 0.79±0.07, n=6, at day 10, P<0.02 and 0.98±0.14, n=6, at day 11, P<0.02. It then declined at day 13 to a value (0.64±0.10, n=6, n.s.) which was not statistically different from that seen at day 8.
Figure 2.
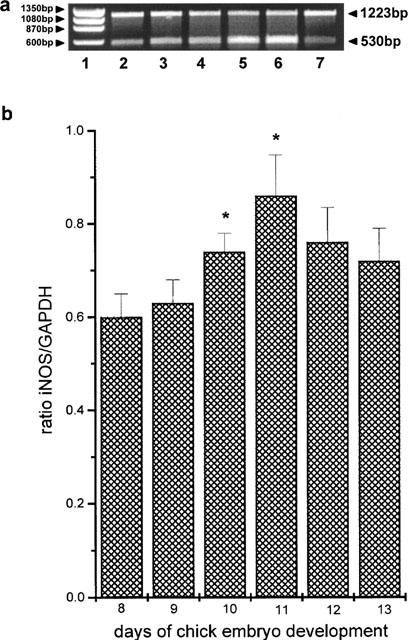
Expression of iNOS mRNA in the chick chorioallantoic membrane between days 8 and 13 of embryo development. (a) Reverse transcriptase-polymerase chain (RT–PCR) reactions for chicken GAPDH and iNOS mRNA. Lane 1: Φ×174 marker. Lanes 2–7: Semicompetitive RT–PCR reactions for the days 8–13 of chicken embryo development, with primers of chicken iNOS cDNA (530 bp band) and the chicken GAPDH cDNA (1200 bp band). (b) The ratio of iNOS/GAPDH electrophoretic band values for the days 8–13 of chicken embryo development. Asterisks denote a statistically significant difference (unpaired t-test) from the ratio at day 8 of chicken embryo development. *P<0.05.
Experiments performed in the presence and absence of calcium, showed that all NOS activity in the CAM was calcium independent (Figure 3). NOS activity, in the presence of calcium, increased significantly from day 8 to day 11 reaching a maximum at day 10 (0.9±0.2, pmol min−1 mg−1 protein, n=6 at day 8 to 1.84±0.2, pmol min−1 mg−1 protein, n=6, P<0.001). From day 11 onwards NOS activity declined to a value of 1.5±0.2, pmol min−1 mg−1 protein, n=6 at day 13. Similar values were observed in the absence of calcium (Figure 3).
Figure 3.
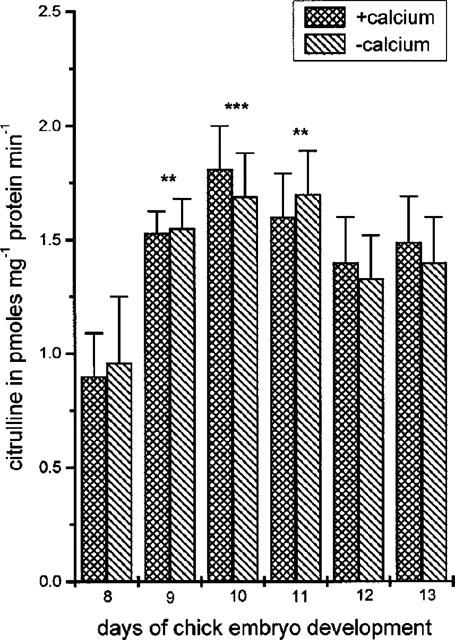
Nitric oxide synthase (NOS) activity in the chick chorioallantoic membrane (CAM) between days 8 and 13 of embryo development, in the presence or absence of EGTA (2 mM). Results show pmoles of citrulline mg−1 protein min−1 and are the mean±s.e.mean, n=6. Asterisks denote a statistically significant difference (unpaired t-test) from NOS activity at day 8 of chicken embryo development. **P<0.01, ***P<0.001.
Likewise NOS activity, nitrite concentration increased significantly from day 8 to day 11 (Figure 4) reaching a maximum at days 10 and 11 (from 0.036±0.006, n=6 nmoles mg−1 protein at day 8 to 0.059±0.004, n=6, P<0.001 at day 10 and 0.057±0.005, n=6 nmoles mg−1 protein, P<0.01 at day 11). From day 11 onwards, nitrite levels slightly declined to values not significantly different from those obtained at day 8 (Figure 4).
Figure 4.
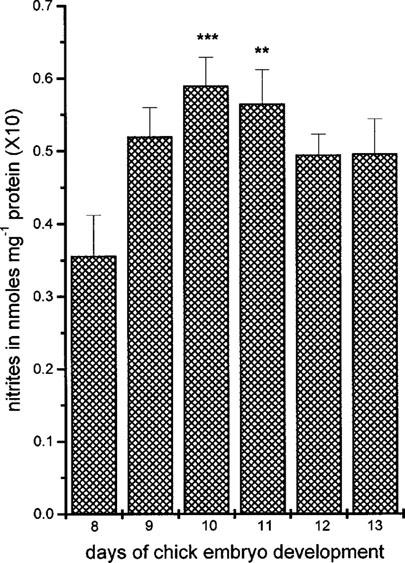
Nitrite production in the chick chorioallantoic membrane (CAM) between days 8 and 13 of embryo development. Results show nmoles of nitrite mg−1 protein and are the mean±s.e.mean, n=6. Asterisks denote a statistically significant difference (unpaired t-test) from nitrite production at day 8 of chicken embryo development. **P<0.01, ***P<0.001.
Since increase in iNOS expression, NOS activity and nitrite production paralleled the increase in angiogenesis, we considered it of importance to investigate whether these increases affected the progress of angiogenesis. LPS is a well known transcriptional activator of iNOS in various biological systems, as well as in the CAM, where it promotes iNOS expression, NOS activity and NO production (Figure 5 and Sakkoula et al., 1997). The effect of LPS on angiogenesis was therefore examined. LPS, applied at day 9 of chick embryo development, from 3.3 to 330 ng disc−1 inhibited angiogenesis in the CAM (expressed as collagenous protein biosynthesis, CPB) dose dependently (Figure 6). Under these conditions, CPB showed a decrease which ranged from 0.5±3.8%, n=11 to 31±5.3%, n=11, compared to controls (0%). To show that this decrease was related to NOS activity, L-NAME (100 nmoles disc−1) was combined with two doses of LPS which gave responses lying in the straight part of the curve, namely 10 and 33 ng disc−1. LPS in combination with L-NAME did no longer inhibit angiogenesis in the CAM (Figure 7a). In the presence of L-NAME, 10 ng disc−1 LPS caused a 38.2±15% increase, n=11 and 33 ng disc−1 LPS caused a 22.6±13% increase in angiogenesis, n=7 compared to controls (0%). Both these values were statistically different from those seen with LPS alone (11±6.3% decrease, n=9, P<0.01 and 28.4±5.7% decrease, n=7, P<0.01). D-NAME (100 nmoles disc−1) or the combination of L-NAME (100 nmoles disc−1) with L-arginine (L-Arg, 150 nmoles disc−1) did not affect the inhibitory effect of LPS (Figure 7b). In the presence of D-NAME, 33 ng disc−1 LPS caused a 23.3±7% decrease, n=7 and in the presence of the combination of L-NAME with L-Arg it caused a 23.4±5% decrease, n=7, compared to controls (0%).
Figure 5.

(a) Effect of 33 ng disc−1 of LPS on the expression of iNOS mRNA in the chick chorioallantoic membrane (CAM). LPS was applied on the CAM at day 9 of embryo development for 6 h. The figure shows the RT–PCR reactions for chicken iNOS mRNA. (b) Effect of 33 ng LPS on nitrite production in the CAM at day 9 of embryo development. Results show nmoles of nitrite mg−1 protein and are the mean±s.e.mean, n=6.
Figure 6.
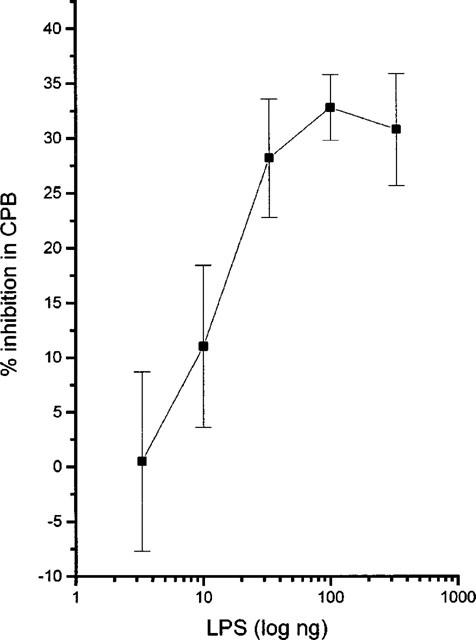
Effect of increasing concentrations of lipopolysaccharide (LPS) on angiogenesis in the chick chorioallantoic membrane in vivo, expressed as collagenous protein biosynthesis (CPB). All results are expressed as mean (±s.e.mean)% of control (n=7–11), **P<0.01.
Figure 7.
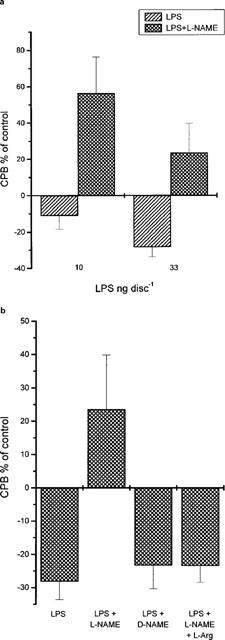
(a) Inhibition of the antiangiogenic effect of LPS by NG-nitro-L-arginine methyl ester (L-NAME) (100 nmoles disc−1) in the chick chorioallantoic membrane in vivo. (b) Effect of L-NAME (100 nmoles disc−1), D-NAME (100 nmoles disc−1) and the combination of L-NAME (100 nmoles disc−1) with L-Arg (150 nmoles disc−1) on the antiangiogenic effect of LPS (33 ng disc−1) in the chick chorioallantoic membrane in vivo. All results are expressed as mean (±s.e.mean)% of control (n=7–11).
Discussion
In the present study it was shown that (a) expression of iNOS mRNA, iNOS activity and NO production, closely follow the progression of angiogenesis in the CAM and (b) a transcriptional activator of iNOS, LPS, inhibits angiogenesis in the same tissue.
In the CAM, NO donors have been shown to inhibit basal angiogenesis and NOS inhibitors to promote it (Pipili-Synetos et al., 1994; 1995). Furthermore, NO mediates the antiangiogenic effect of IL-2 in the CAM and reverses the angiogenic effect of angiogenic substances such as thrombin and PMA (Pipili-Synetos et al., 1994; Sakkoula et al., 1997). It is therefore reasonable to suggest that the increased iNOS expression, iNOS activity and NO production are a means of providing an endogenous brake on the angiogenic process, further supporting the antiangiogenic nature of NO.
The NOS isoform involved in the results presented here is the inducible one. The mRNA expression of the cNOS (the endothelial form) in the CAM was not examined, because at the time when these experiments were performed, there was no information in the literature on the chicken endothelial NOS gene. Since however in the present study the entire enzyme activity in the CAM was calcium-independent, it appears that the constitutive/endothelial form, if any, is not relevant in this tissue. The considerable amounts of nitrites formed by the CAM (μM quantities when expressed in molarity in the incubating vessel) are comparable to the NO released by smooth muscle cells after stimulation with LPS (Fukuo et al., 1995; Goureau et al., 1992), which further suggests that they are the product of iNOS rather than cNOS.
The chicken iNOS, similarly to other species, has been shown to contain transcription factor binding sites including the NF-κB that, as in the mammalian gene, is involved in the induction of chicken iNOS by LPS (Lin et al., 1996). Thus, it is possible that inhibition of angiogenesis caused by LPS is at least in part due to the induction of iNOS. This conclusion is supported by the observation that the antiangiogenic effect of LPS was inhibited by L-NAME in a stereoselective and L-Arg-reversible manner. Moreover, LPS caused increases in iNOS mRNA and NO production in the CAM (Figure 5).
Chicken macrophages and myoblasts can express iNOS (Lin et al., 1996; Shimizu et al., 1998). Thus, because both of these cellular components are contained in the CAM, they may be the source of iNOS in this tissue. In addition, it is now known that endothelial cells which were believed to contain mainly the constitutive NOS isoform, are capable of expressing the inducible form of the enzyme (Kroll & Waltenberger, 1998). However, future experiments are needed to identify the exact tissue elements expressing iNOS in the CAM.
As mentioned above, our present and past data indicate that NO may play the role of an endogenous negative mediator of the angiogenic process being produced at sites and times of increased angiogenesis. Nitric oxide may also serve a negative feedback function in tumours where NOS expression has often been found to correlate with tumour size (Lala & Orucevic, 1998). Negative feedback systems are very common in biology. For example, angiostatin, which itself is a powerful antiangiogenic substance (O'Reilly et al., 1998), is also produced by the tumour along with proangiogenic substances, which will sustain its growth and metastasis.
There is on the other hand a considerable body of evidence suggesting that NO is a proangiogenic mediator (Gallo et al., 1998; Morbidelli et al., 1996; Ziche et al., 1993; 1994; 1997). Most of this work is based upon data showing that growth factors such as the vascular endothelial growth factor upregulate the expression and activity of NOS (Hood et al., 1998; Morbidelli et al., 1996). It is however becoming increasingly evident that although VEGF may indeed increase NO production, the NO produced is mainly shown to negatively regulate expression of VEGF-related parameters and functions (Liu et al., 1998; Shen et al., 1998; Ahmed et al., 1997; Tuder et al., 1995; Tsurumi et al., 1997). The CAM expresses VEGF receptors (Oh et al., 1997). It is therefore possible that, in addition to NO-related events initiated by other angiogenic cytokines, the coupling of VEGF with its receptor may lead to upregulation of iNOS in endothelial cells. This results in NO release which serves as a feedback control mechanism of the angiogenic effects of VEGF during the development of the CAM. The notion of NO being an antiangiogenic substance is supported by the fact that with very few exceptions (Ziche et al., 1993; 1994; Gallo et al., 1998), it inhibits endothelial cell proliferation (Babaei et al., 1998; RayChaudhury et al., 1996; Yang et al., 1994).
In conclusion, the results of the present study show a correlation between angiogenic development and expression of iNOS mRNA, enzyme activity and NO release in the CAM. These findings, coupled to the antiangiogenic activity of LPS and our previous observations showing that NO inhibits angiogenesis (Pipili-Synetos et al., 1994), suggest that NO plays the role of an endogenous brake in neovascularization. NO may therefore be considered as a useful tool in the development of antiangiogenic-antitumour strategies.
Acknowledgments
This work was supported by a grant from the Greek Secretariat of Research and Technology. The authors are grateful to Dr J. Catravas for providing the tetrahydrobiopterin and to Dr P.K. Moore for useful discussion. Sosanna Kritikou is a recipient of a grant from the Greek National Scholarship Foundation.
Abbreviations
- CAM
chick chorioallantoic membrane
- D-NAME
NG-nitro-D-arginine methyl ester
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- iNOS
inducible nitric oxide synthase
- L-Arg
L-arginine
- L-NAME
NG-nitro-L-arginine methyl ester
- LPS
lipopolysaccharide
- NO
nitric oxide
- VEGF
vascular endothelial growth factor
References
- AHMED A., DUNK C., KNISS D., WILKES M. Role of VEGF receptor-1 (Flt-1) in mediating calcium-dependent nitric oxide release and limiting DNA synthesis in human trophoblast cells. Lab. Invest. 1997;76:779–791. [PubMed] [Google Scholar]
- BABAEI S., TEICHERT-KULISZEWSKA K., MONGE J.C., MOHAMED F., BENDECK M.P., STEWART D.J. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ. Res. 1998;82:1007–1015. doi: 10.1161/01.res.82.9.1007. [DOI] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for quantities of proteins, utilising the principle of protein-dye binding. Anal. Biochem. 1976;72:248–253. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium-thiocyanate phenol chloroform extraction. Analyt. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- FOLKMAN J. Tumor angiogenesis. Adv. Cancer Res. 1985;43:172–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- FUKUO K., INOUE T., MORIMOTO S., NAKAHASHI T., YASUDA O., KITANO S., SASADA R., OGIHARA T. Nitric oxide mediates cytotoxicity and basic fibroblast growth factor release in cultured vascular smooth muscle cells. A possible mechanism of neovascularization in atherosclerotic plaques. J. Clin. Invest. 1995;95:669–676. doi: 10.1172/JCI117712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLO O., MASINI E., MORBIDELLI L., FRANCHI A., FINI-STORCHI I., VERGARI W.A., ZICHE M. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl. Cancer Inst. 1998;90:587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- GOUREAU O., LEPOIVRE M., COURTOIS Y. Lipopolysaccharide and cytokines induce a macrophage-type nitric oxide synthase in bovine retinal pigmented epithelial cells. Biochem. Biophys. Res. Commun. 1992;186:854–859. doi: 10.1016/0006-291x(92)90824-5. [DOI] [PubMed] [Google Scholar]
- HOOD J., GRANGER H.J. Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J. Biol. Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- HOOD J.D., MEININGER C.J., ZICHE M., GRANGER H.J. VEGF upregulates ecNOS message, protein and NO production in human endothelial cells. Am. J. Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- KROLL J., WALTENBERGER J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR) Biochem. Biophys. Res. Commun. 1998;252:743–746. doi: 10.1006/bbrc.1998.9719. [DOI] [PubMed] [Google Scholar]
- LALA P.K., ORUCEVIC A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91–106. doi: 10.1023/a:1005960822365. [DOI] [PubMed] [Google Scholar]
- LIN A.W., CHANG C.C., MCCORMICK C.C. Molecular cloning and expression of an avian macrophage nitric-oxide synthase cDNA and the analysis of the genomic 5′-flanking region. J. Biol. Chem. 1996;271:11911–11919. doi: 10.1074/jbc.271.20.11911. [DOI] [PubMed] [Google Scholar]
- LIU Y., CHRISTOU H., MORITA T., LAUGHNER E., SEMENZA G.L., KOUREMBANAS S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5′ enhancer. J. Biol. Chem. 1998;273:15257–15262. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- MARAGOUDAKIS M.E. Angiogenesis Annual of Cardiac surgery 1993London: Current Science; 13–19.Yakoub, M. & Pepper J. (eds.) [Google Scholar]
- MARAGOUDAKIS M.E., SARMONICA M., PANOUTSAKOPOULOU M. Rate of basement membrane biosynthesis as an index to angiogenesis. Tissue Cell. 1988;20:531–539. doi: 10.1016/0040-8166(88)90055-9. [DOI] [PubMed] [Google Scholar]
- MORBIDELLI V., CHANG C.-H., DOUGLAS J.G. , GRANGER H.J., LEDDA F., ZICHE M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am. J. Physiol. 1996;270:H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- OH S.-J., JELTSCH M.M., BIRKENHAGER R., MCCARTHY J.E.G., WEICH H.A., CHRIST B., ALITALO K., WILTING J. VEGF and VEGF-C: Specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- O'REILLY M.S., HOLMGREN L., SHING Y., CHEN C., ROSENTHAL R.A., MOSES M., LANE W.S., CAO Y., SAGE E.H., FOLKMAN J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastasis by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- PAPAPETROPOULOS A., DESAI M.K., RUDIC R.D., MAYER B., ZHANG R., RUIZ-TORRES M.P., GARCIA-GARDENA G., MADRI J.A., SESSA W.C. Nitric synthase inhibitors attenuate transforming growth factor-B1-stimulated capillary organisation in vitro. Am. J. Pathol. 1997;150:1835–1844. [PMC free article] [PubMed] [Google Scholar]
- PARENTI A., MORBIDELLI L., CUI X.L., DOUGLAS J.C., HOOD J.D., GRANGER H.J., LEDDA F., ZICHE M. Nitric oxide is an upstream signal of vascular endothelial growth factor induced extracellular signal regulated kinase 1/2 activation in post capillary endothelium. J. Biol. Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- PIPILI-SYNETOS E., PAPAGEORGIOU A., SAKKOULA E., SOTIROPOULOU G., FOTSIS T., KARAKIOULAKIS G., MARAGOUDAKIS M.E. Inhibition of angiogenesis, tumour growth and metastasis by the NO-releasing vasodilators, isosorbide mononitrate and dinitrate. Br. J. Pharmacol. 1995;116:1829–1834. doi: 10.1111/j.1476-5381.1995.tb16670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPILI-SYNETOS E., SAKKOULA E., HARALABOPOULOS G., ANDRIOPOULOU P., PERISTERIS P., MARAGOUDAKIS M.E. Evidence that nitric oxide is an endogenous antiangiogenic mediator. Br. J. Pharmacol. 1994;11:894–902. doi: 10.1111/j.1476-5381.1994.tb14822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPILI-SYNETOS E., SAKKOULA E., MARAGOUDAKIS M.E. Nitric oxide is involved in the regulation of angiogenesis. Br. J. Pharmacol. 1993;108:855–857. doi: 10.1111/j.1476-5381.1993.tb13476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACHEL L., HANDY C., MOORE P.K. A comparison of the effects of L-NAME, 7-NI and L-NIL on corrageenan-induced hindpaw oedema and NOS activity. Br. J. Pharmacol. 1998;123:1119–1126. doi: 10.1038/sj.bjp.0701735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYCHAUDHURY A., FRISCHER H., MALIK A.B. Inhibition of endothelial cell proliferation and bFGF-induced phenotypic modulation by nitric oxide. J. Cell. Biochem. 1996;63:125–134. doi: 10.1002/(sici)1097-4644(19961101)63:2<125::aid-jcb1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- SAKKOULA E., PIPILI-SYNETOS E., MARAGOUDAKIS M.E. Involvement of nitric oxide in the inhibition of angiogenesis by interleukin-2. Br. J. Pharmacol. 1997;122:793–798. doi: 10.1038/sj.bjp.0701436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN B.-Q., LEE D.Y., GERBER H.-P., KEYT B.A., FERRARA N., ZIONCHECK T.F. Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J. Biol. Chem. 1998;273:29979–29985. doi: 10.1074/jbc.273.45.29979. [DOI] [PubMed] [Google Scholar]
- SHIMIZU T., KINUGAWA K., SUGISHITA Y., SUGISHITA K., HARADA K., MATSUI H., KOHMOTO O., SERIZAWA T., TAKAHASHI T. Molecular cloning and expression of inducible nitric oxide synthase in chick embryonic ventricular myocytes. Cardiovasc. Res. 1998;38:405–413. doi: 10.1016/s0008-6363(98)00005-4. [DOI] [PubMed] [Google Scholar]
- STONE E.M., ROTHBLUN K.N., ALEVY M.C., KUO T.M., SCHWARTZ R.J. Complete sequence of the chicken glyceraldehyde-3-phosphate dehydrogenase gene. Proc. Natl. Acad. Sci. U.S.A. 1985;82:1628–1632. doi: 10.1073/pnas.82.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO C., SOUTHAN G.J., THIEMERMANN C. Beneficial effects and improved survival in rodent models of septic shock with S-methyl-isothiourea sulfate, a novel, potent and selective inhibitor of inducible nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURUMI Y., MUROHARA T., KRASINSKI K., CHEN D., WITZENBICHLER B., KEARNEY M., COUFFINHAL T., ISNER J.M. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nature Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- TUDER R.M., FLOOK B.E., VOELKEL N.F. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J. Clin. Invest. 1995;95:1798–1807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG W., ANDO J., KORENAGA R., TOYO-OKA T., KAMIYA A. Exogenous nitric oxide inhibits proliferation of cultured vascular endothelial cells. Biochem. Biophys. Res. Commun. 1994;203:1160–1167. doi: 10.1006/bbrc.1994.2304. [DOI] [PubMed] [Google Scholar]
- ZICHE M., MORBIDELLI L., CHOUDHURI R., ZHANG H-T., DONNINI S., GRANGER H.J., BICKNELL R. Nitric oxide lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZICHE M., MORBIDELLI E., MASINI H., AMERINI H.J., GRANGER H.J., MAGGI C.A., GEPPETTI P., LEDDA F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZICHE M., MORBIDELLI L., MASINI E., GRANGER H.J., GEPPETTI P., LEDDA F. Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem. Biophys. Res. Commun. 1993;192:1198–1203. doi: 10.1006/bbrc.1993.1543. [DOI] [PubMed] [Google Scholar]


