Abstract
Antihistamine induced cognitive decline was evaluated using positron emission tomography (PET) measurement of histamine H1 receptor (H1R) occupancy and regional cerebral blood flow (rCBF).
Cognitive performance in attention-demanding task deteriorated dose-dependently and the effects were statistically significant after the treatment of 2 mg of d-chlorpheniramine. There was no significant change in subjective sleepiness in the same dose.
The regional blockade of H1R was observed mainly in the frontal, temporal and anterior cingulate cortices, and the intravenous administration of d-chlorpheniramine as a therapeutic dose (2 mg) blocked over 60% of H1R in the frontal cortices.
The results from activation study using visual discrimination tasks demonstrated that enhanced activity in the right prefrontal and anterior cingulate cortices as well as a decreased activity in the left temporal and frontal cortices and midbrain after the treatment of d-chlorpheniramine.
There were no changes in global CBF for the subjects treated with 2 mg d-chlorpheniramine (pre; 44.8±3.3 ml dl−1 min−1 vs post; 44.4±4.7 ml dl−1 min−1).
The results indicated that the attention system of human brain could be altered by therapeutic doses of H1R antagonists. These findings provide the information as to the potential risk of antihistamines in our daily activities.
Keywords: Antihistamines, H1 receptor antagonists, chlorpheniramine, brain, cognition, sedation in man, positron emission tomography, receptor occupancy, cerebral blood flow
Introduction
Allergic conditions such as allergic rhinitis are common, affecting as many as 10% of children and from 20% to 30% of adolescents and adults. Orally administered H1R antagonists, generally called antihistamines, are often used for the relief of symptoms of allergic rhinitis and chronic urticaria (Simons & Simons, 1994). They are also included in the over-the-counter (OTC) agents for the relief of cough and cold symptoms (Hendeles, 1993; Smith & Feldman, 1993). Use of the traditional or first-generation antihistamines, however, may be limited by CNS side effects. Sedation and impaired psychomotor performance are common adverse effects of antihistamines. Drug-induced sedation can interfere with the activities of daily living and with work that requires full alertness. Patients with allergic rhinitis have experienced decreases in verbal learning, decision-making ability and psychomotor speed when taking sedative antihistamines during the allergy season (Nicholson, 1983; Adelsberg, 1997). Impaired performance could potentially be dangerous for people who drive, pilot aircraft, or operate machinery. Although most people taking antihistamines have experienced such sedative effects, the neural correlates of these properties are not well understood in man.
Histaminergic neurons arise from the tuberomammillary nucleus of the posterior hypothalamus and diffusely project to almost all areas of the brain (Schwartz et al., 1991; Watanabe et al., 1984; Wada et al., 1991). The histaminergic system in the hypothalamus is considered to be involved in cortical activation during wakefulness and arousal mechanisms through H1R (Lin et al., 1988). It has been proposed that the effect of sedation of antihistamines result from occupation of central H1R. First-generation antihistamines can impair psychomotor performance without manifestations of subjective sleepiness. It is thought that cortical activities could be regulated by therapeutic dose of antihistamines. However, the brain mechanism of drug-induced sedation is not still understood. There is also little information about the relationship between H1R occupancy and psychomotor activity in humans.
The present study investigates the central mechanism of antihistamine-induced sedation in humans using PET. First, we examined the relationship between H1R occupancy and dose of antihistamine. We also evaluated the relationship between the dose of antihistamine and psychomotor performance in an attention-demanding visual discrimination task. Next, using [15O]H2O-PET and the visual discrimination task, we measured the changes of regional brain activity induced by sedative antihistamine. Furthermore, we examined the global effects of antihistamine on CBF.
Methods
Subjects
All subjects (total 43 men) are healthy male volunteers aged from 20–27 years old. Eight healthy subjects participated in the study for the assessment of changes in task performance and subjective sleepiness. Twenty healthy subjects were selected for the measurement of the H1R occupancy. In addition, 12 healthy male volunteers participated in rCBF measurement using [15O]H2O and 3D-PET. Another three subjects participated in global CBF measurement. Subjects were disqualified from participation if they had a history of neurological or psychiatric disease, recent use of centrally active drugs, chronic antihistamine use, alcohol or drug abuse, or allergy to antihistamines. Informed consent was obtained from all participants in this study. All experiments were performed in compliance with the relevant laws and institutional guidelines.
Task
Previous studies have shown that first-generation antihistamines impair psychomotor and cognitive function. Performance tasks requiring sustained attention and visuo-motor responses are considered to be sensitive to impairment induced by antihistamines (Adelsberg, 1997; Rice & Snyder, 1993; Meltzer, 1990; Seidel et al., 1987; Goetz et al., 1989). Thus, a visual discrimination task was adopted for the assessment of these sedative effects (Figure 1). This task required several types of information processing including signal detection, recognition and decision making. To create a highly attention-demanding condition, the visual stimuli were presented with a near-threshold (5 msec) presentation time in AV tachistoscope (IS701A, Iwatsu, Japan). The viewing distance was 70 cm, the letters subtended a visual angle of approximately 2°, with green characters on a black background. The task was to distinguish a target stimulus from non-target stimuli and to push the button promptly with the right index finger when a target stimulus was presented. Target stimuli were selected from ten kinds of digits and non-target stimuli were selected from 40 kinds of Japanese phonograms (hiragana). Target stimuli were presented randomly with a probability of 20%. The Inter-stimulus interval (ISI) was fixed at 1500 msec and a filled square was continuously displayed on the centre of the screen during these intervals. A total of 90 single digits or letters was serially presented during 135 s of one session. In order to assess the sedative effects of the drug, the reaction time (RT) and accuracy were objectively measured during each session. Accuracy was defined as the proportion of correct responses for target stimuli in all target stimuli presented in a session. Measurement of RT and response accuracy was performed using a NEC personal computer (PC-9801), directly connected to the centre unit of AV tachistoscope.
Figure 1.
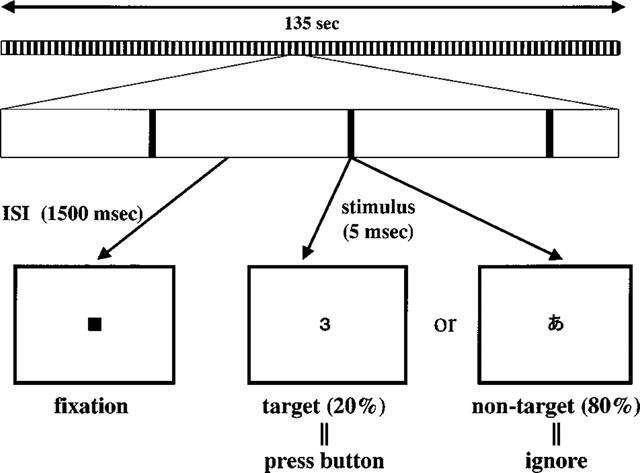
Visual discrimination task. Subjects viewed single visual stimulus at a near-threshold presentation time on the display of an AV tachistoscope (Iwatsu IS701A). The task was to distinguish the target stimulus from non-target stimulus and to press a button with the right index finger when the target stimulus was presented on the centre of the display. Target stimuli were selected from digits and non-target stimuli were selected from 40 kinds of Japanese phonogram (hiragana).
Assessment of subjective sleepiness and task performance
The study was a single-blind interindividual comparison between 1 and 2 mg of d-chlorpheniramine and a placebo (saline). The drug or placebo was intravenously injected on the experimental day, preceded by a 1 week washout period. Eight healthy subjects were assigned at random to the three groups treated with 1 mg or 2 mg of d-chlorpheniramine or placebo. After the 7th and 14th day, the subjects changed to another treatment, as each subject participated once in three different treatments. The order of the three different treatments was randomized. On the experimental day, the subjects entered a dimly-lit room and were instructed about the task and apparatus. After the subjects were trained until they were familiar with task procedure on each experimental day, performance was measured twice both before and after the injection of antihistamine. There was little effect of repeating the same task across three different sessions on the RT and accuracy. To exclude individual differences in the task performance, ratio of task performance between pre-injection and post-injection conditions was compared between these three groups. Subjective sleepiness was also evaluated using the Stanford Sleepiness Scale (SSS) (Hoddes et al., 1973) at a resting condition both before and 20 min after intravenous administration of antihistamine.
H1R occupancy measurements
Twenty healthy subjects were selected for the measurement of the H1R occupancy. For the measurement of H1R occupancy, PET scans were carried out with a CTI931 tomograph using [11C]-doxepin as a ligand of H1R in the human brain as previously described (Yanai et al., 1995). Subjects received 1, 2 or 5 mg of d-chlorpheniramine intravenously 10 min before the administration of [11C]-doxepin. PET data were acquired 30 s after the administration with the subject's eyes closed for 90 min. Binding potential (Bmax/KD) was used for the parameter of density and affinity of a receptor population, and was determined by graphical analysis (Logan et al., 1990). The binding potential on each voxel was determined from the ratio of the slopes for the values of each voxel and the region of interest (ROI) in the cerebellum. The slopes were determined from the last 12 points of the respective regions. H1R occupancy was calculated by the following equation: 100–(binding potential in each treated subject)/(mean value of binding potential in control subjects)×100 (%).
rCBF change during cognitive task
Twelve healthy male volunteers participated in the rCBF measurement using [15O]H2O and 3D-PET. Each subject was randomly assigned to the placebo (n=6) or drug-treated group (n=6). The rCBF images were obtained using a SET-2400W PET scanner (Shimadzu, Japan), with an average axial resolution of 4.5 mm at the full-width of half-maximum and sensitivity for a 20 cm cylindrical phantom of 48.6 k.c.p.s. kBq−1 ml−1 in the 3D-mode (Fujiwara et al., 1997). The advantage of the 3D data acquisition is 6 fold to 8 fold higher than the 2D data acquisition in its sensitivity and this scanner enables us to acquire rCBF images with high spatial resolution and relatively low doses of [15O]H2O. Subjects received approximately 4 mCi (148 MBq) of [15O]H2O intravenously through antecubital vein for each scans and engaged in point fixation or visual discrimination task during measurements of rCBF. First, resting scans were performed during fixation of the point in the centre of the display, and then the scans during visual discrimination task were performed. The visual discrimination task was started at 40 s before the PET scanning and finished at 15 s after the end of the scan. It was reported that plasma half-life of the intravenous administered chlorpheniramine was approximately 15 min during initial decay phase. Subsequently, plasma levels of chlorpheniramine remained stable for an hour (Peets et al., 1972). The 2 mg of d-chlorpheniramine or placebo (saline) was administered intravenously after completion of the pre-treatment scans (resting and task scans), which was followed by a 20 min waiting period before restarting the post-treatment scan (task scan only). A total of three scans were performed before and after injection of drug or placebo. No arterial blood sampling was performed during the measurement of rCBF. Task performance was simultaneously measured during the scans.
Measurement of absolute CBF value
Three subjects participated in the global CBF measurement with arterial blood sampling using [15O]H2O and the SET-2400W PET scanner in 2D-mode. For the measurement of CBF, 25 mCi of [15O]H2O was injected in a period of 30 s. Four dynamic image data were collected in five consecutive frames of 20 s each during 100 s scans. Two scans were performed before the administration of the drugs and the subsequent two scans were done 20 and 40 min after intravenous injection of 2 mg d-chlorpheniramine. Arterial blood was sampled from the radial artery at a flow rate of 4 ml min−1 during scans. Arterial blood radioactivity was monitored with a beta counter set along a blood draining line as previously described (Mejia et al., 1994). The CBF value was calculated using these dynamic images and the arterial radioactivity curve (Herscovitch et al., 1983; Raichle et al., 1983). Standard rCBF images were generated according to the autoradiographic technique with arterial input function.
Data analysis
Performance measurements including RT and accuracy were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. Statistical significance for each analysis is defined as P<0.05. The significance of difference in binding potential of the control, 1, 2 and 5 mg groups were also determined by ANOVA. Statistical significance for each analysis is defined as P<0.01. Calculations of image data were performed on a SPARC workstation (SUN Microsystems, U.S.A.). ANALYZE software was used to calculate ROI data. Statistical parametric mapping (SPM) software (SPM96; Wellcome Department of Cognitive Neurology, London, U.K.) was used for image realignment, normalization, smoothing and to create statistical maps of significant rCBF changes (Friston et al., 1995a,1995b). All CBF images were stereotaxically normalized using nonlinear transformation into a standard space of Talairach & Tournoux (1988). The normalized images were smoothed using a 12×12×12 mm Gaussian filter. The values of rCBF are expressed as ml dl−1 min−1, adjusted using the ANCOVA and scaled to a mean of 50. Condition and covariate effects were estimated according to the general linear model at each voxel. To test hypotheses on regionally specific condition and covariate effects, the estimates were compared using linear compounds or contrasts. The resulting set of voxel values for each contrast constitutes a statistical parametric map of the t-statistic. t-Statistics were computed for each voxel for the comparison: a post-injection task scan minus a pre-injection task scan for d-chlorpheniramine group and placebo group. Correlations for changes between rCBF and RT were also computed by a covariant analysis using the RT of visual discrimination task as a variable of interest. A t-statistic map was calculated to assess whether the slope of the regression at a given voxel was significantly different from zero. Voxels were considered significant if the Z scores were significant at P<0.05 after correction for multiple comparisons. In addition, voxels within the brain regions hypothesized to be involved in the attentional system and visual processing were considered significant at P<0.001 (uncorrected for multiple comparisons). Brain areas hypothesized to be engaged during attention and arousal tasks were the anterior cingulate gyrus, the prefrontal cortex, the inferior parietal cortex, and subcortical areas including the midbrain.
Results
Changes in task performance and subjective sleepiness
Subjective sleepiness was evaluated in the patients treated with antihistamine or placebo. Comparison of the SSS score was performed before and 20 min after the injection. SSS scores tended to increase slightly over the course of time in both the drug-treated group and placebo group, but no significant difference in the changes of SSS scores was observed between the three groups (Figure 2A). Task performance was evaluated using the RT and response accuracy measured during the performance of a visual discrimination task, before and after an intravenous dose of 1 or 2 mg of d-chlorpheniramine or placebo (saline). Significant elongation of RT has been previously reported after treatment with d-chlorpheniramine (Witek et al., 1995). In this study, both RT and accuracy deteriorated dose-dependently, and the effects were statistically significant after the injection of 2 mg of d-chlorpheniramine (Figure 2B,C). The sedative effects of d-chlorpheniramine appeared at relatively lower doses in this study than in a previous report (Meador et al. 1989). These results indicate that the task using visual stimuli at a near-threshold presentation time were sensitive for examining the antihistamine-induced cognitive impairment.
Figure 2.
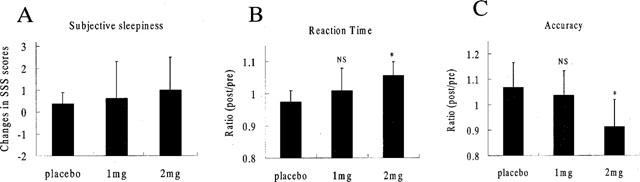
Changes in subjective sleepiness and task performances in the visual discrimination task (5 msec task) after the injection of d-chlorpheniramine and placebo. No significant changes were detected in relative changes of SSS scores (B) in all treated groups. Ratios (post-inj. data / pre-inj. data) of RT (B) and accuracy (C) are shown for the treatment with placebo and d-chlorpheniramine (1 and 2 mg). A significant difference in both RT and the accuracy ratio was observed between the 2 mg and placebo groups (P<0.05). But no significant difference was observed between the 1 mg and placebo groups. The statistical significance of differences in each group were determined by ANOVA followed by Dunnett's multiple comparison test. *P<0.05 (vs placebo group); NS (not significant compared with placebo group).
Relationship between H1R occupancy and impaired performance
We used PET to determine what level of H1R occupancy is required to produce impaired cognition of an attention-demanding task. The data from eight subjects demonstrated that a dose of 2 mg was needed to produce sedative effects during our task. The binding potential (Bmax/KD) of H1R was measured in control subjects and drug-treated subjects injected intravenously with 1, 2 or 5 mg of d-chlorpheniramine. In the anterior cingulate, frontal and temporal cortices, the binding potential significantly decreased after the dose of 2 mg (Figure 3). The H1R occupancies after the treatment of 2 mg, which is a dose often used by allergic patients, reached over 60% in the frontal cortex (Figure 4).
Figure 3.
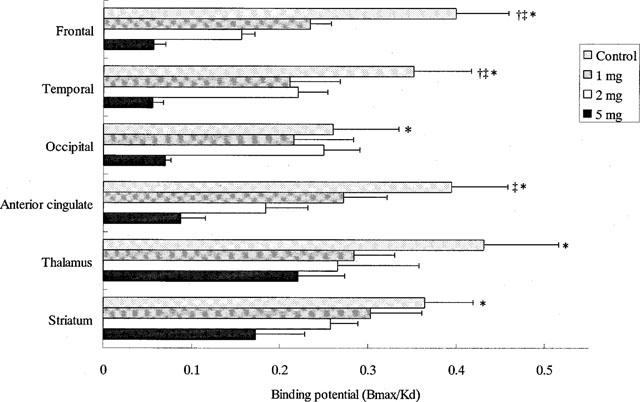
Binding potential (BP) of H1R in the brain after intravenous administration of d-chlorpheniramine. The regions of interest (ROIs) were placed on the frontal, temporal and occipital cortices, the anterior cingulate gyrus, the thalamus and the striatum. The binding potential significantly decreased in all cortical and sub-cortical areas. The values of binding potential at the doses of 0, 1, 2 and 5 mg were 0.400±0.059, 0.234±0.023, 0.156±0.015 and 0.057±0.014, respectively, in the frontal cortex. †P<0.01 (vs 1 mg group); ‡P<0.01 (vs 2 mg group); *P<0.01 (vs 5 mg group).
Figure 4.
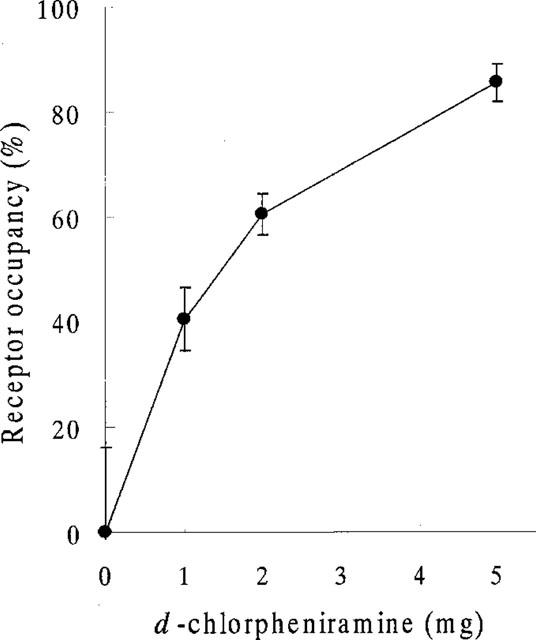
H1R occupancies after administration of 0, 1, 2 and 5 mg of d-chlorpheniramine in the frontal cortex. The H1R occupancies were dose-dependently increased (1 mg; 40.4±6.0%, 2 mg; 60.4±3.8%, 5 mg; 85.5±3.5%) in the frontal cortex.
Effect of antihistamine on cerebral blood flow and task performance
During [15O]H2O-PET scans, task performance was significantly deteriorated after the intravenous treatment of antihistamine. RT was significantly prolonged after treatment with 2 mg of d-chlorpheniramine (pre; 482±83 msec vs post; 552±94 msec, P<0.001). The mean task accuracy also deteriorated after treatment with 2 mg (pre; 84.9±17.2% vs post; 51.9±25.2%, P<0.005) in the d-chlorpheniramine-treated group. However, in the placebo group, no significant change was observed in terms of the RT (pre; 476±65 msec vs post; 494±39 msec) and accuracy (pre; 66.6±22.0% vs post; 76.8±10.8%). There were no significant changes in global CBF in the three subjects treated with 2 mg of d-chlorpheniramine (pre; 44.8±3.3 ml dl−1 min−1 vs post; 44.4±4.7 ml dl−1 min−1). Next we used PET imaging of rCBF as a measure of neuronal activity during the task in 12 human volunteers and compared the patterns of cortical and subcortical activation before and after administration of 2 mg d-chlorpheniramine or placebo (saline). Analysis of covariance was applied voxel by voxel to remove global effects. In the comparison of rCBF images during task between the pre- and post-injection conditions, a significant increase in neuronal activity was observed in the right inferior frontal gyrus, the anterior cingulate gyrus and the left insula (P<0.05, corrected) (Figure 5A, Table 1). On the other hand, significant rCBF decrease was observed in the left middle and superior temporal gyrus, the cingulate gyrus, left inferior and middle frontal gyrus and in the midbrain after treatment of d-chlorpheniramine (P<0.001, uncorrected) (Table 2). In the six placebo-treated subjects, no significant rCBF changes were observed between pre- and post-injection scans. The voxel value in right inferior frontal cortex increased after injection only in drug-treated subjects as shown in Figure 5B. In a correlational analysis of pre- and post-injection task scans of 12 subjects, the rCBF in right frontal cortex was positively correlated with the individual RT during scans (P<0.05, corrected) (Figure 6A,B).
Figure 5.
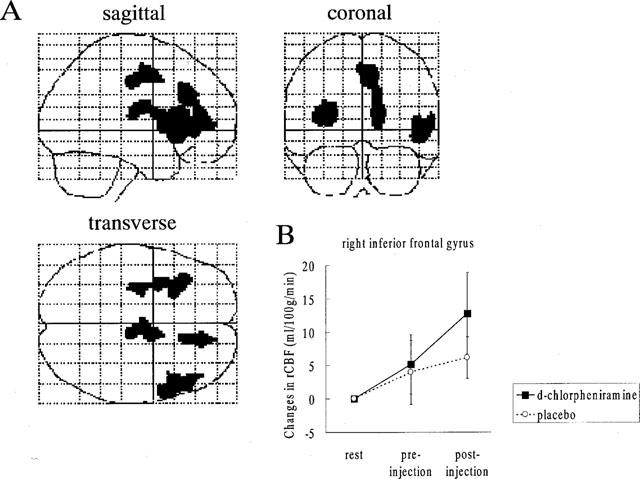
(A) Brain areas where the rCBF activation was relatively increased after the administration of 2 mg d-chlorpheniramine. The rCBF images obtained in the pre-injection scans were subtracted from those in the post-injection scans during visual discrimination task. Areas of significant relative rCBF increases (P<0.05, corrected) are shown as through-projections onto representations of standard stereotactic space. A significant increase in rCBF was observed on the right inferior frontal gyrus (45), the anterior cingulate gyrus (32, 24) and the left insula. The exact coordinates of the local maxima within the areas of activation and their Z statistics are given in Table 1. (B) Relative changes in the right inferior frontal gyrus (48 34 6) in the d-chlorpheniramine (2 mg) group and placebo group. The voxel was selected from the local maxima from the within-group analysis of the drug effect on task. The result indicated that the right inferior frontal cortex was activated only in drug-treated subjects.
Table 1.
Areas of rCBF significantly increased in the post-drug scans compared with the pre-drug scans on 5 msec visual stimuli (P<0.05, corrected).
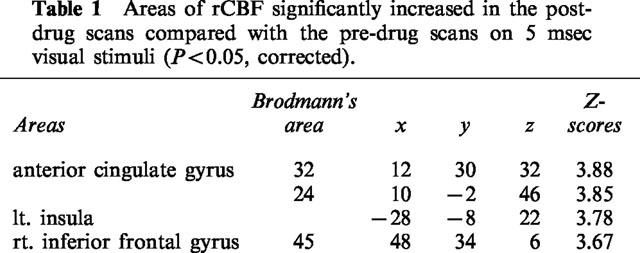
Table 2.
Areas of rCBF significantly decreased in the post-drug scans compared with the pre-drug scans on 5 msec visual stimuli (P<0.001, uncorrected).
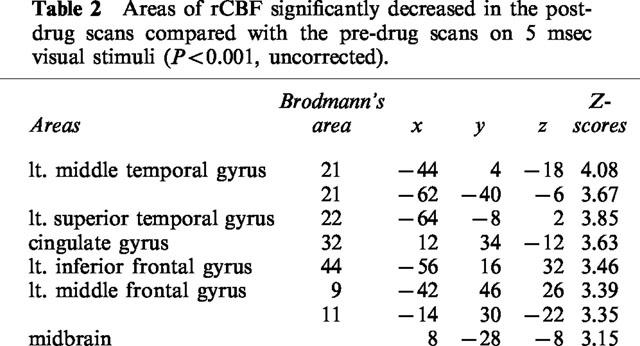
Figure 6.
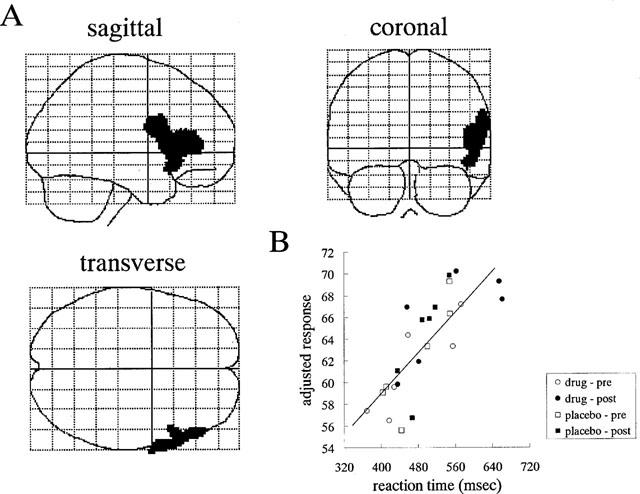
(A) Areas of positive correlation between RT measured during task scans and rCBF both in pre- and post-injection conditions of 12 subjects. Areas were shown as through-projections onto representations of the standard stereotactic space. In the voxel-by-voxel analysis, rCBF in right frontal cortex were significantly correlated with individual RT (P<0.05, corrected). (B) Typical correlation of the adjusted rCBF (adjusted to a mean of 50 ml dl−1 min−1) and individual RT of visual discrimination task in the voxel of right frontal cortex. The voxel was selected from the local maxima within the area of significant positive correlation in the voxel-by-voxel analysis.
Discussion
In this study, the cognitive impairment induced by low doses of d-chlorpheniramine was evaluated using a near-threshold visual discrimination task and rCBF measurement. There are many reports concerning the effect of d-chlorpheniramine on central processing (Adelsberg, 1997; Nolen, 1997). About 20% of patients affected allergic rhinitis complain of subjective sleepiness and tiredness when they take d-chlorpheniramine (Kemp et al., 1985). Previous performance studies demonstrated a deterioration in the visuomotor coordination ability (Nicholson, 1985), elongation of RT, and impaired ability on motor driving (Aso & Sakai, 1988). A study using evoked potential recording also indicated that 8 mg of d-chlorpheniramine prolongs P3 latency, that is a sensitive measure of sustained attention and cerebral processing speed (Kimford et al., 1989). More than 4 mg of d-chlorpheniramine were used for these performance studies. However, there is no report showing impaired cognition at a low dose (2 mg) of d-chlorpheniramine. In the present study, impaired performance and modulated regional activity of the brain were manifested at a dose of 2 mg, which was insufficient for producing subjective sleepiness. These findings strongly suggest that central processes of attention and motor control could be impaired without subjective symptoms. As it appears that there is no consistent relationship between objective performance and the subjective rating of sleepiness, assessment by objective measures in a sedative condition is necessary for the evaluation of drug safety.
However, performance measurements sometimes give a misleading result because the type and difficulty of the task could easily affect the conclusion of whether a drug is sedative or not. The measurement of receptor occupancy in the brain is considered to be an objective method to predict the pharmacological effects of central-acting drugs. Our previous study demonstrated that the receptor occupancy after treatment of first-generation H1R antagonists was about 70% at their therapeutic doses and that over 60% of the H1R blockage were observed after a single oral dose of 2 mg of d-chlorpheniramine (Yanai et al., 1995). In this study, performance measures were deteriorated after an intravenous injection of 2 mg of d-chlorpheniramine and about 60% of H1R was occupied at this dose. As the H1R occupancy after an intravenous administration of 2 mg d-chlorpheniramine was almost similar to that of the oral treatment with classical antihistamines, the results of this study could be extrapolated to the average oral dosing taken by individuals who require these sedative antihistamines medically. In contrast, H1R occupancies by non-sedative antihistamines were relatively lower as previously described (Yanai et al., 1995). These findings suggest that the sedative effects of H1R antagonists are due to the blockade of central H1R. More than 60% of H1R should be blocked to inhibit the arousal effects of neuronal histamine. Therefore, it appears that an occupancy of H1R of over 60% is needed to exhibit the central side effects of an antihistamine. Previous studies about central D2 receptor demonstrated that 70–90% of brain D2 receptor was occupied after treatment at therapeutic doses. Nyberg et al. (1995) examined the relationship between D2 receptor occupancy and RT using a D2 receptor antagonist. Task performance was not affected when there was less than 70% blockade of brain D2 receptor. Our results are similar to these concerning D2 receptor antagonists.
It is generally believed that histaminergic neurons can activate the cortex either directly by their widespread hypothalamocortical projection or indirectly via thalamocortical pathway in animals. Neuronal histamine could also promote cortical activation via the cholinergic system by a dual activation of the substantia innominata and the mesopontine tegmentum (Lin et al., 1996; Khateb et al., 1995). H1R antagonists are reported to inhibit the activation of noradrenergic neurons following central administration of histamine (Fleckenstein et al., 1994). Therefore, task-evoked brain activity might be affected indirectly by the modulation of the noradrenergic or cholinergic neurons. A recent PET study similarly could delineate the noradrenergic modulation of arousal state in human brains (Coull et al., 1997).
In electro-physiological studies, it was reported that H1R antagonists cause a drowsy pattern in spontaneous EEG in animals (Valjakka et al., 1996). A previous study using intracellular recording has shown the direct inhibition of neuronal activity in the cortex by blockade of the H1R (Reiner & Kamondi, 1994). Histamine, acting on the cortical H1R, can enhance behavioural arousal by modulating synaptic transmission of cortical neurons. Since a high density of H1R has been observed in the frontal and temporal cortices, and the cingulate gyrus in human brains (Yanai et al., 1992), a blockade of H1R would be expected to suppress the activity of these cortical neurons. In this view, there is a possibility that the blockade of H1R produces an attenuation of the rCBF increase in the legions activated in response to the task. However, significant rCBF increases were observed in the right frontal cortex and the anterior cingulate gyrus after the injection of d-chlorpheniramine. These structures have strong reciprocal connections with each other (Goldman-Rakic, 1988) and constitute the anterior attention system of the brain (Posner & Peterson, 1990). In recent PET studies it has been reported that the fronto-parietal network is important for sustained attention and visuo-motor control (Coull et al., 1997; Pardo et al., 1991; Johannsen et al., 1997; Lewin et al., 1997). The present study demonstrated a significant correlation between cognitive performance represented by RT and regional activation in the right frontal cortex. The data from performance study also indicated the dose-related changes in RT during task performance. These findings support the concept that right frontal cortex closely involves in attention and visuo-motor control. Previous studies showed that right prefrontal activity is associated with the effort needed to perform a task (Furey et al., 1997). It is also known that activity in anterior cingulate gyrus increases with task difficulty and poor task performance. A recent study demonstrated that a decrease in the activity of the anterior cingulate gyrus was caused by nicotine, reflecting decreasing demands for the task process (Ghatan et al., 1998). In view of these previous findings, our data suggest that the enhanced activity in the right prefrontal cortex and anterior cingulate gyrus represent increasing demands for the cognitive processes of visual discrimination and motor selection.
We observed a significant deactivation in the midbrain after treatment with d-chlorpheniramine. Based on the concept that neuronal histamine could evoke behavioural arousal through the reticular ascending activating system (Jouvet, 1996; Tasaka et al., 1993), we predicted that sedative antihistamines would decrease activity in these subcortical regions. Our results are in agreement with this prediction, although the rCBF decrease in midbrain was less than that in the other cortical regions. A previous PET study demonstrated that the midbrain reticular formation and thalamic intralaminar nuclei were activated when subjects were asked to transit from an awake relaxed state to a state of high vigilance and attention (Kinomura et al., 1996). Therefore, deactivation in the midbrain would reflect a transition from an arousal state to a sedative condition evoked by direct and/or indirect effects of the blockade of endogenous histamine.
The results of this study revealed that the attention system of human brain could be altered by therapeutic doses of H1R antagonists. Our results from the receptor and performance studies suggested that a blockade of more than 70% of H1R by antihistamines could induce central side effects. Increased activation in the right prefrontal and anterior cingulate gyrus in the rCBF analysis was considered to indicate the increase in the attentional demand caused by the therapeutic doses of antihistamines. These findings provide information concerning the potential risk of antihistamines in daily activities.
Acknowledgments
This work was supported by grants-in-aid from the Ministry of Education, Science and Culture, the Ministry of Health and the Shimadzu Science Foundation. We appreciate technical assistance of M. Miyake, Y. Ishikawa, S. Watanuki in PET studies. We also thank Dr H. Arai (Department of Geriatric Medicine) for his helpful comments on the manuscripts.
Abbreviations
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- BP
binding potential
- CNS
central nervous system
- 3D-PET
3-dimensional positron emission tomography
- H1R
histamine H1 receptor
- OTC
over-the-counter
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- ROI
the region of interest
- RT
reaction time
- SPM
statistical parametric mapping
- SSS
Stanford Sleepiness Scale
References
- ADELSBERG B.R. Sedation and performance issues in the treatment of allergic conditions. Arch. Int. Med. 1997;157:494–500. [PubMed] [Google Scholar]
- ASO T., SAKAI Y. Effects of terfenadine on actual driving performance. Jpn. J. Pharmacol. Ther. 1988;19:681–688. [Google Scholar]
- COULL J.T., FRITH C.D., DOLAN R.J., FRACKOWIAK R.S.J., GRASBY P.M. The neural correlates of the noradrenergic modulation of human attention, arousal, and learning. Eur. J. Neurosci. 1997;9:589–598. doi: 10.1111/j.1460-9568.1997.tb01635.x. [DOI] [PubMed] [Google Scholar]
- FLECKENSTEIN A.E., LOOKINGLAND K.J., MOORE K.E. Activation of noradrenergic neurons projecting to the diencephalon following central administration of histamine is mediated by H1 receptors. Brain Res. 1994;638:243–247. doi: 10.1016/0006-8993(94)90656-4. [DOI] [PubMed] [Google Scholar]
- FRISTON K.J., ASHBURNER J., FRITH C.D., POLINE J.P., HEATHER J.D., FRACKOWIAK R.S.J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995a;2:165–189. [Google Scholar]
- FRISTON K.J., HOLMES A.P., WORSLEY K.J., POLINE J.P, , FRITH C.D., FRACKOWIAK R.S.J. Statistical maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995b;2:189–210. [Google Scholar]
- FUJIWARA T., WATANUKI S., YAMAMOTO S., MIYAKE M., SEO S., ITOH M., ISHII K., ORIHARA H., FUKUDA H., SATOH T., KITAMURA K., TANAKA K., TAKAHASHI S. Performance evaluation of a large axial field-of-view PET scanner: SET-2400W. Ann. Nucl. Med. 1997;11:307–313. doi: 10.1007/BF03165298. [DOI] [PubMed] [Google Scholar]
- FUREY M.L., PIETRINI P., HAXBY J.V., ALEXANDER G.E., LEE H.C., VANMETER J., GRADY C.L., SHETTY U., RAPOPORT S.I., SHAPIRO M.B., FREO U. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHATAN P.H., INGVAR M., ERIKSSON L., STONE-ELANDER S., SERRANDER M., EKBERG K., WAHREN J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- GOETZ D.W., JACOBSON J.M., MURNANE J.E., REID M.J., REPPERGER D.W., GOODYEAR C., MARTIN M.E. Prolongation of simple and choice reaction times in a double-blind comparison of twice-daily hydroxyzine versus terfenadine. J. Allergy Clin. Immunol. 1989;84:316–322. doi: 10.1016/0091-6749(89)90414-4. [DOI] [PubMed] [Google Scholar]
- GOLDMAN-RAKIC P.S. Topography of cognition: Parallel distributed networks in primate association cortex. Annu. Rev. Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- HENDELES L. Efficacy and safety of antihistamines and expectorants in nonprescription cough and cold preparations. Pharmacotherapy. 1993;13:154–158. [PubMed] [Google Scholar]
- HERSCOVITCH P., MARKHAM J., RAICHLE M.E. Brain blood flow measured with intravenous H215O. I. Theory and error analysis. J. Nucl. Med. 1983;24:782–789. [PubMed] [Google Scholar]
- HODDES E., ZARCONE V.P., SMYTHE H., PHILLIPS R., DEMENT W.C. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- JOHANNSEN P., JAKOBSEN J., BRUHN P., HANSEN S.B., GEE A., STODKILDE-JORGENSEN H., GJEDDE A. Cortical sites of sustained and devided attention in normal elderly humans. Neuroimage. 1997;6:145–155. doi: 10.1006/nimg.1997.0292. [DOI] [PubMed] [Google Scholar]
- JOUVET M. Waking mechanisms: multiple networks in the mesencephalic reticular formation. Arch. Physiol. Biochem. 1996;104:762–769. doi: 10.1076/apab.104.6.762.12913. [DOI] [PubMed] [Google Scholar]
- KEMP J.P., BUCKLEY C.E., GERSHWIN M.E. Multicenter, double-blind, placebo-controlled trial of terfenadine in seasonal allergic rhinitis and conjunctivitis. Ann. Allergy. 1985;54:502–509. [PubMed] [Google Scholar]
- KHATEB A., FORT P., PEGNA A., JONES B.E., MUHLETHALER M. Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience. 1995;69:495–506. doi: 10.1016/0306-4522(95)00264-j. [DOI] [PubMed] [Google Scholar]
- KIMFORD J., MEADOR M.D., LORING D.W., THOMPSON E.E., THOMPSON W.O. Differential cognitive effects of terfenadine and chlorpheniramine. J. Allergy Clin. Immunol. 1989;84:322–325. doi: 10.1016/0091-6749(89)90415-6. [DOI] [PubMed] [Google Scholar]
- KINOMURA S., LARSON J., GULYAS B., ROLAND P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- LEWIN J.S., FRIEDMAN L., WU D., MILLER D.A., THOMPSON L.A., KLEIN S.K., WISE A.L., HEDERA P., BUCKLEY P., MELTZER H., FRIEDLAND R.P., DUERK J.L. Cortical localization of human sustained attention: detection with functional MR using a visual vigilance paradigm. J. Comput. Assist. Tomogr. 1996;20:695–701. doi: 10.1097/00004728-199609000-00002. [DOI] [PubMed] [Google Scholar]
- LIN J.S., HOU Y., SAKAI K., JOUVET M. Histaminergic descending inputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat. J. Neurosci. 1996;16:1523–1537. doi: 10.1523/JNEUROSCI.16-04-01523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN J.S., SAKAI K., JOUVET M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:1523–1537. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- LOGAN J., FOWLER J.S., VOLKOW N.D., WOLF A.P., DEWEY S.L., SCHLYER D.J., MACGREGOR R.R., HITZEMANN R., BENDTIEM B., GATLEY S.J., CHRISTMAN D.R. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- MEADOR K.J., LORING D.W., THOMPSON E.E., THOMPSON W.O. Differential cognitive effects of terfenadine and chlorpheniramine. J. Allergy Clin. Immunol. 1989;84:322–325. doi: 10.1016/0091-6749(89)90415-6. [DOI] [PubMed] [Google Scholar]
- MEJIA M.A., ITOH M., WATABE H., FUJIWARA T., NAKAMURA T. Specified nonlinearity correction of oxygen-15-water regional cerebral blood flow Images without blood sampling. J. Nucl. Med. 1994;35:1870–1877. [PubMed] [Google Scholar]
- MELTZER E.O. Performance effects of antihistamines. J. Allergy Clin. Immunol. 1990;86:613–619. doi: 10.1016/s0091-6749(05)80225-8. [DOI] [PubMed] [Google Scholar]
- NICHOLSON A.N. Antihistamines and sedation. Lancet. 1983;2:211–212. doi: 10.1016/s0140-6736(83)90185-x. [DOI] [PubMed] [Google Scholar]
- NICHOLSON A.N. Central effects of H1 and H2 antihistamines. Aviat. Space Environ. Med. 1985;56:293–298. [PubMed] [Google Scholar]
- NOLEN T.M. Sedative effects of antihistamines: safety, performance, learning, and quality of life. Clin. Ther. 1997;19:39–55. doi: 10.1016/s0149-2918(97)80071-9. [DOI] [PubMed] [Google Scholar]
- NYBERG S., FARDE L., BARTFAI A., HALLDIN C. Central D2 receptor occupancy and effects of zuclopenthixol acetate in humans. Int. Clin. Psychopharmacol. 1995;10:221–227. doi: 10.1097/00004850-199511000-00003. [DOI] [PubMed] [Google Scholar]
- PARDO J.V., FOX P.T., RAICHLE M.E. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- PEETS E.A., JACKSON M., SYMCHOWICZ S. Metabolism of chlorpheniramine maleate in man. J. Pharmacol. Exp. Ther. 1972;180:464–474. [PubMed] [Google Scholar]
- POSNER M.I., PETERSEN S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- RAICHLE M.E., MARTIN W.R.W., HERSCOVITCH P., MINTUN M.A., MARKKAM J. Brain blood flow measured with intravenous H215O. II. Implementation and validation. J. Nucl. Med. 1983;24:790–798. [PubMed] [Google Scholar]
- REINER P.B., KAMONDI A. A. Mechanism of antihistamine-induced sedation in the human brain: H1 receptor activation reduces a background leakage potassium current. Neuroscience. 1994;59:579–588. doi: 10.1016/0306-4522(94)90178-3. [DOI] [PubMed] [Google Scholar]
- RICE V.J., SNYDER H.L. The effects of Benadryl and Hismanal on psychomotor performance and perceived performance. Aviat. Space Environ. Med. 1993;64:726–734. [PubMed] [Google Scholar]
- SCHWARTZ J.C., ARRANG J.M., GARBARG M., POLLARD H., RUAT M. Histaminergic transmission in the mammalian brain. Physiol. Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- SEIDEL W.F., COHEN S., BLIWISE N.G., DEMENT W.C. Cetirizine effects on objective measures of daytime sleepiness and performance. Ann. Allergy. 1987;59:58–62. [PubMed] [Google Scholar]
- SIMONS F.E.R., SIMONS K.J. The pharmacology and use of H1-receptor-antagonist drugs. N. Engl. J. Pharmacol. 1994;330:1663–1670. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- SMITH M.B.H., FELDMAN W. Over-the-counter cold medications. JAMA. 1993;269:2258–2263. doi: 10.1001/jama.269.17.2258. [DOI] [PubMed] [Google Scholar]
- TALAIRACH J., TOURNEAUX P. A Co-planar Stereotaxic Atlas of a Human Brain. Thieme, Stuttgart; 1988. [Google Scholar]
- TASAKA K., CHUNG Y.H., MIO M., KAMEI C. The pathway responsible for EEG synchronization and effect of histamine on this system. Brain Res. Bull. 1993;32:365–371. doi: 10.1016/0361-9230(93)90201-l. [DOI] [PubMed] [Google Scholar]
- VALJAKKA A., VARTIAINEN J., KOSUNEN H., HIPPELAINEN M., PESOLA P., OLKKONEN H., AIRAKSINEN M.M., TUOMISTO L. Histaminergic modulation of neocortical spindling and slow-wave activity in freely behaving rats. J. Neural Transm. 1996;103:1265–1280. doi: 10.1007/BF01271187. [DOI] [PubMed] [Google Scholar]
- WADA H., INAGAKI N., YAMATODANI A., WATANABE T. Is the histaminergic neuron system a regulatory center for whole-brain activity. Trends Neurosci. 1991;14:415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- WATANABE T., TAGUCHI Y., SHIOSAKA S., TANAKA J., KUBOTA H., TERANO Y., TOHYAMA M., WADA H. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295:13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- WITEK T.J., JR, CANESTRARI D.A., MILLER R.D., YANG J.Y., RIKER D.K. Characterization of daytime sleepiness and psychomotor performance following H1 receptor antagonists. Ann. Allergy, Asthma, Immunol. 1995;74:419–426. [PubMed] [Google Scholar]
- YANAI K., RYU J.H., WATANABE T., IWATA R., IDO T., SAWAI Y., ITO T., ITOH M. Histamine H1 receptor occupancy in human brains after single oral doses of histamine H1 antagonists measured by positron emission tomography. Br. J. Pharmacol. 1995;116:1649–1655. doi: 10.1111/j.1476-5381.1995.tb16386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANAI K., WATANABE T., YOKOYAMA H., MEGURO K., HATAZAWA J., ITOH M., IWATA R., ISHIWATA K., YOKOYAMA H., TAKAHASHI T., IDO T. Histamine H1 receptors in human brain visualized in vivo by [11C]doxepin and positron emission tomography. Neurosci. Lett. 1992;137:145–148. doi: 10.1016/0304-3940(92)90390-s. [DOI] [PubMed] [Google Scholar]


