Abstract
Relaxant and contractile effects of the tethered ligand domain sequences of murine PAR-1, PAR-2, PAR-3 and PAR-4, and of the proteases thrombin and trypsin were examined in mouse isolated tracheal preparations. The epithelium- and cyclo-oxygenase-dependence of these effects and the potential modulatory effects of respiratory tract viral infection were also investigated.
In carbachol-contracted preparations, trypsin, thrombin, and the tethered ligand domain sequences of murine PAR-1 (SFFLRN-NH2), PAR-2 (SLIGRL-NH2) and PAR-4 (GYPGKF-NH2), but not PAR-3 (SFNGGP-NH2), induced transient, relaxant responses that were abolished by the cyclo-oxygenase inhibitor indomethacin.
Repeated administration of SFFLRN-NH2, SLIGRL-NH2 or GYPGKF-NH2 (30 μM) was associated with markedly diminished relaxation responses (homologous desensitization), although there was no evidence of cross-desensitization between these peptides.
The tethered ligand domain sequences for PAR-1 and PAR-4 induced a rapid, transient contractile response that preceded the relaxant response. Contractions were not inhibited by indomethacin and were not induced by either thrombin or trypsin.
Influenza A virus infection did not significantly affect the responses induced by either the proteases or peptides. Furthermore, epithelial disruption caused by mechanical rubbing had no significant effect on responses to these PAR activators in preparations from either virus- or sham-infected mice.
In summary, the proteases trypsin and thrombin, and peptide activators of PAR-1, PAR-2 and PAR-4 induced relaxant responses of mouse isolated tracheal smooth muscle preparations, which were mediated by a prostanoid, probably PGE2. Interestingly, PAR-mediated relaxations were not significantly diminished following acute damage to the epithelium caused by mechanical rubbing and/or the respiratory tract viral pathogen, influenza A.
Keywords: Protease-activated receptors, influenza A, trachea, smooth muscle, trypsin, thrombin, epithelium, prostanoids
Introduction
Protease-activated receptors (PARs) are a subfamily of seven transmembrane domain, G-protein-coupled receptors capable of mediating cellular signalling in response to proteases (Coughlin, 1994). A critical event in the activation of PARs by proteases is the cleavage of an amino terminal extracellular domain of the receptor, which generates a new amino terminus that functions as a tethered ligand and auto-activates the receptor (Vu et al., 1991; Nystedt et al., 1994). Currently, four PARs, termed PAR-1, PAR-2, PAR-3 and PAR-4 have been cloned (Vu et al., 1991; Nystedt et al., 1994; 1995; Ishihara et al., 1997; Xu et al., 1998). PAR-1 is activated by thrombin, a multifunctional serine proteinase that is generated upon tissue injury to promote blood coagulation and platelet aggregation, but which also induces biological responses in many other cell types including vascular smooth muscle and endothelial cells. Thrombin also activates the recently cloned PAR-3 and PAR-4 (Ishihara et al., 1997; Xu et al., 1998). The serine proteinase trypsin is a well-established activator of PAR-2, and a recent study in the airways by Cocks et al. (1999) have revealed that trypsin(ogen) and PAR-2 co-localize immunohistochemically within the airway epithelium. Another potential PAR-2 activator is tryptase (Corvera et al., 1997; Mirza et al., 1997), whose presence in mast cell granules may enable it to reach tissues where PAR-2 is expressed but trypsin is unlikely to be present (Molino et al., 1998). In addition to proteolytic enzymes, the synthetic peptides corresponding to the tethered ligand domains of murine PAR-1 (SFFLRN), PAR-2 (SLIGRL) and PAR-4 (GYPGKF), but not PAR-3 (SFNGGP), also stimulate their respective receptors.
The tissue distribution of PAR-2 has been thoroughly characterized in human tissues (D'Andrea et al., 1998) and strong immunostaining for PAR-2 was found in the endothelium and smooth muscle of the vasculature, which is consistent with recent reports that PAR-2 activation induces prominent changes in vascular smooth muscle tone in vitro and in vivo (Al-Ani et al., 1995; Hollenberg et al., 1996; Magazine et al., 1996; Saifeddine et al., 1996; Glusa et al., 1997; Cheung et al., 1998; Hamilton et al., 1998; Roy et al., 1998; Sobey & Cocks, 1998). Interestingly, strong immunolabelling for PAR-2 was also present in the epithelium and airway smooth muscle in normal human lung (D'Andrea et al., 1998). Moreover, activation of epithelial PAR-2 has been recently shown to cause relaxation of airway preparations from mouse, rat, guinea-pig and humans (Cocks et al., 1999).
The aim of the current study was to investigate the potential spasmogenic and spasmolytic effects of PAR-1, PAR-2, PAR-3 and PAR-4 activators (enzymes and synthetic peptides) in murine isolated tracheal smooth muscle preparations and to determine the extent to which these effects were dependent upon the generation of prostanoid mediators from the tracheal epithelium. The relative importance of an intact epithelium to PAR-mediated responses was established by examining the extent to which the responses were altered following epithelial disruption caused by respiratory tract viral infection and/or by mechanical rubbing, whereas a cyclo-oxygenase inhibitor indomethacin was used to evaluate the intermediary role of prostanoids in these PAR-mediated responses.
Methods
Mouse isolated tracheal preparation
Eight-week-old, male CBA/CaH mice (Animal Resources Center, Perth, Australia) were anaesthetized (methoxyflurane) and inoculated intranasally with 15 μl of fluid containing either influenza A/PR-8/34 virus (10,000 egg-infectious doses) or vehicle (diluted allantoic fluid from embryonated chicken eggs). Two days later, mice were killed with an overdose of pentobarbitone sodium (250 mg kg−1 i.p.) and the upper respiratory tract and associated alimentary tissue removed. The trachea was dissected free from surrounding tissue and cut in half to yield two 2 mm long tracheal smooth muscle preparations.
Isometric tension recordings
Tracheal smooth muscle segments were suspended under a resting tension of 0.5 g in organ baths containing 2 ml of Krebs bicarbonate solution, maintained at 37°C and bubbled continuously with 5% CO2 in O2 (carbogen). The composition of the Krebs bicarbonate solution was (in mM): NaCl 117, KCl 5.36, NaHCO3 25, KH2PO4 1.03, MgSO4.7H2O 0.57, CaCl2 2.5, D-glucose 11.1. Changes in tension were recorded via an isometric force transducer (FTO3, Grass Instruments) connected to a custom-built pre-amplifier and data acquisition system. Following a 45 min equilibration period, during which the tissues were washed every 15 min and tension re-adjusted to 0.5 g, tissues were exposed to the cumulative addition of submaximal (0.2 μM) and supramaximal (10 μM) concentrations of carbachol. The response to 10 μM carbachol was termed Cmax.
Functional studies
Concentration-effect curves to synthetic peptides of the tethered ligand sequence domains; SFFLRN-NH2 (PAR-1), SLIGRL-NH2 (PAR-2), SFNGGP-NH2 (PAR-3) and GYPGKF-NH2 (PAR-4) and to scrambled control peptide sequences (FSFLRN-NH2, LSIGRL-NH2, FSNGGP-NH2, YGPGKF-NH2 and GYPGFK-NH2) were performed by sequential addition of peptide to carbachol-contracted preparations. In these studies, preparations were precontracted with carbachol to 60–70% Cmax and upon reaching a plateau level of contracture, a single concentration of peptide (0.1 μM) was added and the response recorded. Preparations were washed and allowed to re-equilibrate for 15 min, and the process repeated three more times until each of the four concentrations of a single peptide (0.1, 1, 10 and 100 μM) had been tested in the preparation. In all experiments, relaxant and contractile responses were expressed as a percentage of the level of carbachol-induced contraction present immediately prior to addition of the peptide or protease. That is, 100% relaxation (100%R) is equivalent to complete reversal of the carbachol-induced contraction, and 100% contraction (100%C) is equivalent to a doubling of the carbachol-induced contraction.
In studies using the proteolytic activators trypsin (100 enzyme units (U) per ml) and thrombin (30 U ml−1), a single protease-induced response was obtained in each preparation. Responses were also determined to extracts of enzymes that had been boiled for 15 min.
In studies examining the role of cyclo-oxygenase products in PAR-mediated responses, indomethacin (3 μM) was added to the bath 20 min prior to the PAR activators. In other experiments, PAR-mediated effects were examined in epithelium-disrupted preparations. Disruption of the epithelium, which was confirmed histologically, was achieved by rolling the tracheal ring along a silk thread against a moistened (Krebs-bicarbonate solution) piece of tissue paper prior to suspending the preparation in the organ bath.
Desensitization studies
A series of cross-desensitization studies was performed between the tethered ligand sequences for PAR-1, PAR-2 and PAR-4. In these studies, 30 μM of an active PAR peptide (SFFLRN-NH2, SLIGRL-NH2 or GYPGKF-NH2) was added to carbachol-contracted mouse isolated tracheal smooth muscle preparations. After the response had waned and the level of tone had returned to the initial levels of carbachol-induced contracture (5 min), a second 30 μM dose of the same PAR peptide was added. This process was repeated a total of three times, by which stage the response was markedly attenuated (homologous desensitization). Five min after the final desensitizing concentration of peptide had been administered, a single bolus (100 μM) concentration of SFFLRN-NH2, SLIGRL-NH2 or GYPGKF-NH2 was added and the response measured. These responses were compared to control responses obtained simultaneously in preparations that had been repeatedly exposed to 30 μM of the appropriate scrambled peptide sequence.
Data and statistical analyses
Grouped relaxation (%R) and contraction (%C) data are presented as arithmetic mean±s.e.mean and grouped potency data (EC40%R) as geometric mean with associated 95% confidence limits (95% c.l.). Analysis of variance was used to test for differences between treatment groups, as indicated (SigmaStat, Jandel Corporation, San Rafael, CA, U.S.A.). Where appropriate, a modified t-statistic was used to test for differences between pairs of groups (Wallenstein et al., 1980).
Materials
Synthetic PAR peptides (SFFLRN-NH2, SLIGRL-NH2, SFNGGP-NH2 and GYPGKF-NH2) and scrambled sequence peptides (FSFLRN-NH2, LSIGRL-NH2, FSNGGP-NH2, YGPGKF-NH2 and GYPGFK-NH2) were prepared by Dr Richard Lipscombe (Protein Facility, University of Western Australia, Perth, Australia). Thrombin was purchased from CSL Limited (Melbourne, Australia). Other agents used were trypsin, carbachol (carbamylcholine chloride) and indomethacin (Sigma Chemical Company, St Louis, MO, U.S.A.). Influenza A/PR-8/34 virus was propagated in embryonated chicken eggs and stored in allantoic fluid at −85°C. On the day of the experiment virus and reagents were diluted with sterile 0.9% NaCl (w v−1) and stored at 4°C prior to use.
Results
Studies in preparations from sham-infected mice
Responses to the synthetic peptidic sequences
In mouse isolated tracheal smooth muscle preparations precontracted with carbachol to 60–70% Cmax, the PAR-1 activating peptide, SFFLRN-NH2 (100 μM) induced a biphasic response, characterized by a rapid transient contractile phase followed by a transient relaxant phase (Figure 1a). SFFLRN-NH2-induced contractions (16.7±2.9%C) and relaxations (20.0±5.4%R) were both relatively small (Figure 1), but were significantly greater than those induced by the control peptide FSFLRN-NH2 (0.0±0.0%C and 1.1±1.1%R; n=13; P<0.05).
Figure 1.
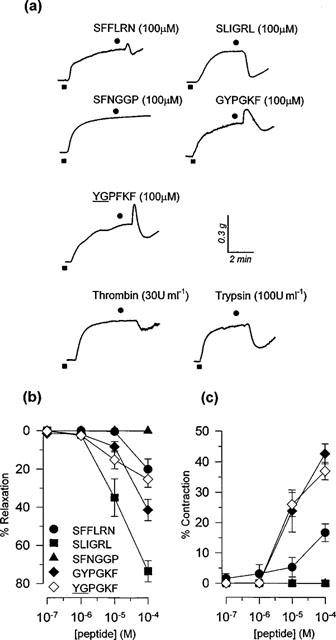
(a) Representative isometric tension recording traces of responses induced by peptide (SFFLRN-NH2, SLIGRL-NH2, SFNGGP-NH2, GYPGKF-NH2, YGPFKF-NH2) and enzyme (trypsin, thrombin) activators of PARs in mouse isolated tracheal smooth muscle preparations pre-contracted with 1 μM carbachol. (b) Peak relaxant responses induced by the PAR peptides SFFLRN-NH2, SLIGRL-NH2, SFNGGP-NH2, GYPGKF-NH2 and YGPGKF-NH2 in mouse isolated tracheal smooth muscle preparations pre-contracted with 1 μM carbachol. Data is presented as mean±s.e.mean (n=9–13). (c) Peak contractile responses induced by the PAR peptides SFFLRN-NH2, SLIGRL-NH2, SFNGGP-NH2, GYPGKF-NH2 and YGPFKF-NH2 in mouse isolated tracheal smooth muscle preparations pre-contracted with 1 μM carbachol. Data is presented as mean±s.e.mean (n=9–13).
The PAR-2 activating peptide, SLIGRL-NH2 induced concentration-dependent transient relaxant responses (Figure 1a,b). At the highest concentration used (100 μM), SLIGRL-NH2 induced 73.5±5.5%R and the concentration that produced 40%R was 12.9 μM (95% confidence limits, 5.0–33.5 μM, n=9). These relaxation responses were significantly larger than those induced by the scrambled peptide sequence LSIGRL-NH2 (6.6±2.4%R, n=6).
The PAR-3 tethered ligand domain peptide SFNGGP-NH2 and the control peptide, FSNGGP-NH2 failed to alter the level of carbachol-induced tone over the entire concentration range studied (0.1–100 μM) (Figure 1).
The PAR-4 activating peptide GYPGKF-NH2 induced a biphasic response, qualitatively similar to that produced by the PAR-1 activating peptide SFFLRN-NH2 (Figure 1a). However, GYPGKF-NH2-induced contractions (42.7±3.3%C) and relaxations (41.3±5.6%R; n=13) were both significantly larger than those induced by SFFLRN-NH2 (Figure 1b,c). Interestingly, the control PAR-4 peptide sequence YGPGKF-NH2 induced biphasic responses that were indistinguishable from those produced by the tethered ligand sequence domain GYPGKF-NH2 (Figure 1b,c). However, a second scrambled sequence control peptide GYPGFK-NH2 did not evoke any significant response in carbachol-contracted preparations (100 μM; 1.0±1.0%R, n=14).
Responses to enzymic PAR activators, thrombin and trypsin
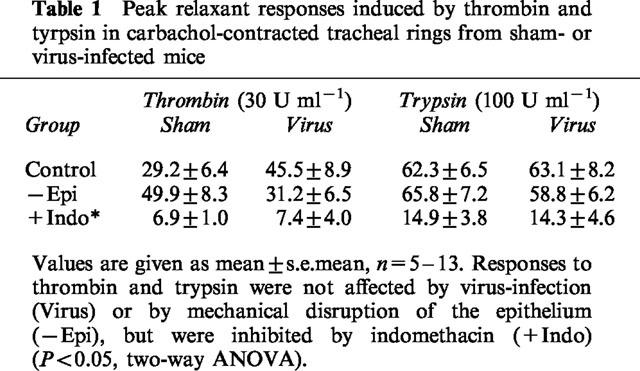
Enzymic stimulants of PARs induced transient relaxant responses, which were not accompanied by any contractile response. As shown in Table 1, responses to thrombin were significantly smaller than those induced trypsin (P<0.05). The relaxation responses induced by boiled thrombin (7.2±2.7%R, n=8) and boiled trypsin (22.6±7.1%R, n=5) were significantly smaller than those induced by the active enzymes respectively (P<0.05).
Table 1.
Peak relaxant responses induced by thrombin and tyrpsin in carbachol-contracted tracheal rings from sham- or virus-infected mice
Desensitization studies
Repeated administration of tethered ligand domain sequences for PAR-1 (30 μM SFFLRN-NH2), PAR-2 (30 μM SLIGRL-NH2) or PAR-4 (30 μM GYPGKF-NH2) resulted in profound desensitization of the relaxant responses to that peptide (Figure 2). However, no cross-desensitization was observed between the PAR-1, PAR-2 and PAR-4 activating peptides SFFLRN-NH2, SLIGRL-NH2 and GYPGKF-NH2. For example, preparations desensitized to the PAR-1 peptide SFFLRN-NH2 were responsive to SLIGRL-NH2 and similarly, SLIGRL-NH2-desensitized preparations remained responsive to SFFLRN-NH2 (Figure 2a,b). In contrast, cross-desensitization was observed between the PAR-4 tethered ligand domain sequence GYPGKF-NH2 and the active, scrambled peptide sequence YGPGKF-NH2; preparations desensitized to GYPGKF-NH2 did not respond to 100 μM YGPGKF-NH2 and vice versa (n=9–10).
Figure 2.
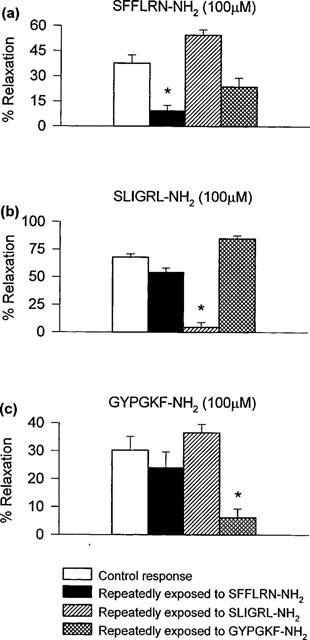
Cross-desensitization studies. Peak relaxant responses induced by 100 μM (a) SFFLRN-NH2, (b) SLIGRL-NH2 and (c) GYPGKF-NH2 in carbachol-contracted preparations following repeated exposure to SFFLRN-NH2, SLIGRL-NH2 or GYPGKF-NH2 to induce desensitization. Data are presented as mean±s.e.mean. *P<0.05, two-way ANOVA followed by modified t-statistic, using the Bonferroni correction for multiple comparisons.
Influence of indomethacin
Contractions induced by SFFLRN-NH2 and GYPGKF-NH2 were not affected by the cyclo-oxygenase inhibitor indomethacin (3 μM). In contrast, indomethacin markedly attenuated relaxation responses to the PAR-1 (SFFLRN-NH2), PAR-2 (SLIGRL-NH2) and PAR-4 (GYPGKF-NH2) tethered ligand domain sequences (Figure 3) and to trypsin and thrombin (Table 1).
Figure 3.
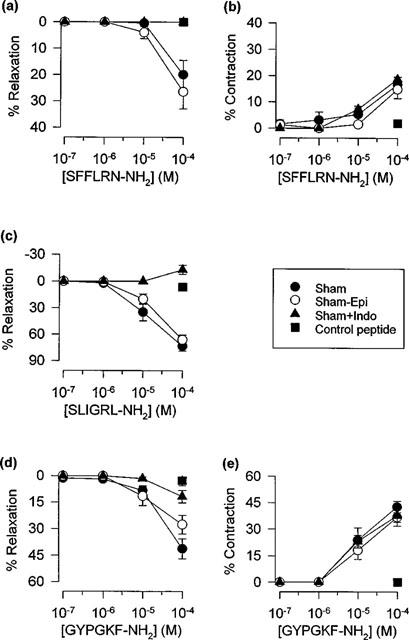
Influence of indomethacin (+Indo) and mechanical disruption of the epithelium (−Epi) on peak relaxant and contractile responses induced by (a,b) SFFLRN-NH2, (c) SLIGRL-NH2 and (d, e) GYPGKF-NH2 in sham-infected mouse tracheal preparations. Data are presented as mean±s.e.mean (n=9–13). Also shown are responses to the respective control peptides, FSFLRN-NH2 (a,b), LSIGRL-NH2 (c) and GYPGFK-NH2 (d,e).
Influence of epithelial disruption caused by mechanical rubbing
Rubbing of the luminal surface of the tracheal preparation with a silk thread caused pronounced disruption of the epithelium (Figure 4). However, mechanical rubbing of the epithelium had no significant effect on contractile or relaxant responses induced by the tethered ligand domain sequences for PAR-1, PAR-2 or PAR-4 (Figure 3) or by the proteases thrombin or trypsin (Table 1).
Figure 4.
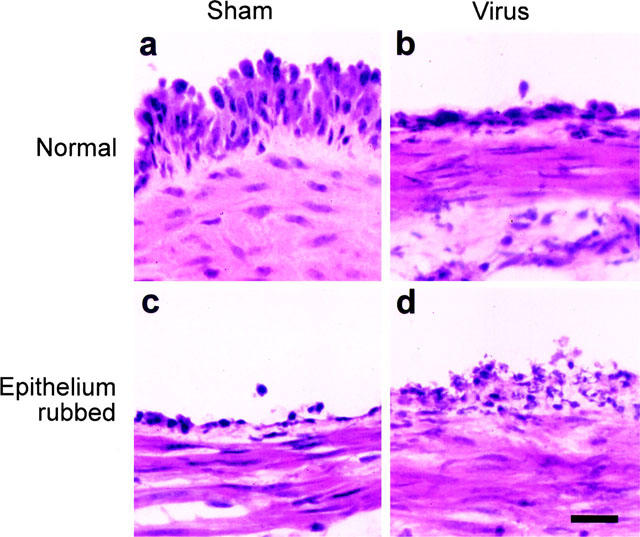
Histological examination of the influence of viral infection and of mechanical rubbing on mouse tracheal epithelium. Lightfield photomicrographs of hematoxylin-stained sections of mouse trachea following (a) sham-infection only, (b) virus-infection only, (c) sham-infection with rubbing, and (d) virus-infection with rubbing. Bar=20 μm.
Studies in preparations from virus-infected mice
Respiratory tract viral infection caused pronounced damage to the epithelium (Figure 4). Nevertheless, contractions and relaxations induced by the peptide activators of PAR-1, PAR-2 and PAR-4 (Figure 5), and by thrombin and trypsin (Table 1) were not significantly different from responses obtained in preparations from sham-infected animals. Furthermore, relaxation responses remained transient, concentration-dependent, sensitive to indomethacin and insensitive to disruption of the epithelium (two-way ANOVA, Table 1; Figure 6).
Figure 5.
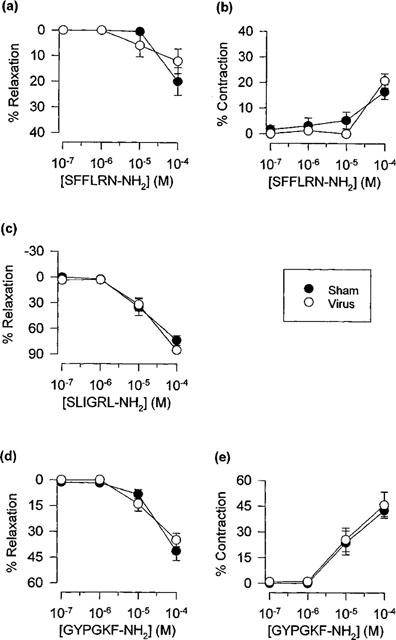
Peak relaxant and contractile responses induced by (a,b) SFFLRN-NH2, (c) SLIGRL-NH2 and (d,e) GYPGKF-NH2 (n=9–13) in carbachol-contracted tracheal preparations from sham- and virus-infected mice. Data are presented as mean±s.e.mean.
Figure 6.
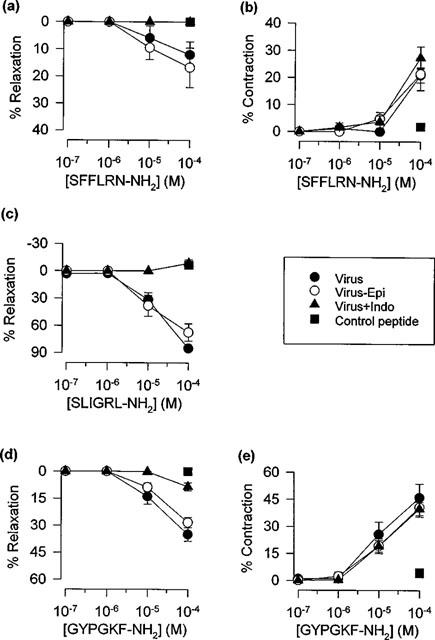
Influence of indomethacin (+Indo) and mechanical disruption of the epithelium (−Epi) on peak relaxant and contractile responses induced by (a,b) SFFLRN-NH2, (c) SLIGRL-NH2 and (d, e) GYPGKF-NH2 (n=9–13) in virus-infected mouse tracheal preparations. Data are presented as mean±s.e.mean. Also shown are responses to the respective control peptides, FSFLRN-NH2 (a, b), LSIGRL-NH2 (c) and GYPGFK-NH2 (d,e).
Discussion
In this study, we established that thrombin and trypsin, as well as synthetic tethered ligand domain sequences for murine PAR-1 (SFFLRN-NH2), PAR-2 (SLIGRL-NH2) and PAR-4 (GYPGKF-NH2), but not PAR-3 (SFNGGP-NH2), induced transient relaxations in carbachol-contracted, murine tracheal smooth muscle. For SFFLRN-NH2 and GYPGKF-NH2, but not other PAR activators tested, the relaxant phase was preceded by a rapid transient contraction. Homologous desensitization of the relaxation response was readily induced by repeated administration of each of the synthetic tethered ligand domain sequences for PAR-1, PAR-2 and PAR-4. However there was little evidence of cross-desensitization between these PAR-activating peptides. All PAR-mediated relaxant responses were inhibited by indomethacin, indicating an intermediary role for a relaxant prostanoid, possibly PGE2. Interestingly, despite the obligatory role of relaxant prostanoid(s) in PAR-mediated relaxation responses, these responses were not significantly attenuated by either mechanical- or virus-induced disruption of the tracheal epithelium.
Stimulation of PARs in vascular and gastrointestinal tissues significantly modulate smooth muscle tone (Hollenberg et al., 1996; Saifeddine et al., 1996; Magazine et al., 1996; Emilsson et al., 1997; Glusa et al., 1997; Hamilton et al., 1998). Moreover, Cocks et al. (1999) have recently established that the PAR-1 and PAR-2 tethered ligand domain sequences SFFLRN-NH2 and SLIGRL-NH2 (as well as thrombin and trypsin) caused relaxation of bronchial smooth muscle from human and several animal species. Consistent with this, the current study has demonstrated that SFFLRN-NH2, SLIGRL-NH2, thrombin and trypsin induced relaxation responses in mouse isolated tracheal preparations, suggesting that activation of either PAR-1 or PAR-2 will induce a relaxant response in this tissue. However, SFFLRN-NH2 has been reported to activate both PAR-1 and PAR-2 (Blackhart et al., 1996; Hollenberg et al., 1997), and thrombin can activate PAR-1, PAR-3 and PAR-4, raising the possibility that the observed relaxant responses to SFFLRN-NH2 and thrombin may have been mediated by PARs other than PAR-1. Nevertheless, additional cross-desensitization studies revealed that mouse isolated tracheal preparations made refractory to PAR-2 activation by repeated exposure to SLIGRL-NH2, were fully responsive to SFFLRN-NH2 and vice versa. These cross-desensitization studies provide strong evidence for the existence of PAR-1 in mouse trachea through which SFFLRN-NH2, and possibly thrombin, can cause relaxation.
PAR-4 also appears to be linked to signal transduction processes that modulate airway smooth muscle tone in murine trachea. The synthetic peptide GYPGKF-NH2, the tethered ligand domain sequence of murine PAR-4, induced a rapid transient contractile response that was followed by a transient relaxant phase. The underlying mechanism for the contractile response is not clear, but it does not appear to be mediated by a spasmogenic prostanoid since it was not inhibited by indomethacin. Cross-desensitization studies between GYPGKF-NH2, SFFLRN-NH2 and SLIGRL-NH2 indicated that GYPGKF-NH2-induced relaxations did not involve any significant action at PAR-1 or PAR-2. The identification of a functional role for PAR-4 in airway smooth muscle is consistent with reports of high levels of PAR-4 mRNA expression in lung (Xu et al., 1998). Although not of central importance to the study, it was of interest to note that whereas the control PAR-1 and PAR-2 peptides (FSFLRN-NH2 and LSIGRL-NH2, respectively) were inactive, the control PAR-4 peptide initially selected for study (YGPGKF-NH2) induced responses indistinguishable from GYPGKF-NH2. Responses to both GYPGKF-NH2 and YGPGKF-NH2 appeared to be mediated via activation of PAR-4 since preparations desensitized to GYPGKF-NH2 were also unresponsive to YGPGKF-NH2, and vice versa. The subsequent finding that the synthetic peptide sequence GYPGFK-NH2 was inactive suggests that the regions of the tethered ligand domains important for the activation of PAR-4 are different from those required for activation of PAR-1 or PAR-2.
In vascular preparations, relaxations induced by PAR activators are generally mediated by endothelium-dependent production of NO (Antonaccio et al., 1993; Hollenberg et al., 1996; Glusa et al., 1997; Sobey & Cocks, 1998; Hamilton et al., 1998), whereas in gastrointestinal preparations, cyclo-oxygenase products (Saifeddine et al., 1996), perhaps PGE2 (Kong et al., 1997) appear to mediate the effects induced by PAR activators. The finding that relaxations induced by each of the PAR activators examined in the current study were abolished by indomethacin indicate that prostanoids also mediated PAR-induced relaxations in airway smooth muscle and are consistent with recent findings by Cocks et al. (1999). Although the identity of the relaxant prostanoid is not certain, PGE2 is a likely candidate because it is synthesized within the airways, can inhibit bronchoconstriction through its effects on airway smooth muscle, nerves and mast cells (Jacoby, 1995) and, more specifically, induces relaxation of mouse isolated tracheal smooth muscle preparations precontracted with methacholine (Li et al., 1998).
Strong PAR-2 immunoreactivity has been detected on bronchial epithelium and smooth muscle from human lung (D'Andrea et al., 1998) and PGE2 is a cyclo-oxygenase product of airway epithelial (Churchill et al., 1989; Salari & Chan-Yeung, 1989; Duniec et al., 1989; Liedtke, 1988; Widdicombe et al., 1989) and smooth muscle cell cultures (Delamere et al., 1994; Barry et al., 1995; Vadas et al., 1996; Vigano et al., 1997; Pang & Knox, 1997a,1997b; Belvisi et al., 1997; Pyne et al., 1997; Schmidlin et al., 1998). Thus, two potential sources of PAR-induced prostanoids are the epithelium and the airway smooth muscle. In an attempt to elucidate the source of the relaxant prostanoid(s), the current study investigated the influence of disrupting the epithelium on PAR mediated relaxations. Rubbing the luminal surface of the trachea with a silk thread caused widespread and marked disruption of the epithelium, as verified by histological examination, but did not attenuate the relaxation responses to any of the PAR-activators tested. Furthermore, virus-induced disruption of the epithelium, either alone or in combination with mechanical disruption, also failed to significantly attenuate PAR-mediated relaxations. Together, these findings suggest that in murine trachea, relaxations induced by exogenously applied PAR-activators were mediated via non-epithelium-derived prostanoids.
However, it is important to note that although the mechanical and viral insults applied in the current study caused extensive epithelial disruption, neither protocol caused complete denudation of the epithelium. Murine tracheal epithelium typically contains three predominant cell types, ciliated cells, serous cells and basal cells (Ramphal et al., 1979; Evans et al., 1989). Mechanical disruption effectively removed the majority of the tracheal epithelium, except for a number of small cells resting on the basal lamina, characteristic of basal cells. Similarly, infection with influenza virus has been shown in scanning and transmission electron microscopy studies to denude the murine tracheal surface of ciliated and serous cells, without adversely affecting the basal cell layer (Ramphal et al., 1979). Consistent with these latter findings, parainfluenza virus infects ciliated cells and secretory cells but not basal cells of rat tracheal epithelium (Massion et al., 1993). Thus, it is possible that the PAR-mediated, indomethacin-sensitive relaxation responses observed in tracheal preparations whose epithelium has been disrupted by mechanical or viral means, is due to the actions of a prostanoid(s) released from basal epithelial cells. This latter postulate is consistent with the findings that removal of murine bronchial epithelial cells by a detergent Triton X-100 is associated with an abolition of PAR-2-mediated relaxations (Cocks et al., 1999). However, it is currently unclear as to whether basal cells (as opposed to other epithelial cell types) are able to synthesize and release prostanoids in situ and, moreover, whether basal epithelial cells express PARs that are linked to prostanoid production.
As indicated above, another potential source of relaxant prostanoids is airway smooth muscle (Delamere et al., 1994; Vigano et al., 1997; Pang & Knox, 1997a,1997b; Belvisi et al., 1997). Although PAR-2 immunoreactivity has been detected on airway smooth muscle (D'Andrea et al., 1998), it is not yet clear whether PARs are linked to prostanoid production in these cells. Irrespective of the source of prostanoids, the current study has clearly demonstrated that respiratory tract viral infection induced by influenza A has no significant effect on PAR-mediated relaxations in murine trachea. Although viral infection did not appear to modulate in vitro responses to PAR activators, it would be of interest to determine the in vivo bronchodilatory actions of these agents is modulated during viral infection. These in vitro studies suggest that the bronchoconstrictor effects associated with virus-induced hyperresponsiveness may be functionally antagonized by relaxant airway PARs, especially PAR-2.
Acknowledgments
The authors wish to acknowledge the National Health and Medical Research Council of Australia and The Asthma Foundation of Western Australia for their financial support.
Abbreviations
- Cmax
contractile response to 10 μM carbachol
- EC40R
concentration of drug producing 40%R determined by interpolation
- PAR
protease-activated receptor
- PGE2
prostaglandin E2
- %C
drug-induced contractile response expressed as a percentage of the carbachol-induced pre-contraction
- %R
drug-induced relaxant response expressed as a percentage of carbachol-induced pre-contraction
References
- AL-ANI B., SAIFEDDINE M., HOLLENBERG M.D. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can. J. Physiol. Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- ANTONACCIO M.J., NORMANDIN D., SERAFINO R., MORELAND S. Effects of thrombin and thrombin activating peptides on rat aortic vascular smooth muscle. J. Pharmacol. Exp. Ther. 1993;266:125–132. [PubMed] [Google Scholar]
- BARRY T., DELAMERE F., HOLLAND E., PAVORD I., KNOX A. Production of PGE2 by bovine cultured airway smooth muscle cells: regulation by cAMP. J. Appl. Physiol. 1995;78:623–628. doi: 10.1152/jappl.1995.78.2.623. [DOI] [PubMed] [Google Scholar]
- BELVISI M.G., SAUNDERS M.A., HADDAD EL-B., HIRST S.J., YACOUB M.H., BARNES P.J., MITCHELL J.A. Induction of cyclooxygenase-2 by cytokines in human cultured airway smooth muscle cells: novel inflammatory role of this cell type. Br. J. Pharmacol. 1997;120:910–916. doi: 10.1038/sj.bjp.0700963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- CHEUNG W.M., ANDRADE-GORDON P., DERIAN C.K., DAMIANO B.P. Receptor-activating peptides distinguish thrombin receptor (PAR-1) and protease activated receptor 2 (PAR-2) mediated hemodynamic responses in vivo. Can. J. Physiol. Pharmacol. 1998;76:16–25. doi: 10.1139/cjpp-76-1-16. [DOI] [PubMed] [Google Scholar]
- CHURCHILL L., CHILTON F.H., RESAU J.H., BASCOM R., HUBBARD W.C., PROUD D. Cyclooxygenase metabolism of endogenous arachidonic acid by cultured human tracheal epithelial cells. Am. Rev. Respir. Dis. 1989;140:449–459. doi: 10.1164/ajrccm/140.2.449. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.M., ANDERSON G.P., FRAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., MCCONALOGUE K., BOHM S.K., KHITIN L.M., CAUGHEY G.H., PAYAN D.G., BUNNETT N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUGHLIN S.R. Protease-activated receptors start a family. Proc. Natl. Acad. Sci. 1994;91:9200–9202. doi: 10.1073/pnas.91.20.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANDREA M.R., DERIAN C.K., LETURCQ D., BAKER S.M., BRUNMARK A., LING P., DARROW A.L., SANTULLI R.J., BRASS L.F., ANDRADE-GORDON P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J. Histochem. Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- DELAMERE F., HOLLAND E., PATEL S., BENNETT J., PAVORD I., KNOX A. Production of PGE2 by bovine cultured airway smooth muscle cells and its inhibition by cyclooxygenase inhibitors. Br. J. Pharmacol. 1994;111:983–988. doi: 10.1111/j.1476-5381.1994.tb14840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNIEC Z.M., ELING T.E., JETTEN A.M., GRAY T.E., NETTESHEIM P. Arachidonic acid metabolism in normal and transformed rat tracheal epithelial cells and its possible regulation of cell proliferation. Exp. Lung Res. 1989;15:391–408. doi: 10.3109/01902148909087867. [DOI] [PubMed] [Google Scholar]
- EMILSSON K., WAHLESTEDT C., SUN M.-K., NYSTEDT S., OWMAN C., SUNDELIN J. Vascular effects of proteinase-activated receptor 2 agonist peptide. J. Vasc. Res. 1997;34:267–272. doi: 10.1159/000159233. [DOI] [PubMed] [Google Scholar]
- EVANS M.J., COX R.A., SHAMI S.G., WILSON B., PLOPPER C.G. The role of basal cells in attachment of columnar cells to the basal lamina of the trachea. Am. J. Respir. Cell Mol. Biol. 1989;1:463–469. doi: 10.1165/ajrcmb/1.6.463. [DOI] [PubMed] [Google Scholar]
- GLUSA E., SAFT A., PRASA D., STURZEBECHER J. Trypsin- and SLIGRL-induced vascular relaxation and the inhibition by benzamidine derivatives. Thromb. Haemost. 1997;78:1399–1403. [PubMed] [Google Scholar]
- HAMILTON J.R., NGUYEN P.B., COCKS T.M. Atypical protease-activated receptor mediates endothelium-dependent relaxation of human coronary arteries. Circ. Res. 1998;82:1306–1311. doi: 10.1161/01.res.82.12.1306. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B. Proteinase-activated receptor-2 in rat aorta: structural requirements for agonist activity of receptor-activating peptides. Mol. Pharmacol. 1996;49:229–233. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., KAWABATA A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- JACOBY D.B. Mediator functions of epithelial cells Asthma and Rhinitis 1995573–583.In: Busse W.W. & Holgate S.T. (eds)Boston: Blackwell Scientific Publications, pp
- KONG W., MCCONALOGUE K., KHITIN L.M., HOLLENBERG M.D., PAYAN D.G., BOHM S.K., BUNNETT N.W. Luminal trypsin may regulate enterocytes through proteinase-activated receptor-2. Proc. Natl. Acad. Sci. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI L., VAALI K., PAAKKARI I., VAPAATALO H. Involvement of bradykinin B1 and B2 receptors in relaxation of mouse isolated trachea. Br. J. Pharmacol. 1998;123:1337–1342. doi: 10.1038/sj.bjp.0701741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEDTKE C.M. Differentiated properties of rabbit tracheal epithelial cells in primary culture. Am. J. Physiol. 1988;255:C760–C770. doi: 10.1152/ajpcell.1988.255.6.C760. [DOI] [PubMed] [Google Scholar]
- MAGAZINE H.I., KING J.M., SRIVASTAVA K.D. Protease activated receptors modulate aortic vascular tone. Int. J. Cardiol. 1996;53:S75–S80. doi: 10.1016/0167-5273(96)02569-7. [DOI] [PubMed] [Google Scholar]
- MASSION P.P., FUNARI C.C.P., UEKI I., MCDONALD D.M., NADEL J.A. Parainfluenza (Sendai) virus infects ciliated cells and secretory cells but not basal cells of rat tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 1993;9:361–370. doi: 10.1165/ajrcmb/9.4.361. [DOI] [PubMed] [Google Scholar]
- MIRZA H., SCHMIDT V.A., DERIAN C.K., JESTY J., BAHOU W.F. Mitogenic esponses mediated through the proteinase-activated receptor-2 are induced by expressed forms of mast cell α- or β-tryptases. Blood. 1997;90:3914–3922. [PubMed] [Google Scholar]
- MOLINO M., RAGHUNATH P.N., KUO A., AHUJA M., HOXIE J.A., BRASS L.F., BARNATHAN E.S. Differential expression of functional protease-activated receptor-2 (PAR-2) in human vascular smooth muscle cells. Arterioscl. Thromb. Vasc. Biol. 1998;18:825–832. doi: 10.1161/01.atv.18.5.825. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., LARSSON A.K., ABERG H., SUNDELIN J. The mouse proteinase-activated receptor-2 cDNA and gene. Molecular cloning and functional expression. J. Biol. Chem. 1995;270:5950–5955. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- PANG L., KNOX A.J. PGE2 release by bradykinin in human airway smooth muscle cells: involvement of cyclooxygenase-2 induction. Am. J. Physiol. 1997a;273:L1132–L1140. doi: 10.1152/ajplung.1997.273.6.L1132. [DOI] [PubMed] [Google Scholar]
- PANG L., KNOX A.J. Effect of interleukin-1β, tumour necrosis factor-α and interferon-γ on the induction of cyclooxygenase-2 in cultured human airway smooth muscle cells. Br. J. Pharmacol. 1997b;121:579–587. doi: 10.1038/sj.bjp.0701152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYNE N.J., TOLAN D., PYNE S. Bradykinin stimulates cAMP synthesis via mitogen-activated protein kinase-dependent regulation of cytosolic phospholipase A2 and prostaglandin E2 release in airway smooth muscle. Biochem. J. 1997;328:689–694. doi: 10.1042/bj3280689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMPHAL R., FISCHLSCHWEIGER W., SHANDS J.W., SMALL P.A. Murine influenzal tracheitis: A model for the study of influenza and tracheal epithelial repair. Am. Rev. Respir. Dis. 1979;120:1313–1324. doi: 10.1164/arrd.1979.120.6.1313. [DOI] [PubMed] [Google Scholar]
- ROY S.S., SAIFEDDINE M., LOUTZENHISER R., TRIGGLE C.R., HOLLENBERG M.D. Dual endothelium-dependent vascular activities of proteinase-activated receptor-2-activating peptides: evidence for receptor heterogeneity. Br. J. Pharmacol. 1998;123:1434–1440. doi: 10.1038/sj.bjp.0701726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALARI H., CHAN-YEUNG M. Release of 15-hydroxyeicosatetraenoic acid (15-HETE) and prostaglandin E2 (PGE2) by cultured human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1989;1:245–250. doi: 10.1165/ajrcmb/1.3.245. [DOI] [PubMed] [Google Scholar]
- SCHMIDLIN F., SCHERRER D., DAEFFLER L., BERTRAND C., LANDRY Y., GIES J.P. Interleukin-1beta induces bradykinin B2 receptor gene expression through a prostanoid cyclic AMP-dependent pathway in human bronchial smooth muscle cells. Mol. Pharmacol. 1998;53:1009–1015. [PubMed] [Google Scholar]
- SOBEY C.G., COCKS T.M. Activation of protease-activated receptor-2 (PAR-2) elicits nitric oxide-dependent dilatation of the basilar artery in vivo. Stroke. 1998;29:1439–1444. doi: 10.1161/01.str.29.7.1439. [DOI] [PubMed] [Google Scholar]
- VADAS P., STEFANSKI E., WLOCH M., GROUIX B., VAN DEN BOSCH H., KENNEDY B. Secretory non-pancreatic phospholipase A2 and cyclooxygenase-2 expression by tracheobronchial smooth muscle cells. Eur. J. Biochem. 1996;235:557–563. doi: 10.1111/j.1432-1033.1996.t01-1-00557.x. [DOI] [PubMed] [Google Scholar]
- VIGANO T., HABIB A., HERNANDEZ A., BONAZZI A., BORASCHI D., LEBRET M., CASSINA E., MACLOUF J., SALA A., FOLCO G. Cyclooxygenase-2 and synthesis of PGE2 in human bronchial smooth-muscle cells. Am. J. Respir. Crit. Care Med. 1997;155:864–868. doi: 10.1164/ajrccm.155.3.9117018. [DOI] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WALLENSTEIN S., ZUCKER C.L., FLEISS J.L. Some statistical methods useful in circulation research. Circ. Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J.H., UEKI I.F., EMERY D., MARGOLSKEE D., YERGEY J., NADEL J.A. Release of cyclooxygenase products from primary cultures of tracheal epithelia of dog and human. Am. J. Physiol. 1989;257:L361–L365. doi: 10.1152/ajplung.1989.257.6.L361. [DOI] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]


