Abstract
The direct impact of ethanol on native, non-NMDA glutamate receptors was examined in acutely isolated MS/DB neurons from rat. The impact of ethanol functional tolerance and physical dependence on non-NMDA receptor function was also determined.
Non-NMDA receptors were defined pharmacologically as predominantly the AMPA subtype, because both AMPA- or kainate-activated currents were blocked by GYKI 52466, a selective AMPA receptor antagonist. The relative magnitude of potentiation of AMPA-activated currents by 10 or 100 μM cyclothiazide was consistent with recombinant AMPA flop-subtype receptors. Finally, the selective kainate receptor agonist, SYM 8021, induced little current in MS/DB neurons.
AMPA receptor currents when activated by kainate were sensitive to ethanol, showing inhibition of ∼5–50% when 10–300 mM ethanol and kainate were briefly co-applied (3 s). Ethanol (100 mM) also inhibited both the initial transient peak and sustained currents activated by AMPA. Inhibition was sustained during continuous ethanol superfusions of 5 min, suggesting a lack of acute tolerance to ethanol-induced AMPA receptor blockade.
Rapid application of 3–3000 μM kainate activated concentration-dependent currents in MS/DB neurons from Control and Ethanol Dependent animals that were not significantly different. Also, direct ethanol inhibition (300 mM) of kainate-activated currents was not reduced by ethanol dependence, suggesting a lack of functional tolerance.
These results suggest that native AMPA receptors on MS/DB neurons are inhibited by pharmacologically-relevant concentrations of ethanol. However, these receptors, unlike NMDA receptors, do not undergo adaptation with sustained ethanol exposure sufficient to induce physical dependence.
Keywords: Medial septum/diagonal band, non-NMDA glutamate receptors, AMPA receptor, ethanol intoxication, tolerance and dependence, patch-clamp electrophysiology
Introduction
Glutamate serves as the principal excitatory neurotransmitter in the mammalian central nervous system (Watkins & Evans, 1981; Dingledine et al., 1999), generating fast excitatory postsynaptic potentials by activation of ionotropic receptors of the α-amino-3-hydroxy-5-methyl-4-isoxalonepropionic acid (AMPA; subunits GluR1-4), kainate (subunits GluR5-7, KA-1&2) and N-methyl-D-aspartate (NMDA; subunits NR1-3) subtypes and modulating neurotransmitter release through activation of metabotropic G-protein receptors (mGluR1-8) or kainate subtype receptors (Nakanishi et al., 1998; Dingledine et al., 1999). Glutamate neurotransmission generally appears to be a target for ethanol and likely plays important roles in aspects of ethanol intoxication such as cognitive impairment, blackouts and ethanol withdrawal excitability, as well as in excitotoxic mechanisms involved in longer-term brain damage in foetal alcohol syndrome and Wernicke-Korsakoffs syndrome (Tsai et al., 1995; Faingold et al., 1998).
At a cellular level, ethanol exerts a well characterized acute inhibition of both native (Peoples et al., 1997; Thomas et al., 1998; Bhave et al., 1999) and recombinant (Dildy-Mayfield & Harris, 1992; Koltchine et al., 1993) NMDA receptors, an action which in some model systems may reverse within minutes of continuing exposure in a manner consistent with acute tolerance (Grover et al., 1994; Miyakawa et al., 1997). Under some conditions, ethanol induces functional receptor up-regulation during chronic treatment over several days (Trevisan et al., 1994; Chen et al., 1997; Grover et al., 1998; Kumari & Ticku, 1998; Thomas et al., 1998), although changes in binding sites for NMDA receptor ligands are not consistently robust (see for review Rudolph et al., 1997). Such ethanol-induced increases in NMDA receptor function likely make significant contributions to functional tolerance, withdrawal hyperexcitability, calcium-dependent excitotoxicity and long-term brain damage in chronic alcoholism (Tsai et al., 1995; Grover et al., 1998; Thomas et al., 1998). Interestingly, NMDA receptors containing certain subunits, show greater relative inhibition by ethanol (Yang et al., 1996; Bhave et al., 1999), and potentially may further increase the vulnerability of neurons expressing them to chronic ethanol toxicity.
Ethanol, also inhibits, acutely, the activity of native and recombinant non-NMDA ionotropic receptors. Overall, a similar degree of inhibition is observed for both recombinant AMPA or kainate receptor subtypes in expression systems (Lovinger, 1993; Minami et al., 1998b; Valenzuela & Cardoso, 1999). Whether there are receptor subtype selective actions of ethanol for native neuronal AMPA/kainate receptors is less certain (Lovinger et al., 1990; Dildy-Mayfield & Harris, 1992; Lovinger, 1993; Martin et al., 1995; Yang et al., 1996), in part due to a lack until recently of selective pharmacological tools for distinguishing native AMPA and kainate subtypes (Johansen et al., 1995; Wilding & Huettner, 1995; Donevan et al., 1998; Dingledine et al., 1999; Savidge et al., 1999; Weiner et al., 1999). Evidence that in vitro or in vivo chronic ethanol exposure can up-regulate non-NMDA receptors is mixed (Trevisan et al., 1994; Molleman & Little, 1995; Chandler et al., 1999), and specific effects on pharmacologically characterized native AMPA or kainate receptor subtypes largely remains to be determined. Finally, the actions of acute or chronic ethanol on either native or recombinant metabotropic glutamate receptors have received even less attention than NMDA, AMPA or kainate receptors and their interactions with ethanol largely remain to be defined or confirmed (Minami et al., 1998a).
The purpose of the present investigation was to define the impact of immediate in vitro ethanol application and in vivo tolerance and dependence-inducing chronic ethanol treatment on the function of native non-NMDA inotropic receptors found on medial septum/diagonal band (MS/DB) neurons. Previously, ethanol was shown, acutely to inhibit native NMDA receptors on MS/DB neurons from rat, while induction of physical dependence up-regulated peak NMDA receptor currents in these cells and induced resistance to inhibitory actions of immediate ethanol application (Grover et al., 1998). In the present study, currents activated by either AMPA or kainate in MS/DB neurons were studied and shown pharmacologically to be mediated by AMPA subtype glutamate receptors. AMPA receptor currents were inhibited by pharmacologically-relevant concentrations of ethanol (10–300 mM). However, unlike NMDA receptors, AMPA receptors on MS/DB neurons were not up-regulated following induction of physical ethanol dependence and did not show functional tolerance to immediate ethanol inhibition. Together, these findings are consistent with the idea that AMPA receptors, which are the primary carriers of fast excitatory neurotransmission, are relatively resistant to the sustained inhibitory influence of ethanol when compared with NMDA receptors, which have a prominent role in synaptic plasticity and thus undergo adaptive up-regulation in response to ethanol blockade.
Methods
Experimental animals
Male Sprague-Dawley rats, 100–135 g (Timco-Harlan Industries, Houston, TX, U.S.A.) were maintained at the College of Medicine's Animal Care Facility, which is fully accredited by AAALAC, International. Animals were housed at 22–25°C and on a 12 h light cycle (lights on 0700 to 1900 h) and maintained on standard rat chow with water ad lib until utilized. To induce physical dependence on ethanol the liquid diet method of (Frye et al., 1981; Grover et al., 1998) was used. Animals were randomly assigned to ‘Ethanol Dependent' or ‘Control' groups and housed individually. On day 1 they received rat chow, water ad lib and 35 ml of nutritionally complete liquid diet. On the 2nd day, rat chow was removed and an additional 35 ml of liquid diet and water were provided. Beginning the third day, the ‘Ethanol Dependent' group received water and liquid diet with ethanol partially replacing dextrose, isocalorically (1 g of ethanol=1.75 g dextrose). Ethanol was increased from 0.07–0.08 g ml−1, after 6 days to compensate for the development of metabolic tolerance. Animals were sacrificed after 12 days of ethanol treatment while still intoxicated. Although blood ethanol concentrations were not measured in the present study, we have previously shown this regimen induces daily ethanol consumption of 12–16 g kg−1, and maintains up to 2 mg ml−1 of ethanol in the blood (Frye et al., 1981). The animals show weight gain (1–2 g day−1) and marked withdrawal signs (including: susceptibility to audiogenic seizures and forelimb tremor) indicating the presence of physical dependence on ethanol (Frye et al., 1981). ‘Controls' were fed 35 ml of liquid diet without ethanol throughout the 14 day treatment period, an amount previously found to be calorically and nutritionally equivalent to average daily consumption by ethanol-treated animals. ‘Naive' animals were fed ad lib standard rat chow throughout the treatment period under initial housing conditions described above.
Acutely dissociated neurons and whole cell recordings
To collect MS/DB neurons, the brain was cooled in iced cutting solution, [(mM): NaCl, 118; KCl, 3; MgCl2, 6; CaCl2, 0.5; N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulphonic acid] (HEPES), 5; D-glucose, 11; NaHCO3, 25; bubbled with 95% O2+5% CO2], the forebrain was blocked coronally and sliced (400 μm) on a Vibratome (Polysciences Inc.). After micro-disection, MS/DB segments were incubated in trypsin (Sigma Type XI, ∼0.7 mg ml−1) in a ‘PIPES' buffer [(mM): NaCl, 120; KCl, 5; MgCl2, 1; CaCl2, 1; 1,4-piperazinediethanesulphonic acid (PIPES), 20; D-glucose, 25; pH 7.0 with NaOH, bubbled with 100% O2] at 35°C for ∼1 h. After rinsing, slices were stored in the same buffer up to 5 h. Neurons were dissociated by gentle mechanical trituration with a fire polished Pasteur pipette in Dulbecco's Modified Eagle Medium (D-MEM, Gibco Laboratories) and dispersed onto a cover slip (previously submerged and then rinsed free of 0.1% Alcian blue, to increase adherence) in a recording chamber on the stage of an inverted microscope (Axiovert 100, Zeiss). Neurons which attached to the cover slip within 4 min were continuously perfused ∼1–2 ml min−1 with ‘bath' solution [(mM): NaCl, 140; KCl, 3; MgCl2, 2; CaCl2, 2; HEPES, 10; D-glucose, 33; pH 7.4 with NaOH; 315–320 mOsm] at 22–25°C. Whole-cell patch-clamp recordings techniques were used for all experiments as previously described (Grover et al., 1998). Patch pipettes were pulled from 1.5 mm o.d. glass capillary tubing (#7052, Garner Glass Co.), and the tips wax coated to reduce stray capacitance before fire-polishing to a resistance of 2–8 MΩ. Pipettes were filled with a solution kept on ice until used [(mM): CsCl, 130; MgCl2, 2; ethylene glycol-bis(β-aminoethyl ether), N,N,N′,N′-tetraacetic acid (EGTA), 10; HEPES, 10; Mg-ATP, 4; GTP, 0.1; pH 7.2 with CsOH; 285–300 mOsm]. An Axopatch 200A amplifier, pClamp 6 software (Axon Instr., Foster City, CA, U.S.A.) and a strip chart recorder (Gould Instr., Valley View, OH, U.S.A.) were used to acquire and analyse current recordings from individual neurons voltage clamped at −60 mV. Estimates of cell surface area were obtained indirectly as the value required for offsetting cell capacitance (pF) and were read directly from the amplifier potentiometer.
Drug applications
Drugs dissolved in the ‘bath' solution were applied directly to isolated neurons by a dual pipette superfusion system, in which a pair of identical pipettes (760 μm i.d., PEEK tubing #1533, Upchurch Scientific, Oak Harbor, WA, U.S.A.) cemented side by side and positioned immediately adjacent to the cell being recorded. The upstream pipette continually delivered tetrodotoxin (0.5 μM) containing solution to block voltage-gated Na+ channels. This barrel was briefly displaced by the drug pipette which delivered tetrodotoxin-containing solution from a bank of reservoirs also containing one or more of the test drugs including, AMPA hydrobromide, SYM 2081, GYKI 52466 hydrochloride and cyclothiazide (Tocris), kainate and 6,7-dinitroquinoxaline-2,3-dione (DNQX; Sigma) or ethanol (Aaper Chemical). AMPA/kainate-activated currents reached maximum values in ∼100 ms and reversed on a similar time scale after returning the upstream pipette over the cell. Baseline responses to 100 μM AMPA or kainate (as appropriate) obtained immediately before and after each drug test were used to standardize results for each neuron. Data are expressed as mean±s.e.mean for the neurons indicated by n and were evaluated by analysis of variance (ANOVA) and/or paired t, or independent t or Chi square (two tailed) as appropriate with P values ⩽0.05 accepted as evidence of significant differences. An estimate of the maximum response, EC50, and slope (Hill coefficient) were calculated for individual neurons where sufficient data were collected to allow a concentration-response curve to be fit with
where I was the kainate-induced current, Imax the maximum kainate current, ‘kainate' the concentration of agonist, Kd the apparent dissociation constant and n the slope. All data for Ethanol Dependent and Control group concentration-response curves were compared by evaluating the F statistic generated from a goodness of fit analysis using equation 1 for these data separately and when combined (Motulsky & Ransnas, 1987).
Results
AMPA- and kainate-activated currents in MS/DB neurons
Application of 100 μM AMPA or kainate consistently activated currents in all 78 MS/DB neurons tested. AMPA currents generally were marked by an initial transient current spike followed by a more sustained current, showing little desensitization (Figure 1A; cells 1 and 3) and were quite stable throughout experiments lasting up to 60 min. 100 μM kainate-activated currents without an initial current transient or desensitization during the 3 s application (Figure 2A). In a few neurons, AMPA-activated currents also did not exhibit a prominent initial transient (Figure 1A; cell 2 and Figure 2A). Simultaneous application of 100 μM GYKI 52466, a relatively selective non-competitive AMPA receptor antagonist (Johansen et al., 1995; Wilding & Huettner, 1995), largely blocked the sustained current component after 100 μM AMPA, which was reduced to less than 3% of control (Figure 1B; see ‘sustained'; n=6). The initial peak current at the onset of AMPA application was less sensitive to GYKI 52466, since 61% of total peak current remained (Figure 1B; see ‘transient'; n=6). No effort was made to determine whether the transient current reflected a subset of rapidly desensitizing receptors not blocked by GYKI 52466, or a kinetic difference in the rate at which the simultaneously applied AMPA and antagonist interacted with the receptor. However, in a few cells coapplication of GYKI 52466 and AMPA unmasked a transient current component that was not visible above the sustained current when AMPA was applied alone (Figure 1A; cell 2). Application of the relatively selective agonist, SYM 2801 (100 μM), which has similar efficacy, but a greater affinity (>1000 fold) than AMPA for the kainate receptor subtype (Donevan et al., 1998; Savidge et al., 1999), induced currents on average ∼10% of those activated by 100 μM AMPA (Figure 1A; cells 2 and 3 and Figure 1B; n=6). This observation reinforced the suggestion that the predominant native non-NMDA receptor subtype on MS/DB neurons is AMPA. Finally, application of 100 μM cyclothiazide which inhibits rapid desensitization of AMPA subtype receptors (Partin et al., 1994) significantly potentiated AMPA-activated currents by 427% (Figure 1A; cells 1 and 2 and Figure 1B; n=9). An initial peak current was no longer apparent. Cyclothiazide (10 μM) was much less effective in potentiating AMPA responses (131% increase; Figure 1B; n=6) than 100 μM cyclothiazide. This may suggest that flop-type subunit splice variants are predominant components of AMPA receptors in the MS/DB neurons.
Figure 1.
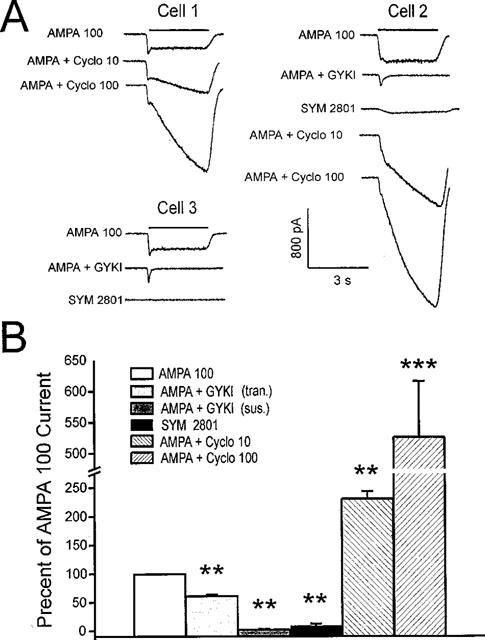
Characterization of AMPA subtype glutamate receptors on MS/DB neurons. In (A), application of 3 s pulses of 100 μM AMPA activates inward currents in three MS/DB neurons. Note initial transient peak currents in cells 1 and 3, but not cell 2. Simultaneous tests of AMPA combined with GYKI 52466 (GYKI; 100 μM) blunts initial rising phase of AMPA currents and largely blocks the subsequent sustained current (cells 2 and 3). SYM 2801 (100 μM) tested alone for 3 s activates little or no current in cells 2 and 3, while AMPA applied in combination with cyclothiazide (Cyclo; 10 or 100 μM) causes currents to increase during the test in cells 1 and 2. In (B), are shown mean±s.e.mean results for GYKI 52466 (100 μM) inhibition of initial ‘transient' (tran.) and ‘sustained' (sus.) AMPA currents; of SYM 2801-activated (100 μM) current; and of cyclothiazide potentiation of AMPA currents for 5–9 neurons is shown. Paired t, two-tailed, **P<0.01; ***P<0.001 when compared with 100 μM AMPA alone.
Figure 2.
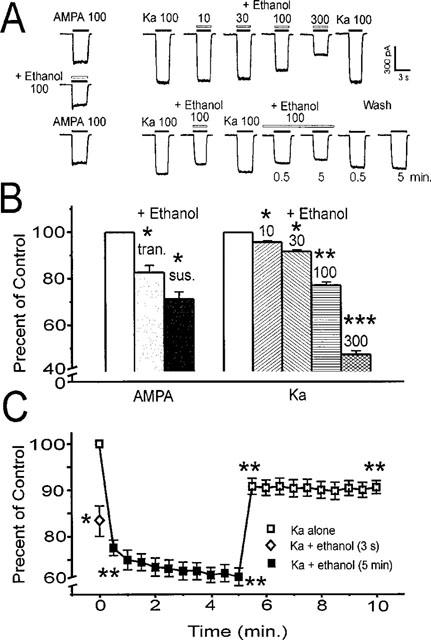
Ethanol inhibits AMPA and kainate-activated currents in MS/DB neurons. Traces in (A) show 100 μM AMPA current is reversibly inhibited by 100 mM ethanol applied in combination for 3 s. On the left, kainate (Ka; 100 μM) activates currents without initial transients. Co-application with 10–300 mM ethanol (top traces) for 3 s shows increasing, but reversible inhibition. Lower traces from another cell show that 3 s 100 mM ethanol also inhibits kainate current, and that sustained ethanol exposure (pair of traces representing 0.5 and 5 min in ethanol), causes slightly greater inhibition, but no acute tolerance. Removal of ethanol partially reverses the inhibition, although recovery is not complete even after 5 min (‘Wash' pair of traces represents 0.5 and 5 min after ethanol). In (B) are shown mean±s.e.mean results for 100 mM ethanol inhibition of initial transient (tran.) and subsequent sustained (sus.) AMPA 100 μM current as well as concentration-dependent inhibition of 100 μM kainate current by ethanol 10–300 mM. Mean results are shown in (C) for kainate (100 μM) current and brief (3 s) and sustained (5 min) ethanol 100 mM exposure and the 5 min wash out of ethanol. Data represent results from 6–9 neurons. Paired t, two-tailed, *P<0.05; **P<0.01; ***P<0.001 compared with the appropriate initial 100 μM AMPA or kainate alone in (B); in (C) *P<0.05 for 3 s ethanol inhibition vs 0.5 min; **P<0.01 for ethanol+kainate at 0.5 or 5 min vs initial kainate alone; also for washout 5.5 or 10 min kainate alone vs earlier 5 min ethanol+kainate.
Acute ethanol inhibits AMPA- or kainate-activated currents
Brief 3 s applications of 100 mM ethanol combined with either AMPA or kainate consistently inhibited currents activated by these agents in acutely isolated MS/DB neurons from ethanol naive animals (Figure 2A). Inhibition was fully reversible after these short applications. For AMPA-activated currents, both the initial transient and subsequent sustained currents were blunted ∼20–30% (Figure 2B). Ethanol (100 mM) also inhibited 100 μM AMPA currents when the responses were potentiated to 422±44% of control by 100 μM cyclothiazide (ethanol inhibition=16.7±2.3%; two-tailed paired t; P<0.001; n=7; data not shown). Ethanol inhibition (10–300 mM) was concentration-dependent (Figure 2A; see upper right traces) ranging from ∼5–50% of 100 μM kainate currents (Figure 2B; n=9). Continuous exposure of neurons to 100 mM ethanol in the superfusion solution for periods of up to 5 min also resulted in significant inhibition of current activated by 3 s kainate test pulses (Figure 2A; see lower right traces).
Kainate-activated current was reduced on average by ∼25% across the 5 min ethanol exposure period (Figure 2C; single factor ANOVA, P<0.001; n=8). Interestingly, inhibition during the brief 3 s ethanol test was only slightly less than that after more sustained exposure (Figure 2C; ethanol inhibition: 3 s=16.4±3.1%; after 1 min=25.1±2.1%; n=8), indicating that inhibition occurred instantaneously. During the 5 min ethanol superfusion, kainate-activated currents continued to show sustained inhibition, suggesting that acute tolerance did not rapidly develop, as previously observed (Grover et al., 1994) for evoked NMDA responses in hippocampal slices that quickly lost sensitivity to ethanol inhibition. Following removal of ethanol from the superfusate, kainate-activated currents swiftly recovered towards baseline, but still remained significantly blunted (∼10% smaller than pre-ethanol control; single factor ANOVA, P<0.01) and did not show rebound hyperactivity up to 5 min after withdrawal of ethanol (Figure 2C).
Chronic ethanol marginally increases peak kainate-activated currents
In a separate set of experiments, kainate was used to activate AMPA receptors in acutely isolated MS/DB neurons from ethanol dependent or control animals. In most dissociated MS/DB neurons, kainate (3–3000 μM) activated relatively stable, peak currents which showed little or no desensitization at the highest kainate concentrations (Figure 3A; lower traces), as previously reported (Paternain et al., 1995). The threshold for current activation was between 3–10 μM kainate with maximum responses occurring between 1000–3000 μM and the results were concentration-dependent for both treatment groups (single factor ANOVA, Ps<0.001). As shown in Figure 3A (top traces) a few cells showed a substantial rate of desensitization at kainate concentrations of 100 μM or above. A subset of neurons showing enhanced desensitization (>10% loss of initial peak current during the 3 s kainate test) was found in both treatment groups, but appeared unrelated to ethanol dependence (fraction desensitizing: Control=3 of 13; Ethanol Dependent=2 of 8; two-tailed uncorrected χ2 P>0.80). Thirty μM kainate-activated currents were largely blocked by 30 μM DNQX (Figure 3A,B), as expected for non-NMDA subtype glutamate receptors. There was no difference in the extent of inhibition between groups (inhibition by DNQX: Control=90.6±5.4%; Ethanol Dependent=92.8±2.7%; two-tailed independent t, P=0.67). Efforts to fit a sigmoid relationship to data for all neurons (Figure 3B) showed that Control and Ethanol Dependent data were fit with greater efficiency by two distinct relationships rather than one common curve, suggesting a significant overall difference between groups (F3,120=2.79 P<0.05). However, no specific differences were found at individual kainate concentrations (for example, 3000 μM kainate (pA pF−1): Control=72±7.3; Ethanol Dependent=91±18.3; two-tailed independent t, P=0.36), suggesting group differences overall were not marked. One additional analysis compared a subset of neurons from Control and Ethanol Dependent animals (n=16 and 10, respectively), where sufficient data were recorded to allow sigmoid relationship to be fit for each cell individually, generating estimates of kinetic parameters. Once again, there was a trend towards larger ‘maximum' estimates with chronic ethanol treatment as was present in Figure 3B, but as before there were no significant differences in the means of any concentration-response curve parameters between groups (Control vs Ethanol Dependent, two-tailed independent t: ‘maximum response' (pA pF−1)=71±8 vs 89±16, P=0.32; ‘EC50 (μM)'=89±12 vs 109±17, P=0.35; ‘Hill coefficient'=1.47±0.08 vs 1.37±0.07, P=0.37). These results when taken together with the earlier best fit analysis suggests a general trend toward a small enhancement of AMPA receptor activation by kainate following chronic ethanol treatment which might contribute to CNS excitability during ethanol withdrawal, but under the present test conditions, the apparent shift is not robust.
Figure 3.
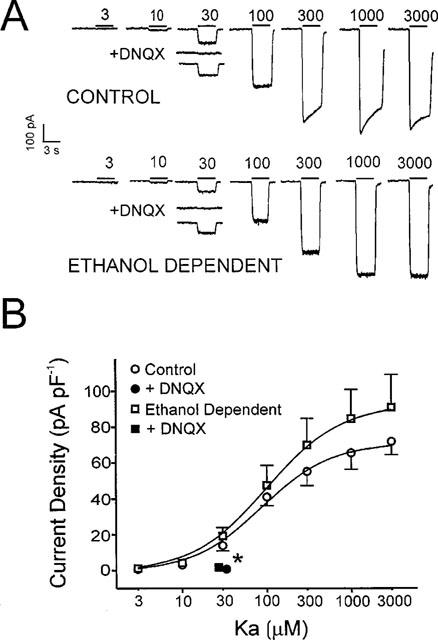
Effect of chronic in vivo ethanol treatment on the sensitivity of MS/DB neurons to kainate. (A) shows individual recordings from two neurons, from a Control and an Ethanol Dependent animal, where similar concentration-dependent activation of inward current was stimulated by kainate (Ka; 3–3000 μM). Note 30 μM DNQX completely blocks 30 μM kainate currents in both cells. Also currents from the Control neuron show some desensitization above 100 μM kainate, which is not seen in the Ethanol Dependent cell. (B) shows mean±s.e.mean results for 3–3000 μM kainate currents normalized to neuronal capacitance for ten Ethanol Dependent and 16 Control neurons and inhibition by 30 μM DNQX of 30 μM kainate currents. Smooth curves were fit with equation 1. Paired t, two-tailed, *P<0.05 when DNQX+kainate is compared to kainate alone for both groups.
Ethanol inhibition of kainate current is not blunted after in vivo ethanol treatment
To further characterize changes in AMPA receptor function following chronic ethanol treatment, the impact of 3 s ethanol co-application on 100 μM kainate currents was re-examined (Figure 4). Acute ethanol application caused concentration-dependent inhibition of kainate responses in all three groups (single factor ANOVAs, all Ps<0.001; n=7–9). Ethanol significantly inhibited kainate current at concentrations of 10 mM and above for Naive, Control and Ethanol Dependent groups. However, no inhibition occurred in any group at 3 mM ethanol. The extent of inhibition by 300 mM ethanol, the highest concentration tested, was not different across the groups at ∼48% (single factor ANOVA; Ethanol Dependent, n=8; Control, n=9; Naive, n=9; P=0.84), suggesting that functional tolerance to the immediate inhibitory effect of ethanol had not developed. It is important to note that it is highly unlikely that these results were influenced by residual ethanol that remained from the in vivo treatment of ethanol dependent neurons, since isolated tissues were incubated and superfused in ethanol-free media for well over an hour before testing.
Figure 4.
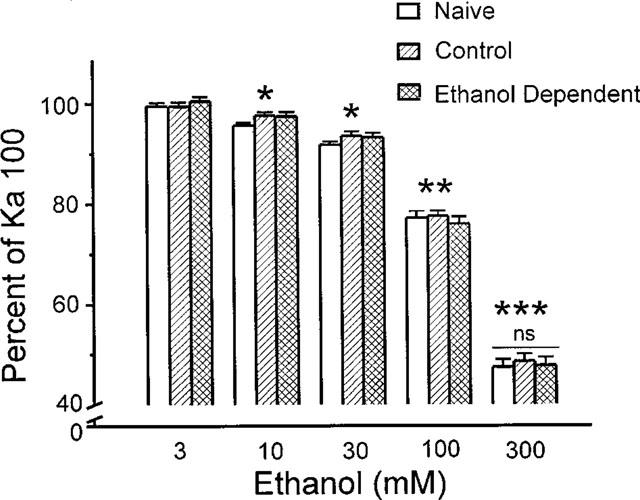
Ethanol causes comparable inhibition of kainate currents in cells from Naive, Control and Ethanol Dependent treatments. Brief application (3 s) of ethanol (3–300 mM) in combination with kainate (Ka; 100 μM) caused consistent concentration-dependent inhibition in 8–9 neurons from each treatment group. All ethanol treatments except 3 mM caused significant inhibition with paired t, two-tailed, *P<0.05; **P<0.01 or ***P<0.001 when compared with kainate alone; ‘ns' signifies no difference in the extent of inhibition with 300 mM ethanol across groups.
Discussion
The present report supports earlier observations that suggested non-NMDA glutamate receptors on acutely isolated MS/DB neurons are predominantly of the AMPA subtype (Kumamoto & Murata, 1995; Jasek & Griffith, 1998; Waters & Allen, 1998). AMPA activated currents exhibited a rapidly desensitizing initial peak followed by a steady state response, while currents induced by low (<100 μM) kainate generally exhibited stable peak responses that did not desensitize. However, in contrast to earlier reports, ∼25% of MS/DB neurons (5 of 21 cells) showed significant desensitization of peak kainate-activated currents (i.e., kainate⩾100 μM caused >10% decrease during 3 s applications; see Figure 3A), suggesting some differences in receptor composition or function relative to the majority of cells. Kainate desensitization was present both in Ethanol Dependent (2 of 8 cells) and Control groups (3 of 13 cells) at similar frequencies (X2 uncorrected; P>0.80). The predominant presence of AMPA receptors on MS/DB neurons is also supported by nearly complete inhibition of AMPA- or kainate-activated currents by GYKI 52466 and GYKI 53655 (see Figure 1; Waters & Allen, 1998), selective AMPA receptor antagonists (Paternain et al., 1995). Magnocellular basal forebrain neurons also have a small complement of kainate receptors (Waters & Allen, 1998). The neuronal population examined here was not limited to these large neurons, and GYKI 52466 inhibited ∼98% steady state AMPA-activated current (Figure 1B), suggesting little contribution from kainate receptors. Concanavalin A was not used here to enhance kainate receptor responses by blocking desensitization (Paternain et al., 1995), since it was not required to visualize synaptically evoked kainate receptor mediated currents (Weiner et al., 1999). The small response to 100 μM SYM 2081 (∼10% of 100 μM AMPA currents; Figure 1B) is also consistent with the predominance of AMPA receptors, since kainate receptors should be maximally activated by as little as 1 μM SYM 2081 (Donevan et al., 1998). Data in Figure 1 indicate that current density induced by 100 μM SYM 2081 (2.0±0.7 pA pF−1; n=5) was equivalent to an EC15 for kainate (i.e., ∼10 μM; see Figure 3B, Control curve) in MS/DB neurons. Finally, substantial cyclothiazide potentiation, which blocks AMPA receptor desensitization, was also consistent with AMPA receptors being the prominent non-NMDA glutamate receptor type on MS/DB neurons, since cyclothiazide does not enhance the activity of kainate receptors (Partin et al., 1994). Furthermore, differential responses to cyclothiazide suggested that AMPA receptors were likely composed predominately of subunits with the ‘flop' splice variant as has been suggested previously (Jasek & Griffith, 1998), since significantly less potentiation occurred with 10 relative to 100 μM cyclothiazide (Figure 1B). Recombinant AMPA receptors composed predominately of ‘flip' type subunits exhibit similar potentiation by 10 and 100 μM cyclothiazide, but ‘flop' containing receptors are potentiated much less by 10 than 100 μM cyclothiazide (Partin et al., 1994; Johansen et al., 1995). Whether this applied to native AMPA receptors is unknown. Also AMPA receptor subunits of individual MS/DB neurons have yet to be defined in conjunction with receptor function.
Native AMPA subtype glutamate receptors on MS/DB neurons are inhibited by pharmacologically relevant concentrations of ethanol as are NMDA receptors (Grover et al., 1998), exhibiting reduced function of ∼20–25% at ethanol levels (∼100 mM), which cause significant signs of intoxication such as anaesthesia. This inhibitory effect is immediate and does not depend on ethanol diffusion into the neuronal plasma membrane. Ethanol inhibition of these receptors on MS/DB neurons is sustained and does not diminish over several minutes as previously observed for NMDA-mediated synaptic potentials in hippocampus, where an acute tolerance developed (Grover et al., 1994). Interestingly, MS/DB neurons differentially regulate AMPA and NMDA receptors during much longer in vivo ethanol exposures that induce functional tolerance and physical dependence. Acute ethanol inhibition of NMDA receptors is blunted ∼50% in neurons from ethanol dependent rats, consistent with development of a functional tolerance (Grover et al., 1998), but there is no loss of acute ethanol inhibition evident for kainate-activated AMPA receptors (Figure 4). In addition, MS/DB neurons show significantly enhanced maximum responses to NMDA, suggesting functional receptor up-regulation that could play a role in withdrawal hyperexcitability (Grover et al., 1998). AMPA receptors exhibit only a slight non-significant trend towards an adaptive increase, suggesting that they are more resistant to up-regulation by chronic ethanol inhibition. This difference between NMDA and AMPA receptors may relate to their synaptic roles. Constant levels of AMPA receptors would likely be maintained to sustain fast synaptic transmission. A role in modulating synaptic efficacy and plasticity might drive NMDA receptor up-regulation to overcome sustained ethanol inhibition of synaptic transmission.
Native AMPA receptors are generally less sensitive to ethanol inhibition than recombinant receptors. AMPA receptors on acutely isolated MS/DB neurons show similar sensitivity to 100 mM ethanol inhibition as receptors on hippocampal or cerebral cortical neurons (∼14–35%; see Figures 2 and 4; Lovinger, 1993). These native receptors are somewhat less sensitive to ethanol than recombinant GluR1-4 receptors expressed in oocytes or HEK 293 cells (∼35–50% inhibition; Lovinger, 1993; Dildy-Mayfield & Harris, 1995). The reason for variations in ethanol sensitivity of AMPA receptors is not clear. Interestingly, in more intact tissue slice preparations (Lovinger et al., 1990; Weiner et al., 1999) where synaptic receptor populations are studied using evoked transmitter release, ethanol is even less effective in inhibiting AMPA/kainate receptors. These studies suggest native synaptic AMPA receptors in intact neurons may be less vulnerable to ethanol inhibition and ethanol-evoked adaptation, consistent with the lack of increased receptor function here (Figure 3B).
The kainate sensitivity of neurons from control liquid diet treated animals (EC50 (μM)=89; Hill coefficient=1.47) was similar in the present study to kainate-activated currents previously reported for acutely isolated or cultured MS/DB neurons (EC50 (μM)=75–138 μM; Hill coefficient=1.24–1.62) (Kumamoto & Murata, 1995; Jasek & Griffith, 1998; Waters & Allen, 1998). Although non-linear analysis of ‘Control' and ‘Ethanol Dependent' kainate current data (Figure 3B) showed a small significant reduction in variance with distinct curves, point to point comparison or estimates across individual neurons were not different. Thus up-regulation of AMPA receptors by chronic ethanol treatment remains to be convincingly established, despite earlier evidence for increased NMDA receptor function in MS/DB neurons (Grover et al., 1998). Taken together, these results are consistent with an earlier report that hippocampal NMDA receptor NR1 subunit peptide was increased by in vivo chronic ethanol treatment, but AMPA receptor GluR1 or GluR2 subunit peptide was not altered (Trevisan et al., 1994). Interestingly, ethanol up-regulated AMPA or NMDA receptor subunits on cerebral cortical cells cultured in 0.1 mM, but not 2 mM glutamine (Chandler et al., 1999), suggesting high glutamate levels from glutamine metabolism may offset ethanol inhibition and stop receptor up-regulation. NMDA receptor up-regulation in vitro by antagonists, MK-801 and AP-5, also reverses with NMDA receptor stimulation (Follesa & Ticku, 1996). Perhaps chronic ethanol differentially alters NMDA and AMPA receptors (Grover et al., 1998; Figure 3B) on MS/DB neurons in vivo because ambient glutamate can overcome AMPA, but not NMDA receptor inhibition by ethanol. Overall, up-regulation resistant AMPA receptors may be less likely than NMDA receptors to play a significant role in ethanol withdrawal hyperexcitability.
In summary, native non-NMDA receptors in acutely isolated MS/DB neurons from the rat were defined pharmacologically as predominantly of the AMPA flop-subtype. Kainate-activated currents in these cells were sensitive to ethanol, showing inhibition of ∼5–50% with acute application of 10–300 mM ethanol. Inhibition was sustained during continuous ethanol superfusions of 5 min, suggesting a lack of acute tolerance to ethanol-induced AMPA receptor blockade. Comparison of concentration-dependent kainate-activated currents in MS/DB neurons suggested only a slight trend towards receptor up regulation with physical dependence on ethanol. Also direct ethanol inhibition of kainate-activated currents was not reduced by chronic in vivo ethanol treatment, suggesting a lack of functional tolerance to ethanol inhibition. Thus native AMPA receptors on MS/DB neurons are vulnerable to inhibition by pharmacologically-relevant concentrations of ethanol, but unlike NMDA receptors, resist adaptation during functional tolerance and physical dependence development.
Acknowledgments
The authors appreciate the assistance of Dustin DuBois, William Griffith, Shu-Huei Hsiao and Brian McCool for helpful comments concerning manuscript. This work was supported in part by Public Health Service grant AA10067 (G.D. Frye).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxalonepropionic acid
- ANOVA
analysis of variance
- Cyclo
cyclothiazide
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- GYKI
GYKI 52466
- HEPES
N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulphonic acid]
- Ka
kainate
- MS/DB
medial septum/diagonal band
- NMDA
N-methyl-D-aspartate
- PIPES
1,4-piperazinediethanesulphonic acid
References
- BHAVE S.V., SNELL L.D., TABAKOFF B., HOFFMAN P.L. Ethanol sensitivity of NMDA receptor function in developing cerebellar granule neurons. Eur. J. Pharmacol. 1999;369:247–259. doi: 10.1016/s0014-2999(99)00071-0. [DOI] [PubMed] [Google Scholar]
- CHANDLER L.J., NORWOOD D., SUTTON G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alc. Clin. Exp. Res. 1999;23:363–370. [PubMed] [Google Scholar]
- CHEN X.Y., MICHAELIS M.L., MICHAELIS E.K. Effects of chronic ethanol treatment on the expression of calcium transport carriers and NMDA/glutamate receptor proteins in brain synaptic membranes. J. Neurochem. 1997;69:1559–1569. doi: 10.1046/j.1471-4159.1997.69041559.x. [DOI] [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., HARRIS R.A. Comparison of ethanol sensitivity of rat brain kainate, D,L-alpha-amino-3-hydroxy-5-methyl-4-isoxalone proprionic acid and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1992;262:487–494. [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., HARRIS R.A. Ethanol inhibits kainate responses of glutamate receptors expressed in Xenopus oocytes: Role of calcium and protein kinase C. J. Neurosci. 1995;15:3162–3171. doi: 10.1523/JNEUROSCI.15-04-03162.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLEDINE R., BORGES K., BOWIE D., TRAYNELIS S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- DONEVAN S.D., BEG A., GUNTHER J.M., TWYMAN R.E. The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J. Pharmacol. Exp. Ther. 1998;285:539–545. [PubMed] [Google Scholar]
- FAINGOLD C.L., GOUEMO P., RIAZ A. Ethanol and neurotransmitter interactions: From molecular to integrative effects. Prog. Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- FOLLESA P., TICKU MK. NMDA receptor upregulation: Molecular studies in cultured mouse cortical neurons after chronic antagonist exposure. J. Neurosci. 1996;16:2172–2178. doi: 10.1523/JNEUROSCI.16-07-02172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYE G.D., CHAPIN R.E., VOGEL R.A., MAILMAN R.B., KILTS C.D., MUELLER R.A., BREESE G.R. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: A comparison with ethanol. J. Pharmacol. Exp. Ther. 1981;216:306–314. [PubMed] [Google Scholar]
- GROVER C.A., FRYE G.D., GRIFFITH W.H. Acute tolerance to ethanol inhibition of NMDA-mediated EPSPs in the CA1 region of the rat hippocampus. Brain Res. 1994;642:70–76. doi: 10.1016/0006-8993(94)90906-7. [DOI] [PubMed] [Google Scholar]
- GROVER C.A., WALLACE K.A., LINDBERG S.A., FRYE G.D. Ethanol inhibition of NMDA currents in acutely dissociated medial septum/diagonal band neurons from ethanol dependent rats. Brain Res. 1998;782:43–52. doi: 10.1016/s0006-8993(97)01001-9. [DOI] [PubMed] [Google Scholar]
- JASEK M.C., GRIFFITH W.H. Pharmacological characterization of ionotropic excitatory amino acid receptors in young and aged rat basal forebrain. Neurosci. 1998;82:1179–1194. doi: 10.1016/s0306-4522(97)00337-0. [DOI] [PubMed] [Google Scholar]
- JOHANSEN T.H., CHAUDHARY A., VERDOORN T.A. Interactions among GYKI-52466, cyclothiazide, and aniracetam at recombinant AMPA and kainate receptors. Mol. Pharmacol. 1995;48:946–955. [PubMed] [Google Scholar]
- KOLTCHINE V., ANANTHARAM V., WILSON A., BAYLEY H., TREISTMAN S.N. Homomeric assemblies of NMDAR1 splice variants are sensitive to ethanol. eurosci. Lett. 1993;152:13–16. doi: 10.1016/0304-3940(93)90471-v. [DOI] [PubMed] [Google Scholar]
- KUMAMOTO E., MURATA Y. Excitatory amino acid-induced currents in rat septal cholinergic neurons in culture. Neurosci. 1995;69:477–493. doi: 10.1016/0306-4522(95)00260-p. [DOI] [PubMed] [Google Scholar]
- KUMARI M., TICKU M.K. Ethanol and regulation of the NMDA receptor subunits in fetal cortical neurons. J. Neurochem. 1998;70:1467–1473. doi: 10.1046/j.1471-4159.1998.70041467.x. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M. High ethanol sensitivity of recombinant AMPA-type glutamate receptors expressed in mammalian cells. Neurosci. Lett. 1993;159:83–87. doi: 10.1016/0304-3940(93)90804-t. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., WHITE G., WEIGHT F.F. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J. Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN D., TAYYEB M.I., SWARTZWELDER H.S. Ethanol inhibition of AMPA and kainate receptor-mediated depolarizations of hippocampal area CA1. Alc. Clin. Exp. Res. 1995;19:1312–1316. doi: 10.1111/j.1530-0277.1995.tb01617.x. [DOI] [PubMed] [Google Scholar]
- MINAMI K., GEREAU R.W., MINAMI M., HEINEMANN S.F., HARRIS R.A. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol. Pharmacol. 1998a;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- MINAMI K., WICK M.J., STERN-BACH Y., DILDY-MAYFIELD J.E., BROZOWSKI S.J., GONZALES E.L., TRUDELL J.R., HARRIS R.A. Sites of volatile anesthetic action on kainate (Glutamate receptor 6) receptors. J. Biol. Chem. 1998b;273:8248–8255. doi: 10.1074/jbc.273.14.8248. [DOI] [PubMed] [Google Scholar]
- MIYAKAWA T., YAGI T., KITAZAWA H., YASUDA M., KAWAI N., TSUBOI K., NIKI H. Fyn-kinase as a determinant of ethanol sensitivity: Relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- MOLLEMAN A., LITTLE H.J. Increases in non-N-methyl-D-aspartate glutamatergic transmission, but no change in γ-aminobutyric acidB transmission, in CA1 neurons during withdrawal from in vivo chronic ethanol treatment. J. Pharmacol. Exp. Ther. 1995;274:1035–1041. [PubMed] [Google Scholar]
- MOTULSKY H.J., RANSNAS L.A. Fitting curves to data using nonlinear regression: A practical and nonmathematical review. FASEB J. 1987;1:365–374. [PubMed] [Google Scholar]
- NAKANISHI S., NAKAJIMA Y., MASU M., UEDA Y., NAKAHARA K., WATANABE D., YAMAGUCHI S., KAWABATA S., OKADA M. Glutamate receptors: brain function and signal transduction. Brain Res. Rev. 1998;26:230–235. doi: 10.1016/s0165-0173(97)00033-7. [DOI] [PubMed] [Google Scholar]
- PARTIN K.M., PATNEAU D.K., MAYER M.L. Cyclothiazide differentially modulates desensitization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol. Pharmacol. 1994;46:129–138. [PubMed] [Google Scholar]
- PATERNAIN A.V., MORALES M., LERMA J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- PEOPLES R.W., WHITE G., LOVINGER D.M., WEIGHT F.F. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurones: Whole-cell patch-clamp analysis. Br. J. Pharmacol. 1997;122:1035–1042. doi: 10.1038/sj.bjp.0701483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDOLPH J.G., WALKER D.W., IIMURO Y., THURMAN R.G., CREWS F.T. NMDA receptor binding in adult rat brain after several chronic ethanol treatment protocols. Alc . Clin. Exp. Res. 1997;21:1508–1519. [PubMed] [Google Scholar]
- SAVIDGE J.R., STURGESS N.C., BRISTOW D.R., LOCK E.A. Characterisation of kainate receptor mediated whole-cell currents in rat cultured cerebellar granule cells. Neuropharmacol. 1999;38:375–382. doi: 10.1016/s0028-3908(98)00202-0. [DOI] [PubMed] [Google Scholar]
- THOMAS M.P., MONAGHAN D.T., MORRISETT R.A. Evidence for a causative role of N-methyl-D-aspartate receptors in an in vitro model of alcohol withdrawal hyperexcitability. J. Pharmacol. Exp. Ther. 1998;287:87–97. [PubMed] [Google Scholar]
- TREVISAN L., FITZGERALD L.W., BROSE N., GASIC G.P., HEINEMANN S.F., DUMAN R.S., NESTLER E.J. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J. Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- TSAI G.C., GASTFRIEND D.R., COYLE J.T. The glutamatergic basis of human alcoholism. Am. J. Psychiatry. 1995;152:332–340. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- VALENZUELA C.F., CARDOSO R.A. Acute effects of ethanol on kainate receptors with different subunit compositions. J. Pharmacol. Exp. Ther. 1999;288:1199–1206. [PubMed] [Google Scholar]
- WATERS D.J., ALLEN T.G. Ca2+-permeable non-NMDA glutamate receptors in rat magnocellular basal forebrain neurones. J. Physiol. 1998;508:453–469. doi: 10.1111/j.1469-7793.1998.453bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS J.C., EVANS R.H. Excitatory amino acid transmitters. Annu. Rev. Pharmacol. Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- WEINER J.L., DUNWIDDIE T.V., VALENZUELA C.F. Ethanol inhibition of synaptically evoked kainate responses in rat hippocampal CA3 pyramidal neurons. Mol. Pharmacol. 1999;56:85–90. doi: 10.1124/mol.56.1.85. [DOI] [PubMed] [Google Scholar]
- WILDING T.J., HUETTNER J.E. Differential antagonism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol. Pharmacol. 1995;47:582–587. [PubMed] [Google Scholar]
- YANG X.H., CRISWELL H.E., SIMSON P., MOY S., BREESE G.R. Evidence for a selective effect of ethanol on N-methyl-D-aspartate responses: Ethanol effects a subtype of the ifenprodil-sensitive N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 1996;278:114–124. [PubMed] [Google Scholar]


