Abstract
We examined the effects of nine different tricyclic antidepressant drugs on the glycine uptake mediated by the glycine transporter 1b (GLYT1b) and glycine transporter 2a (GLYT2a) stably expressed in human embryonic kidney 293 cells. Desipramine, imipramine, clomipramine, nomifensine and mianserin had no effect on the activity of the glycine transporters. Doxepin, amitriptyline and nortriptyline inhibited the two transporter subtypes to a similar extent.
Amoxapine displayed a selective inhibition of GLYT2a behaving as a 10 fold more efficient inhibitor of this isoform than of GLYT1b.
Kinetic analysis of the initial rates of glycine uptake by GLYT2a as a function of either glycine, chloride or sodium concentration, in the absence and presence of amoxapine indicated that amoxapine behaved as a competitive inhibitor of both glycine and chloride and a mixed-type inhibitor with respect to sodium.
A kinetic model was developed which explains adequately these data, and gives information about the order of binding of sodium and chloride ions to GLYT2a.
Our results may contribute to the development of the glycine transporter pharmacology. Additionally, the inhibition of the glycine uptake by GLYT2 is suggested to have some role in the sedative and psychomotor side effects of amoxapine.
Keywords: Glycine transporters, stable expression, amoxapine, tricyclic antidepressants, human embryonic kidney cells, transport mechanism
Introduction
Glycine is a major inhibitory neurotransmitter in the spinal cord and the brain stem of vertebrates, where it participates in a variety of motor and sensory functions. In addition, glycine could potentiate the action of glutamate, the main excitatory neurotransmitter in the brain, on postsynaptic N-methyl-D-aspartate (NMDA) receptors. The re-uptake of glycine into presynaptic nerve terminals and surrounding glial processes plays a major role in the maintenance of low synaptic levels of the transmitter (Iversen, 1971; Kanner & Shuldiner, 1987). Glycine transporters are members of the Na+- and Cl−-dependent neurotransmitter transporter gene family (Liu et al., 1992b; Amara & Kuhar, 1993; Shafqat et al., 1993; Malandro & Kilberg, 1996), a group of integral glycoproteins (Nuñez & Aragón, 1994; Tate & Blakely, 1994; Olivares et al., 1995) which share a common structure with 12 transmembrane domains (Kanner & Kleinberger-Doron, 1994). Several neurotransmitter uptake systems, including those for glycine, present an unexpected molecular heterogeneity. By now, two glycine transporter genes (GLYT1 and GLYT2) (Liu et al., 1992a, 1993; Smith et al., 1992; Borowsky et al., 1993; Kim et al., 1994; Adams et al., 1995) have been cloned. GLYT1 presents three isoforms (GLYT1a, GLYT1b and GLYT1c) that differ in their amino terminal sequences and are generated both by alternative promoter usage and by alternative splicing (Smith et al., 1992; Liu et al., 1993; Adams et al., 1995; Borowsky & Hoffman, 1998). Recently, a second GLYT2 isoform (GLYT2b) has been isolated, cloned and characterized in our laboratory (Ponce et al.,1998). The GLYT1 variants are pharmacologically distinguishable from the GLYT2 ones by their higher sensitivity to the inhibition by sarcosine (Liu et al., 1993) but no GLYT2-specific inhibitors are available to date.
Amoxapine is a tricyclic dibenzoxazepine (an N-aryl piperazine) which acts similarly to several other tricyclic antidepressants. The clinical effectiveness of some tricyclic antidepressants is now thought to be due to their inhibitory effects on the presynaptic monoamine re-uptake systems. In the case of amoxapine, it has been demonstrated to inhibit norepinephrine transport (Edwards et al., 1988). However, most of the tricyclic antidepressants have a considerable variety of undesired side effects, a pervasive property which can be due to interactions with neurotransmitter receptors.
In this paper we examined the effects of several tricyclic antidepressant compounds on the activities of the high affinity glycine transporters GLYT1b and GLYT2a stably expressed in human embryonic kidney 293 (HEK 293) cells. We found that among the tricyclic antidepressants tested, amoxapine inhibited, in a specific manner, the GLYT2a isoform of the glycine transporters. Kinetic analysis of the GLYT2a-mediated glycine uptake in the presence or absence of amoxapine was performed to study the mechanism of amoxapine inhibition. A model was presented which satisfactorily explains the experimental data. Our results indicate that the simultaneous binding of amoxapine and either glycine or chloride to the transporter is not possible although ternary complexes between Na+, inhibitor and transporter may exist, therefore suggesting that Na+ may bind glycine transporter before Cl− and glycine.
Methods
Stable cell lines
Two ClaI/NotI restriction fragments containing the full length cDNAs of GLYT1b (Rb20 clone) (Smith et al., 1992) or GLYT2a (Liu et al., 1993) were released from pBluescript SK (Stratagene) and subcloned into the ClaI/NotI-digested pIRES1hyg bicistronic vector (Clontech) modified as previously described (López-Corcuera et al., 1998). The recombinant plasmids (10 μg) containing GLYT1b or GLYT2a under the cytomegalovirus (CMV) promoter were calcium-phosphate transfected using the ProFection mammalian transfection system-calcium phosphate kit (Promega) into HEK 293 cells at 50% confluence in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat inactivated foetal bovine serum (FBS), at 37°C, 5% CO2. Twenty-four hours after transfection cells were diluted and switched to a medium containing 0.25 mg ml−1 hygromycin. Resistant colonies were isolated from transfected plates 4 weeks later during which the medium was replaced every 4 days. Single cells were used to generate clonal lines. Selected clones for GLYT1b or GLYT2a stable cell lines express the highest levels of protein that immunoreacts with specific antibodies against GLYT1 or GLYT2, respectively (Zafra et al., 1995a,1995b). The clonal cell lines used in the experiments reported here show saturable, high-affinity, and Na+ and Cl−-dependent glycine transport which is absent in the parental HEK 293 cells. This glycine uptake retains all the functional properties that are characteristic of every glycine transporter isoform (López-Corcuera et al., 1998).
Transport assays
Subconfluent cells growing in polylysine-covered 24-well plates were washed at 37°C with 1 ml of HEPES-buffer saline (HBS; mM) NaCl 150, HEPES-Tris 10, CaCl2 1, KCl 2.5, MgSO4 2.5, glucose 10, pH 7.4. The washing solution was removed and cells incubated for the indicated times at 37°C in 0.3 ml of an uptake solution that contained an isotopic dilution of 3H-labeled glycine in HBS yielding a 10 μM final glycine concentration in the presence of the tricyclic antidepressant tested or its solvent (water, except for amoxapine dissolved in methanol, and nomifensine dissolved in dimethyl sulphoxide). Transport was stopped with two 1 ml washes of ice-cold HBS, and cells dissolved in 0.25 ml of 0.2 N NaOH. Aliquots of each well were taken for scintillation counting and protein concentration determination. In the experiments in which different Na+ and Cl− concentrations were used, NaCl was isotonically replaced by choline chloride or sodium acetate to achieve the indicated Na+ or Cl− concentrations, respectively. All the transport measurements were done in triplicate or quadruplicate.
Protein concentration
Protein concentration was determined by the method of Bradford (1976).
Materials
[2-3H]Glycine (1.6 TBq mmol−1 in ethanol/water 2:98) was supplied by DuPont NEN (U.S.A.). Ligase and restriction enzymes were from Boehringer Mannheim (Germany). All the tricyclic antidepressants tested were supplied by RBI Natick, MA, (U.S.A.). All other reagents were obtained in the purest form available.
Data analysis
Transport activity data as a multidimensional function of glycine, chloride, sodium and inhibitor concentration was fitted to the equation (see Results) by an iterative, non-linear, unweighted least-squares algorithm, using the SigmaPlot package (Jandel Co.). IC50 was obtained by fitting of the inhibition data to the following equation:
Results
In this report we present a comparative study on the effects of several antidepressant drugs on the uptake of glycine mediated by the glycine transporter isoforms GLYT1b and GLYT2a. HEK 293 cell lines stably expressing either GLYT1b or GLYT2a which were previously generated in our laboratory as described in the Methods section, were used in this study. Part of the biochemical and electrophysiological characterization of these cell lines has already been reported (López-Corcuera et al., 1998).
Figure 1 is a time course showing the uptake of glycine by the clonal cell lines expressing GLYT1b (Figure 1a) or GLYT2a (Figure 1b) compared with the substrate accumulation exhibited by the parental HEK 293 cells. After 15 min incubation in the presence of radiolabelled glycine (10 μM final concentration), clonal cell lines exhibited transport activities of approximately 16 fold (GLYT1b) or 7 fold (GLYT2a) over the background of the parental cells. The glycine uptake by the glycine transporters was Na+ and Cl−-dependent and increased linearly with time up to approximately 10 min. The basal glycine uptake by the HEK 293 cell line is Na+ but not Cl−-dependent and therefore, the Na+-and Cl−-dependent glycine uptake exhibited by the stable cell lines is due solely to the activities of the recombinant transporters GLYT1b and GLYT2a (0.35±0.03 and 0.32±0.02 nmol glycine.mg protein−1 5 min−1 at 10 μM glycine in the presence and in the absence of Cl−, respectively for the basal glycine uptake by HEK 293 cells). All the experiments performed in this study were corrected by subtracting the non-specific basal glycine transport from every data point.
Figure 1.

Time course of glycine transport by stable cell lines. Transport assays were performed as described in Methods for the indicated times. Each point represents the mean of three determinations and error bars represent s.e.
Nine clinically active antidepressant drugs were tested in this study, and their effects on the activity of the glycine transporters are shown on Table 1. Some of these compounds produced no inhibition or showed partially inhibitory effects on the transport activity of the two glycine transporters. Doxepin, amitriptyline and nortriptyline inhibited GLYT1b and GLYT2a by the same extent. However, amoxapine was found to have a selective inhibitory effect on GLYT2a, having no effect on GLYT1b transport activity. This remarkable finding prompted us to study in more detail the differential effects of amoxapine on the two glycine transporter subtypes.
Table 1.
Sensitivity of stably expressed glycine transporters to different tricyclic antidepressants

Figure 2 depicts a dose-response relationship for the amoxapine effect on the high-affinity glycine uptake by the stable cell lines. As shown, amoxapine inhibited GLYT2a transport activity displaying an IC50 of 92±8 μM, whereas GLYT1b inhibition had an IC50 in the mM range (around 1 mM). Therefore, amoxapine was 10 fold more efficient in inhibiting GLYT2a than GLYT1b.
Figure 2.
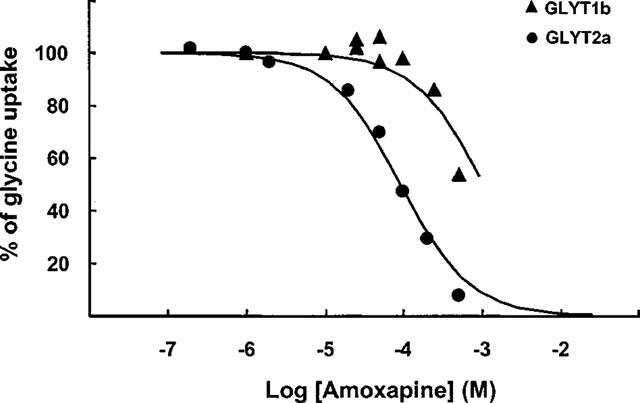
Dose-response relationship for the amoxapine effect on the glycine uptake by the stable cell lines. GLYT1b or GLYT2a transport activities were measured as described in Methods in the presence of increasing concentrations of amoxapine. Values represent the percentage of control values measured in the presence of the vector solvent. Specific control glycine uptakes were 20.0±0.7 and 6.2±0.2 nmol glycine.mg of protein−1.5 min−1 for GLYT1b and GLYT2a, respectively. Each value represents the mean±s.e.mean of triplicate determinations. Error bars are smaller than symbols. Curves were computer-generated according to equation 1, using the best-fit IC50 parameter determined in each case.
Kinetic analysis of the initial rates of glycine uptake by the GLYT2a transporter measured over 5 min, as a function of either glycine, chloride or sodium concentration, in the absence and presence of amoxapine is shown in Figure 3. Eadie-Hofstee plots were also performed in order to help determine the nature of amoxapine inhibition (Figure 3, insets). In absence of inhibitor, the rate of glycine transport was a simple hyperbolic function of either glycine or chloride concentration, indicating that a single molecule of glycine and a single chloride ion are involved in the transport process. Transport rate was a sigmoidal function of the sodium concentration, suggesting a possible stoichiometry of at least two sodium ions. These results are similar to our previously published data (López-Corcuera et al., 1998). When studying amoxapine inhibition, the curves suggested that amoxapine had a clear effect on the apparent kinetic constants of glycine and chloride, and did not apparently affect their maximum transport rates (Figure 3a and c), thus suggesting that amoxapine behaved as a competitive inhibitor of both glycine and chloride. On the contrary, amoxapine clearly affected the maximum transport rate when it was expressed as a function of sodium concentration, appearing to have only a slight effect on the apparent kinetic constant (Figure 3b). Therefore, amoxapine behaved as a mixed-type inhibitor with respect to the sodium.
Figure 3.

Effects of amoxapine on the kinetics of the glycine uptake by GLYT2a as a function of glycine, Na+ and Cl− concentrations. Glycine uptake was measured as described in Methods in the presence of amoxapine (200 μM, a, b; 50 μM, c) or its solvent at the equivalent concentration (125 mM methanol, control). Transport rates were measured over 5 min in a standard HBS uptake solution containing increasing concentrations of glycine (a), or at 10 μM glycine in a solution were NaCl was isotonically replaced with either choline chloride (b) or sodium acetate (c) to achieve the indicated Na+ or Cl− concentrations. Insets, Eadie-Hofstee plots. V, rate of uptake; [S], substrate concentration. Each value represents the mean±s.e.mean of triplicate determinations. Error bars are smaller than symbols. All the curves were computer-generated, according to the equation, using the following best-fit kinetic constants: KGly=108 μM, KCl=37 mM, KNa=86 mM, KI=23 μM and α=0.8; these parameters were obtained with a standard error of about 10%.
A whole kinetic model was then developed to explain these results. Since no information is presently known concerning the order in which sodium, chloride and glycine bind this transporter before the translocation process, we assumed the most general hypothesis, i.e., a random model where the three substrates may independently bind the transporter, as shown in Figure 4. We firstly assumed a 1 : 1 stoichiometry for glycine, chloride and inhibitor and a 3 : 1 stoichiometry for sodium (López-Corcuera et al., 1998). As a first approximation, we assumed that any combination of the transporter with any of the substrate or inhibitor molecules was possible, and that the transporter affinity for the substrates was not affected by the binding of any other molecule, i.e. there are no allosteric effects. In these conditions, the rate of glycine transport may be expressed as:
where
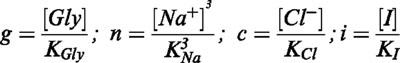 |
Kj being the equilibrium dissociation constant of specie j, P a parameter which accounts for the transport turnover rate, and Tt the total concentration of the transporter. The expression in the denominator expands to generate a sum of terms; these terms correspond to each of the possible states of transporter molecule. This model predicts that transport activity follows a pure hyperbolic dependence of glycine and chloride concentrations, and a sigmoidal dependence of sodium concentration. With respect to the glycine, equation 2 may be arranged in the following manner:
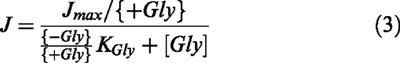 |
where Jmax is the maximum transport rate and {+Gly} and {−Gly} represent the terms which correspond to the transporter species which have and do not have a bound glycine molecule, respectively. From the equation it is evident that, according to this model, the inhibitor will necessarily have an effect on Jmax. Since the inhibitor was found to behave with respect to glycine in a competitive manner, not affecting Jmax, we had to assume that no inhibitor-dependent terms are included in {+Gly}, i.e., that the transporter cannot simultaneously bind one molecule of glycine and one molecule of inhibitor. The same argument is applicable to chloride. On the contrary, the inhibitor was experimentally found to affect sodium's apparent kinetic constant; therefore, we had to assume the existence of the transporter species simultaneously bound to sodium and the inhibitor. Eliminating the terms corresponding to transporter species which simultaneously contain glycine and inhibitor or chloride and inhibitor, equation 2 becomes:
This model now predicts a pure competitive behaviour of the inhibitor with respect to glycine and chloride, and a pure non-competitive behaviour with respect to sodium.
Figure 4.

Scheme of the kinetic model proposed for the inhibition by amoxapine of the glycine uptake mediated by the GLYT2a transporter. T, GLYT2a transporter; I, inhibitor (amoxapine); J, glycine transport rate, KNa+, KCl−, Kgly, equilibrium constants. KI, inhibition constant. The model corresponds to equation 5 (Results). For further details, see the text.
Finally, since some effect of the inhibitor was detected on sodium's apparent kinetic constant, we had to assume that binding of the inhibitor to the transporter produces some allosteric effect on the binding of sodium. This was taken into account by the model introducing the α factor, which measures the relative variation in the sodium equilibrium constant when the inhibitor is bound to the transporter. Thus, binding of the inhibitor is assumed to diminish KNa by a factor of α1/3, and consistently, binding of sodium diminishes KI by a factor of α. The equation is transformed as follows:
Equation 5 now predicts a mixed-type competitive behaviour of inhibitor with respect to sodium, and corresponds to the kinetic model schematized in Figure 4.
From Equation 5, a relationship between IC50 value from the inhibition curves and the inhibitor equilibrium constant may be obtained:
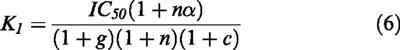 |
All the data presented in Figure 3 were simultaneously fitted to Equation 5, obtaining the equilibrium constants for glycine, chloride and sodium, as well as the inhibition constant for amoxapine. As shown in the computer-generated curves of Figure 3, fitting of the model to all the experimental data was very satisfactory using a single set of kinetic constants. Kinetic constants for glycine, chloride and sodium were similar to those previously reported (López-Corcuera et al., 1998). Inhibition constant for amoxapine was 23±2 μM (best fit parameter±s.d.), and the α parameter was 0.8±0.1, suggesting that sodium affinity for the transporter diminished by only 8% (α1/3=0.92) upon binding of the inhibitor.
Inhibitor constant of amoxapine was also calculated by introducing the above-obtained equilibrium constants for glycine, chloride and sodium into equation 6, and the known IC50 of the inhibitor (Figure 2), getting a KI for amoxapine of 14±1 μM. This value is in good agreement with that obtained by the simultaneous fitting. These values of KI are 5–10 times lower than our estimation of Kgly (about 100 μM, Figure 3), indicating that the affinity of the inhibitor for the transporter is greater than that of its natural substrate.
Discussion
Amoxapine is used clinically as an effective antidepressant having a rapid onset of action with few anticholinergic or cardiovascular side effects (Jue et al., 1982). This drug belongs to the family of tricyclic antidepressants and acts preferentially by inhibiting norepinephrine re-uptake in presynaptic neurons. A basic premise for the action of tricyclic antidepressants, such as amoxapine, is that these drugs act acutely by blocking transport, thus elevating extracellular concentrations of norepinephrine and/or serotonin and to a lesser extent dopamine, and thereby potentiating the activation of postsynaptic receptors (Barker & Blakely, 1996; Amara & Sonders, 1998). However, little evidence is available regarding the effects of tricyclic antidepressants on the amino acid neurotransmitter transporters.
Since these tricyclic antidepressants were either discovered accidentally or were selected on the basis of quite different criteria, their pharmacological profiles and their side effects at therapeutically used doses are generally unique for each one. However, most of them have been classified in groups according to their different clinical effects. So, doxepin, amitriptyline and nortriptyline are classified in the group having sedative effects (Kielholz, 1966). Because it is known that amoxapine not only produces sedation but also decreases locomotor activity and produces ptosis (Jue et al., 1982), we decided to examine its effects on the glycine transporters.
The results included herein, demonstrate that amoxapine clearly inhibits in a dose-response manner the transport of glycine mediated by the GLYT2a isoform, whereas it had little effect on the activity of the GLYT1b transporter. Although GLYT1 and GLYT2 show relevant differences related to their structure and brain localization, there are no known pharmacological differences between their transport activities except for the sensitivity of the GLYT1 isoforms to the inhibition by sarcosine, which is a substrate of these variants. The GLYT2-specific effect of amoxapine presented here is the first selective inhibitory effect on GLYT2 activity reported so far. Since amoxapine behaves as a 10 fold more efficient inhibitor of GLYT2, the two transporter isoforms must interact differentially with the drug. This isoform-specific properties must arise from structural differences affecting the active sites of the GLYT1 and GLYT2 proteins. Thus, the construction and analysis of chimeric proteins between these two transporters would provide valuable information on the domains that contribute to the unique features of each carrier, such as its sensitivity to selective inhibitors as amoxapine.
On the basis of its brain localization, it has been suggested that GLYT1 could be associated with the NMDA receptors (Kim et al., 1994). The other glycine transporter, GLYT2, is specifically associated with inhibitory glycinergic neurotransmission, being found in the presynaptic aspects of glycinergic synapses (Zafra et al., 1995a,1995b). Provided that GLYT2 plays an important role in the modulation of glycine concentration at the synaptic cleft of glycinergic neurons (Poyatos et al., 1997), the inhibitory effect of amoxapine may result in an enhancement of glycine neurotransmission in the spinal cord and the brain stem. Thus, although the sedative and psychomotor effects of amoxapine as an antidepressant might not be totally explained by the modulation of the GLYT2 glycine transporter, the side effects of amoxapine above the therapeutic dosages might be due to the increase in glycine levels within the synaptic cleft caused by the inhibition of the glycine uptake by GLYT2.
In this report we have performed a detailed kinetic analysis of the inhibition by amoxapine of GLYT2a activity. A model was presented which satisfactorily explains the experimental data. Our results indicate that the simultaneous binding of amoxapine and either glycine or chloride to the transporter is not possible; this may be due either to a conformational change of transporter upon inhibitor binding which prevents binding of chloride or glycine, or to a complete or partial overlap between amoxapine, glycine and chloride binding sites. Since one of the main biochemical differences between GLYT1 and GLYT2 is the higher affinity of the former by chloride (López-Corcuera et al.,1998), our results suggest that the different sensitivity of the transporters to amoxapine reflect a structural difference between the two transporter isoforms which is somehow related to the Cl− binding site.
Similarly, our model predicts the existence of ternary complexes between sodium, inhibitor and transporter, and thus strongly suggests that sodium may bind glycine transporter independently from the binding of chloride and glycine. Therefore, our results provide the first experimental information about the possible order of binding of sodium and chloride ions to the glycine transporters. An interaction of sodium ions with the GAT1 transporter in the absence of substrate has also been proven (Mager et al., 1993). Consistently with our random-order model, we cannot discard the possibility that either glycine or chloride bind the transporter independently from sodium. However, it can be shown that an ordered model where sodium binds first, followed by either chloride or glycine may also fit our experimental data. Given that chloride, but not glycine, is present in the extracellular space at high concentrations, the transport process would probably be more efficient if chloride binds transporter before glycine (Rudnick & Clark, 1993; Humphreys et al., 1994). In the summary, the results presented in this report not only may be useful for understanding the mechanism of the inhibition of glycine transport by amoxapine but may also enlighten some aspects of the mechanism of glycine transport itself, as well as contribute to the development of glycine transporter pharmacology.
Acknowledgments
This work was supported by grants from the Spanish Dirección General de Investigación Científica y Técnica (PM95-0026), the BIOMED program of the European Union (BMH4-CT95-0571), the European Union TMR Program (FMRX-CT98-0228), and an institutional grant from the Fundación Ramón Areces.
Abbreviations
- CMV
cytomegalovirus
- DMEM
Dulbecco's modified Eagle's medium
- FBS
foetal bovine serum
- GLYT1 and GLYT2
glycine transporters 1 and 2
- HBS
HEPES-buffered saline
- HEK
human embryonic kidney
- NMDA
N-methyl-D-aspartate.
References
- ADAMS R.H., SATO K., SHIMADA S., TOHYAMA M., PUSCHEL A.W., BETZ H. Gene structure and glial expression of the glycine transporter GlyT1 in embryonic and adult rodents. J. Neurosci. 1995;15:2524–2532. doi: 10.1523/JNEUROSCI.15-03-02524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMARA S.G., KUHAR M.J. Neurotransmitter transporters: recent progress. Annu. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- AMARA S.G., SONDERS M.S. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Dep. 1998;51:87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- BARKER E.L., BLAKELY R.D. Identification of a single amino acid, phenylalanine 586, that is responsible for high affinity interactions of tricyclic antidepressants with the human serotonin transporter. Mol. Pharmacol. 1996;50:957–965. [PubMed] [Google Scholar]
- BOROWSKY B., HOFFMAN B.J. Analysis of a gene encoding two glycine transporter variants reveals alternative promoter usage and a novel gene structure. J. Biol. Chem. 1998;273:29077–29085. doi: 10.1074/jbc.273.44.29077. [DOI] [PubMed] [Google Scholar]
- BOROWSKY B., MEZEY E., HOFFMAN B.J. Two glycine transporter variants with distinct localization in the CNS and peripheral tissues are encoded by a common gene. Neuron. 1993;10:851–863. doi: 10.1016/0896-6273(93)90201-2. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;7:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- EDWARDS D.J., SORISIO D.A., SEDLOCK M.L. Decreases in tyrosine and p-hydroxyphenylglycol caused by various tricyclic antidepressants. Biochem. Pharmacol. 1988;37:2069–2075. doi: 10.1016/0006-2952(88)90558-8. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS C.J., WALL S.C., RUDNICK G. Ligand binding to the serotonin transporter: equilibria, kinetics, and ion dependence. Biochemistry. 1994;33:9118–9125. doi: 10.1021/bi00197a014. [DOI] [PubMed] [Google Scholar]
- IVERSEN L.L. Role of transmitter uptake mechanisms in synaptic transmission. Br. J. Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUE S.G., DAWSON G.W., BRODGEN R.N. Amoxapine: A review of its pharmacology and efficacy in depressed states. Drugs. 1982;24:1–23. doi: 10.2165/00003495-198224010-00001. [DOI] [PubMed] [Google Scholar]
- KANNER B.I., KLEINBERGER-DORON N. Structure and function of sodium-coupled neurotransmitter transporters. Cell. Physiol. Biochem. 1994;4:174–284. doi: 10.1159/000173821. [DOI] [PubMed] [Google Scholar]
- KANNER B.I., SHULDINER S. Mechanism of transport and storage of neurotransmitters. CRC Crit. Rev. Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- KIELHOLZ P. Der heugite stand der medicamentose depressions-behandlung. Nervenartz. 1966;37:118–121. [PubMed] [Google Scholar]
- KIM K.M., KINGSMORE S.F., HAN H., YANG FENG T.L., GODINOT N., SELDIN M.F., CARON M.G., GIROS B. Cloning of the human glycine transporter type 1: molecular and pharmacological characterization of novel isoform variants and chromosomal localization of the gene in the human and mouse genomes. Mol. Pharmacol. 1994;45:608–617. [PubMed] [Google Scholar]
- LIU Q.R., LÓPEZ CORCUERA B., MANDIYAN S., NELSON H., NELSON N. Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features. J. Biol. Chem. 1993;268:22802–22808. [PubMed] [Google Scholar]
- LIU Q.R., LÓPEZ CORCUERA B., NELSON H., MANDIYAN S., NELSON N. Cloning and expression of a cDNA encoding the transporter of taurine and beta-alanine in mouse brain. Proc. Natl. Acad. Sci. U.S.A. 1992a;89:12145–12149. doi: 10.1073/pnas.89.24.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Q.R., MANDIYAN S., NELSON H., NELSON N. A family of genes encoding neurotransmitter transporters. Proc. Natl. Acad. Sci. U.S.A. 1992b;89:6639–6643. doi: 10.1073/pnas.89.14.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LÓPEZ-CORCUERA B., MARTÍNEZ-MAZA R., NUÑEZ E., ROUX M., SUPPLISSON S., ARAGÓN C. Differential properties of two stably expressed brain-specific glycine transporters. J. Neurochem. 1998;71:2211–2219. doi: 10.1046/j.1471-4159.1998.71052211.x. [DOI] [PubMed] [Google Scholar]
- MAGER S., NAEVE J., QUICK M., LABARCA C., DAVIDSON N., LESTER H.A. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- MALANDRO M.S., KILBERG M.S. Molecular biology of mammalian amino acid transporters. Ann. Rev. Biochem. 1996;65:305–336. doi: 10.1146/annurev.bi.65.070196.001513. [DOI] [PubMed] [Google Scholar]
- NUÑEZ E., ARAGÓN C. Structural analysis and functional role of the carbohydrate component of glycine transporter. J. Biol. Chem. 1994;269:16920–16924. [PubMed] [Google Scholar]
- OLIVARES L., ARAGÓN C., GIMÉNEZ C., ZAFRA F. The role of N-glycosylation in the targeting and activity of the GLYT1 glycine transporter. J. Biol. Chem. 1995;270:9437–9442. doi: 10.1074/jbc.270.16.9437. [DOI] [PubMed] [Google Scholar]
- PONCE J., POYATOS I., ARAGÓN C., GIMÉNEZ C., ZAFRA F. Characterization of the 5′ region of the rat brain glycine transporter GLYT2 gene: identification of a novel isoform. Neurosci. Lett. 1998;242:25–28. doi: 10.1016/s0304-3940(98)00037-8. [DOI] [PubMed] [Google Scholar]
- POYATOS I., PONCE J., ARAGÓN C., GIMÉNEZ C., ZAFRA F. The glycine transporter GLYT2 is a reliable marker for glycine-immunoreactive neuron. Mol. Brain Res. 1997;49:63–70. doi: 10.1016/s0169-328x(97)00124-1. [DOI] [PubMed] [Google Scholar]
- RUDNICK G., CLARK J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim. Biophys. Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- SHAFQAT S., VELAZ-FAIRCLOTH M., GUADAÑO-FERRAZ A., FREMAU R.T. , JR Molecular characterization of neurotransmitter transporters. Mol. Endocrinol. 1993;7:1517–1529. doi: 10.1210/mend.7.12.7908408. [DOI] [PubMed] [Google Scholar]
- SMITH K.E., BORDEN L.A., HARTIG P.R., BRANCHEK T., WEINSHANK R.L. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- TATE C.G., BLAKELY R.D. The effect of N-linked glycosylation on activity of the Na(+)- and Cl(−)-dependent serotonin transporter expressed using recombinant baculovirus in insect cells. J. Biol Chem. 1994;269:26303–26310. [PubMed] [Google Scholar]
- ZAFRA F., ARAGÓN C., OLIVARES L., DANBOLT N.C., GIMÉNEZ C., STORM-MATHISEN J. Glycine transporters are differentially expressed among CNS cells. J. Neurosci. 1995a;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAFRA F., GOMEZA J., OLIVARES L., ARAGÓN C., GIMÉNEZ C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur. J. Neurosci. 1995b;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]


