Introduction
Adenosine mediates a wide range of physiological functions by activation of at least four cell surface receptors named A1, A2A, A2B and A3. Each receptor subtype has been cloned and shows unique ligand binding properties and a distinct pattern of tissue expression (Fredholm et al., 1994; Linden, 1994; Alexander & Peters, 1997; Ralevic & Burnstock, 1998). Adenosine is a highly active biologic compound with a variety of effects on numerous tissues, including heart muscle, coronary arteries, smooth muscle cells, platelets and cells involved in immune and inflammatory reactions. Through interaction with A2A receptors, adenosine is involved in platelet antiaggregatory effects, neutrophil antiinflammatory responses as well as in modulation of immune cell function (Salmon & Cronstein, 1990; Sullivan et al., 1990; Hoskin et al., 1994; MacKenzie et al., 1994). The presence of A2A receptors on all these different cell types i.e. monocytes, lymphocytes, neutrophils, basophils, mast cells should not be surprising because the cells involved in the immune and inflammatory responses arise from a common stem cell source in the bone marrow. Studies on the effects of adenosine and adenosine analogues on blood cells are important due to the proposed role of adenosine (1) in the pathogenesis of diseases such as severe combined immunodeficiency (ADA SCID) (Giblett et al., 1972; Huang et al., 1997), (2) in the regulation of normal immune processes (Bouma et al., 1997; Apasov et al., 1997), (3) as an endogenous anti-aggregatory and anti-inflammatory agent (Cronstein, 1995; Sullivan & Linden, 1998) and (4) in the use of adenosine analogues as pharmacologic agents (Jacobson et al., 1992; Olah & Stiles, 1995; Poulsen & Quinn, 1998). On this basis the present review summarizes the pharmacological, biochemical and functional data of A2A receptors expressed in human platelets, lymphocytes and neutrophils of peripheral blood and describes the signal transmission mechanisms and the functional responses responsible for adenosine's biological effects.
Human platelets
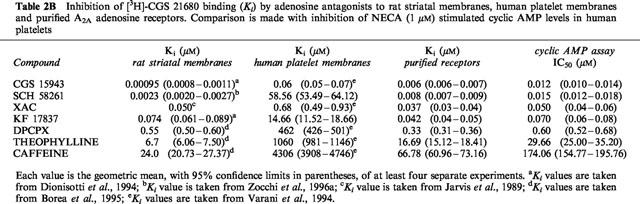
Since 1963 adenosine has been postulated to be an endogenous inhibitor of platelet aggregation and secretion (Born & Cross, 1963). The putative efficacy of dipyridamole as an antiaggregatory agent could be explained at least in part by its effect to block the nucleoside transport system, and thereby increase the extracellular concentration of adenosine and potentiate its effects on membrane receptors. Platelets, though non-nucleated and, thus, strictly speaking, not entitled to be classified as cells, represent a uniform tissue possessing A2 receptors (now known to be the A2A receptor subtype) on the external membrane (Haslam & Cusack, 1981). It is believed that the antiaggregatory action of adenosine depends upon the inhibition, coupled with adenylate cyclase activation, of both calcium influx and mobilization of internal stores (Paul et al., 1990). Considering the relevance of platelet aggregation in a variety of cardiovascular and cerebrovascular disorders, several studies have been performed to characterize A2A adenosine receptors in these blood components. The lack of a selective A2A radioligand had hampered the direct characterization of the A2A receptor subtype. The non-selective agonist NECA (A1, A2A, A2B, A3) was the first pharmacological tool to be used for labelling the A2A receptor in the rat brain but it was also found to bind to different affinity states and subtypes of adenosine receptors (Yeung & Green 1984; Bruns et al., 1986). As in other peripheral tissues, in human platelet membranes the binding properties of [3H]-NECA did not agree with the pharmacology of A2A receptors (Hütteman et al., 1984; Ukena et al., 1984; Lohse et al., 1988; Nakata & Fujisawa, 1988; Keen et al., 1989). Specifically, while only 10% of [3H]-NECA binding to human platelet membranes is displaced by N6-substituted adenosine derivatives, which are known to both activate adenylate cyclase and inhibit platelet aggregation, about 95% of this binding could be displaced with unlabelled NECA suggesting that the majority of the [3H]-NECA binding to human platelet membranes is to non-receptor sites. This ubiquitous adenosine A2-like binding site has been separated chromatographically and distinguished pharmacologically from the A2A adenosine receptor (Lohse et al., 1988). Subsequently, the NECA-binding site has been purified from human placental (Hutchison et al., 1990) and human platelet membranes (Fein et al., 1994) and was named ‘adenotin', a low affinity binding protein of 98 kDa whose biological significance has not been clarified, and precluded the direct characterization of A2A receptors in platelet membranes. With the aim to overcome the disadvantages observed with NECA, different radioligands have been proposed for the characterization of the human platelet A2A receptor. The A1-selective antagonist [3H]-XAC, having high levels of non-specific binding (Ukena et al., 1986), does not permit satisfactory binding studies of A2A receptors. The A2A selective agonist [3H]-CGS 21680, which has been reported to interact with the high affinity A2A adenosine receptor in both binding and adenylyl cyclase assays (Jarvis et al., 1989; Johansson et al., 1992; Mathot et al., 1995), has also been used by Varani et al. (1994) in human platelet membranes. Although saturation experiments showed that [3H]-CGS 21680 interacts with one recognition site only, the affinity of this agonist for the receptor was in the micromolar range, therefore suggesting that this radioligand like NECA, labels also the low affinity non-receptor site (Table 1). Moreover, as previously reported for the platelet [3H]-NECA binding (Hütteman et al., 1984), the displacement of [3H]-CGS 21680 binding by a series of adenosine receptor agonists and antagonists revealed Ki values substantially higher than those obtained for either stimulation or inhibition of cyclic AMP levels (Table 2A,B). Thus, although CGS 21680 interacts with the platelet A2A adenosine receptor, like NECA, it proved to be unsatisfactory for the characterization of A2A human platelet receptors. This fact has led some authors to postulate that the adenosine receptor expressed in platelets may be different from the A2A subtype identified in brain striatum (Cristalli et al., 1994). To overcome the many difficulties associated with the interaction between the A2A adenosine receptors and adenotin sites it was attempted to characterize a purified platelet A2A adenosine receptor preparation by using the selective radioligand [3H]-CGS 21680 (Varani et al., 1996). The method employed (Zolnierowicz et al., 1990) as an alternative to the gel filtration chromatography (Lohse et al., 1988), avoids high non-specific binding by removing most of the adenotin binding protein using extraction of membranes with CHAPS followed by PEG precipitation. In the purified protein preparation [3H]-CGS 21680 interacts with one recognition site only, with an affinity in the nanomolar range (Kd=285 nM) and a binding capacity of 2 pmol mg−1 protein. These values are not far from those obtained for [3H]-NECA in both solubilized A2A receptors (Lohse et al., 1988) and partially purified preparation (Zolnierowicz et al., 1990) and disagree with data obtained for [3H]-CGS 21680 binding in human platelet membranes (Varani et al., 1994) suggesting the presence in this case of a large component of low affinity binding to non-receptor sites (Table 1). Moreover, the affinities of all tested compounds for the purified receptor are systematically higher, by one to three orders of magnitude, than their affinities for platelet membranes and are very similar to those obtained in rat striatum suggesting that, only under these experimental conditions, the agonist CGS 21680 appears to be an adequate radioligand to study purified A2A adenosine receptors in human platelets (Table 2A,B). It is worth nothing that the discrepancies found between the affinity data obtained in cyclic AMP and receptor binding assays for many adenosine receptor ligands in human platelet membranes are markedly reduced in the partially purified platelet preparation in which Ki, EC50 or IC50 values are very similar for all compounds studied (Table 2A,B). These data indicate that in the purified platelet membrane preparation the presence of adenosine receptors is prevalent with respect to the non-receptor component (adenotin site).
Table 1.
Comparison between Kd and Bmax values obtained for [3H]-NECA and [3H]-CGS 21680 binding in human platelet membranes and in two different A2A receptor preparations
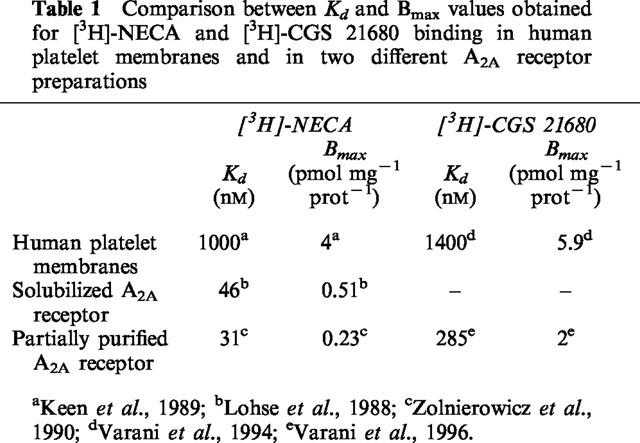
Table 2a.
Inhibition of [3H]-CGS 21680 binding (Ki) by adenosine agonists to rat striatal membranes, human platelet membranes and partially purified A2A adenosine receptors. Comparison is made with stimulation of cyclic AMP levels in human platelets
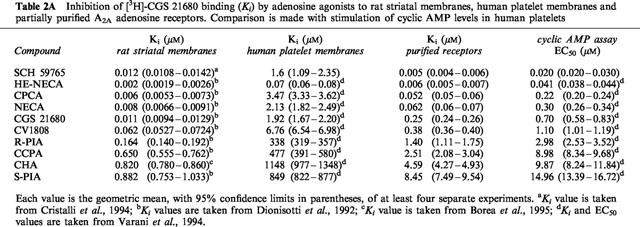
Table 2b.
Inhibition of [3H]-CGS 21680 binding (Ki) by adenosine antagonists to rat striatal membranes, human platelet membranes and purified A2A adenosine receptors. Comparison is made with inhibition of NECA (1 μM) stimulated cyclic AMP levels in human platelets
Agonists at G protein-coupled receptors are not ideal radioligands (Stiles & Jacobson, 1987). Indeed, as in the case of the binding between agonist radioligands and other G protein-linked receptors, the binding of [3H]-CGS 21680 to A2A receptors is influenced by several factors including the state of the G proteins (Johansson et al., 1992). Thus, the availability of antagonist radioligands, having high A2A receptor affinity and selectivity, represents a step forward in the characterization of this adenosine receptor subtype. Important progress has been made with the development of selective A2A adenosine receptor antagonists (Ongini & Fredholm, 1996; Ongini et al., 1999). One of them, the non-xanthine compound SCH 58261 (Baraldi et al., 1994), has been widely characterized in a variety of binding and functional assays (Zocchi et al, 1996a) and has been shown not to interact with the adenotin binding site (Varani et al., 1996). The tritium-labelled form, [3H]-SCH 58261, has been found to label A2A receptors in the rat brain (Zocchi et al., 1996b; Fredholm et al., 1998), in peripheral tissue membranes such as porcine coronary arteries (Belardinelli et al., 1996), and human cloned receptors transfected in mammalian cells (Dionisotti et al., 1997). SCH 58261 has low affinity for A1 receptors measured in rat brain cortex, does not interact with A2B receptors and has low affinity (μM range) for either rat or human A3 receptors (Zocchi et al., 1996a). On the basis of this pharmacological profile [3H]-SCH 58261 has been used to characterize A2A receptors in human platelet membranes.
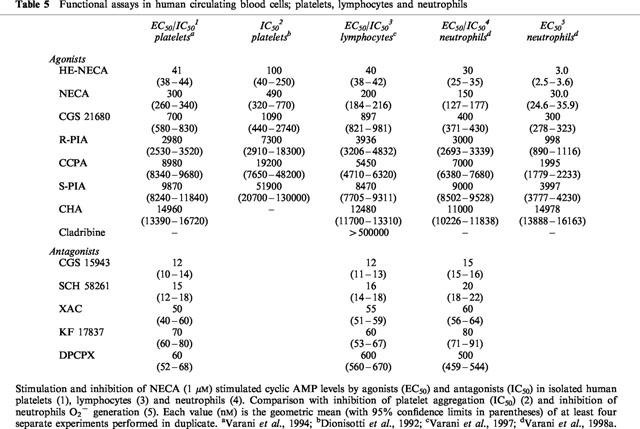
In saturation studies, [3H]-SCH 58261 labels one class of binding sites only, with Kd of 0.85 nM (Dionisotti et al., 1996; Varani et al., 1998b) a value similar to that observed in rat striatal membranes (Kd=0.70 nM) (Zocchi et al., 1996b), but very different from that obtained with [3H]-NECA in human platelet membranes (Table 3). In competition experiments, a series of typical adenosine ligands bound A2A platelet receptors with an affinity and a rank order of potency similar to that found in rat striatal membranes (Table 4). Moreover Ki values of adenosine agonists and antagonists correlate well with results from functional studies such as stimulation of cyclic AMP levels or platelet aggregation inhibition (Dionisotti et al., 1992) (Table 5). Thus, [3H]-SCH 58261 is the first radioligand that has allowed the binding characterization of the A2A receptor subtype in human platelets. Recently, in A2A receptor-knockout mice, it was reported that platelet aggregation was increased, indicating the importance of this receptor subtype in platelet function (Ledent et al., 1997). Activation of A2A receptors in platelets causes an increase in cyclic AMP accumulation and a decrease in platelet aggregation. During ischemia and/or hypoxia, extracellular and plasma levels of adenosine increase to levels suficient to activate A2A receptors and hence decrease platelet aggregability. The availability of [3H]-SCH 58261 should stimulate further the elucidation of the changes, such as up or downregulation, in A2A platelet receptors under a variety of physiologic and pathologic conditions.
Table 3.
Comparison between Kd and Bmax values obtained for [3H]-SCH 58261 binding in rat striatal membranes and in human circulating blood cells; platelets, lymphocytes and neutrophils
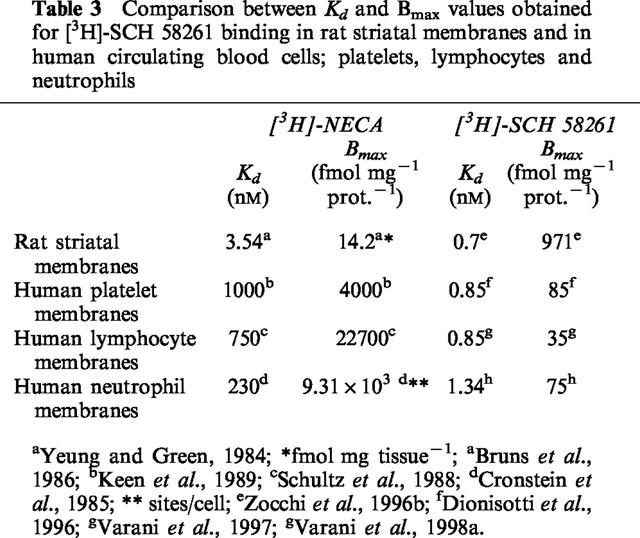
Table 4.
Inhibition of [3H]-SCH 58261 binding, (Ki), by adenosine agonists and antagonists to rat striatal, human platelet, lymphocyte and neutrophil membranes
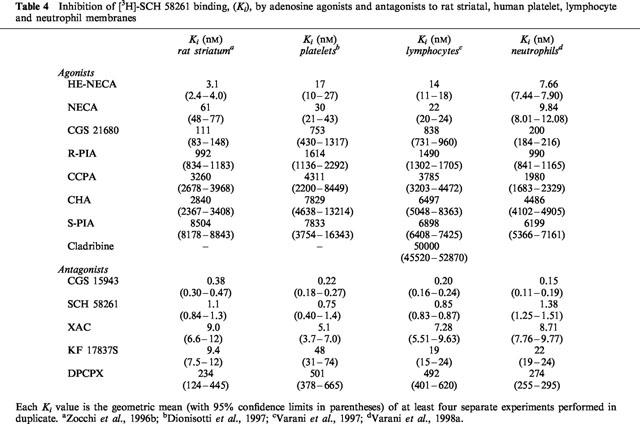
Table 5.
Functional assays in human circulating blood cells; platelets, lymphocytes and neutrophils
Human lymphocytes
The interest in the immunomodulatory effects of adenosine arose after the discovery that hereditary deficiency of the enzyme adenosine deaminase (ADA) was associated with severe combined immune deficiency disease (SCID) (Giblett et al., 1972). SCID is a disease characterized by severe lymphocytopenia, affecting both B and T cells and marked susceptibility to infection. ADA SCID has been hypothesized to be due to the accumulation of intracellular products of adenosine metabolism leading to the depletion of lymphocytes (Hirschorn, 1995; Blackburn et al., 1996). However, the studies aimed at elucidating the mechanisms by which absence of ADA leads to immunodeficiency, first suggested the presence of adenosine receptors on lymphocytes to suppress or dampen the immune response (Wolberg et al., 1975). Subsequently, other studies reported that adenosine causes cyclic AMP accumulation in lymphocytes by interacting with a specific external membrane receptor and that the effect of a series of adenosine analogues to inhibit lymphocyte cytotoxicity was correlated with the potency of the same compounds to increase the cellular content of cyclic AMP (Marone et al., 1978; Schwartz et al., 1978; Bonnafous et al., 1981). Recently, it has been reported that exposure of T lymphocytes to extracellular adenosine causes inhibition of T cell effector functions. These immunosuppressive effects of adenosine in cytotoxic T lymphocytes (CTL) actions may be explained by activation of A2A receptors followed by sustained increases in cyclic AMP that, in turn, antagonize T cell receptor (TCR)-triggered signaling including the FasL mRNA upregulation. The effect of the selective A2A agonist CGS 21680 to inhibit the lysis of antigen-specific target cells by CTL strongly supports the identification of A2A receptor as the major expressed receptor in T lymphocytes (Koshiba et al., 1997). Similarly, the effect of adenosine to inhibit T-cell proliferation and IL-2 receptor (CD 25) expression, is mimicked by low concentrations of 2-chloroadenosine and CGS 21680 suggesting the involvement of the A2A subtype (Huang et al., 1997). The expression of CD25 is an important indicator of lymphocyte activation, because failure of the production of either IL-2 or its receptor results in a failure of the T-cell immune response (Waldmann, 1986; Crabtree, 1989). The predominant expression of A2A receptors, which has been established in functional assays using selective agonists and antagonists of A2A receptors, was confirmed by Northern blot studies of A1, A2A and A3 mRNA expression in lymphoid tissues: A2A receptor mRNA, but not A1 and A3 receptor mRNA, was detected (Koshiba et al., 1997; Huang et al., 1997).
Thus, the accumulation of adenosine in ADA SCID or under hypoxic conditions causes an increased ‘signalling' through adenosine receptors on T cells and immunosuppression providing a novel target for immunomodulation. Several authors propose that T cell depletion, immunodeficiency and autoimmunity could be due to extracellular adenosine-induced ‘signalling', which in turn inhibits the TCR ‘signaling', affecting the TCR-driven positive and negative selection of thymocytes (Sitkovsky, 1998; Smith et al., 1998; Koshiba et al., 1997). This is of interest in view of the potential pharmacologic use of adenosine analogues as possible antileukemic and antiinflammatory agents, but the development of new drugs that target specific adenosine receptors must be done with a full understanding of the role of these receptors in the immune system.
A binding study has been performed in lymphocyte membranes to characterize the affinity (Kd) and the density (Bmax) of A2A receptors (Schultz et al., 1988). The radioligand used was the non selective agonist NECA which had a Kd of 0.75 μM and a Bmax of 22.7 pmol/mg of protein (Table 3). However, NECA has also been found to interact with non-receptor binding proteins in peripheral tissues (Hutchison et al., 1990) thus, due to the high amount of non-receptor NECA binding, the A2A fraction of the binding could not be well characterized. The availability of [3H]-SCH 58261 has facilitated the characterization of lymphocyte A2A receptors. In human lymphocytes, [3H]-SCH 58261 labelled a single class of recognition sites with affinity (Kd=0.85 nM) very similar to that observed in rat striatal and platelet membranes (Kd=0.7 and 0.85 nM, respectively; Table 2) (Varani et al., 1997, 1998b). In competition studies, typical adenosine receptor agonists and antagonists bound the lymphocyte A2A receptor with a rank order of potency and affinities similar to those observed in [3H]-SCH 58261 binding to human platelet membranes and in agreement also with those found in rat striatal membranes (Table 4). Functional data derived from the agonists-stimulation and antagonists-inhibition of cyclic AMP levels correlate well with binding parameters (Table 5). Finally, to evaluate the forces driving the coupling of A2A human lymphocyte receptors with a selective antagonist, thermodynamic studies were performed and the enthalpic (ΔH°) and entropic (ΔS°) contributions to the standard free energy (ΔG°) of the binding equilibrium were determined. The linearity of the van't Hoff plot for [3H]-SCH 58261 binding in human lymphocytes indicates that the ΔCp° values of the drug-interaction is nearly zero which means that ΔH° and ΔS° values were not significantly affected by temperature variations, at least over the temperature range investigated (Borea et al., 1995). It is notable that such a linearity of van't Hoff plots in a restricted range of temperatures (usually 0–25/30°C) appears to be a common feature of practically all membrane receptor ligands so far studied, from a thermodynamic point of view (Gilli et al., 1994). Thermodynamic data obtained from the van't Hoff plot, indicate that [3H]-SCH 58261 binding is entropy and enthalpy driven (ΔS°=38.87±4.51 Jmol−1 K−1, ΔH°=−36.74±3.42 kJ mol−1), a behaviour found to be typical of the interaction of antagonists with A2A receptors in rat striatum (Borea et al., 1995). Altogether all these data suggest that adenosine receptors present on lymphocytes have a pharmacological and biochemical profile typical of the A2A receptor subtype. Information about the lymphocyte subset-specific expression of adenosine receptors would be useful, but it has been difficult to obtain sufficient quantities of cells to analyse expression of adenosine receptors in biochemical or radioligand binding assays, particularly in the minor subpopulations of lymphocytes. The role of A2A adenosine receptors in the regulation of immune response has been investigated by determining the expression levels of this receptor in different subsets of functional lymphocytes (Koshiba et al., 1999). A monoclonal anti-A2A receptor antibody was used to develop a flow cytometric assay. The levels of expression of A2A receptors are much higher among T than B cells. T cells subsets are distinguished by the expression of TCR coreceptor molecules CD8+ and CD4+ involved in recognition of class I and class II major histocompatibility complex, respectively. More CD4+ than CD8+ T cells express A2A adenosine receptors, but activation of T cells increases A2A expression predominantly in CD8+ T cells. CD8+ T cells are mostly cytotoxic effector cells, whereas CD4+ cells have been implicated in T helper cell activities. Studies of T helper cell subsets (TH1 and TH2) reveal that lymphokine-producing cells are much more likely to express A2A receptors than are cells that do not produce lymphokines. A possible explanation is that inhibitory A2A receptors are induced selectively in cells that produce cytokines, as a mean of limiting cytokine release (Koshiba et al., 1999).
Interestingly, on the basis that a deficiency of ADA produces combined immunodeficiency disease in humans, the lymphopenia observed in affected children has been attributed to the toxic effects of deoxyadenosine. Thus, cladribine which is an adenosine deaminase-resistant analogue of deoxyadenosine, has recently been proposed for the treatment of various leukemias and lymphomas (Carrera et al., 1994). Cladribine is an A1/A2 non-selective receptor agonist and it has been investigated in [3H]-SCH 58261 binding assay to verify whether its mechanism of action underlying the antilimphoprolipherative activity is due to the interaction with A2A receptors. However, cladribine showed a low affinity (Ki=50 μM) in the binding assay and was unable to fully stimulate cyclic AMP accumulation (EC50>500 μM) suggesting that the interaction with A2A receptors is not the only (or main) mechanism of action of this compound. Recently, the involvement of adenosine in the intrathymic apoptotic deletion of T-cells during development has been reported (Barbieri et al., 1998). In order to characterize the role of adenosine, the effect of both 2-chloro-adenosine and cladribine to trigger apoptosis of T-cells has been evaluated. The data suggest that, for 2-chloro-adenosine, apoptosis is partially induced by the activation of the A2A receptor subtype, whereas no role has emerged for adenosine receptors in cladribine-dependent apoptosis. Moreover, in these cells, apoptosis could also be triggered through the activation of the A3 receptor using selective agonists, but this mechanism is not involved in either 2-chloro-adenosine or cladribine-induced apoptosis. Thus, there are at least three different ways by which adenosine derivatives may induce apoptosis including the A2A-like extracellular membrane receptor interaction, or the activation of the A3 receptors, or the entry of nucleoside into cells and direct activation of intracellular events involved in the apoptotic process and this is the proposed mechanism of action of cladribine. In the future it would be of interest to study deoxyadenosine congeners resistant to deamination, in the hope that these agents might possess antileukemic and antilymphocyte activity.
Human neutrophils
Neutrophils are the most abundant white cells in the peripheral blood and are usually the first cells to arrive at an injured or infected site. For more than a decade adenosine, by interacting with specific receptors on the surface of neutrophils, has been recognized as an endogenous antiinflammatory agent (Schrier & Imre, 1986; Cronstein et al., 1990, 1992a; Nolte et al., 1992; Sullivan et al., 1995). Like in other physiological systems, the effects of adenosine on inflammatory process are not all in one direction. Neutrophils have been found to express both A1 and A2A adenosine subtypes and the antiinflammatory actions of adenosine related to A2A receptors are balanced by the proinflammatory effects mediated through the A1 receptor subtype (Salmon & Cronstein, 1990; Fredholm et al., 1996). Thus, during the initiation of an inflammatory response low concentrations of adenosine promote neutrophil chemotaxis, phagocytosis (Cronstein et al., 1990; Salmon & Cronstein 1990) and endothelial cell adhesion (Becker et al., 1992; Schwartz et al., 1993); at the higher concentrations present in traumatizated tissues, acting via A2A receptors, adenosine inhibits cellular adhesion and superoxide anion generation (Cronstein & Haines, 1992; Sullivan & Linden, 1998). Tissue damage induced by inflammation stems in part from the migration of neutrophils to the site of infection followed by the release of membrane-damaging oxygen radicals. Adenosine inhibits both activities and therefore could be important in limiting abnormal and excessive inflammatory reactions (Cronstein, 1994). More recently other antiinflammatory effects of adenosine acting at its receptors, have been documented. For example, activation of A2A receptors seems to be associated with inhibition of tumour necrosis factor (TNF)-α, IL-6 and IL-8 release by activated mononuclear phagocytes. TNF-α and other cytokines are important in the pathogenesis of sepsis (Fong & Lowry, 1990) and ischemia-reperfusion injury (Seekamp et al., 1993) and the well-established beneficial effects of adenosine on ischemia-reperfusion injury have been attributed mainly to direct inhibition of neutrophil function (Marts et al., 1993). The antiinflammatory effects of adenosine and its receptor analogues were first suggested in 1983 when Cronstein et al. (1983) demonstrated that adenosine, released from neutrophils in suspension, selectively inhibited superoxide anion generation stimulated by FMLP, concanavalin A and the calcium ionophore A 23187. This effect was enhanced by dipyridamole which prevents adenosine uptake into the cells. The prevention of the oxidative burst by adenosine results from its interaction with a specific membrane receptor that has been identified as the A2 subtype (now known to be the A2A subtype) on the basis of some important characteristics such as the IC50 of adenosine for inhibition of O2− generation, the order of potency of adenosine analogues, the effect of methylxanthines to antagonize the actions of adenosine and the binding parameters of a radiolabelled adenosine analogue to intact cells (Cronstein et al., 1985). In general, occupancy of A2A adenosine receptors stimulates accumulation of intracellular cyclic AMP, which acts as a second messenger to alter cellular function (Londos et al., 1980; Daly et al., 1981; Fredholm et al., 1994; Sullivan & Linden, 1998). However, there is reason to think that the actions of adenosine in mediating the inhibition of superoxide anion generation might not be due to cyclic AMP. This hypothesis is based on the observation that a non-methylxanthine phosphodiesterase inhibitor (Ro 20-1724) enhances intracellular cyclic AMP levels and inhibits superoxide anion generation (Cronstein et al., 1988). Both Ro 20-1724 and NECA inhibit superoxide anion generation in an additive way but Ro 20-1724 does not potentiate the functional effect of NECA; in addition it has been reported that KT 5720, an agent which inhibits cyclic AMP-dependent protein kinase A, reverses the effects of cell-soluble analogues of cyclic AMP (dibutyril cyclic AMP) on superoxide anion production but not of NECA (Cronstein et al., 1992b) supporting the hypothesis that cyclic AMP does not act as the intracellular messenger for adenosine inhibition of O2− generation. In contrast, there are studies in which KT 5720 and other protein kinase A blockers, such as Rp-cyclic adenosine 3′,5′-phosphorothioate (Rp-cAMP), are able to reduce the potency of adenosine (Sullivan et al., 1995, 1998; Fredholm et al., 1996). Thus, although the role of cyclic AMP in mediating the effects of adenosine analogues is in doubt, this last finding suggests that cyclic AMP may at least contribute and is in agreement with data reported by Varani et al. (1998a). The effects of adenosine on other steps in the activation pathway of the neutrophils have also been explored. Chemoattractant-stimulated generation of inositol 1,4,5-triphosphate leads to mobilization of intracellular Ca2+ in neutrophils. Adenosine does not affect the early and rapid increase in free cytosolic Ca2+ in stimulated neutrophils but inhibits the sustained increase in intracellular Ca2+ that follows stimulation by using chemoattractants suggesting that adenosine does not interfere with the early steps in cell activation (Thiel & Bardenheuer, 1992). Pasini et al. (1985) have reported that adenosine may act as a Ca2+ channel blocker in neutrophils because it prevents superoxide anion generation in response to the calcium ionophore A23187. In contrast, other authors observed that adenosine inhibited chemoattractant-stimulated O2− generation even in the absence of extracellular Ca2+ and, as a consequence, the role of adenosine on Ca2+-dependent step in neutrophils activation remains unclear (Cronstein et al., 1988, 1997).
More recent studies indicate that adenosine receptor activation could also interfere with a subsequent step in signal transduction for chemoattractant receptors. It has been demonstrated that the direct activation of G proteins with NaF determines superoxide anion generation in neutrophils and the adenosine receptor occupancy does not reduce superoxide anion generation stimulated by NaF suggesting that adenosine inhibits the interaction between occupied chemotactic receptors and G proteins (Cronstein et al., 1990; Burkey & Webster, 1993). Possibly, the activation of adenosine receptors may determine the uncoupling of FMLP receptors from the signal transduction mechanism and the occupancy of adenosine receptors produces the association of chemoattractant receptors with the cytoskeleton, promoting their premature desensitization (Jesaitis et al., 1989; Cronstein & Haines, 1992; Revan et al., 1996).
Recent pharmacological studies comparing [3H]-SCH 58261 binding with functional data in human neutrophils have shed some light on the signal transduction mechanism responsible for the adenosine-mediated inhibition of O2− production and the role of cyclic AMP (Varani et al., 1998a,1998b). In the past, radioligand binding studies in neutrophils have been performed with the non-selective agonist NECA (Cronstein et al., 1985) that showed a high background binding, interacting also with the adenotin site (Table 3). [3H]-SCH 58261 has been found to label in human neutrophils a single class of recognition sites with Kd and Bmax values (1.34 nM and 75 fmol mg−1 prot., respectively) with the same order of magnitude as that determined in human platelet and lymphocyte membranes (Table 3). In competition studies, typical adenosine agonists and antagonists bound the A2A receptor with a rank order of potency and affinity range similar to that observed in [3H]-SCH 58261 binding to human platelet and lymphocyte membranes (Table 4). Interestingly, in the stimulation of cyclic AMP accumulations, the compounds studied exhibited a rank order of potency similar to that observed in binding experiments (Table 5). Thus, like A2A receptors in other mammalian tissues, those present on neutrophils are coupled with adenylate cyclase stimulation. Moreover, the studies of inhibition of superoxide anion production revealed that the receptor on neutrophils is of the A2A subtype. Adenosine analogues were similar in both their effects to inhibit superoxide anion generation and to inhibit the binding of [3H]-SCH 58261 (Tables 4 and 5). Similarly, the data on antagonists of HE-NECA actions in human neutrophils were in reasonable agreement with those obtained in binding assays. An excellent correlation was found also between cyclic AMP accumulation data and inhibition of O2− generation by adenosine receptor agonists investigated in this study, reported for comparison in Table 5, (Spearman rank correlation coefficient=1.00, P<0.01) suggesting that cyclic AMP could be involved in the action of A2A receptors to inhibit superoxide anion generation. Finally, thermodynamic analysis of [3H]-SCH 58261 binding was performed and the enthalpic (ΔH°) and entropic (ΔS°) contribution to the standard free energy (ΔG°) of the binding equilibrium were determined. Data obtained from this study indicate that [3H]-SCH 58261 binding to human neutrophils is entropy and enthalpy-driven (ΔS°=38.46±3.52 J mol−1 K−1, ΔH°= −35.67±3.38 kJ mol−1), a behaviour similar to that found in rat striatum, which is typical of A2A receptor antagonists (Borea et al., 1995) and in agreement with that found in human lymphocytes.
Conclusions
In conclusion, the therapeutic use of the antiaggregatory and antiinflammatory properties of adenosine as well as of its immunosuppressive effects requires a satisfactory characterization of the receptor subtype involved and the development of ligands selective for this receptor. Altogether, it can be stated that [3H]-SCH 58261 has helped to characterize A2A receptors which are present in tissues where they have been known to have a functional role such as platelets, lymphocytes and neutrophils. Thus, obstacles due to labelling of the adenotin site by [3H]-NECA or [3H]-CGS 21680 have been successfully overcome. A general conclusion from the most recent binding and functional data is that A2A receptors have similar sensitivity in different tissues and animal species. This finding is in agreement with the molecular biology data showing that the A2A receptor is a highly conserved structure in various animal species (Jacobson, 1995).
The available studies demonstrating the presence of A2A adenosine receptors in human circulating blood cells, strongly suggest that adenosine could play an important role in modulating aggregatory, immune and inflammatory processes and that the activation of A2A receptors may have therapeutic potential. For example, adenosine has been reported as a natural protectant of cells in ischemic tissue and although several regulatory mechanisms are involved, the activation of extracellular adenosine receptors is the most relevant. The activation of A2A receptors prevents platelet aggregation, affects the immune response in cancer, auto-immune and neurodegenerative diseases and decreases the inflammatory reactions. This is particularly important based on recent evidence suggesting the presence of an inflammatory component in a variety of neurological disorders, including trauma, ischemia, sclerosis and various forms of encephalopathies (Abbracchio & Burnstock, 1998). The therapeutic uses of adenosine and its analogues are limited by severe side effects like hypotension and bradycardia that are due to the ubiquitous nature of the adenosine receptors as well as a short half life (Belardinelli et al., 1989). As an alternative strategy, agents that enhance endogenous adenosine concentrations at sites of inflammation might be useful. Recent studies indicate that enhanced release of adenosine mediates the antiinflammatory effects of both methotrexate and sulphasalazine, the two most widely used agents for the treatment of rheumatoid arthritis (Cronstein & Weissmann 1993; Gadangi et al., 1996). Both methotrexate and sulphasalazine inhibit 5 aminoimidazole-4-carboxamide ribonucleotide (AICAR) transformylase with consequent intracellular acumulation of its substrate, AICAR. AICAR accumulation augments the release of adenosine from the endothelium damaged by the presence of adherent and activate PMN and the end-point of this biochemical interference is a reduced PMN adhesion. Therefore, in the future, site and receptor selective targeting strategies are needed to develop novel pharmacologic agents having optimal efficacy with minimal unwanted effects for the treatment of a variety of diseases linked with adenosine-signalling pathways.
Table 6.
Correlation among Ki values of tested agonists and antagonists in the [3H]-SCH 58261 binding to human neutrophil membranes and corresponding Ki values, EC50 and IC50 values
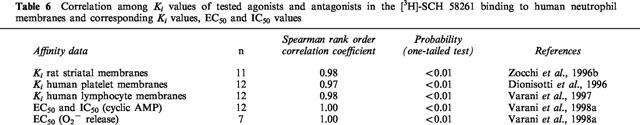
Table 7.
Correlation among EC50 and IC50 of adenosine receptor agonists and antagonists, respectively, obtained values in cyclic AMP assays using human blood cells

Abbreviations
- CCPA
2-chloro-N6-cyclopentyladenosine
- CHA
N6-cyclohexyladenosine
- CHAPS
3-[(3-cholamido-propyl)dimethylammonio]-1-propanesulphonate
- CGS 21680
2-[p-(2-carboxyethyl)-phenethylamino]-5′-N-ethylcarboxamidoadenosine
- CGS 15943
5-amino-9-chloro-2-(2-furyl)1,2,4-triazolo[1,5-c]quinazoline
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- FMLP
N-formyl-L-methionyl-L-leucyl-L-phenylalanine
- HE-NECA
2-hexynyl-5′-N-ethyl-carboxamidoadenosine
- KF 17837
(E)-1,3-dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine
- NECA
5′-N-ethylcarboxamidoadenosine
- PEG
polyethylene glycol 8000
- R-PIA and S-PIA (R(−) and S(+)-N6-(2- phenylisopropyl)-adenosine; SCH 58261
5-amino-7-(phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine
- XAC
8-[4-[[[[(2-aminoethyl)amino]-carbonyl]-methyl]oxy]-phenyl]-1,3dipropylxanthine
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinergic signalling: pathophysiological roles. Jpn. J. Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., PETERS J.A. Receptor & Ion Channel Nomenclature Supplement, Adenosine receptors. Trends Pharmacol. Sci. 1997;10 Suppl:7–8. [Google Scholar]
- APASOV S., KOSHIBA M., CHUSED T.M., SITKOVSKY M. Effects of extracellular ATP and adenosine on different thymocytes subsets: possible role of ATP-gated channels and G protein-coupled purinergic receptor. J. Immunol. 1997;158:5095–5105. [PubMed] [Google Scholar]
- BARALDI P.G., MANFREDINI S., SIMONI D., ZAPPATERRA L., ZOCCHI C., DIONISOTTI S., ONGINI E. Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine and 1,2,3-triazolo[1,5-c]pyrimidine displaying potent and selective activity as A2A adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 1994;4:2539–2544. [Google Scholar]
- BARBIERI D., ABBRACCHIO M.P., SALVIOLI S., MONTI D., COSSARIZZA A., CERUTI S., BRAMBILLA R., CATTABENI F., JACOBSON K.A., FRANCESCHI C. Apoptosis by 2-chloro-2′-deoxy-adenosine and 2-chloro-adenosine in human peripheral blood mononuclear cells. Neurochem. Int. 1998;32:493–504. doi: 10.1016/s0197-0186(97)00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER B.F., ZAHLER S., RASCHKE P., SCHWARTZ L.M., BEBLO S. Adenosine enhances neutrophil sticking in the coronary system: a novel mechanism contributing to cardiac reperfusion damage. Pharm. Pharmacol. Lett. 1992;2:8–11. [Google Scholar]
- BELARDINELLI L., LINDEN J., BERNE R.M. The cardiac effects of adenosine. Prog. Cardiovasc. Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- BELARDINELLI L., SHRYOCK J.C., RUBLE J., MONOPOLI A., DIONISOTTI S., ONGINI E., DENNIS D.M., BAKER S.P. Binding of the novel non-xanthine A2A adenosine receptor antagonist [3H]-SCH 58261 to coronary artery membranes. Circ. Res. 1996;79:1153–1160. doi: 10.1161/01.res.79.6.1153. [DOI] [PubMed] [Google Scholar]
- BLACKBURN M.R., DATTA S.K., WAKAMIYA M., VARTABEDIAN B.S., KELLEMS R.E. Metabolic and immunologic consequences of limited adenosine deaminase expression in mice. J. Biol. Chem. 1996;271:15203–15210. doi: 10.1074/jbc.271.25.15203. [DOI] [PubMed] [Google Scholar]
- BONNAFOUS J.C., DORNAND J., FAVERO J., MANI J.C. Lymphocyte membrane adenosine receptors coupled to adenylate cyclase properties and occurrence in various lymphocyte subclasses. J. Receptor Res. 1981;2:347–366. doi: 10.3109/107998981809038872. [DOI] [PubMed] [Google Scholar]
- BOREA P. A., DALPIAZ A., VARANI K., GUERRA L., GILLI G. Binding thermodinamics of adenosine A2A receptor ligands. Biochem. Pharmacol. 1995;49:461–469. doi: 10.1016/0006-2952(94)00464-w. [DOI] [PubMed] [Google Scholar]
- BORN G.V.R., CROSS M.J. The aggregation of blood platelets. J. Physiol. 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUMA M.G., JEUNHOMME T.M.M.A., BOYLE D.L., DENTENER M.A., VOITENOK N.N., VAN DEN WILDENBERG F.A.J.M., BUURMAN W.A. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J. Immunol. 1997;158:5400–5408. [PubMed] [Google Scholar]
- BRUNS R.F., LU G.H., PUGSLEY T.A. Characterization of the A2 adenosine receptor labeled by [3H]-NECA in rat striatal membranes. Mol. Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- BURKEY T.H., WEBSTER R.O. Adenosine inhibits FMLP-stimulated adherence and superoxide anion generation by human neutrophils at an early step in signal transduction. Biochim. Biophys. Acta. 1993;1175:312–318. doi: 10.1016/0167-4889(93)90223-c. [DOI] [PubMed] [Google Scholar]
- CARRERA J.C., SAVEN A., PIRO L.D. Purine metabolism of lymphocytes. New Drug Therapy. 1994;8:357–380. [PubMed] [Google Scholar]
- CRABTREE GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- CRISTALLI G., VITTORI S., THOMPSON R.D., PADGETT W.L., SHI D., DALY J.W., OLSSON R.A. Inhibition of platelet aggregation by adenosine receptor agonists. Naunyn. Schmied. Arch. Pharmacol. 1994;349:644–650. doi: 10.1007/pl00004904. [DOI] [PubMed] [Google Scholar]
- CRONSTEIN B.N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- CRONSTEIN B.N. A novel approach to the development of antiinflammatory agents: Adenosine release at the inflammed sites. J. Invest. Med. 1995;43:50–57. [PubMed] [Google Scholar]
- CRONSTEIN B.N. Adenosine regulation of neutrophil function and inhibition of inflammation via adenosine receptors Purinergic Approaches in Experimental Therapeutics 1997New York: Wiley-Liss, Inc; 285–299.In: Jacobson, K.A. & Jarvis, M.F (eds) [Google Scholar]
- CRONSTEIN B.N., DAGUMA L., NICHOLS D., HUTCHISON A.J., WILLIAMS M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J. Clin. Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONSTEIN B.N., HAINES K.A. Stimulus-response uncoupling in the neutrophil: adenosine A2-receptor occupancy inhibits the sustained but not the early, events of stimulus transduction in human neutrophils by a mechanism independent of actin-filament formation. Biochem. J. 1992;281:631–635. doi: 10.1042/bj2810631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONSTEIN B.N., HAINES K.A., KOLASINSKI S., REIBMAN J. Occupancy of G alpha s-linked receptors uncouples chemoattractant receptors from their stimulus-transduction mechanisms in the neutrophil. Blood. 1992b;80:1052–1057. [PubMed] [Google Scholar]
- CRONSTEIN B.N., KRAMER S.B., ROSENSTEIN E.D., KORCHAK H.M., WEISSMANN G., HIRSCHHORN R. Occupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarization. Biochem. J. 1988;252:709–715. doi: 10.1042/bj2520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONSTEIN B.N., KRAMER S.B., WEISSMAN G., HIRSCHHORN R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J. Exp. Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONSTEIN B.N., LEVIN R.I., PHILIPS M.R., HIRSCHHORN R., ABRAMSON S.B., WEISSMANN Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J. Immunol. 1992a;148:2201–2206. [PubMed] [Google Scholar]
- CRONSTEIN B.N., ROSENSTEIN E.D., KRAMER S.B., WEISSMAN G., HIRSCHHORN R. Adenosine: a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J. Immunol. 1985;135:1366–1371. [PubMed] [Google Scholar]
- CRONSTEIN B.N., WEISSMANN G. The adhesion molecules of inflammation. Arthritis Rheum. 1993;36:147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- DALY J.W., BRUNS R.F., SNYDER S.H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981;28:2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- DIOCEE B.K., SOUNESS J.E. Characterization of 5′-N-ethylcarboxamido[3H]adenosine binding to pig aorta smooth muscle membranes. Biochem. Pharmacol. 1987;36:3621–3627. doi: 10.1016/0006-2952(87)90011-6. [DOI] [PubMed] [Google Scholar]
- DIONISOTTI S., CONTI A., SANDOLI D., ZOCCHI C., GATTA F., ONGINI E. Effects of the new A2 adenosine receptor antagonist 8FB-PTP, an 8 substituted pyrazolo-triazolo-pyrimidine, on in vitro functional models. Br. J. Pharmacol. 1994;112:659–665. doi: 10.1111/j.1476-5381.1994.tb13126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIONISOTTI S., FERRARA S., MOLTA C., ZOCCHI C., ONGINI E. Labeling of A2A adenosine receptors in human platelets using the new non-xanthine antagonist radioligand [3H]-SCH 58261. J. Pharmacol. Exp. Ther. 1996;298:726–732. [PubMed] [Google Scholar]
- DIONISOTTI S., ONGINI E., ZOCCHI C., KULL B., ARSLAN G., FREDHOLM B.B. Characterization of human A2A adenosine receptors using the antagonist radioligand [3H]-SCH 58261. Br. J. Pharmcol. 1997;121:353–360. doi: 10.1038/sj.bjp.0701119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIONISOTTI S., ZOCCHI C., VARANI K., BOREA P.A., ONGINI E. Effects of adenosine derivatives on human and rabbit platelet aggregation. Correlation of adenosine receptor affinities and antiaggregatory activity. Naunyn Schmied. Arch. Pharmacol. 1992;346:673–676. doi: 10.1007/BF00168741. [DOI] [PubMed] [Google Scholar]
- FEIN T., SCHULZE E., BAR J., SCHWABE U. Purification and characterization of an adenotin-like adenosine binding protein from human platelets. Naunyn. Schmied. Arch. Pharmacol. 1994;349:374–380. doi: 10.1007/BF00170883. [DOI] [PubMed] [Google Scholar]
- FONG Y., LOWRY S.F. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin. Immunol. Immunopathol. 1990;55:157. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., LINDSTROM K., DIONISOTTI S., ONGINI E. [3H]-SCH 58261, a selective adenosine A2A receptor antagonist, is a useful ligand in autoradiographic studies. J. Neurochem. 1998;70:1210–1216. doi: 10.1046/j.1471-4159.1998.70031210.x. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ZHANG Y., VAN DER PLOEG I. Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leukocytes. Naunyn. Schmied. Arch. Pharmacol. 1996;354:262–267. doi: 10.1007/BF00171056. [DOI] [PubMed] [Google Scholar]
- GADANGI P., LONGAKER M., NAIME D., LEVIN R.I., RECHT P.A., CARLIN G., CRONSTEIN B.N. The antiinflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J. Immunol. 1996;156:1937–1941. [PubMed] [Google Scholar]
- GIBLETT E.R., ANDERSON J.E., COHEN F., POLLARA B., MEUWISSEN H.J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2:1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- GILLI P., FERRETTI V., GILLI G., BOREA P.A. Enthalpy-entropy compensation in drug-receptor binding. J. Phys. Chem. 1994;98:1515–1518. [Google Scholar]
- HASLAM R.J., CUSACK N.J. Blood platelet receptors for ADP and for adenosine Purinergic receptors 1981Chapman & Hall, London New York; 223–285.In: Burnstock G (ed) [Google Scholar]
- HIRSCHORN R. Adenosine deaminase deficiency: molecular basis and recent developments. Clin. Immunol. Immunopathol. 1995;76 Pt 2:S219–227. doi: 10.1016/s0090-1229(95)90288-0. [DOI] [PubMed] [Google Scholar]
- HOSKIN D., REYNOLDS T., BLAY J. 2-Chloroadenosine inhibits the MHC-unrestricted activity of anti-CD3 activated killer cells: Evidence for the involvement of a non-A1/A2 cell-surface adenosine receptor. Cell. Immunol. 1994;159:85–93. doi: 10.1006/cimm.1994.1297. [DOI] [PubMed] [Google Scholar]
- HUANG S., APASOV S., KOSHIBA M., SITKOVSKY M. Role of A2A extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- HUTCHISON K. A., NERVIS B., PERINI F., FOX I. H. Soluble and membrane-associated human low-affinity adenotin binding protein (adenotin): properties and homology with mammalian and avian stress proteins. Biochemistry. 1990;29:5138–5144. doi: 10.1021/bi00473a020. [DOI] [PubMed] [Google Scholar]
- HÜTTEMAN E., UKENA D., LEUSCHOW V., SCHWABE U. Adenosine receptors in human platelets. Characterization by 5′-N-ethylcarboxamido[3H]adenosine binding in relation to adenylate cyclase activity. Naunyn. Schmied. Arch. Pharmacol. 1984;325:226–233. doi: 10.1007/BF00495948. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., VAN GALEN P.J.M., WILLIAMS M. Perspective, adenosine receptors: Pharmacology, structure activity relationships and therapeutic potential. J. Med. Chem. 1992;35:407–22. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON M.A. Molecular biology of adenosine receptors Adenosine and adenine nucleotides: from molecular biology to integrative physiology 1995Boston, U.S.A.: Kluwer Academic Publishers; 241–248.In: Belardinelli, L., & Pelleg, A. (eds) [Google Scholar]
- JARVIS M.F., SCHULZ R., HUTCHISON A.J., DO U.H., SILLS M.A., WILLIAMS M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J. Pharmacol. Exp. Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- JESAITIS A.J., TOLLEY J.O., BOKOCK G.M., ALLEN R.A. Regulation of chemoattractant receptor interaction with transducing proteins by organizational control in the plasma membrane of human neutrophils. J. Cell. Biol. 1989;109:2783–2790. doi: 10.1083/jcb.109.6.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON B., PARKINSON F.E., FREDHOLM B.B. Effects of mono- and divalent ions on the binding of the adenosine analogue CGS 21680 to adenosine A2 receptors in rat striatum. Biochem Pharmacol. 1992;44:2365–2370. doi: 10.1016/0006-2952(92)90681-8. [DOI] [PubMed] [Google Scholar]
- KEEN M., KELLY E., NOBBS P., MAC DERMOT J. A selective binding site for [3H]-NECA that is not an adenosine A2 receptor. Biochem. Pharmacol. 1989;21:3827–3833. doi: 10.1016/0006-2952(89)90592-3. [DOI] [PubMed] [Google Scholar]
- KOSHIBA M., KOJIMA H., HUANG S., APASOV S., SITKOVSK Y. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J. Biol. Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- KOSHIBA M., ROSIN D.L., HATASHI N., LINDEN J., SITKOVSKY M.V. Patterns of A2A extracellular adenosine receptor expression in different functional subset of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol. Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- LEDENT C., VAUGEOIS J.M., SCHIFFMAN S.N., PEDRAZZINI T., EL YACOUBI M., VANDERHAEGHEN J.J., COSTENTIN J., HEATH J.K., VASSART G., PARMENTIER M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2A receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- LINDEN J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends. Pharmacol. Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- LOHSE M. J., ELGER B., LINDEBORN-FOTINOS J., KLOTZ K.N., SCHWABE U. Separation of solubilized A2 adenosine receptors of human platelets from non-receptor [3H]-NECA binding sites by gel filtration. Naunyn Schmied. Arch. Pharmacol. 1988;337:64–68. doi: 10.1007/BF00169478. [DOI] [PubMed] [Google Scholar]
- LONDOS C., COOPER M.F.D., WOLFF J. Subclasses of external adenosine receptors. Proc. Natl. Acad. Sci. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE W.M., HOSKIN D.W., BLAY J. Adenosine inhibits the adhesion of anti-CD3-activated killer lymphocytes to adenocarcinoma cells through the A3 receptor. Cancer. Res. 1994;54:3521–3526. [PubMed] [Google Scholar]
- MARONE G., PLAUT M., LICHTENSTEIN L.M. Characterization of a specific adenosine receptor on lymphocytes. J. Immunol. 1978;121:2153–2159. [PubMed] [Google Scholar]
- MARTS B.C., BAUDENDISTEL L.J., NAUNHEIM K.S., DAHMS T.E. Protective effect of 2-chloroadenosine on lung ischemia reperfusion injury. J. Surg. Res. 1993;54:523. doi: 10.1006/jsre.1993.1081. [DOI] [PubMed] [Google Scholar]
- MATHOT R.A.A., CLETON A., SOUDIJN W., IJZERMAN A.P., DANHOF M. Pharmacokinetic modelling of the haemodynamic effects of the A2A adenosine receptor agonist CGS 21680C in conscious normotensive rats. Br. J. Pharmacol. 1995;114:761–768. doi: 10.1111/j.1476-5381.1995.tb13270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H., FUJISAWA H. Adenosine binding sites of rat pheochromocytoma PC 12 cell membranes: partial characterization and solubilization. J. Biochem. 1988;104:457–460. doi: 10.1093/oxfordjournals.jbchem.a122489. [DOI] [PubMed] [Google Scholar]
- NOLTE D., LORENZEN A., LEHR H.A., ZIMMER F.J., KLOTZ K.N., MESSMER K. Reduction of postischemic leukocyte endothelium interaction by adenosine via A2 receptor. Naunyn. Schmied. Arch. Pharmacol. 1992;347:234–237. doi: 10.1007/BF00165307. [DOI] [PubMed] [Google Scholar]
- OLAH M.E., STILES G.L. Adenosine receptor subtypes: Characterization and Therapeutic Regulation. Ann. Rev. Pharmacol. Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- ONGINI E., DIONISOTTI S., GESSI S., IRENIUS E., FREDHOLM B. B. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn. Schmied. Arch. Pharmacol. 1999;359:7–10. doi: 10.1007/pl00005326. [DOI] [PubMed] [Google Scholar]
- ONGINI E., FREDHOLM B. B. Pharmacology of adenosine A2A receptors. Trends Pharmacol. Sci. 1996;17:364–372. [PubMed] [Google Scholar]
- PASINI F.L., CAPECCHI P.L., ORRICO A., CECCATELLI L., DIPIERRI T. Adenosine inhibits polymorphonuclear leukocyte in vitro activation: a possible role as an endogenous calcium entry blocker. Int. J. Immunopharmol. 1985;7:203–215. doi: 10.3109/08923978509047634. [DOI] [PubMed] [Google Scholar]
- PAUL S., FEOKTISTOV I., HOLLISTER A.S., ROBERTSON D., BIAGGIONI I. Adenosine inhibits the rise in intracellular calcium and platelet aggregation produced by thrombin: evidence that both effects are coupled to adenylate cyclase. Mol. Pharmacol. 1990;37:870–875. [PubMed] [Google Scholar]
- POULSEN S-A., QUINN R.J. Adenosine receptors: new opportunities for future drugs. Bioorg. & Med. Chem. 1998;6:619–641. doi: 10.1016/s0968-0896(98)00038-8. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- REVAN S., MONTESINOS M.C., NAIME D., LANDAU S., CRONSTEIN B.N. Adenosine A2 receptor occupancy regulates stimulated neutrophil function via activation of a serine/threonin protein phosphate. J. Biol. Chem. 1996;271:17114–17118. doi: 10.1074/jbc.271.29.17114. [DOI] [PubMed] [Google Scholar]
- SALMON J.E., CRONSTEIN B.N. Fcg-receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. J. Immunol. 1990;145:2235–2240. [PubMed] [Google Scholar]
- SCHRIER D.J., IMRE K.M. The effects of adenosine on human neutrophil function. J. Immunol. 1986;137:3284–3289. [PubMed] [Google Scholar]
- SCHULTZ L.A., KAMMER G.M., RUDOLPH S.A. Characterization of the human T lymphocyte adenosine receptor: comparison of normal and systemic lupus erythematosus cells. FASEB J. 1988;2:244–250. doi: 10.1096/fasebj.2.3.3258258. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ L.M., RASCHKE P., BECKER B.F., GERLACH E. Adenosine contributes to neutrophil-mediated loss of myocardial function in postischemic guinea pig hearts. J. Mol. Cell. Cardiol. 1993;25:927–938. doi: 10.1006/jmcc.1993.1105. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ L.M., STERN R.C., POLMAR S.H. Demonstration of an adenosine receptor on human lymphocytes in vitro and its possible role in the adenosine deaminase-deficient form of severe combined immunodeficiency. Clin. Immunol. Immunopathol. 1978;9:499–505. doi: 10.1016/0090-1229(78)90146-0. [DOI] [PubMed] [Google Scholar]
- SEEKAMP A., WARREN J.S., REMICK D.G., TILL G.O., WARD P.A. Requirements for tumor necrosis factor-a and interleukin-1 in limb ischemia/reperfusion injury and associated lung injury. Am. J. Pathol. 1993;143:453. [PMC free article] [PubMed] [Google Scholar]
- SMITH P.T., ARMSTRONG J., KOSHIBA M., HUANG S., APASOV S., SITKOVSKY M. Studies of expression and possible functional role of purinergic receptors in cell-mediated immunity: experimental approaches, controls, and caveats. Drug. Dev. Res. 1998;45:229–244. [Google Scholar]
- SITKOVSKY M.V. Extracellular purines and their receptors in immunoregulation. Review of recent advances. Nippon Ika Daigaku Zasshi. 1998;65:351–357. doi: 10.1272/jnms1923.65.351. [DOI] [PubMed] [Google Scholar]
- STILES G.L., JACOBSON K.A. A new affinity, iodinated adenosine receptor antagonist as a radioligand/photoaffinity crosslinking probe. Mol. Pharmacol. 1987;32:184–188. [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN G.W., CARPER H.T., MANDELL G.L. The specific type IV phosphodiesterase inhibitor rolipram combined with adenosine reduces tumor necrosis factor-α-primed neutrophil oxidative activity. Int. J. Immunopharmacol. 1995;17:793–803. doi: 10.1016/0192-0561(95)00073-b. [DOI] [PubMed] [Google Scholar]
- SULLIVAN G., LINDEN J. Role of A2A adenosine receptors in inflammation. Drug Dev. Res. 1998;45:103–112. [Google Scholar]
- SULLIVAN G., LINDEN J., HEWLETT E., CARPER H., HYLON J., MANDELL G. Adenosine and related compounds counteract tumor necrosis factor-alpha inhibition of neutrophil migration: implication of a novel cyclic AMP-independent action on the cell surface. J. Immunol. 1990;145:1537–1544. [PubMed] [Google Scholar]
- THIEL M., BARDENHEUER H. Regulation of oxygen radical production of human polymorphonuclear leukocytes by adenosine: the role of calcium. Pfluegers Arch. 1992;420:522–528. doi: 10.1007/BF00374628. [DOI] [PubMed] [Google Scholar]
- UKENA D., BOHME E., SCHWABE U. Effects of several 5′-carboxamide derivatives of adenosine on adenosine receptors of human platelets and rat fat cells. Naunyn. Schmied. Arch. Pharmacol. 1984;327:36–42. doi: 10.1007/BF00504989. [DOI] [PubMed] [Google Scholar]
- UKENA D., JACOBSON K.A., KIRK K.L., DALY J.W. A [3H] amine congener of 1,3-dipropyl-8-phenylxanthine. A new radioligand for A2 adenosine receptors of human platelets. FEBS Lett. 1986;199:269–274. doi: 10.1016/0014-5793(86)80493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARANI K., BOREA P.A., GUERRA L., DIONISOTTI S., ZOCCHI C., ONGINI E. Binding characteristics of the adenosine A2 receptor ligand [3H]-CGS 21680 to human platelet membranes. Biochem. Pharmacol. 1994;48:1658–1661. doi: 10.1016/0006-2952(94)90212-7. [DOI] [PubMed] [Google Scholar]
- VARANI K., GESSI S., DALPIAZ A., BOREA P.A. Pharmacological and biochemical characterization of purified A2A adenosine receptor in human platelet membranes by [3H] CGS 21680 binding. Br. J. Pharmacol. 1996;117:1693–1701. doi: 10.1111/j.1476-5381.1996.tb15341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARANI K., GESSI S., DALPIAZ A., BOREA P.A. Characterization of A2A adenosine receptor in human lymphocyte membranes by [3H] SCH 58261 binding. Br. J. Pharmacol. 1997;122:386–392. doi: 10.1038/sj.bjp.0701378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARANI K., GESSI S., DIONISOTTI S., ONGINI E., BOREA P.A. [3H] SCH 58261 labelling of functional A2A adenosine receptors in human neutrophil membranes. Br. J. Pharmacol. 1998a;123:1723–1731. doi: 10.1038/sj.bjp.0701758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARANI K., GESSI S., MERIGHI S., ONGINI E., BOREA P.A. Adenosine A2A receptors of human circulating blood elements. Drug Dev. Res. 1998b;45:253–260. [Google Scholar]
- WALDMANN T.A. The structure, function and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986;232:727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- WOLBERG G., ZIMMERMAN T.P., HIEMSTRA K., WINSTON M., CHU L.C. Adenosine inhibition of lymphocyte-mediated cytolysis: possible role of cyclic adenosine monophosphate. Science. 1975;87:957–959. doi: 10.1126/science.167434. [DOI] [PubMed] [Google Scholar]
- YEUNG S.H., GREEN R.D. [3H]5'-N-ethylcarboxamide adenosine binds to both Ra and Ri adenosine receptors in striatum. Naunyn. Schmied. Arch. Pharmacol. 1984;325:218–225. doi: 10.1007/BF00495947. [DOI] [PubMed] [Google Scholar]
- ZOCCHI C., ONGINI E., CONTI A., MONOPOLI A., NEGRETTI A., BARALDI P.G., DIONISOTTI S. The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2A adenosine receptor antagonist. J. Pharmacol. Exp. Ther. 1996a;276:398–404. [PubMed] [Google Scholar]
- ZOCCHI C., ONGINI E., FERRARA S., BARALDI P.G., DIONISOTTI S. Binding of the radioligand [3H]-SCH 58261, a new non-xanthine A2A adenosine receptor antagonist, to rat striatal membranes. Br. J. Pharmacol. 1996b;117:1381–1386. doi: 10.1111/j.1476-5381.1996.tb15296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOLNIEROWICZ S., WORK C., HUTCHISON K., FOX I.H. Partial separation of platelet and placental adenosine receptor from adenosine A2-like binding protein. Mol. Pharmacol. 1990;37:554–559. [PubMed] [Google Scholar]


