Abstract
Irritable bowel syndrome is characterized by visceral hyperalgesia commonly associated with stress and inflammatory processes. We investigated the role of tachykinin NK2 receptors in the ability of trinitrobenzenesulphonic acid (TNBS) and stress to enhance the sensitivity of the rat rectum to distension using a selective tachykinin NK2 receptor antagonist (MEN 11420).
Rats were fitted with electrodes implanted in the striated muscles of the abdomen. Rectal distension (RD) was performed with a balloon inflated by steps of 0.4 ml from 0 to 1.6 ml. Five groups were submitted to RD performed 3 days before and after intrarectal instillation of TNBS. Fifteen minutes before RD, rats were treated with saline or MEN 11420 (5–100 μg kg−1 i.v.). Two other groups, submitted to 2 h restraint or sham stress sessions were randomly treated i.v. with saline or MEN 11420 (10–200 μg kg−1) prior to RD applied 20 min later.
The basal response to RD was characterized by a significant increase in the number of abdominal contractions. This response occurred with a threshold volume of 0.8 ml and was dose-dependently reduced by MEN 11420 (5–100 μg kg−1 i.v.). Rectal inflammation lowered the volume of distension producing abdominal contractions to 0.4 ml (allodynia). This effect was either reduced or suppressed by MEN 11420. A similar allodynia was observed after a stress session and this effect was reduced (49%) or suppressed by MEN 11420 at 200 and 100 μg kg−1, respectively.
Tachykinin NK2 receptors are involved in rectal hypersensitivity associated with inflammation and stress.
Keywords: Tachykinins, neurokinin A, NK2 receptors, stress, inflammation, rectal sensitivity, pain
Introduction
Chronic abdominal pain and/or discomfort are probably the most common symptoms observed in patients with irritable bowel syndrome (IBS). Recent reports suggest that previous experiences of gut inflammation might trigger an exacerbation of IBS symptoms, particularly in patients experiencing stressful life events (Gwee et al., 1996).
Inflammatory bowel disease is associated with a reduced threshold for pain sensation in response to colonic or rectal distension in man (Rao et al., 1987). Moreover, acute physical and mental stress stimuli affect the perception of pain distension-induced in both healthy and IBS patients (Erckenbrecht et al., 1988; Ford et al., 1995; Métivier et al., 1996). In rats, colonic or rectal irritation with chemicals induces a drastic increase in sensitivity to gradual distension (Ness et al., 1991; Morteau et al., 1994a). It has also been demonstrated that restraint stress increases nociception due to rectal distension (RD), while simultaneously inducing hypoalgesia for cutaneous thermic stimuli (Porro & Carly, 1988; Gué et al., 1997).
Numerous reports have implicated tachykinins such as substance P (SP) and neurokinin A (NKA) in the transmission of visceral nociception via tachykinin NK1 and NK2 receptors (Julia et al., 1994; Holzer & Holzer-Petsche, 1997; Maggi, 1997). It has been reported that SR 48968, a selective tachykinin NK2 receptor antagonist, reduces the number of abdominal contractions induced by gradual rectal distension in awake rats (Julia et al., 1994). Pronounced changes in the content of neuropeptides such as tachykinins and in innervation patterns have been observed in the inflamed gut of patients with inflammatory bowel disease (Eysselein & Nast, 1991; Anton & Shanahan, 1998). The mechanisms by which stress affects viscerosensitivity have not been fully investigated. However, recent findings suggest that stress may activate mast cells within the gut, facilitating the release of mast cell-derived mediators in response to rectal distension (Gué et al., 1997). Moreover, tachykinins induce an immediate and transient release of histamine from mast cells (Joos & Pauwels, 1993; Saban et al., 1997).
Although it is intriguing to postulate that interactions between the immune and nervous systems exist and play a role in visceral hyperalgesia, in vivo studies blocking or mimicking neuropeptide actions are needed to prove this bidirectional communication.
The aim of this study was to investigate the possible role of tachykinin NK2 receptors in visceral hypersensitivity by studying the effect of a potent and selective tachykinin NK2 receptor antagonist, MEN 11420 (Nepadutant) (Catalioto et al., 1998), in rat models of visceral hyperalgesia induced by inflammation or stress.
Methods
Animal preparation
Male and female Wistar rats (Elevage Janvier, Le Genest Saint Isle, France) weighing 200–300 g were used in these experiments. The animals were housed individually in polypropylene cages (37.5×17×15 cm), kept in a temperature-controlled room (21±1°C) on a 12 : 12 h light–dark cycle (lights on 08 00h) and fed with a standard laboratory diet (A03, UAR, Epinay, France) given ad libitum. Six groups of eight rats were surgically prepared for electromyography according to a previously described technique (Ruckebusch & Fioramonti, 1975). Rats were anaesthetized with acepromazine (Calmivet, Vétoquinol, Lure, France; 0.5 mg kg−1) and ketamine (Imalgene 1000, Rhône-Mérieux, Lyon, France; 120 mg kg−1) administered intraperitoneally (i.p.). Under general anaesthesia, three groups of three electrodes of nichrome wire (60 cm long×80 mm diameter) were implanted bilaterally in the abdominal external oblique musculature just superior to the inguinal ligament. Electrodes were exteriorized on the back of the neck and protected by a glass tube (6 mm outer diameter, 20 cm length) attached to the skin.
Electromyographic recordings
Electromyographic (EMG) recordings began 5 days after surgery. The electrical activity of abdominal striated muscles was recorded with an electroencephalograph machine (Mini-huit, Alvar, Paris, France) using a short time constant (0.03 s) to remove low-frequency signals (<3 Hz) and a paper speed of 3.6 cm min−1.
Inflammation procedure
Trinitrobenzenesulphonic acid (TNBS, 80 mg kg−1 in 0.3 ml 50% ethanol) was administered intrarectally through a silicone rubber catheter introduced 1 cm into the anus under light diethyl-ether anaesthesia, as previously described (Morteau et al., 1994a).
Stress procedure
Partial restraint stress (PRS), a relatively mild, non-ulcerogenic model of restraint (Williams et al., 1988), was used. Briefly, the animals were lightly anaesthetized with diethyl ether and their foreshoulders, upper forelegs and thoracic trunk were wrapped in a confining harness of paper tape to restrict, but not prevent body movement. The animals were then placed in their home cage for 2 h. The rats recovered from diethyl ether anaesthesia within 2–3 min and immediately moved about in their cages and ate and drank, but the mobility of their forelegs was restricted, thus preventing grooming of the face, upper head and neck. Control animals (sham) were anaesthetized but were not wrapped. After recovering from the anaesthesia, control rats groomed the face, head and abdomen. Partial restraint stress was always performed between 1000 and 1200 h.
Rectal distension procedure
To prevent recording artefacts owing to movement during distension, rats were accustomed, 3 days before distension, to be placed in a polypropylene tube (6 cm diameter×22 cm long). A balloon consisting of an arterial embolectomy catheter (Fogarty, Edwards Laboratories, Inc.) was introduced into the rectum 1 cm from the anus and fixed at the base of the tail. The balloon (2 mm diameter×2 cm long) was progressively inflated with water by steps of 0.4 ml, from 0 to 1.6 ml, each inflation step lasting 5 min. To detect possible leakage, the volume of water introduced in the balloon was checked by complete removal with a syringe at the end of distension period.
Experimental protocol
Rectal sensitivity
The number of abdominal contractions during each 5 min periods of distension was a reproducible criterion of nociception due to rectal distension (Morteau et al., 1994b).
In a first series of experiments performed on four groups of eight male rats fitted with electrodes, rectal distension was performed 3 days before and after intrarectal instillation of trinitrobenzenesulphonic acid. Fifteen minutes before rectal distension, the animals of each group were treated intravenously (i.v.) with saline (0.2 ml NaCl 0.9%) or MEN 11420 at doses of 5, 20 or 100 μg kg−1, respectively.
In a second series of experiments performed on two other groups of eight female rats also fitted with electrodes, one group was submitted to a 2 h restraint stress session, while the second group was submitted to a ‘sham stress' session. Ten minutes after the end of this period, the animals were treated i.v. in a randomized order with saline (0.2 ml NaCl 0.9%) or MEN 11420 at doses of 10, 100 or 200 μg kg−1. Twenty minutes later, the animals were submitted to step-by-step rectal distension. Each stress session was performed at 3 day intervals.
Colorectal compliance
In a third series of experiments, in order to check the effect of MEN 11420 on rectal compliance, one group of six male rats not fitted with electrodes was used. A latex balloon (2 cm length) was designed for pressure measurements. The balloon was connected to an electronic pressure transducer built in the laboratory (Barostat, INRA, Toulouse, France). Preliminary testing in room air showed that the pressure in the balloon remained at zero for inflation volumes up to 2.0 ml. Therefore, within the range of volumes used in our study (0–1.6 ml), the balloon compliance may be considered infinite. The rats were placed in a tunnel and a balloon was rectally inserted, in a minimally invasive manner, at 1 cm from the anus. The balloon was increasingly inflated with air in an intermittent manner in steps of 0.4 ml for 10 s at 60 s intervals, up to 1.6 ml and the corresponding pressure expressed in millimetres of mercury was determined for each volume. In order to avoid excessive pain, the balloon inflation was stopped when the maximal value of 80 mmHg was reached. Each rat was subjected to a series of three pressure measurements which were performed 15 min after i.v. administration of vehicle (0.2 ml NaCl 0.9%) or MEN 11420 at doses of 100 or 200 μg kg−1, in a randomized order. Each pressure measurement was performed at 3 day intervals.
Chemicals
MEN 11420 ((Asn (2-AcNH-b-D-Glc)-Asp-Trp-Phe-Dap-Leu) c(2b-5β)) was synthesized at Menarini Laboratories, Florence, Italy, by conventional solid-phase methods.
Analysis of data
Statistical analysis of the number of abdominal contractions occurring during each 5 min period during rectal distension was performed by one-way ANOVA followed by Student's paired or unpaired t-test. Additional inter-group comparisons were performed before and after TNBS and stress. Values were expressed as means±s.e.mean. Differences were considered to be significant when P<0.05.
Results
Basal rectal sensitivity
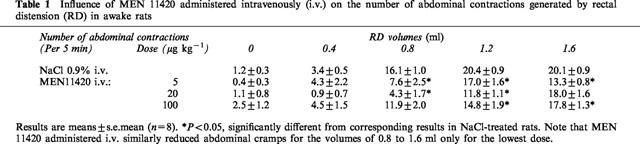
Gradual rectal distension increased the frequency of abdominal contractions in a volume-dependent manner. In rats treated with vehicle (NaCl 0.9% i.v.), the first volume of distension increasing significantly the number of abdominal contractions beyond the pre-distension level was 0.8 ml (Table 1), which in our experimental conditions is considered the pain threshold to distension in the basal state, i.e. normalgesia. Injected i.v. at a dose of 5 μg kg−1, 15 min before the beginning of rectal distension, MEN 11420 significantly (P<0.05) reduced the abdominal response for all volumes of distension from 0.8 to 1.6 ml as compared to vehicle treated rats (Table 1). The number of abdominal contractions for the distension volumes of 0.8 and 1.2 ml was also reduced after pretreatment with MEN 11420 at a dose of 20 μg kg−1 (i.v.). However, this dose did not significantly (P>0.05) affect the number of contractions at the highest volume of distension, i.e. 1.6 ml (Table 1). At a dose of 100 μg kg−1 i.v., MEN 11420 also attenuated significantly (P<0.05) the abdominal response to high volumes of distension, i.e. 1.2 and 1.6 ml (Table 1). No inter-group difference in the abdominal responses to each volume of distension was observed.
Table 1.
Influence of MEN 11420 administered intravenously (i.v.) on the number of abdominal contractions generated by rectal distension (RD) in awake rats
TNBS-induced allodynia
In the control group (vehicle), 3 days after rectal inflammation with TNBS, a significant increase in the number of abdominal contractions was observed for a volume of 0.4 ml, compared to the basal state (10.4±5.3 vs 3.4±0.5 before inflammation) (Figure 1). Furthermore, the response to 0.4 ml distension was significantly different from the control (predistension) EMG recording. However, for higher volumes of distension (0.8–1.6 ml) the number of abdominal contractions did not significantly differ from that observed at the same volumes before TNBS (Figure 1).
Figure 1.
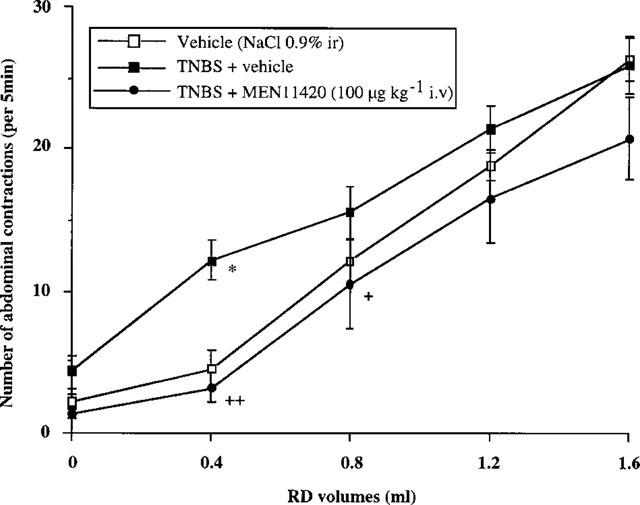
Effect of MEN 11420 (100 μg kg−1 i.v.) on the abdominal response to rectal distension (RD) in rats intrarectally (i.r.) instilled with vehicle or TNBS (80 mg kg−1) (mean±s.e.mean; n=8). *Significantly different from control values (P<0.05). +Significantly different from TNBS values at P<0.05 (+) and P<0.01 (++).
Three days after rectal instillation of TNBS, MEN 11420 at all doses tested suppressed the TNBS-induced increase in abdominal response to rectal distension with a volume of 0.4 ml when compared with vehicle (Figure 2). Moreover, similarly to what was seen before TNBS, MEN 11420 at 5 μg kg−1 i.v. significantly (P=0.005) decreased by 41.2% the number of abdominal contractions observed for the volume of 0.8 ml. At higher doses, i.e. 20 and 100 μg kg−1, MEN 11420 also suppressed the TNBS-induced allodynia at 0.4 ml without affecting the response for higher volumes of distension, except at the dose of 100 μg kg−1 for the volume of 0.8 ml (Figure 1).
Figure 2.
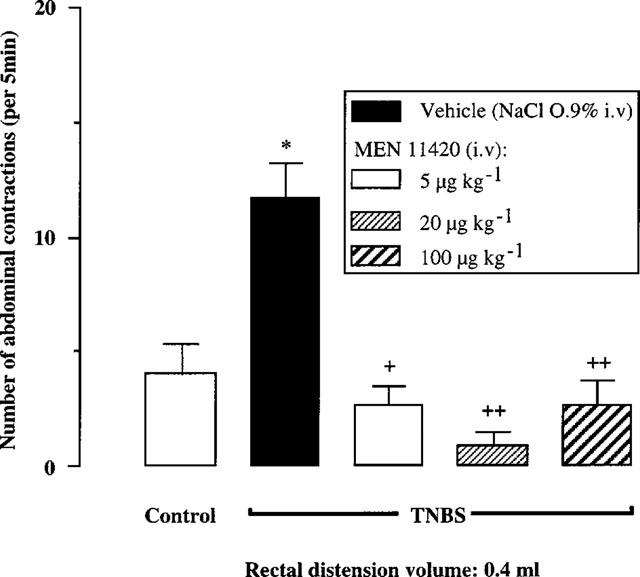
Influence of increasing i.v. doses of MEN 11420 on the abdominal response to rectal distension in vehicle- and TNBS (80 mg kg−1)-treated rats (mean±s.e.mean; n=8). *Significantly different from control values (P<0.01). +Significantly different from TNBS values at P<0.05 (+) and P<0.01 (++).
Stress-induced rectal hyperalgesia
In rats submitted to PRS and in contrast to ‘sham-stress' animals, we observed a significant increase in the number of abdominal contractions that prior to RD were evoked only by the presence of the tube supporting the balloon in the rectum (6.8±1.7 vs 1.8±0.8 in sham stressed rats). In sham stressed rats, the first volume of distention increasing significantly the number of abdominal contractions to RD was 0.8 ml (16.5±1.9 vs 1.8±0.8). PRS applied during 2 h reduced to 0.4 ml the volume of distension increasing significantly the abdominal response to RD (12.3±1.9 vs 6.8±1.7 in control EMG recordings without distension). Moreover, PRS significantly (P<0.05) increased the number of abdominal contractions observed for all the other volumes of distension (Figure 3, Table 2).
Figure 3.
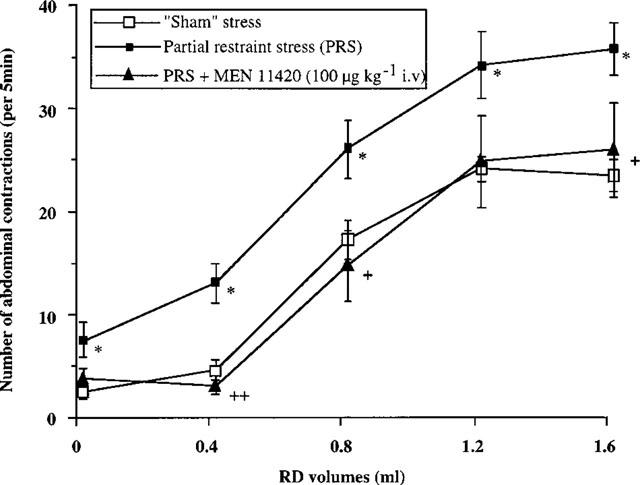
Effect of MEN 11420 (100 μg kg−1 i.v.) on the abdominal response to rectal distension (RD) in stressed female rats (mean±s.e.mean; n=8). *Significantly different from ‘sham' values (P<0.05). +Significantly different from ‘stress' values at P<0.05 (+) and P<0.01 (++).
Table 2.
Influence of MEN 11420 on stress-induced increase in abdominal contractions generated by increasing volumes of rectal distension (RD) in female rats
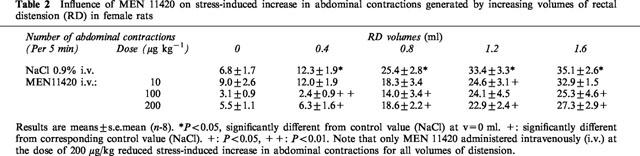
Administered 10 min after the end of PRS, MEN 11420 at doses of 100 and 200 μg kg−1 (i.v.) suppressed the PRS-induced enhancement of abdominal contractions at a volume of 0.4 ml, restoring a normal sensitivity to RD (Figure 4). Moreover, at these two dosages, MEN 11420 also reduced or suppressed the PRS-induced increase in the abdominal response to the other volumes of distension, except for 1.2 ml at the dose of 100 μg kg−1 (Table 2). At a lower dose, i.e. 10 μg kg−1 (i.v.), MEN 11420 significantly (P<0.05) reduced the number of abdominal contractions only for the volume of 1.2 ml (Table 2).
Figure 4.
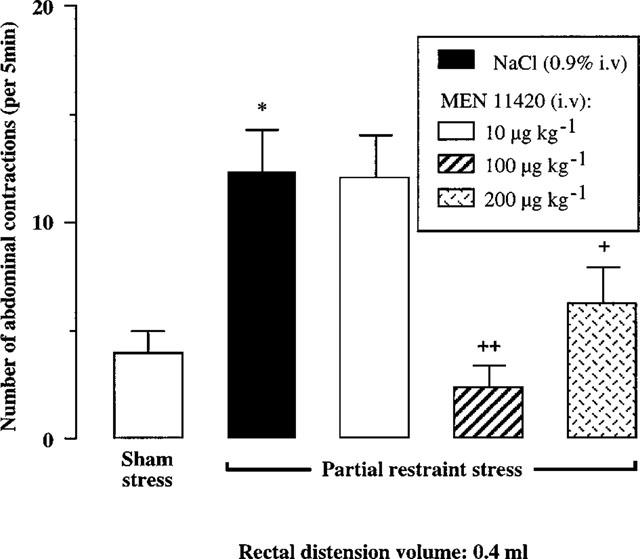
Influence of increasing i.v. doses of MEN 11420 (10, 100 and 200 μg kg−1) on the abdominal response to rectal distension with 0.4 ml in rats submitted to partial restraint stress (mean±s.e.mean; n=8). *Significantly different from ‘sham' values (P<0.05). +Significantly different from ‘stress+vehicle' values at P<0.05 (+) and P<0.01 (++).
Intrarectal pressure and rectal compliance
Reproducible basal pressure responses to increasing volumes of inflation were observed after vehicle (NaCl 0.9% i.v.) administration. No significant intra-individual or inter-individual variation was observed. The i.v. administration of MEN 11420 at doses of 100 and 200 μg kg−1 did not significantly (P>0.05) modify the pressure-volume response curve observed after vehicle administration (Figure 5). This indicates that rectal compliance was not affected by the administration of MEN 11420 at the doses tested.
Figure 5.
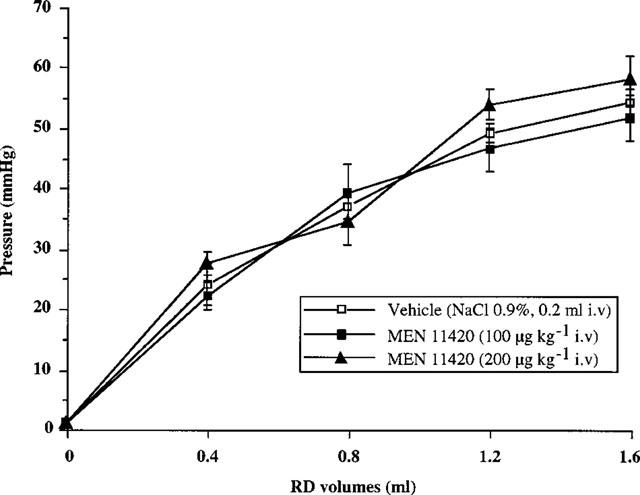
Intrarectal pressure response to increasing RD volumes: effect of MEN 11420 (100, 200 μg kg−1 i.v.) on rectal compliance in male rats (mean±s.e.mean; n=6).
Discussion
As it was described previously (Morteau et al., 1994b), rectal distension induces a volume-related increase in the occurrence of abdominal contractions, which are considered to be an index of visceral pain, since the distension threshold of the response is lowered after local inflammation (Morteau et al., 1994a) and stress (Gué et al., 1997). Our results indicate that the selective tachykinin NK2 receptor antagonist MEN 11420 (Catalioto et al., 1998), administered i.v. at doses of 5–100 μg kg−1, influences normal rectal sensitivity in basal conditions. This effect of MEN 11420 is not related to a change in rectal compliance. Previous studies have shown that, in the same rat model, the behavioural pain response to RD involves NK2 receptors (Julia et al., 1994). The possibility that MEN 11420 has a direct effect on the striated muscle of the abdomen–related to NK2 antagonist or other properties – cannot be suspected since toxicological studies have shown that in rats, in contrast to mice (Catalioto et al., 1998), no decrease in locomotor activity or any clinical sign of altered behaviour is observed at doses up to 50 mg kg−1 i.v. The present results demonstrate that the efficacy of MEN 11420 was enhanced after inflammation and stress. Considering the high selectivity of MEN 11420 for tachykinin NK2 receptors (Catalioto et al., 1998), we are confident that at all doses tested its effects are ascribable to blockade of tachykinin NK2 receptors (Lecci et al., 1997).
The induction of rectal inflammation by TNBS increases the sensitivity to rectal distension, which is evident from a reduced volume threshold for the abdominal contractions 3 days after rectal induction. This hypersensitivity is not related to a change in rectal compliance (Morteau et al., 1994a). The first main point in our findings is that the hypersensitivity to distension induced by rectocolonic inflammation can be partially or totally reversed by the tachykinin NK2 receptor antagonist (5–100 μg kg−1 i.v.). Our result is in agreement with a previous observation concerning inflammatory somatic pain in rats (Sluka et al., 1997). These authors showed that the development of heat hyperalgesia and pain-related behaviour ratings induced by acute inflammation (intraarticular injection of kaolin and carrageenan into the knee joint) are dose-dependently inhibited by systemic injection of a tachykinin NK2 receptor antagonist (SR48968). Our results denote variability of the effect of MEN 11420 for the highest volumes of distension, i.e. 1.2 ml and 1.6 ml before and after rectal inflammation. We suppose that this is linked to inter-individual variations, limiting for some volumes the significance of the results.
PRS increases the abdominal response to rectal distension (hypersensitivity), previously shown to be unrelated to rectal compliance (Gué et al., 1997). Interestingly, our results show that MEN 11420 (100–200 μg kg−1 i.v.) dose-dependently reduces or suppresses the stress-induced enhancement of abdominal contractions. Even though an anxiolytic effect of a tachykinin NK2 antagonist injected systemically has been suggested in one published work (Walsh et al., 1995), this activity was described for a treatment performed before the stress session. In our study, the test compound has been injected after the initiation of hyperalgesia by the stress session. Also, our results are the expression of an anti-hyperalgesic action of MEN 11420, rather than an anxiolytic effect, since MEN 11420 was administered after the stress session.
In this study, MEN 11420 reduces the rectal hyperalgesia induced by local inflammation and stress to a greater extent than it affects the basal sensitivity, suggesting that tachykinin NK2 receptor antagonists may not be considered as agents producing visceral analgesia, such as opioids, but rather as agents which correct visceral hyperalgesia.
Effect of MEN 11420 on inflammation-induced visceral hyperalgesia
The origin of inflammation-induced visceral hyperalgesia is not fully understood. There is mounting evidence that an unbalanced function of peptidergic neurons contributes to motor, secretory, vascular and immunological disturbances in inflammation (Holzer, 1998): transient mucosal inflammation may result in long-lasting sensitization of visceral afferent pathways containing neuropeptides such as tachykinins (Bernstein et al., 1996).
In principle, there are at least four basic mechanisms through which tachykinin NK2 receptor antagonists could be active in correcting visceral hyperalgesia associated with inflammation. First tachykinin NK2 receptor antagonists may act by reversing the immune/inflammatory changes produced by TNBS instillation: among others, these include activation of mast cells known to express tachykinin NK2 receptors which are involved in mast cell degranulation-induced histamine release (Krumins & Broomfield, 1992; Lilly et al., 1995).
Second, there is mounting evidence that the expression of NK2 receptors in primary afferents may be affected in inflammatory situations. Indeed, the expression of tachykinin NK2 receptors on vagal afferents is upregulated after induction of allergic reactions/inflammation (Weinreich et al., 1997; Moore et al., 1999), and we can speculate that after inflammation an upregulation of these receptors also occurs on spinal primary afferents innervating the rectum. Accordingly, McLean et al. (1997) presented evidence suggestive of up-regulation of tachykinin NK2 receptors in nematode post-infected rats, since the ED50 of SR 48968 in reducing a pseudoaffective response (increase in blood pressure due to jejunal distension), indicative of intestinal hyperalgesia was five times lower than needed to produce the same effect in control animals.
A third possibility may involve the occurrence of inflammation-related plastic changes in tachykinin release/tachykinin receptor expression at spinal cord level, resulting in a relatively more important role in NK2 receptors in the processing of nociceptive stimuli after induction of inflammation (Lecci et al., 1994).
Finally, the possibility of an anti-inflammatory effect of NK2 receptors cannot be neglected since in TNBS treated rats daily administration of SR 48968 (5 mg kg−1) reduces colonic inflammation (Mazelin et al., 1998). However, in our experiments, the NK2 receptor antagonist (5–100 μg kg−1) was administered acutely, just prior to distension and with a limited time to act as an anti-inflammatory agent.
Effect of MEN 11420 on visceral hyperalgesia induced by stress
Although the causes and mechanisms of irritable bowel syndrome (IBS) are not well understood, it is admitted that stress might contribute to visceral hypersensitivity because most patients with IBS suffer from increased levels of anxiety and psychological distress (Drossman et al., 1988). More recently, it has been demonstrated that IBS symptoms appear more frequently after severe infectious diarrhoea in stressed patients suggesting that stress and gut inflammation act synergistically to trigger gut hyperalgesia (Gwee et al., 1996).
As for TNBS-induced hyperalgesia, tachykinin NK2 receptors may act at different levels ranging from local immunocytes to the spinal cord level, in preventing stress-induced rectal hyperalgesia. Furthermore, the expression of tachykinin NK2 receptors has been reported at brain level and particularly in hypothalamic nuclei, a brain structure involved in the modulation of nociceptive messages from the gut in animals and humans (Dinan et al., 1990). Tachykinins, in addition to playing a role as nociceptive and pain transmitters in the spinal cord, may act in the brain as neurotransmitters and/or neuromodulators within the neuronal circuits mediating central responses to stress (Culman & Unger, 1995). Central NK2 receptors have been shown to participate in defence and stress reactions and their activation may trigger the release of central corticotrophin-releasing factor involved in mechanisms by which stress induces rectal hyperalgesia (Gué et al., 1997).
Difference in efficacy and site of action
The difference in the range of active doses of MEN 11420 in the TNBS and stress models, led us to speculate that the tachykinin NK2 receptor antagonist might act preferentially at the periphery to suppress TNBS-induced allodynia, and at the central level to suppress stress-induced altered visceral sensitivity. In fact, the higher efficacy of MEN 11420 (lower active dose) in the TNBS model, compared to the stress model, may be related to different levels of the implication of tachykinin NK2 receptors: peripheral sensitization of primary afferents or facilitation at the spinal cord level in the TNBS model and short-term sensitization modulated by brain pathways in the stress-induced hypersensitivity.
However, our results do not enable us to affirm whether the effects of MEN 11420 are produced centrally or peripherally (or at both levels), since multiple sites of actions and mechanisms could account for the ability of this NK2 receptor antagonist to modulate rectal hypersensitivity induced by inflammation or stress.
Role of tachykinin NK2 receptor antagonists in IBS
Our experiments have clearly shown the involvement of tachykinin NK2 receptors in the visceral hyperalgesia induced by both inflammation and stress. This involvement is not fully selective for hyperalgesia states. However, the greater efficacy of NK2 receptor antagonists in these circumstances led us to speculate that the role of NK2 receptors becomes more important during visceral hyperalgesia, which is in keeping with previous observations made in Nippostrongylus brasiliensis post-infected rats (McLean et al., 1997).
Whatever the mechanism involved, the present data strongly support the concept that tachykinin NK2 receptor antagonists such as MEN 11420 can be viewed as promising agents in the treatment of functional gastrointestinal disorders characterized by visceral pain, such as IBS.
Acknowledgments
The authors thank Isabelle Dupré for her helpful technical assistance, and Ecole Supérieure d'Agriculture de Purpan (ESAP), and Menarini Richerche SpA for their financial contribution to this work.
Abbreviations
- EMG
electromyographic
- IBS
irritable bowel syndrome
- i.p.
intraperitoneal
- i.v.
intravenous
- NKA
neurokinin A
- PRS
partial restraint stress
- RD
rectal distension
- SP
substance P
- TNBS
trinitrobenzenesulphonic acid
References
- ANTON P.A., SHANAHAN F. Neuroimmunomodulation in inflammatory bowel disease. How far from ‘bench' to ‘bedside'. Ann. N.Y. Acad. Sci. 1998;840:723–734. doi: 10.1111/j.1749-6632.1998.tb09611.x. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN C.N., NIAZI N., ROBERT M., MERTZ H., KODNER A., MUNAKATA J., NALIBOFF B., MAYER E.A. Rectal afferent function in patients with inflammatory and functional intestinal diseases. Pain. 1996;66:151–161. doi: 10.1016/0304-3959(96)03062-x. [DOI] [PubMed] [Google Scholar]
- CATALIOTO R.M., CRISCUOLI M., CUCCHI P., GIACHETTI A., GIANNOTTI S., GIULIANI S., LECCI A., LIPPI A., PATACCHINI R., QUARTARA L., RENZETTI A.R., TRAMONTANA M., ARCAMONE F., MAGGI C.A. MEN 11420 (Nepadutant) a novel glycosylated bicyclic peptide tachykinin NK-2 receptor antagonist. Br. J. Pharmacol. 1998;123:81–91. doi: 10.1038/sj.bjp.0701587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CULMAN J., UNGER T. Central tachykinins: mediators of defense reaction and stress reactions. Can. J. Physiol. Pharmacol. 1995;73:885–891. doi: 10.1139/y95-122. [DOI] [PubMed] [Google Scholar]
- DINAN T.G., BARRY S., AHKION S., CHUA A., KEELING P.W. Assessment of central noradrenergic functioning in irritable bowel syndrome using a neuroendocrine challenge test. J. Psychosom. Res. 1990;34:575–580. doi: 10.1016/0022-3999(90)90032-y. [DOI] [PubMed] [Google Scholar]
- DROSSMAN D.A., MCKEE D.C., SANDLER R.S., MITCHELL C.M., CRAMER E.M., LOWMAN B.C., BURGER A.L. Psychological factors in the irritable bowel syndrome. A multivariate study of patients and non patients with irritable bowel syndrome. Gastroenterol. Clin. Biol. 1988;95:701–708. doi: 10.1016/s0016-5085(88)80017-9. [DOI] [PubMed] [Google Scholar]
- ERCKENBRECHT J.F., HOEREN C., SKODA G., ENCK P., BERGES W., WIENBECK M. Differential effects of mental and physical stress on perception of gastrointestinal distension. Gastroenterology. 1988;94:A115. [Google Scholar]
- EYSSELEIN V.E., NAST C.C. Neuropeptides and inflammatory bowel disease. Z. Gastroenterol. Verh. 1991;26:253–257. [PubMed] [Google Scholar]
- FORD M.J., CAMILLERI M., ZINSMEISTER A.R., HANSON R.B. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology. 1995;109:1772–1780. doi: 10.1016/0016-5085(95)90743-2. [DOI] [PubMed] [Google Scholar]
- GUÉ M., DELRIO-LACHÈZE C., EUTAMÈNE H., THÉODOROU V., MORÉ J., FIORAMONTI J., BUÉNO L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol. Mot. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- GWEE K.A., GRAHAM J.C., MCKENDRICK M.W., COLLINS S.M., MARSHALL J.S., WALTERS S.J., READ N.W. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion. 1998;59:269–283. doi: 10.1159/000007504. [DOI] [PubMed] [Google Scholar]
- HOLZER P., HOLZER-PETSCHE U. Tachykinins in the gut. Part II. Roles in neural excitation, secretion and inflammation. Pharmacol. Ther. 1997;73:219–263. doi: 10.1016/s0163-7258(96)00196-9. [DOI] [PubMed] [Google Scholar]
- JOOS G.F., PAUWELS R.A. The in vivo effect of tachykinins on airway mast cells of the rat. Am. Rev. Respir. Dis. 1993;148:922–926. doi: 10.1164/ajrccm/148.4_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- JULIA V., MORTEAU O., BUÉNO L. Involvement of neurokinin 1 and 2 receptors in viscerosensitive response to rectal distension in rats. Gastroenterology. 1994;107:94–102. doi: 10.1016/0016-5085(94)90065-5. [DOI] [PubMed] [Google Scholar]
- KRUMINS S.A., BROOMFIELD C.A. Evidence of NK1 and NK2 tachykinin receptors and their involvement in histamine release in a murine mast cell line. Neuropeptides. 1992;21:65–72. doi: 10.1016/0143-4179(92)90516-y. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., SANTICIOLI P., MAGGI C.A. Involvement of spinal tachykinin NK-1 and NK-2 receptors in detrusor hyperreflexia during chemical cystisis in anaesthetized rats. Eur. J. Pharmacology. 1994;259:129–135. doi: 10.1016/0014-2999(94)90501-0. [DOI] [PubMed] [Google Scholar]
- LECCI A., TRAMONTANA M., GIULIANI S., MAGGI C.A. Role of tachykinin NK-1 and NK-2 receptors on colonic motility in anesthetized rats: effect of agonists. Can. J. Physiol. Pharmacol. 1997;75:552–557. [PubMed] [Google Scholar]
- LILLY C.M., HALL A.E., RODGER I.W., KOBZIK L., HALEY K.J., DRAZEN J.M. Substance P-induced histamine release in tracheally perfused guinea pig lungs. J. Appl. Physiol. 1995;78:1234–1241. doi: 10.1152/jappl.1995.78.4.1234. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinin as peripheral modulators of primary afferent nerves and visceral sensitivity. Pharmacol. Res. 1997;36:153–169. doi: 10.1006/phrs.1997.0219. [DOI] [PubMed] [Google Scholar]
- MAZELIN L., THEODOROU V., MORE J., EDMONDS-ALT X., FIORAMONTI J., BUENO L. Comparative effects of nonpeptide tachykinin receptor antagonists on experimental gut inflammation in rats and guinea-pigs. Life. Sci. 1998;63:293–304. doi: 10.1016/s0024-3205(98)00271-9. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., PICARD C., GARCIA-VILLAR R., MORÉ J., FIORAMONTI J., BUÉNO L. Effects of nematode infection on sensitivity to intestinal distension: Role of tachykinins NK2 receptors. Eur. J. Pharmacol. 1997;337:279–282. doi: 10.1016/s0014-2999(97)01275-2. [DOI] [PubMed] [Google Scholar]
- MÉTIVIER S., DELVAUX M., LOUVEL D., LAGIER E., FIORAMONTI J., BUÉNO L., FREXINOS J. Influence of stress on sensory threshold to rectal distension in healthy volunteers. Gastroenterology. 1996;110:A717. [Google Scholar]
- MOORE K.A., TAYLOR G.E., WEINREICH D. Serotonin unmasks functional NK2 receptors in vagal sensory neurons of the guinea-pig. J. Physiol. 1999;514:111–124. doi: 10.1111/j.1469-7793.1999.111af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTEAU O., HACHET T., CAUSSETTE M., BUÉNO L. Experimental colitis alters visceromotors response to colorectal distension in awake rats. Dig. Dis. Sci. 1994a;39:1239–1248. doi: 10.1007/BF02093789. [DOI] [PubMed] [Google Scholar]
- MORTEAU O., JULIA V., EECKHOUT C., BUÉNO L. Influence of 5-HT3 receptor antagonists in visceromotor and nociceptive responses to rectal distension before and during experimental colitis in rats. Fundam. Clin. Pharmacol. 1994b;8:553–562. doi: 10.1111/j.1472-8206.1994.tb00837.x. [DOI] [PubMed] [Google Scholar]
- NESS T.J., RANDICH A., GEBHART G.F. Further behavioral evidence that colorectal distension is a ‘noxious' visceral stimulus in rats. Neurosci. Lett. 1991;131:113–116. doi: 10.1016/0304-3940(91)90349-x. [DOI] [PubMed] [Google Scholar]
- PORRO C.A., CARLI G. Immobilization and restraint effects on pain reactions in animals. Pain. 1988;32:289–307. doi: 10.1016/0304-3959(88)90041-3. [DOI] [PubMed] [Google Scholar]
- RAO S.C., READ N.W., DAVISON P.A., BANNISTER J.J., HOLDSWORTH C.D. Anorectal sensitivity and responses to rectal distension in patients with ulcerative colitis. Gastroenterology. 1987;93:1270–1275. doi: 10.1016/0016-5085(87)90255-1. [DOI] [PubMed] [Google Scholar]
- RUCKEBUSCH M., FIORAMONTI J. Electrical spiking activity and propulsion in small intestine in fed and fasted rats. Gastroenterology. 1975;68:1500–1508. [PubMed] [Google Scholar]
- SABAN M.R., SABAN R., BJORLING D.E. Kinetics of peptide-induced release of inflammatory mediators by the urinary bladder. Br. J. Urol. 1997;80:742–747. doi: 10.1046/j.1464-410x.1997.00415.x. [DOI] [PubMed] [Google Scholar]
- SLUKA K.A., MILTON M.A., WILLIS W.D., WESTLUND K.N. Differential roles of neurokinin 1 and neurokinin 2 receptors in the development and maintenance of heat hyperalgesia induced by acute inflammation. Br. J. Pharmacol. 1997;120:1263–1273. doi: 10.1038/sj.bjp.0701044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSH D.M., STRATTON S.C., HARVEY F.J., BERESFORD I.J., HAGAN R.M. The anxiolytic-like activity of GR159897, a non-peptide NK2 receptor antagonist, in rodent and primate models of anxiety. Psychopharmacology. 1995;121:186–191. doi: 10.1007/BF02245629. [DOI] [PubMed] [Google Scholar]
- WEINREICH D., MOORE K.A., TAYLOR G.E. Allergic inflammation in isolated vagal sensory ganglia unmasks silent NK2 tachykinin receptors. J. Neurosci. 1997;17:7683–7693. doi: 10.1523/JNEUROSCI.17-20-07683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C.L., VILLAR R.G., PETERSON J.M., BURKS T.F. Stress-induces changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–621. doi: 10.1016/0016-5085(88)90231-4. [DOI] [PubMed] [Google Scholar]


