Abstract
The present study investigated whether or not there may be differences in the direct cardiac actions of the novel, highly β1-selective adrenoceptor antagonist nebivolol (NEB) in comparison to metoprolol (MET), bisoprolol (BIS), carvedilol (CAR) and bucindolol (BUC) in human myocardium (n=9).
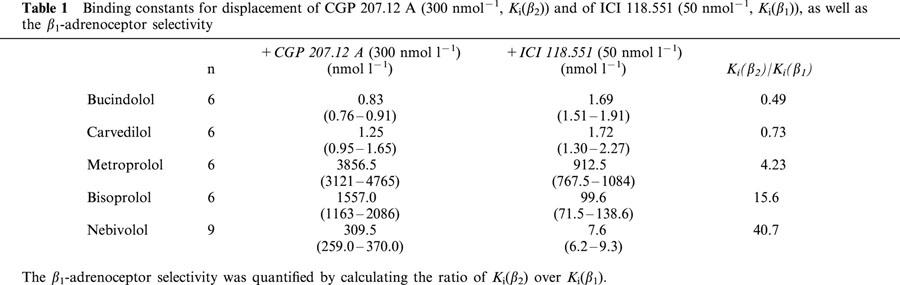
The rank order of β1-selectivity as judged by competition experiments to 3H-CGP 12.1777 in the presence of CGP 207.12 A (300 nmol l−1, Kiβ2) or ICI 118.551 (50 nmol l−1, Kiβ1) were NEB(Kiβ2/Kiβ1: 40.7)>BIS(15.6)>MET(4.23)>CAR(0.73)>BUC(0.49).
The rank order of the negative inotropic potency of the β-adrenoceptor antagonists measured in left ventricular trabeculae (dilated cardiomyopathy, DCM) as judged by the concentration needed to induce a 50% decrease in isoprenaline (1 μmol l−1)-stimulated force (IC50) was: MET (0.6 μmol l−1)>CAR (4.1 μmol l−1)>NEB (7.0 μmol l−1).
NEB, BUC, MET and CAR did not not exert an intrinsic sympathomimetic activity (ISA) as determined by measurements of force development in forskolin (0.3 μmol l−1) pre-treated left ventricular trabeculae, nor by measuring adenylate cyclase activity in forskolin (0.3 μmol l−1)-stimulated assays (crude membranes). This also holds true for radioligand binding assays with or without guanine nucleotide guanyl-5′-yl imidodiphosphate (Gpp(NH)p).
Although all studied β-adrenoceptor antagonists lack intrinsic sympathomimetic activity (ISA), they differ in the β1-selectivity as well as in their direct negative inotropic action. These differences as well as the mode of extracardiac action may have an impact on outcome of patients treated with β-adrenoceptor antagonists.
Keywords: Nebivolol, bucindolol, carvedilol, metoprolol, bisoprolol, heart failure, human myocardium, intrinsic sympathomimetic activity, β1-selectivity
Introduction
It has been shown by clinical trials that β-adrenoceptor antagonists (βAAs) improve survival of patients suffering from heart failure (MERIT-HF Study Group, 1999; CIBIS II Investigators and Committees, 1999; Packer et al., 1996). Whether the beneficial effects hold true for all βAAs in an ongoing matter of debate, since the βAA bucindolol improved symptoms but did not improve outcome of heart failure patients (BEST Steering Committee, 1995; Bristow, 2000). In addition, it is unclear, whether or not differences in the direct cardiac action have an impact on prognostic benefit of βAAs or not. On one hand, this has been due to their negative inotropic and bronchiconstructive effects via inhibition of β2-adrenoceptors. On the other hand, it has been shown previously that βAAs with intrinsic sympathomimetic activity (ISA, e.g. xamoterol (Schwinger et al., 1990b)) are contraindicated in human heart failure, because of the detrimental increase in heart rate (The Xamoterol in Severe Heart Failure Study Group, 1990). In contrast, βAAs with high inverse agonistic efficacy may cause receptor up-regulation (Milligan et al., 1995), and may therefore contribute to the restoration of the blunted β-adrenoceptor adenylate cyclase system in human heart failure. This holds true for metoprolol, whereas carvedilol does not lead to receptor up-regulation (Gilbert et al., 1996). Thus, when treating patients with already compromised left ventricular function, it may be wise to use a βAA with minor cardiodepressant effects, high β1-selectivity and no ISA.
The novel βAA nebivolol increases survival in cardiomyopathic hamsters with congestive heart failure (Ver Donck et al., 1991). Due to the altered β-adrenoceptor-adenylate cyclase coupling in failing human myocardium (e.g. downregulation of the β-adrenoceptors (Bristow et al., 1982; 1986; Schwinger et al., 1990a; 1991); upregulation of Gi (Feldman et al., 1988); up-regulation of the β-adrenoceptor kinase (Ungerer et al., 1993), the inotropic responsiveness of βAAs in failing myocardium might be different from that in animal models. Therefore, the present study investigates the direct cardiac effects on force of contraction, β1-selectivity and ISA of the βAA nebivolol in comparison to metoprolol and carvedilol. The non-selective βAA bucindolol (Hershberger et al., 1990) and the β1-selective βAA bisoprolol were also studied. The chemical structures of the different β-adrenoceptor antagonists are shown in Figure 1.
Figure 1.
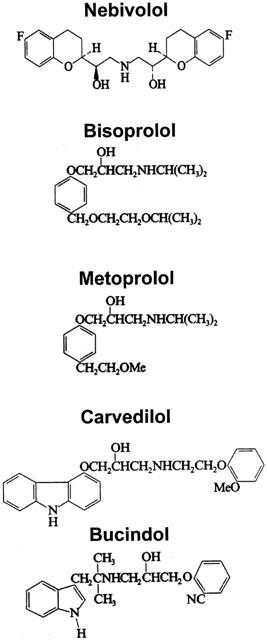
Chemical structures of the different β-adrenoceptor antagonists.
Methods
Myocardial tissue
Human left ventricular trabeculae were obtained from seven non-failing donor hearts (mean age: 54±4 years, one woman, six men) that could not be transplanted for technical reasons and from five hearts with end-stage heart failure due to dilated cardiomyopathy at the time of heart transplantation (mean age: 52±6 years, one woman, four men). None of the donors had a history of heart disease and all had normal left ventricular function as measured by the attending cardiologist. Mean ejection fraction of the heart failure group was 27.3±3.3%, mean cardiac index 2.1±0.1 l min m−2, mean left ventricular end-diastolic pressure 16.2± 2.4 mmHg. Medication consisted of nitrates, diuretics, angiotensin-converting enzyme inhibitors, and digoxin. None of the patients have received Ca2+ channel antagonists or Ca2+ channel agonists within 7 days of surgery, or β-adrenoceptor agonists 48 h before surgery. Drugs used for general anaesthesia were flunitrazepam, fentanyl, and pancuronium bromide with isoflurane. The investi- gation conforms with the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee.
Contraction experiments
The experiments were performed on isolated, electrically driven, isometrically contracting muscle preparations. The experiments were performed as previously described (Schwinger et al., 1990b).
β1- and β2-adrenoceptor binding studies
Membrane preparation
For the investigation of the β1-adrenoceptor selectivity, human non-failing left ventricular myocardium was used. Due to the described β1-adrenoceptor downregulation in human failing myocardium (Bristow et al., 1982; 1986; Schwinger et al., 1990a; 1991), failing left ventricular cardiac tissue may not be suitable for the determination of β1-adrenoceptor selectivity. The tissue of the left ventricle from human non-failing mycardium were chilled in 15 ml ice-cold homogenization buffer (in mmol l−1: Tris/HCl 40, EDTA 1, dithiotreitol 1, pH 8.0). Connective tissue was trimmed away and myocardial tissue was minced with scissors and homogenized with a motor-driven glass-teflon potter for 1 min. Afterwards, the crude membrane preparation was homogenized by hand for 1 min with a glass-glass potter. The homogenate was centrifuged at 480 g for 15 min. The supernatant was diluted with an equal volume of ice-cold 1 M KCl, stored on ice for 10 min, and then centrifuged at 100,000×g for 45 min. The pellet was re-suspended in incubation buffer (in mmol l−1: Tris-HCl 50, MgCl2 10, pH 7.4), and homogenized for 1 min with a glass-glass potter. This suspension was centrifuged at 100,000×g for 45 min. The pellet was finally re-suspended in incubation buffer and stored at −80°C.
Radioligand binding assay
β-adrenoceptors in cardiac tissue homogenates were investigated using 3H-CGP 12.177 [(−)-4-(3-t-butylamino-2-hydroxy-propoxy)-(5,7-3H) benzimidazol-2-one)] as the radiolabelled ligand (specific activity 50 Ci mmol l−1). Specific binding was determined as the difference of binding in the absence and presence of 10 μmol l−1 DL-propranolol β-adrenoceptor subtypes were determined by competition experiments using the β1-selective antagonist CGP 207.12 A (0.3 μmol l−1) and the β2-selective antagonist ICI 118.551 (0.05 μ mol l−1). β2 and β1 ratio was calculated as described previously (de lean et al., 1982). Experiments were performed as described previously (Schwinger et al., 1991).
Adenylate cyclase activity
Particulate membrane fractions from human non-failing hearts were obtained as described in the preparation for cardiac β1- and β2-receptors except that after the first centrifugation step at 100,000×g, the pellet was resuspended in a hypotonic medium (in mmol l−1: ATP 2.5, MgCl2 2.5, KHCO3 1, and Tris/HCl 2, pH 7.4) and that it was finally resuspended in KHCO3 (1 mmol l−1). Adenylate cyclase activity was determined as described previously (Schmidt et al., 1995).
Guanine-nucleotide modulated binding
Particulate membrane fractions from human non-failing hearts were obtained as described in the preparation for cardiac β1- and β2-adrenoceptors. A competition curve of the βAAs was performed as described above (radioligand binding assay) in the presence and in the absence of a high concentration of the non-hydrolysable guanine nucleotide guanyl-5′-yl imidodiphosphate (Gpp(NH)p, 30 μmol l−1). Experiments were performed as described previously (Schmidt et al., 1995; Bristow et al., 1992).
Materials
Nebivolol was generously provided by Berlin-Chemie AG, Berlin, Germany; metoprolol by Astra GmbH, Wedel, Germany; carvedilol by Boehringer Mannheim, Mannheim, Germany; bisoprolol by Merck, Darmstadt, Germany. Bucindolol was kindly given by Knoll AG, Mannheim, Germany and CGP 20712 by Novartis, Basel, Switzerland. 3H-CGP 12.177 was obtained from Amersham, Braunschweig, Germany, and ICI 118.551 from Tocris, Bristol, U.K.
Statistics
All values are mean±s.e.mean. Statistical significance was analysed with the student's t-test for unpaired or paired observations as well as by using analysis of variance followed by a protected t-test (Bonferroni) or paired t-test.
Results
β1-selectivity
To study the β1-selectivity of nebivolol, carvedilol and metoprolol, competition experiments to 3H-CGP 12.1777 binding were performed in the presence of ICI 118.551 (50 nmol l−1) in order to obtain a homogeneous population of β1-adrenoceptors, as well as in the presence of CGP 207.12 A (300 nmol l−1) to determine competition of β2-adrenoceptors. The non-selective βAA bucindolol and the β1-selective βAA bisoprolol were studied for comparison.
Figure 2 shows competition curves obtained with nebivolol (upper part, A), bisoprolol (middle part, left side, B), metoprolol (middle part, right side, C), bucindolol (lower part, left side, D) and carvedilol (lower part, right side, E) in crude membrane preparations of human non-failing left ventricular myocardium. Figure 3 summarizes the results. Nebivolol exerts the highest β1-selectivity in human myocardium, displaying a 40.7-fold selectivity ratio (Table 1). Metoprolol and bisoprolol also displayed β1-selectivity but less than nebivolol. Bucindolol and carvedilol were not selective for β1-adrenoceptors (Table 1).
Figure 2.
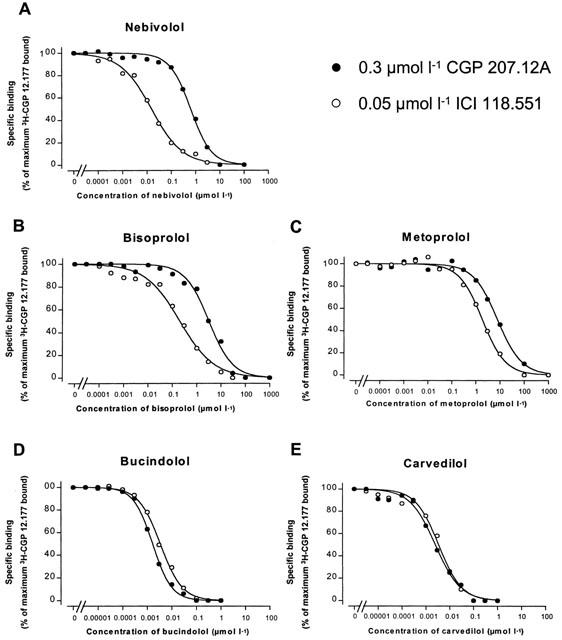
β-adrenoceptor selectivity. Experiments with nebivolol (A), bisoprolol (B), metoprolol (C), bucindolol (D) and carvedilol (E) were performed in the presence of 0.3 μmol l−1 CGP 207.12A or 0.05 μmol l−1 ICI 118.551 in order to obtain competition at a homogeneous population of β2- or β1-adrenoceptors on left ventricular myocardial membranes obtained from human myocardium.
Figure 3.
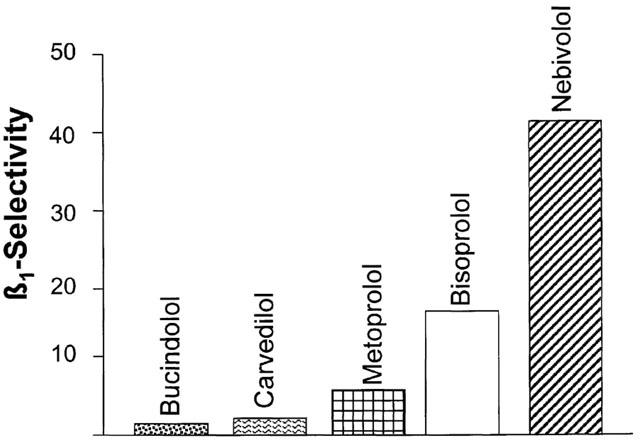
Rank order of β1-adrenoceptor selectivity of nebivolol, bisoprolol, metoprolol, bucindolol and carvedilol.
Table 1.
Binding constants for displacement of CGP 207.12 A (300 nmol−1, Ki(β2)) and of ICI 118.551 (50 nmol−1, Ki(β1)), as well as the β1-adrenoceptor selectivity
Inhibition of β-agonist-stimulated muscle contraction
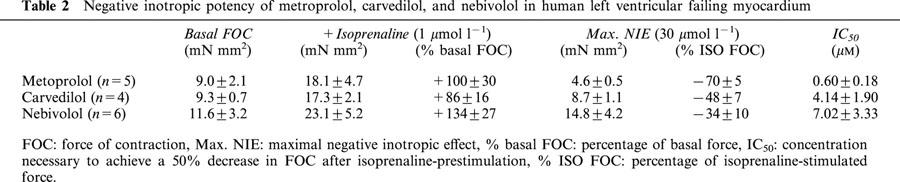
Figure 4 shows original tracings of the force of contraction under basal conditions, after application of isoprenaline (1 μmol l−1), and after application of a low (0.3 μmol l−1) and high (10 μmol l−1) concentration of the βAAs nebivolol (upper panel), carvedilol (middle panel) and metoprolol (lower panel). Figure 5 gives the percentage changes of isoprenaline pre-stimulated force of contraction obtained in human left ventricular failing myocardium after application of the different βAAs. Basal as well as isoprenaline (1 μmol l−1)-stimulated force of contraction measured at a stimulation frequency of 1 Hz and an extracellular Ca2+-concentration of 1.8 mM was similar in all groups (Table 2). The rank order of negative inotropic efficacy as judged from the negative inotropic effect on isoprenaline pre-stimulated force of contraction measured at 30 μM of the respective β-blockers was: metoprolol>carvedilol ⩾nebivolol (Table 2). By analysing the concentration necessary to induce a 50% decrease on isoprenaline pre-stimulated force of contraction (IC50) the following rank of negative inotropic potency order was obtained: metoprolol⩾carvedilol>nebivolol (Table 2).
Figure 4.
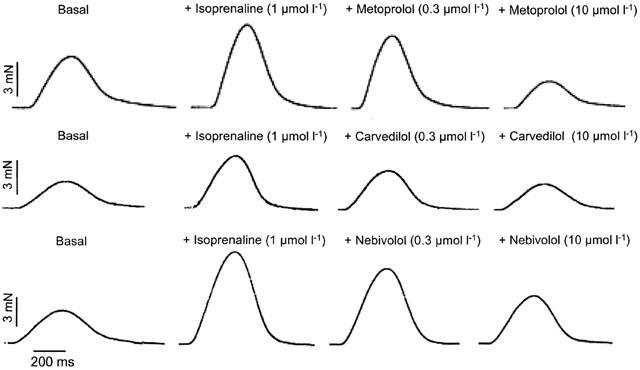
Original tracings illustrating the effect of a low (0.3 μmol l−1) and high (10 μmol l−1) concentration of metoprolol, carvedilol and nebivolol in isoprenaline (1 μmol l−1) pre-stimulated isolated, electrically driven (1 Hz) trabeculae of human failing myocardium.
Figure 5.
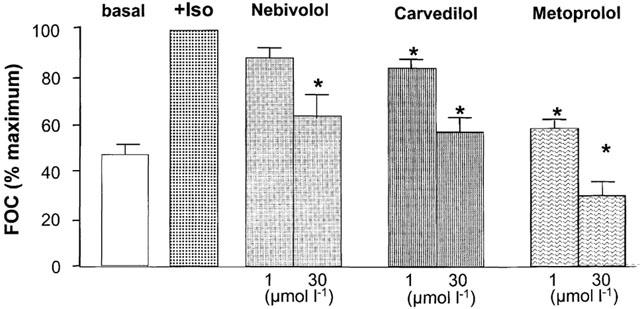
Percentage changes of isoprenaline pre-stimulated force of contraction obtained in human left ventricular failing myocardium after application of metoprolol, carvedilol and nebivolol.
Table 2.
Negative inotropic potency of metroprolol, carvedilol, and nebivolol in human left ventricular failing myocardium
Intrinsic sympathomimetic activity
To functionally study the ISA of metoprolol, carvedilol, bucindolol, and nebivolol in human myocardium, cumulative concentration-response curves were obtained measuring force of contraction in isolated trabeculae of human non-failing hearts in the presence of forskolin (Figure 6). Forskolin has been shown to be a tool for the detection of ISA mediated by βAAs (Jasper et al., 1988). In isolated left ventricular trabeculae of human non-failing left ventricular myocardium, application of forskolin (0.3 μmol l−1) induced similar positive inotropic effects in all groups studied (nebivolol-group: +38±4%, n=5; metoprolol-group: +40±5%, n=5; carvedilol-group: +35±6%, n=4; bucindolol-group: +39± 8%, n=5). Neither metoprolol, nor carvedilol, bucindolol or nebivolol increased force of contraction after forskolin pretreatment, indicating that none of the βAAs studied possessed ISA.
Figure 6.
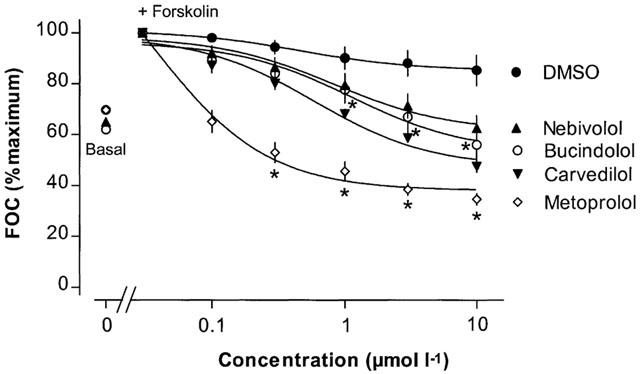
Concentration-response curves for the cardiac effects of the β-adrenoceptor antagonists metoprolol, carvedilol and nebivolol in human left ventricular non-failing myocardium after pre-stimulation with forskolin.
The ISA of nebivolol in comparison to bucindolol was also studied in crude membrane preparations of human non-failing left ventricular myocardium by measuring the adenylate cyclase activity after pre-stimulation with forskolin (0.3 μmol l−1). Bucindolol (10−10–10−5 mol l−1) as well as nebivolol (10−9–10−4 mol l−1) did not change adenylate cyclase activity under these conditions (Figure 7).
Figure 7.
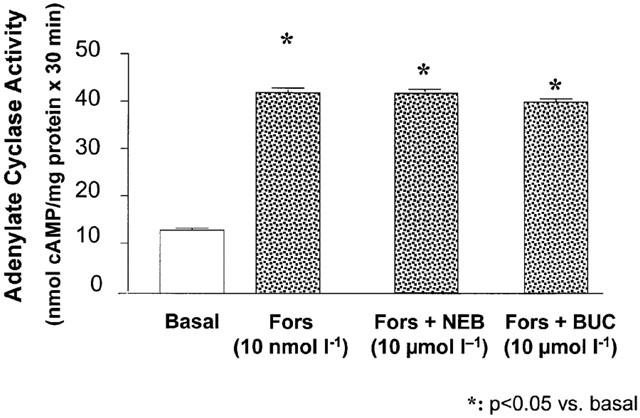
Influence of bucindolol and nebivolol on adenylate cyclase activity in crude membrane preparations of human left ventricular non-failing myocardium.
To further investigate whether the βAAs were capable of binding to the agonist binding site and inducing ISA, radioligand binding experiments with the β1-selective agonist 3H-CGP 12.177 (0.6 nmol l−1) were performed in the presence as well as in the absence of Gpp(NH)p (30 μmol l−1) in crude membrane preparations of human non-failing myocardium. Gpp(NH)p is able to induce alterations in the β-adrenoceptors when the receptors are coupled to the adenylate cyclase by virtue of agonist binding resulting in a rightward shift of the binding displacement curve. Figure 8 presents the results obtained for the guanine nucleotide modulated binding studies for nebivolol (Figure 8B), carvedilol (Figure 8C), and bucindolol (Figure 8D). The β1-adrenoceptor agonist isoprenaline was studied for comparison (Figure 8A). Only the binding displacement curve of isoprenaline was shifted to the right in the presence of Gpp(NH)p (Figure 8A). Thus, all experiments investigating whether nebivolol, metoprolol, carvedilol, and bucindolol possess β-adrenoceptor agonistic moiety demonstrate that these compounds are devoid of intrinsic sympathomimetic properties.
Figure 8.
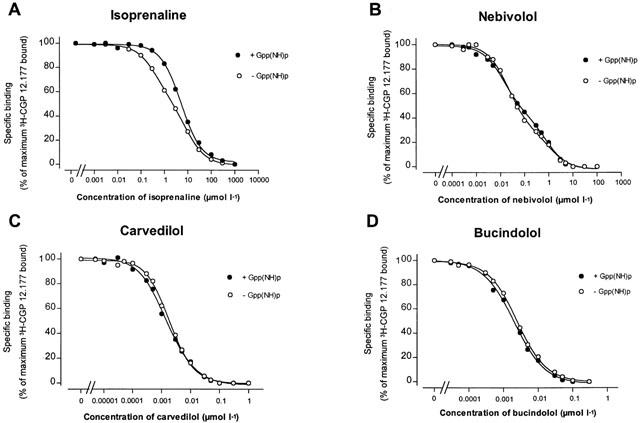
Effect of Gpp(NH)p on 3H-CGP 12.177 binding experiments in the presence of isoprenaline, nebivolol, carvedilol and bucindolol in human non-failing left ventricular myocardium.
Discussion
Some, but not all βAAs improve symptoms and prolong survival of heart failure patients (MERIT-HF Study Group, 1999; CIBIS II Investigators and Committees, 1999; Packer et al., 1996). Initiation of βAA therapy is often limited by worsening congestive heart failure, which may manifest as a decrease in haemodynamics (Kukin et al., 1999) or by β2-mediated bronchoconstriction. Therefore, low doses of the βAA are a necessity, especially in the beginning of a β-adrenoceptor blocker treatment. Thus, it is important to know whether βAAs differ in their inotropic action. The present study investigated the direct cardiac effects of the βAAs nebivolol, metoprolol, carvedilol, bucindolol and bisoprolol in human myocardium.
Nebivolol was the βAA with the highest β1-selectivity in human myocardium as compared with bisoprolol and metoprolol. A high β1-selectivity may be of special benefit for the βAA-treatment of heart failure patients due to a reduction of undesired side-effects (e.g. bronchoconstriction). More importantly, recent studies have shown that blockade with β1-adrenoceptor-selective antagonists improves survival in patients with congestive heart failure (MERIT-HF Study Group, 1999; CIBIS II Investigators and Committees, 1999). It is not yet clear whether this also holds true for non-selective βAAs. As shown in the BEST trial, bucindolol produced a non-significant reduction of 10% in total mortality (BEST Steering Committee, 1995; Bristow, 2000). The question whether non-selectivity of carvedilol is critical to its benefit in chronic heart failure patients is currently being addressed in the Carvedilol and Metoprolol European Trial (COMET), comparing metoprolol and carvedilol. The influence of nebivolol-treatment on heart failure is currently being investigated in the SENIOR trial.
β1-selectivity did not correlate with the negative inotropic potency or efficacy of the studied βAAs. The negative inotropic efficacy and potency of metoprolol, for example, is much more pronounced than that of carvedilol, although metoprolol is a β1-selective, and carvedilol a non-selective βAA. Therefore, especially for the βAAs of the second and third generation, the direct cardiac action of these drugs may also be critically dependent on the additional mode of action provoked by these βAAs. Application of metoprolol, for example, has been shown to block the calcium current in ventricular myocytes of guinea-pigs (Sanchez-Chapula, 1992). Accordingly, initial application of metoprolol given in patients receiving background triple therapy for mild to severe heart failure produced significant decreases in cardiac output, cardiac index and stroke volume (Michel et al., 1988). The minor cardio-depressant effects of nebivolol are in agreement with the favourable effects of nebivolol on left ventricular function seen in patients with dilated heart disease (Wisenbaugh et al., 1993) and may be of importance for the therapeutical benefit in heart failure patients.
It has been shown recently that βAAs with ISA, like xamoterol, are contraindicated in human heart failure, because of the detrimental increase in heart rate (The Xamoterol in Severe Heart Failure Study Group, 1990). Previous studies showed that patients treated with β1-selective antagonists without ISA had more adrenoceptors than control subjects (Motomura et al., 1990) and that the adenylate cyclase activation by the β-adrenoceptor agonist isoprenaline is also enhanced in this situation (Trochu et al., 1999). The ISA is therefore an important criterion for the therapeutical usefulness of βAAs in heart failure patients. In the present study no ISA was found for bucindolol and carvedilol in crude membrane preparations of human ventricular myocardium, which is in agreement with previous studies in rat as well as in human myocardium (Hershberger et al., 1990; Bristow et al., 1992). In human atrial myocardium, however, small amounts of partial agonism of bucindolol could be detected after the muscle had been depleted of catecholamines (Trochu et al., 1999). These variable results may be due to the use of different experimental setups, with differences in the basal activation states of the β-adrenoceptors in the respective tissues (Schwinger et al., 1990b). Nebivolol also did not reveal ISA in the present study, which is in line with experiments in reserpinized dogs and spontaneously hypertensive rats. Thus, besides its high β1-adrenoceptor selectivity and its small negative inotropy, the lack of ISA may be of special benefit for the therapeutical use of nebivolol in human heart failure.
Limitation of the study
The present study was performed under in vitro conditions, experiments were either performed on isolated trabeculae or on crude membrane preparations of human heart. It cannot be excluded that in the in vivo effects of the studied βAAs may differ from those observed in vitro. Due to the altered β-adrenoceptor-adenylate cyclase coupling, this study of the cardiac effects of βAAs may be of advantage compared to those performed in various animal models.
Conclusions
The cardiac effects of βAAs are varying in human cardiac tissue. The cardiodepressant effects of a βAA may not correlate with its β1-selectivity but result from the combined effects of β1-selectivity, intrinsic sympathomimetic properties and inverse agonism. The pharmacodynamical profile of nebivolol in human myocardium (high β1-selectivity, lack of ISA and inverse agonistic activity) may be favourable for the treatment of heart failure patients. In consequence, whether or not nebivolol improves symptoms and outcome in patients with heart failure will be studied in a placebo controlled trial (SENIORS Study).
Acknowledgments
We are indebted to all colleagues of the Departments of Cardiothoracic Surgery of the Universities of Cologne and Munich for providing us with human myocardial samples. This work contains data from the doctoral thesis of A. Bundkirchen. Experimental work was supported by the Deutsche Forschungsgemeinschaft (DFG, Dr Schwinger) and the Leslee Fortune Project (Drs Schwinger and Brixius).
Abbreviations
- βAA
β-adrenoceptor antagonists
- BIS
bisoprolol
- BUC
bucindolol
- CAR
carvedilol
- Gpp(NH)p
guanine nucleotide guanyl-5′yl-imidodiphosphate
- ISA
intrinsic sympathomimetic activity
- MET
metoprolol
- NEB
nebivolol
References
- BEST STEERING COMMITTEE Design of the Beta-Blocker Evaluation Survival Trial (BEST) Am. J. Cardiol. 1995;75:1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R. β-Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., GINSBURG R., MINOBE W., CUBICIOTTI R.S., SAGEMAN W.S., LURIE K., BILLINGHAM M.E., HARRISON D.E., STINSON E.B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in the failing human hearts. N. Engl. J. Med. 1982;302:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., GINSBURG R., UMANS V., FOWLER M., MINOBE W., RASMUSSEN R., ZERA P., MENLOVE R., SHAH P., JAMIESON S. Beta1- and beta2-adrenergic receptor subpopulation in nonfailing and failing human myocardium: coupling of both receptor subtypes to muscle contraction and selective beta1-receptor downregulation in heart failure. Circ. Res. 1986;56:2987–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., LARRABEE P., MINOBE W., RODEN R., SKERL L., KLEIN J., HANDWERGER D., PORT J.D., MÜLLER-BECKMANN B. Receptor pharmacology of carvedilol in the human heart. J. Cardiovasc. Pharmacol. 1992;19 Suppl. 1:S68–S80. doi: 10.1097/00005344-199219001-00014. [DOI] [PubMed] [Google Scholar]
- CIBIS II INVESTIGATORS AND COMMITTEES The Cardiac Insufficiency Bisoprolol Study II (CIBIS II): A randomized trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- DE LEAN A., HANCOCK A.A., LEFKOWITZ R.J. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol. Pharmacol. 1982;21:5–16. [PubMed] [Google Scholar]
- FELDMAN A.M., CATES A.E., VEAZEY W.B., HERSCHBERGER R.E., BRISTOW M.R., BAUGHMAN K.L., BAUMGARTNER W.A., VAN DOP C. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J. Clin. Invest. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBERT E.M., ABRAHAM W.T., OLSEN S., HATTLER B., WHITE M., MEALY P., LARRABEE P., BRISTOW M.R. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94:2817–2825. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- HERSHBERGER R.E., WYNN J.R., SUNDBERG L., BRISTOW M.R. Mechanism of action of bucindolol in human ventricular myocardium. J. Cardiovasc. Pharmacol. 1990;15:959–967. doi: 10.1097/00005344-199006000-00014. [DOI] [PubMed] [Google Scholar]
- JASPER J.R., MICHEL M.C., INSEL P.A. Molecular mechanism of beta-adrenergic receptor blockers with intrinsic sympathomimetic activity. FASEB J. 1988;2:2891–2894. doi: 10.1096/fasebj.2.13.2901994. [DOI] [PubMed] [Google Scholar]
- KUKIN M.L., FREUDENBERGER R.S., MANNINO M.M., KALMAN J., STEINMETZ M., BUCHHOLZ-VARLEY C., OCAMPO O.N. Short-term and long-term hemodynamics and clinical effects of metoprolol alone and combined with amlodipine in patients with chronic heart failure. Am. Heart J. 1999;138:261–268. doi: 10.1016/s0002-8703(99)70110-9. [DOI] [PubMed] [Google Scholar]
- MERIT-HF STUDY GROUP Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure. Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- MICHEL M.C., PINGSMANN A., BECKERINGH J.J., ZERKOWSKI H.R., DOETSCH N., BRODDE O.E. Selective regulation of beta1- and beta2-adrenoceptors in the human heart by chronic beta-adrenoceptor antagonist treatment. Br. J. Pharmacol. 1988;94:685–692. doi: 10.1111/j.1476-5381.1988.tb11576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLIGAN G., BOND R.A., LEE M. Inverse agonism: pharmacological curiosity or potential therapeutic strategy. TiPS. 1995;16:10–13. doi: 10.1016/s0165-6147(00)88963-4. [DOI] [PubMed] [Google Scholar]
- MOTOMURA S., DEIGHTON N.M., ZERKOWSKI H.R., DOETSCH N., MICHEL M.C., BRODDE O.E. Chronic beta1-adrenoceptor antagonist treatment sensitizes beta2-adrenoceptors, but desensitizes M2-muscarinic receptors in the human right atrium. Br. J. Pharmacol. 1990;101:363–369. doi: 10.1111/j.1476-5381.1990.tb12715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER M., BRISTOW M.R., COHN J.N., COLUCCI W., FOWLER M.B., GILBERT E.M., SHUSTERMAN N.H., FOR THE U.S. CARVEDILOL HEART FAILURE STUDY GROUP The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N. Engl. J. Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-CHAPULA J. Effects of metoprolol on action potential and membrane currents in guinea-pig ventricular myocytes. Naunyn-Schmiedeberg's Arch Pharmacol. 1992;345:342–348. doi: 10.1007/BF00168696. [DOI] [PubMed] [Google Scholar]
- SCHMIDT U., SCHWINGER R.H.G., BÖHM M. Interaction of halothane with inhibitory G-proteins in the human myocardium. Anesthesiology. 1995;83:353–360. doi: 10.1097/00000542-199508000-00016. [DOI] [PubMed] [Google Scholar]
- SCHWINGER R.H.G., BÖHM M., ERDMANN E. Evidence against spare or uncoupled beta-adrenoceptors in the human heart. Am. Heart J. 1990a;119:899–904. doi: 10.1016/s0002-8703(05)80329-1. [DOI] [PubMed] [Google Scholar]
- SCHWINGER R.H.G., BÖHM M., ERDMANN E. The effect of xamoterol in failing human myocardium. Eur. Heart J. 1990b;11:323–327. doi: 10.1093/oxfordjournals.eurheartj.a059705. [DOI] [PubMed] [Google Scholar]
- SCHWINGER R.H.G., BÖHM M., PIESKE B., ERDMANN E. Different β-adrenoceptor-effector coupling in human ventricular and atrial myocardium. Eur. J. Clin. Pharmacol. 1991;21:443–451. doi: 10.1111/j.1365-2362.1991.tb01393.x. [DOI] [PubMed] [Google Scholar]
- THE XAMOTEROL IN SEVERE HEART FAILURE STUDY GROUP Xamoterol in severe heart failure. Lancet. 1990;336:1–6. [PubMed] [Google Scholar]
- TROCHU J.N., ERFANIAN M., KHANDOUDI N., BARON O., BRIL A., GAUTHIER C. Carvedilol produces contractile effects different from those of bucindolol in human atria. Circulation. 1999;100 Suppl.:1–439. [Google Scholar]
- UNGERER M., BÖHM M., ELCE J.S., ERDMANN E., LOHSE M.J. Altered expression of beta-adrenergic receptor kinase and beta1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- VER DONCK L., WOUTERS L., OLBRICH H.G., MUTSCHLER E., BORGERS M. Nebivolol increases survival in cardiomyopathic hamsters with congestive heart failure. J. Cardiovasc. Pharmacol. 1991;18:1–3. doi: 10.1097/00005344-199107000-00001. [DOI] [PubMed] [Google Scholar]
- WISENBAUGH T., KATZ I., DAVIS J., ESSOP R., SKOULARIGIS J., MIDDLEMOST S., ROTHLISBERGER C., SKUDICKY D., SARELI P. Long-term (3 month) effects of a new beta-blocker (nebivolol) on cardiac performance in dilated cardiomyopathy. J. Am. Coll. Cardiol. 1993;21:1094–1100. doi: 10.1016/0735-1097(93)90230-x. [DOI] [PubMed] [Google Scholar]


