Abstract
The aim of this work was to evaluate the role of leukotrienes in brain damage in vivo in a model of focal cerebral ischaemia in the rat, obtained by permanent occlusion of middle cerebral artery.
A significant (P<0.01) elevation of LTC4, LTD4 and LTE4 (cysteinyl-leukotrienes) levels occurred 4 h after ischaemia induction in the ipsilateral cortices of ischaemic compared to sham-operated animals (3998±475 and 897±170 fmol g−1 tissue, respectively, P<0.01).
The NMDA receptor antagonist MK-801 and the adenosine A2A receptor antagonist SCH 58261 were administered in vivo at doses known to reduce infarct size and compared with the leukotriene biosynthesis inhibitor MK-886.
MK-886 (0.3 and 2 mg kg−1 i.v.) and MK-801 (3 mg kg−1 i.p.) decreased cysteinyl-leukotriene levels (−78%, P<0.05; −100%, P<0.01; −92%, P<0.01, respectively) 4 h after permanent occlusion of the middle cerebral artery, whereas SCH 58261 (0.01 mg kg−1 i.v.) had no significant effects.
MK-886 (2 mg kg−1 i.v.) was also able to significantly reduce the cortical infarct size by 30% (P<0.05).
We conclude that cysteinyl-leukotriene formation is associated with NMDA receptor activation, and that it represents a neurotoxic event, the inhibition of which is able to reduce brain infarct area in a focal ischaemic event.
Keywords: Leukotrienes, cysteinyl-leukotrienes, cerebral infarct, focal cerebral ischaemia, MK-886, MK-801, SCH 58261
Introduction
Cysteine-containing LTs (cysteinyl-LTs), namely LTC4, LTD4, LTE4 are metabolites of arachidonic acid formed through the 5-lipoxygenase (5-LOX) pathway (Samuelsson, 1983). Cysteinyl-LTs are known mainly for their potent action as constrictors of smooth muscle (Feuerstein, 1985). However, their profile of action is much broader; they possess pro-inflammatory characteristics (Hay et al., 1995) and, in particular, they increase postcapillary venule tone and permeability, thus causing oedema (Dahlen et al., 1981).
Leukotrienes are synthesized in different areas of the central nervous system both in vitro and in vivo (Dembinska-Kiec et al., 1984; Lindgren et al., 1984). The concentration of free arachidonic acid, which is usually very low in the brain, increases greatly following various stimuli, including ischaemia (Bosisio et al., 1976). Increased LT formation from brain homogenates has been demonstrated in different species where global ischaemia had been induced by bilateral occlusion of common carotid artery, especially after reperfusion (Dempsey et al., 1986a,1986b; Mabe et al., 1990; Minamisawa et al., 1988; Moskowitz et al., 1984). In addition, elevated cysteinyl-LT levels have been found in cerebrospinal fluid of patients within 72 h from acute cerebral ischaemia (Aktan et al., 1991).
Furthermore, it has been recently suggested that eicosanoids might be associated with neuronal injury after hypoxia or trauma. Indeed, mice deficient in cytosolic phospholipase A2 (cPLA2), one of the enzymes responsible for arachidonic acid release, had smaller cerebral infarct volume following transient ischaemia (Bonventre et al., 1997) and the excitotoxic aminoacid glutamate enhances PLA2 activity (Bonventre, 1997). However, there is only indirect evidence, based on the use of the dual cycloxygenase/lipoxygenase inhibitor BW755C, that lipoxygenase products might be involved in neurotoxicity (Baran et al., 1994; Chen et al., 1995).
For a better understanding of the involvement of leukotrienes in ischaemic damage and neurotoxicity, we have chosen a rat model of brain ischaemia induced by the permanent occlusion of the middle cerebral artery (pMCAo). This experimental model is relevant to human stroke, as this pathology is most frequently caused by thrombotic occlusion of the same vessel. The histopathological consequence is a necrotic area involving both neurons and glial cells (the infarct core) which develops around the site of occlusion of the MCA. This area is surrounded by the so called penumbra, where a secondary damage develops.
In this model, we examined the effects of two neuroprotectant agents, MK-801 and SCH 58261, on the levels of immunoreactive cysteinyl-LTs (i-cysteinyl-LTs) in vivo. MK-801 is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist (Wong et al., 1986) and SCH 58261 is a potent and selective adenosine A2A receptor antagonist (Zocchi et al., 1996). Both these compounds have been shown to have neuroprotective properties in models of focal cerebral ischaemia (Monopoli et al., 1998; Park et al., 1988). Finally, we wanted to see if the biosynthesis of i-cysteinyl-LT triggered by cerebral ischaemia is able per se to induce cell death. With this aim we designed experiments with MK-886 (Gillard et al., 1989), a potent and selective inhibitor of the 5-lipoxygenase (IC50: 2.5 nM in isolated human leucocytes), in cerebral ischaemia.
The present study provides the first evidence of neuroprotection obtained by post-ischaemic reduction of leukotriene levels and of the deleterious effects of these lipid mediators, in a model of focal cerebral ischaemia.
Methods
Materials
3H-LTC4 (specific activity 110.5 Ci mmol−1) were from NEN Life Science Products, Boston, MA, U.S.A. and Sep-Pak C18 solid phase cartridges from Waters Associates, Milford, MA, U.S.A.; n-hexane, EtOAc, MeOH and EtOH for liquid chromatography were purchased from Merck, Darmstadt, Germany; Ultrapure H2O (MilliQ) was from Millipore Co., Bedford, MA, U.S.A.; A23187 was from Sigma, St. Louis, MO, U.S.A. Reagents for enzyme immunoassay (EIA) were obtained from Cayman Chemical Co., Ann Arbor, MI, U.S.A. except for cysteinyl-LT antibody which was from Perspective Biosystems Inc., Framingham, MA, U.S.A. MK-801 was from R.B.I., Natick, MA, U.S.A.; SCH 58261 was synthesized at the Schering-Plough Research Institute and MK-886 was a kind gift from Dr A. Ford-Hutchinson (Merck-Frosst Canada Inc., Pointe-Claire, Dorval, Quebec, Canada). Ultima Gold was from Packard Instruments Co., Meriden, CT, U.S.A.
Animal housing and surgery
Experiments were conducted in male Sprague-Dawley rats (Charles River, Calco, Como, Italy), weighing 250–275 g. Procedures involving animals and their care were conducted in conformity with the institutional guidelines, in compliance with the European Economic Community Council Directive 86/609 (OJ L 358, 1, December 12, 1987). The animals were caged for at least 3 days before surgery, with free access to food (until 12 h before surgery) and water, and maintained on a 12 light/12 dark schedule (lights on at 0700 h).
Focal cerebral ischaemia was induced by permanent, unilateral occlusion of the left middle cerebral artery (pMCAo) in rats anaesthetized with chloral hydrate (400 mg kg−1 i.p.). The pMCAo was performed according to methods described elsewhere with minor changes (Shigeno et al., 1985). Briefly, all rats underwent subtemporal subperiosteal craniectomy (with intact zygoma) and exposure of the main trunk of MCA under 16×magnification of an operating stereomicroscope (M351, Leica Instruments, Nussloch, Germany). The exposed artery was electrocoagulated close to its origin at the junction with the olfactory branch. Each rat was allowed to breath spontaneously and body temperature was maintained at 37°C (36.5–37.5°C) with a homeothermic heating blanket. All necessary care was taken to perform surgery under sterile conditions.
The experimental groups were:
Control rats which have been anaesthetized but not operated (both brain hemispheres)
Sham-operated rats which have been anaesthetized and sham-operated (craniectomy and exposure of the main trunk of left MCA).
Ischaemic rats which have been anaesthetized and operated (craniectomy and permanent occlusion of the left MCA).
Drug administration
MK-886 was dissolved in a saline solution with 10% DMSO, and administered in the femoral vein at two doses (0.3 mg kg−1 or 2 mg kg−1). MK-801 was dissolved in a saline solution and given at the dose of 3 mg kg−1 i.p.; SCH58261 was dissolved in saline solution additioned with 10% DMSO and administered i.p. at the dose of 0.01 mg kg−1. Rats were given drugs or their vehicle in a volume of 5 ml kg−1 within 10 min after the pMCAo.
Infarct size analysis
Rats were sacrificed by decapitation 24 h after pMCAo in order to have the ischaemic damage completly developed (Kirino et al., 1988). The brains were rapidly removed and fixed in Carnoy (60% EtOH, 30% chloroform, 10% acetic acid glacial). Infarct volume was determined on paraffin-embedded coronal slices (10 μm) stained with cresyl violet to determine the cortical and striatal damage. Sections were sampled at a distance of 1 mm starting from 3.2 mm from bregma, following a rostro-caudal direction, for eight levels. Total brain and infarct areas were measured by using an image analyser (Image-Pro Plus, Media Cybernetics, MD, U.S.A.). The volume of infarction was calculated with trapezoid's estimator of morphometric volume (Rosen & Harry, 1990) and corrected for oedema. The amount of ischaemic damage was expressed in absolute values (mm3).
Oedema was evaluated indirectly as percentage increase of the ischaemic hemisphere volume with respect to the control hemisphere, using the following formula: [(Vipsi − Vcontra)/Vipsi]×100, where Vipsi=volume of the ipsilateral (ischaemic) hemisphere, Vcontra=volume of the contralateral (healthy) hemisphere.
LT extraction and analysis
Cerebral tissue
Male rats were sacrificed by decapitation at different times (1, 4, 6 and 24 h) after the operation. The head was immediately transferred on ice; brain cortex and hippocampus, both ipsi- and contralateral with respect to the operated hemisphere, were quickly removed, weighed and homogenized in ice-cold absolute EtOH (1 : 3, w v−1 for the cortex; 1 : 9, w v−1 for the hippocampus). In order to have detectable amounts of i-cysteinyl-LTs under all conditions, brain areas from two animals were combined and the samples were centrifuged at 10,000×g for 15 min at 4°C. The supernatants were removed and, after addition of 3H-LTC4 (50,000 d.p.m./sample) for recovery calculation, they were stored at −20°C until analysis and in any case not longer than 15 days.
For LT analysis, each sample was diluted with Ultrapure H2O to obtain a final EtOH concentration of 15% and extracted using a Sep-Pak C18 solid phase cartridge, previously washed with 3 ml MeOH and 3 ml H2O. The column was eluted with 3 ml hexane (discarded), then with 1 ml EtOAc to elute LTB4 and finally with 1 ml MeOH to elute cysteinyl-LTs.
MeOH fractions were separately dried and reconstituted in buffer (0.1 M K2HPO4, 0.1 M KH2PO4, 1.5 mM NaN3, 0.4 M NaCl, 1 mM EDTA, 1 g l−1 bovine serum albumin) just before enzyme immunoassay (Pradelles et al., 1985; 1990). Solid phase EIA was performed on 96-well microplates with the Titertek apparatus (Flow Laboratories, Helsinki, Finland), using an antibody with a high (50–90%) cross-reactivity between LTC4, LTD4 and LTE4, in order to be able to measure all the cysteinyl-LTs (immunoreactive cysteinyl-LTs, i-cysteinyl-LTs). The antibody displayed negligible interaction with the prostanoids and fatty acids (cross-reactivity <0.01 and 0.12%, respectively). The detection limit was 10–15 fmol. The total recovery for cysteinyl-LTs was approximately 50%.
Samples containing the radioactive standards, but not tissue, were processed in parallel with the others and represent the blank of the procedure.
Lung parenchyma
Macroscopically normal human lung tissue was obtained at the time of resection. Tissue fragments of approximately 100 mg each were incubated overnight in gassed Tyrode's buffer (mM): NaCl 140, MgCl2 0.5, KCl 2.7, CaCl2 1.7, NaH2PO4 0.36, glucose 5, NaHCO3 12, pH 7.4 at 25°C. The next day, the lung fragments were washed and resuspended in Tyrode's buffer 1 : 10 w v−1. After 15 min at 30°C, they were treated with either the drugs under study (30 nM SCH 58261, 10 μM MK-801, 1 μM MK-886) or their vehicle; 15 min later, they were challenged with A23187 for 20 min. The tissue was then eliminated and the supernatant was frozen. The samples were analysed for LT content as described above.
Data analysis
LT levels are expressed as fmol g−1 of tissue, mean±s.e. Infarct volume and percentage of infarction are presented as mean±s.e. Statistical evaluation was carried out by analysis of variance, one or two way ANOVA, according to the experimental design. A P value <0.05 was considered to be statistically significant.
Results
Basal levels of i-cysteinyl-LTs in cerebral cortex
In control animals, which had undergone anaesthesia but no surgery, there were detectable levels of i-cysteinyl-LT after 2 h (425±85 fmol g−1 tissue, n=4; Figure 1). These levels were not different from those in contralateral cortices from both ischaemic and sham-operated animals. The two latter conditions yielded i-cysteinyl-LT levels not significantly different from one another at any time up to 24 h after pMCAo; for this reason, such values were pooled at each time point (Figure 1) and compared with the levels in ipsilateral cortices from sham-operated animals. As shown in Figure 1, in the ipsilateral cortices the surgical procedure caused i-cysteinyl-LT levels to be significantly higher (P<0.05) than in the contralateral areas at 2 and 4 h after occlusion. Therefore, the values of the ipsilateral cortices of sham-operated animals, which include the basal production, were taken as reference values to evaluate the effect of ischaemia.
Figure 1.
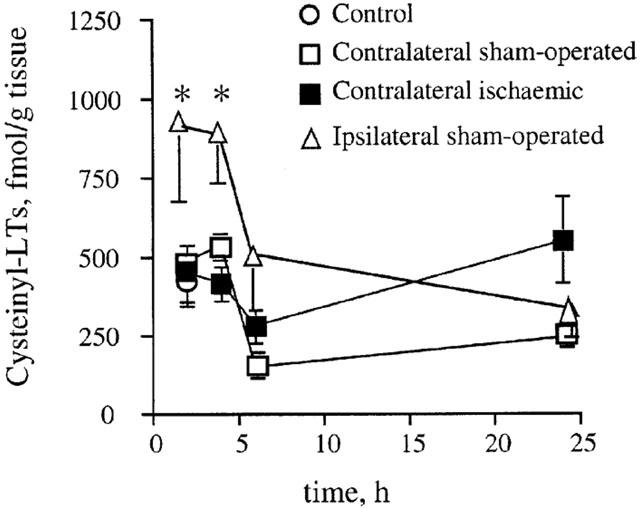
Time course of the variation of cysteinyl-LT levels in the contralateral cortices from sham-operated and ischaemic animals and in the ipsilateral cortices from sham-operated animals. Data are means±s.e.mean, n=3–5. (*P<0.05 vs contralateral cortices). Statistical analysis was performed by two-way ANOVA followed by Bonferroni's test.
Cysteinyl-LT levels after pMCAo
Cysteinyl-LT levels in cerebral cortex following pMCAo were different from values obtained in sham-operated animals. Following ischaemia, i-cysteinyl-LT levels peaked at 4 h and at this time point were approximately four times higher than reference values (P<0.01) (Figure 2).
Figure 2.
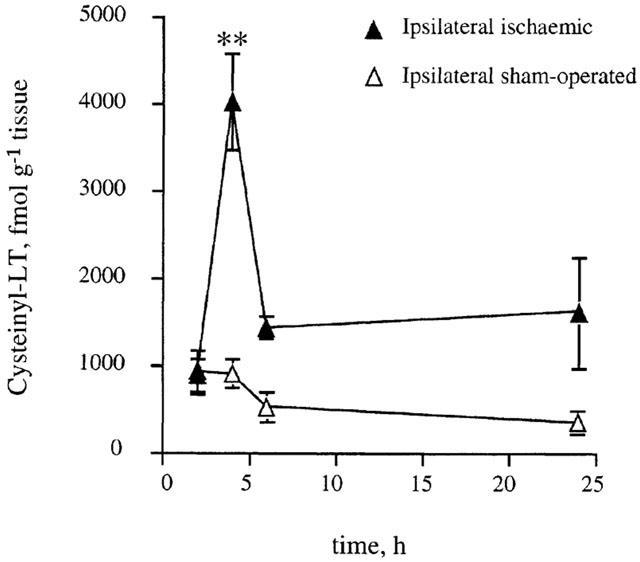
Time course of the variation of cysteinyl-LT levels in the ipsilateral cortices from ischaemic animals compared with reference values (ipsilateral sham-operated cortices). Data are means±s.e.mean, n=4–9. (**P<0.01 vs sham-operated cortices). Statistical analysis was performed by two-way ANOVA followed by Bonferroni's test.
On the contrary, the levels of i-cysteinyl-LTs in the hippocampus, an area which is not involved in ischaemic damage upon pMCAo (Shigeno et al., 1985), in ischaemic animals (3970±1009 fmol g−1 tissue, n=3) were not significantly different from those either in contralateral areas (2006±197 fmol g−1 tissue, n=3) of the same animals or in hippocampi of sham-operated ones (2908±640 fmol g−1 tissue, n=3).
Pharmacological modulation of i-cysteinyl-LT levels after pMCAo
When rats were treated with the NMDA receptor antagonist MK-801 (3 mg kg−1 i.p.) after pMCAo, ischaemia-induced i-cysteinyl-LT increase measured 4 h after the occlusion was reduced by 92.4% (P<0.01, Figure 3). The adenosine A2A receptor antagonist SCH 58261 (0.01 mg kg−1 i.v.) showed a trend to reduce the increase in i-cysteinyl-LT levels (−48%) after ischaemia, but this inhibition did not attain statistical significance. The 5-LOX inhibitor MK-886 at both doses (0.3 and 2 mg kg−1 i.v.) under the same conditions inhibited i-cysteinyl-LT increase by 78% (P<0.05) and 100% (P<0.01), respectively (Figure 3). Administration of drug vehicles did not modify i-cysteinyl-LT levels in ischaemic cortices.
Figure 3.
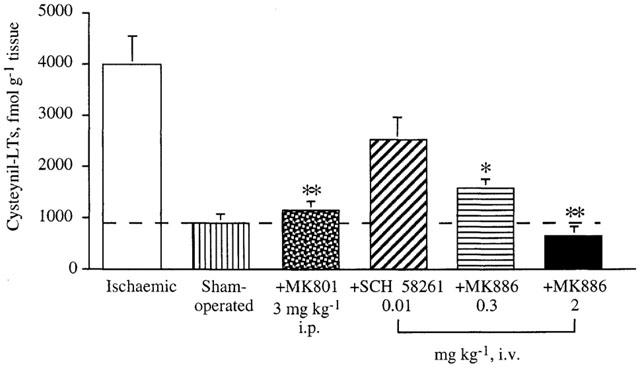
Variation of cysteinyl-LT levels in ischaemic cortices induced by in vivo administration, of MK-801 (3 mg kg−1 i.p.), SCH 58261 (0.01 mg kg−1 i.v.) and MK-886 (0.3 and 2 mg kg−1 i.v.). Cysteinyl-LT levels were assayed 4 h after pMCAo. Inhibition was evaluated by taking cysteinyl-LT formation in ipsi-lateral sham-operated cortices as basal value. Data are means±s.e.mean, n=4–10. (*P<0.05 and **P<0.01 vs ischaemic cortices). Statistical analysis was performed out by one-way ANOVA followed by Bonferroni's test.
In order to evaluate whether MK-801 and SCH 58261 were able to directly inhibit i-cysteinyl-LT formation, we measured the effect of MK-801 (10 μM) and SCH 58261 (30 nM) in comparison to MK-886 (1 μM) in human lung parenchyma, a tissue less rich in both NMDA and adenosine A2A receptors. The ratio of SCH 58261 or MK-801 concentrations to MK-886 concentration was higher than those used in vivo. In this preparation, the challenge with 10 μM A23187 caused the production of 333±25 fmol mg−1 tissue. Neither MK-801 nor SCH 58261 inhibited i-cysteinyl-LT production significantly (673±100 and 472±59 fmol mg−1 tissue, respectively), whereas MK-886 induced an almost complete inhibition (37±1 fmol mg−1 tissue, −92%, P<0.01).
Effects of the leukotriene synthesis inhibitor MK-886 on infarct size after pMCAo
The permanent occlusion of left MCA resulted in a reproducible ischaemic damage within the territory of the artery, i.e. in the dorsolateral cortex and in the neostriatum, as well as an increase in the volume of the lesioned hemispheres, representing the occurence of oedema. The administration of MK-886 at the lowest dose (0.3 mg kg−1 i.v.) 10 min after the pMCAo did not significantly reduce the volume of ischaemic brain damage in the cortex and striatum (infarct volume: total: 84.8±9.5 vs 77.3±9.1 mm3; cortical: 60.6±7.6 vs 54.9±7.2 mm3; striatal: 24.2±3.0 vs 21.7±2.9 mm3; (n=10–11)). At the higher dose (2 mg kg−1 i.v.), MK-886 significantly reduced total infarct volume by 25% and cortical infarct volume by 30% (P<0.05) (Figure 4), whereas striatal infarct volume was not significantly affected by drug treatment.
Figure 4.
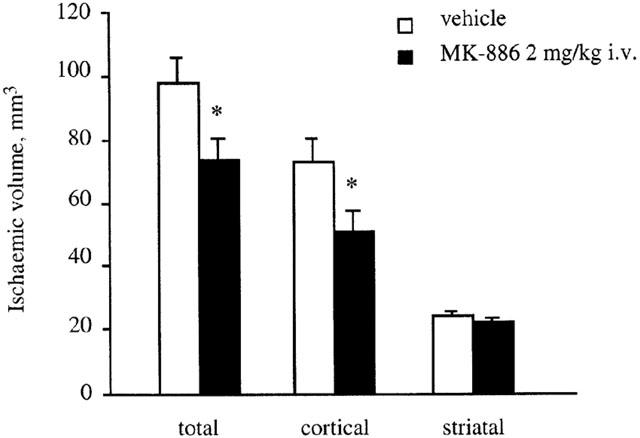
Total, cortical and striatal infarct volume 24 h after pMCAo in rats treated with either MK-886 (2 mg kg−1 i.v.) or vehicle after pMCAo. Data are means±s.e.mean, n=10–13. (*P<0.05 vs vehicle; statistical analysis was performed by two-way ANOVA followed by Dunnett's test for multiple comparison).
On the contrary, MK-886 did not significantly decrease oedema (oedema: vehicle 16%±2.5; MK-886 10%±2), although a tendency toward inhibition was observed.
No changes in physiological parameters, such as mean arterial blood pressure and heart rate, were observed during surgical and pharmacological treatments (data not shown).
Discussion
The present study shows that i-cysteinyl-LT levels are increased in rat brain cortex in a model of permanent focal ischaemia, upon middle cerebral artery occlusion (pMCAo). Previous evidence of increased LT formation in brain ischaemia had been obtained only in models of global ischaemia (Dempsey et al., 1986a,1986b; Mabe et al., 1990; Minamisawa et al., 1988; Moskowitz et al., 1984), which, as already mentioned, are not as relevant to human stroke as the focal ischaemia model.
Treatment with the 5-lipoxygenase inhibitor MK-886 (Gillard et al., 1989) significantly inhibited ischaemia-induced i-cysteinyl-LT formation at both doses tested. In particular, the higher dose (2 mg kg−1 i.v.) completely abolished LT formation. Although no direct data exist on the ability of this drug to cross blood-brain barrier, our results indicate that such passage very likely occurs, at least under conditions where the blood brain barrier is partially altered. The effect of MK-886 was compared with that of the known neuroprotective drugs MK-801, a glutamate NMDA receptor antagonist, and SCH 58261, an adenosine A2A antagonist, at doses shown to be effective in decreasing the infarct size (Hatfield et al., 1992; Monopoli et al., 1998). Unexpectedly, MK-801 significantly decreased i-cysteinyl-LT levels suggesting that stimulation of NMDA receptors leads to activation of 5-LOX in the brain. To our knowledge, this is the first evidence that the glutamate NMDA receptor is associated with the formation of cysteinyl-LTs.
Cerebral ischaemia induced by pMCAo in the rat has gained increasing acceptance as a model of focal infarction in humans (Shigeno et al., 1985; Tamura et al., 1981). In this ischaemia model, surgery and the subsequent tissue manipulation induces an increase in the levels of the inflammatory mediators cysteinyl-LTs, as demonstrated by their levels in sham-operated animals. However, such levels are significantly higher in the ipsilateral cortices of ischaemic rats. This indicates that the ischaemic event per se triggers the formation of a considerable amount of i-cysteinyl-LTs. The reperfusion period following an ischaemic event is considered to be the major player for the inflammatory response in the brain infarcted areas. There is evidence that also in pMCAo a strong inflammatory reaction occurs, accompanied by cytokine release and inflammatory cells infiltration (Garcia et al., 1994; Liu et al., 1993; 1994; Schroeter et al., 1994; Stroemer & Rothwell, 1998).
In our model, the i-cysteinyl-LT levels peak at 4 h after pMCAo and rapidly decline thereafter. Such relatively rapid increase in levels suggests that i-cysteinyl-LTs are not formed by infiltrating cells. Indeed, the influx of monocytes and neutrophils becomes relevant at a much later time (Zhang et al., 1994; Zhang & Chopp, 1997). It is likely that i-cysteinyl-LTs are synthesized by resident brain cells, although the precise source has not yet been fully clarified. Anterior pituitary cells and astroglial cells in culture are able to form cysteinyl-LTs (Hartung & Toyka, 1987; Kiesel et al., 1991; Petroni et al., 1991; Seregi et al., 1990) and more recently, it has been shown that purified microglial cells are mainly involved, rather than astrocytes (Matsuo et al., 1995). On the other hand, the release of glutamate following pMCAo peaks much earlier (about 2 h post ischaemia induction) (Matsumoto et al., 1992; Melani et al., 1999) and thus it could be an up-stream event with respect to cysteinyl-LT formation.
Because cysteinyl-LTs are well-known inflammatory mediators, able to cause oedema (Bochnowicz & Underwood, 1995; Dahlen et al., 1981; Evans et al., 1989), the effect of MK-886 on ischaemia-induced oedema was evaluated. However, MK-886 had no effect on ischaemia induced oedema. In a model of global ischaemia, the administration of other 5-LOX inhibitors (nordihydroguaiaretic acid and AA-861) actually reduced oedema to a significant extent (Dempsey et al., 1986a; Mabe et al., 1990; Watanabe & Egawa, 1994). These first-generation lipoxygenase inhibitors possess also anti-oxidant activity (Steinhilber, 1999), which might contribute to reduction of oedema independently from 5-LOX inhibition. This does not apply to the more specific inhibitors, such as MK-886, which inhibit 5-LOX activity by interacting with FLAP (Five Lipoxygenase Activating Protein) (Dixon et al., 1990). The use of a different inhibitor together with the type of analysis we applied, based on an indirect evaluation of water content, could explain the discrepancy with our results.
Interestingly, MK-886, at the dose of 2 mg kg−1, significantly reduced the infarct size in the cerebral cortex (about 30%). Such an effect is comparable with that obtained with the reference compounds MK-801 (−39%) and SCH 58261 (−30%) in our same laboratories (Monopoli et al., 1998). Ischaemic damage in striatum was marginally influenced by the administration of the drug. In the core region (caudate putamen and lower frontoparietal somatosensory cortex), where the reduction of blood flow is more severe, energy failure occurs rapidly, followed by neuronal death. In surrounding at-risk areas, mainly frontal and parietal cortex, neurones remain viable and may be salvaged by restoration of blood flow. The neuroprotective effects of MK-886 was most prominent in these cortical areas.
In our experimental conditions, MK-886-induced neuroprotection was observed only at a dose which inhibited i-cysteinyl-LT formation completely. This might reflect the multifactorial nature of the ischaemic damage, where other mediators besides cysteinyl-LTs, possibly released with a different time-course, might play a role. An alternative explanation might reside in the very high potency of these lipid mediators, such that, with a partial inhibition, concentrations high enough to be fully active are mantained, as previously observed with another inhibitor of LT formation, loratadine (Letari et al., 1994). Accordingly, MK-801, a neuroprotective agent, reduced i-cysteinyl-LT levels more than 90%. To the contrary, SCH 58261 which significantly decreases the infarct size at the same dose tested in the present study (Monopoli et al., 1998), might act through mechanisms which only partially involve cysteinyl-LTs. In our experiments it did not lower i-cysteinyl-LT levels significantly.
Although unidentified lipoxygenase products have been suggested to be neurotoxic (Baran et al., 1994; Chen et al., 1995), this is the first in vivo evidence that cysteinyl-LTs might be involved in the development of ischaemia-induced neurotoxicity, as evaluated by infarct size analysis. Thus, this suggests that anti-LT drugs might have neuroprotective properties.
With regard to neuroprotection, there are some considerations of interest: first, so far all the studies on the inhibition of cerebral leukotriene synthesis had been performed using the global model of cerebral ischaemia and with oedema formation as the only end-point (Mabe et al., 1990). In addition, a neuroprotective action of 5-LOX inhibitors had been previously shown only in in vitro models of traumatic or hypoxic neuronal injury (Girard et al., 1996; Wallis & Panizzon, 1993). To our knowledge, this is the first demonstration that the inhibition of leukotriene synthesis is able to decrease the infarct size, although we cannot exclude that, in our model, neuroprotection derives from the reduction of brain oedema (which however did not attain statistical significance), due to lower levels of cysteinil-LT.
Finally, the 5-LOX inhibitor MK-886 provided neuroprotection when administered post-ischaemia and by an acute injection, aspects relevant to possible therapeutic use (Jonas et al., 1997).
In conclusion, our results suggest that an increase in cysteinyl-LT levels following cerebral ischaemia is mainly associated with the activation of the NMDA receptor by glutamate, although we cannot exclude that other mechanisms, activated by the ischaemic event, such as spreading depression, may be involved in LT production. From these findings, it appears that these lipid mediators may play an important role in development of brain damage. In view of this, potential neuroprotective properties of anti-LT compounds need to be further investigated.
Acknowledgments
The authors wish to thank Ms Laura Nogueira for skillful technical assistance, Dr A.W. Ford-Hutchinson (Merck-Frosst Canada Inc.) for kindly providing us with the 5-LOX inhibitor MK-886 and Prof G.C. Folco, Prof A. Sala, Prof G.E. Rovati (Department of Pharmacological Sciences, University of Milan), Prof C. Patrono (University of Chieti) and Dr E. Ongini (Schering-Plough Research Institute, San Raffaele Science Park, Milan) for helpful suggestions and comments.
Abbreviations
- 5-LOX
5-lipoxygenase
- cPLA2
cytosolic phospholipase A2
- cysteinyl-LT
cysteinyl-leukotriene
- DMSO
dimethyl sulphoxide
- FLAP
five lipoxygenase activating protein
- pMCAo
permanent occlusion of the middle cerebral artery
References
- AKTAN S., AYKUT C., ERCAN S. Leukotriene C4 and prostaglandin E2 activities in the serum and cerebrospinal fluid during acute cerebral ischaemia. Prostaglandins Leukot. Essent. Fatty Acids. 1991;43:247–249. doi: 10.1016/0952-3278(91)90037-6. [DOI] [PubMed] [Google Scholar]
- BARAN H., VASS K., LASSMANN H., HORNYKIEWICZ O. The cyclooxygenase and lipoxygenase inhibitor BW755C protects rats against kainic acid-induced seizures and neurotoxicity. Brain. Res. 1994;646:201–206. doi: 10.1016/0006-8993(94)90078-7. [DOI] [PubMed] [Google Scholar]
- BOCHNOWICZ S., UNDERWOOD D.C. Dose-dependent mediation of leukotriene D4-induced airway microvascular leakage and bronchoconstriction in the guinea pig. Prostaglandins Leukot. Essent. Fatty Acids. 1995;52:403–411. doi: 10.1016/0952-3278(95)90069-1. [DOI] [PubMed] [Google Scholar]
- BONVENTRE J.V. Roles of phospholipases A2 in brain cell and tissue injury associated with ischaemia and excitotoxicity. J. Lipid Mediat. Cell. Signal. 1997;17:71–79. doi: 10.1016/s0929-7855(97)00021-7. [DOI] [PubMed] [Google Scholar]
- BONVENTRE J.V., HUANG Z., TAHERI M.R., O'LEARY E., LI E., MOSKOWITZ M.A., SAPIRSTEIN A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- BOSISIO E., GALLI C., GALLI G., NICOSIA S., SPAGNUOLO C., TOSI L. Correlation between release of free arachidonic acid and prostaglandin formation in brain cortex and cerebellum. Prostaglandins. 1976;11:773–781. doi: 10.1016/0090-6980(76)90186-6. [DOI] [PubMed] [Google Scholar]
- CHEN J., WEINSTEIN P.R., GRAHAM S.H. Attenuation of postischemic brain hypoperfusion and reperfusion injury by the cyclooxygenase-lipoxygenase inhibitor BW755C. J. Neurosurg. 1995;83:99–104. doi: 10.3171/jns.1995.83.1.0099. [DOI] [PubMed] [Google Scholar]
- DAHLEN S.E., BJORK J., HEDQVIST P., ARFORS K.E., HAMMARSTROM S., LINDGREN J.A. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc. Nat. Acad. Sci. U.S.A. 1981;78:3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMBINSKA-KIEC A., SIMMET T., PESKAR B.A. Formation of leukotriene C4-like material by rat brain tissue. Eur. J. Pharmacol. 1984;99:57–62. doi: 10.1016/0014-2999(84)90431-x. [DOI] [PubMed] [Google Scholar]
- DEMPSEY R.J., ROY M.W., COWEN D.E., MALEY M.E. Lipoxygenase metabolites of arachidonic acid and the development of ischaemic cerebral oedema. Neurol. Res. 1986a;8:53–56. doi: 10.1080/01616412.1986.11739731. [DOI] [PubMed] [Google Scholar]
- DEMPSEY R.J., ROY M.W., MEYER K., COWEN D.E., TAI H.H. Development of cyclooxygenase and lipoxygenase metabolites of arachidonic acid after transient cerebral ischaemia. J. Neurosurg. 1986b;64:118–124. doi: 10.3171/jns.1986.64.1.0118. [DOI] [PubMed] [Google Scholar]
- DIXON R.A., DIEHL R.E., OPAS E., RANDS E., VICKERS P.J., EVANS J.F., GILLARD J.W., MILLER D.K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- EVANS T.W., ROGERS D.F., AURSUDKIJ B., CHUNG K.F., BARNES P.J. Regional and time-dependent effects of inflammatory mediators on airway microvascular permeability in the guinea pig. Clin. Sci. 1989;76:479–485. doi: 10.1042/cs0760479. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN G. Autonomic pharmacology of leukotrienes. J. Auton. Pharmac. 1985;5:149–168. doi: 10.1111/j.1474-8673.1985.tb00116.x. [DOI] [PubMed] [Google Scholar]
- GARCIA G.H., LIU K.F., YOSHIDA Y., ZHANG Z.G., LIAN J., CHEN S., DEL ZOPPO G.J. Influx of leucokytes and platelets in an evolving brain infart (Wistar rat) Am. J. Pathol. 1994;144:188–199. [PMC free article] [PubMed] [Google Scholar]
- GILLARD J., FORD-HUTCHINSON A.W., CHAN C., CHARLESON S., DENIS D., FOSTER A., FORTIN R., LEGER S., MCFARLANE C.S., MORTON H., PIECHUTA H., RIENDEAU D., ROUZER C.A., ROKACH J., YOUNG R., MACINTYRE D.E., PETERSON L., BACH T., EIERMANN G., HOPPLE S., HUMES J., HUPE L., LUELL S., METZGER J., MEURER R., MILLER D.K., OPAS E., PACHOLOK S. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2-dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can. J. Physiol. Pharmacol. 1989;67:456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- GIRARD J., PANIZZON K., WALLIS R.A. Azelastine protects against CA1 traumatic neuronal injury in the hippocampal slice. Eur. J. Pharmacol. 1996;300:43–49. doi: 10.1016/0014-2999(95)00804-7. [DOI] [PubMed] [Google Scholar]
- HARTUNG H.P., TOYKA K.V. Leukotriene production by cultured astroglial cells. Brain. Res. 1987;435:367–370. doi: 10.1016/0006-8993(87)91627-1. [DOI] [PubMed] [Google Scholar]
- HATFIELD R.H., GILL R., BRAZELL C. The dose-response relationship and therapeutic window for dizocilpine (MK-801) in a rat focal ischaemia model. Eur. J. Pharmacol. 1992;216:1–7. doi: 10.1016/0014-2999(92)90201-e. [DOI] [PubMed] [Google Scholar]
- HAY D.W.P., TORPHY T.J., UNDEM B.J. Cysteinyl leukotrienes in asthma: old mediators up to new tricks. Trends Pharmacol. Sci. 1995;16:304–309. doi: 10.1016/s0165-6147(00)89059-8. [DOI] [PubMed] [Google Scholar]
- JONAS S., TRAN A.Q., EISENBERG E., AZAM M., VIERA D., GRUMET S. Does effect of a neuroprotective agent on volume of experimental animal cerebral infarct predict effect of the agent on clinical outcome in human stroke. Ann. N.Y. Acad. Sci. U.S.A. 1997;825:281–287. doi: 10.1111/j.1749-6632.1997.tb48439.x. [DOI] [PubMed] [Google Scholar]
- KIESEL L., PRZYLIPIAK A.F., HABENICHT A.J.R., PRZYLIPIAK M.S., RUNNEBAUM B. Purification of leukotrienes in gonadotropin-releasing hormone-stimulated pituitary cells: potential role in luteinizing hormone release. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8801. doi: 10.1073/pnas.88.19.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRINO T., TAMURA A., SANO K.Early and late neuronal damage following cerebral ischaemia Advance in Behavioural Biology: Mechanism of Cerbral Hypoxia and Stroke. 1988New York: Plenum Press; 23–24.ed. Somien, G. pp [Google Scholar]
- LETARI O., MIOZZO A., FOLCO G.C., BELLONI P.A., SALA A., ROVATI G.E., NICOSIA S. Effects of loratadine on cytosolic Ca2+ levels and leukotriene release: novel mechanisms of action independent of the anti-histamine activity. Eur. J. Pharmacol. - Mol. Pharmacol. Sect. 1994;266:219–227. doi: 10.1016/0922-4106(94)90130-9. [DOI] [PubMed] [Google Scholar]
- LINDGREN J.A., HOKFELT T., DAHLEN S.E., PATRONO C., SAMUELSSON B. Leukotrienes in the rat central nervous system. Proc. Natl. Acad. Sci. U.S.A. 1984;81:6212–6216. doi: 10.1073/pnas.81.19.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T., CLARK R.K., MCDONNELL P.C., YOUNG P.R., WHITE R.F., BARONE F.C., FEUERSTEIN G.Z. Tumor necrosis factor-α expression in ischaemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- LIU T., MCDONNELL P.C., YOUNG P.R., WHITE R.F., SIREN A.L., HALLENBECK J.M., BARONE F.C., FEUERSTEIN G.Z. Interleukin 1-β mRNA expression in ischaemic rat cortex. Stroke. 1993;24:1746–1751. doi: 10.1161/01.str.24.11.1746. [DOI] [PubMed] [Google Scholar]
- MABE H., NAGAI H., SUZUKA T. Role of brain tissue leukotriene in brain oedema following cerebral ischaemia: effect of a 5-lipoxygenase inhibitor, AA-861. Neurol. Res. 1990;12:165–168. doi: 10.1080/01616412.1990.11739937. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO K., GRAF R., ROSNER G., SHIMADA N., HEISS W.D. Flow threshold for extracellular purine catabolite elevation in cat focal ischaemia. Brain. Res. 1992;579:309–314. doi: 10.1016/0006-8993(92)90066-i. [DOI] [PubMed] [Google Scholar]
- MATSUO M., HAMASAKI Y., FUJIYAMA F., MIYAZAKI S. Eicosanoids are produced by microglia, not by astrocytes, in rat glial cell cultures. Brain. Res. 1995;685:201–204. doi: 10.1016/0006-8993(95)00490-h. [DOI] [PubMed] [Google Scholar]
- MELANI A., PANTONI L., CORSI C., BIANCHI L., MONOPOLI A., BERTORELLI R., PEPEU G., PEDATA F. Striatal outflow of adenosine, excitatory amino acids g-aminobutirric acid, and taurine in awake freely moving rats after middle cerebral artery occlusion. Stroke. 1999;30:2448–2455. doi: 10.1161/01.str.30.11.2448. [DOI] [PubMed] [Google Scholar]
- MINAMISAWA H., TERASHI A., KATAYAMA Y., KANDA Y., SHIMIZU J., SHIRATORI T., INAMURA K., KASEKI H., YOSHINO Y. Brain eicosanoid levels in spontaneously hypertensive rats after ischaemia with reperfusion: leukotriene C4 as a possible cause of cerebral edema. Stroke. 1988;19:372–377. doi: 10.1161/01.str.19.3.372. [DOI] [PubMed] [Google Scholar]
- MONOPOLI A., LOZZA G., FORLANI A., MATTAVELLI A., ONGINI E. Blokade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. Neuroreport. 1998;9:3955–3959. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A., KIWAK K.J., HEKIMIAN K., LEVINE L. Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischaemia and reperfusion. Science. 1984;224:886–889. doi: 10.1126/science.6719118. [DOI] [PubMed] [Google Scholar]
- PARK C.K., NEHLS D.G., GRAHAM D.I., TEASDALE G.M., MCCULLOCH J. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann. Neurol. 1988;24:543–551. doi: 10.1002/ana.410240411. [DOI] [PubMed] [Google Scholar]
- PETRONI A., BLASEVICH M., VISIOLI F., ZANCOCCHIA B., CARUSO D., GALLI C. Arachidonic acid cycloxygenase and lipoxygenase pathways are differently activated by platelet activating factor and the calcium-ionophore A23187 in a primary culture of astroglial cells. Brain Res. Dev. Brain Res. 1991;63:221–227. doi: 10.1016/0165-3806(91)90081-s. [DOI] [PubMed] [Google Scholar]
- PRADELLES P., ANTOINE C., LELLOUCHE J.P., MACLOUF J.Enzyme immunoassays for leukotrienes C4 and E4 using acetylcholinesterase Methods in Enzymology. 1990San Diego, New York: Academic Press Inc; 82–89.ed. Murphy, R.C. & Fitzpatrick, F.A. pp [DOI] [PubMed] [Google Scholar]
- PRADELLES P., GRASSI J., MACLOUF J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Anal. Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- ROSEN G.D., HARRY J.D. Brain volume estimation from serial section measurements:a comparison of methodologies. J. Neurosci. Method. 1990;35:115–124. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B.Leukotrienes: a new class of mediators of immediate hypersensitivity reactions and inflammation Advances in prostaglandin, thromboxane and leukotriene research. 1983New York: Raven Press; 1–13.ed. Samuelsson, B., Paoletti, R. & Ramwell, P. pp [PubMed] [Google Scholar]
- SCHROETER M., JANDER S., WITTE O.W., STOLL G. Local immunoresponses in the rat cerebral cortex after middle cerebral artery occlusion. J. Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- SEREGI A., SIMMET T., SCHOBERT A., HERTTING G. Characterization of cysteinyl-leukotriene formation in primary astroglial cell cultures. J. Pharm. Pharmacol. 1990;42:191–193. doi: 10.1111/j.2042-7158.1990.tb05383.x. [DOI] [PubMed] [Google Scholar]
- SHIGENO T., MCCULLOCH J., GRAHAM D.I., MENDELOW A.D., TEASDALE G.M. Pure cortical ischaemia versus striatal ischaemia. Circulatory, metabolic, and neuropathologic consequences. Surg. Neurol. 1985;24:47–51. doi: 10.1016/0090-3019(85)90063-1. [DOI] [PubMed] [Google Scholar]
- STEINHILBER D. 5-Lipoxygenase: a target for antiinflammatory drugs revisited. Curr. Med. Chem. 1999;6:71–85. [PubMed] [Google Scholar]
- STROEMER R.P., ROTHWELL N.J. Exacerbation of ischemic brain damage by localized striatal injection of interleukin-1β in the rat. J. Cereb. Blood Flow Metab. 1998;18:833–839. doi: 10.1097/00004647-199808000-00003. [DOI] [PubMed] [Google Scholar]
- TAMURA A., GRAHAM D.I., MCCULLOCH J., TEASDALE G.M. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- WALLIS R.A., PANIZZON K.L. Protection from hypoxic and N-methyl-D-aspartate injury with azelastine, a leukotriene inhibitor. Eur. J. Pharmacol. 1993;238:165–171. doi: 10.1016/0014-2999(93)90844-8. [DOI] [PubMed] [Google Scholar]
- WATANABE T., EGAWA M. Effects of an antistroke agent MCl-186 on cerebral arachidonate cascade. J. Pharmacol. Exp. Ther. 1994;271:1624–1629. [PubMed] [Google Scholar]
- WONG E.H., KEMP J.A., PRIESTLEY T., KNIGHT A.R., WOODRUFF G.N., IVERSEN L.L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc. Natl. Acad. Sci. U.S.A. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG R.L., CHOPP M., CHEN H., GARCIA J.H. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J. Neurol. Sci. 1994;125:3–10. doi: 10.1016/0022-510x(94)90234-8. [DOI] [PubMed] [Google Scholar]
- ZHANG Z.G., CHOPP M. Measurement of myeloperoxidase immunoreactive cells in ischemic brain. Neurosci. Res. Commun. 1997;20:85–90. [Google Scholar]
- ZOCCHI C., ONGINI E., CONTI A., MONOPOLI A., NEGRETTI A., BARALDI P.G., DIONISOTTI S. The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2a adenosine receptor antagonist. J. Pharmacol. Exp. Ther. 1996;276:398–404. [PubMed] [Google Scholar]


