Abstract
In the present study, the roles of nitric oxide (NO) and superoxide anions (O2−) in allergen-induced airway hyperreactivity (AHR) after the late asthmatic reaction (LAR) were investigated ex vivo, by examining the effects of the NO synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) and superoxide dismutase (SOD) on the responsiveness to methacholine of isolated perfused guinea-pig treacheae from unchallenged (control) animals and from animals 24 h after ovalbumin challenge.
At 24 h after allergen challenge, the animals developed AHR in vivo, as indicated by a mean 2.63±0.54 fold (P<0.05) increase in sensitivity to histamine inhalation.
Compared to unchallenged controls, tracheal preparations from the ovalbumin-challenged guinea-pigs displayed a significant 1.8 fold (P<0.01) increase in the maximal response (Emax) to methacholine, both after intraluminal (IL) and extraluminal (EL) administration of the agonist. No changes were observed in the sensitivity (pEC50) to the agonist. Consequently, the ΔpEC50 (EL-IL), as a measure of epithelial integrity, was unchanged.
In the presence of L-NAME (100 μM, IL), tracheae from control guinea-pigs showed a 1.6 fold (P<0.05) increase in the Emax of IL methacholine. By contrast, the Emax of IL methacholine was significantly decreased in the presence of 100 u ml−1 EL SOD (54% of control, P<0.01).
Remarkably, the increased responsiveness to IL methacholine at 24 h after allergen challenge was reversed by L-NAME to control (P<0.01), and a similar effect was observed with SOD (P<0.01).
The results indicate that both NO and O2− are involved in the tracheal hyperreactivity to methacholine after the LAR, possibly by promoting airway smooth muscle contraction through the formation of peroxynitrite.
Keywords: Nitric oxide, superoxide anion, peroxynitrite, methacholine, late asthmatic reaction, airway hyperreactivity, tracheal perfusion, guinea-pig
Introduction
Airway hyperreactivity (AHR) to chemical, physical and pharmacological stimuli, including histamine and methacholine, is a hallmark of allergic asthma. In allergic asthmatics as well as in sensitized guinea-pigs, AHR can be developed both after the early asthmatic reaction (EAR) and the late asthmatic reaction (LAR), which presumably involves airway inflammation due to influx of inflammatory cells into the airways (Durham et al., 1988; Aalbers et al., 1993; Santing et al., 1994).
Endogenous nitric oxide (NO) plays a key role in the physiological regulation of airway function and has been implicated in the pathophysiology of inflammatory airway diseases, including bronchial asthma (Barnes & Belvisi, 1993; Moncada & Higgs, 1993; Barnes, 1998).
Thus, NO has a potent bronchodilator action by inducing relaxation of airway smooth muscle (Gruetter et al., 1989; Dupuy et al., 1992), is an important immunomodulator by promoting the proliferation of Th2 lymphocytes (Barnes & Liew, 1995) and, at high concentrations, may have deleterious effects in the airways by causing mucosal swelling, infiltration of inflammatory cells and epithelial damage (Kuo et al., 1992; Flak & Goldman, 1996; Schuiling et al., 1998a).
In the airways, NO is synthesized by constitutive NO synthase isoforms (cNOS) mainly expressed in inhibitory nonadrenergic noncholinergic nerves (neuronal NOS or nNOS), endothelial cells (endothelial NOS or eNOS) and airway epithelium (nNOS and eNOS) (Fischer et al., 1993; Kobzik et al., 1993; Asano et al., 1994), which are primarily involved in the regulation of airway and vascular tone by the local production of small amounts of NO in reponse to physiological stimuli (Barnes & Belvisi, 1993). The inducible isoform of NOS (iNOS), producing much larger amounts of NO, may be induced by proinflammatory cytokines during airway inflammation, particularly in inflammatory and epithelial cells (Barnes & Belvisi, 1993; Hamid et al., 1993; Asano et al., 1994), and may be involved in the detrimental effects described above.
Studies in vitro and in vivo have indicated that endogenous NO is involved in the regulation of airway reactivity to bronchoconstrictor stimuli. Thus, in isolated guinea-pig tracheal tube preparations it was demonstrated that nonselective NOS inhibitors such as Nω-nitro-L-arginine methyl ester (L-NAME) and NG-monomethyl-L-arginine (L-NMMA) cause enhanced muscarinic agonist-, histamine-, substance P- and bradykinin-induced airway constriction in vitro (Nijkamp et al., 1993; de boer et al., 1996; Figini et al., 1996; 1997). In vivo, inhaled NO reversed histamine and methacholine-induced bronchoconstriction in mild asthmatics (Kacmarek et al., 1996), guinea-pigs (Dupuy et al., 1992) and dogs (Brown et al., 1994), while administration of L-NAME or L-NMMA caused enhanced bronchoconstriction in response to allergen (Persson et al., 1993), histamine (Nijkamp et al., 1993; Schuiling et al., 1998b), bradykinin (Ricciardolo et al., 1994) and NKA (Ricciardolo et al., 2000) in the guinea-pig and to methacholine, bradykinin, histamine and adenosine 5′-monophosphate in mild asthmatics (Ricciardolo et al., 1997; Taylor et al., 1998).
In a guinea-pig model of acute allergic asthma, characterized by allergen-induced early and late asthmatic reactions, airway inflammation and AHR after both reactions (Santing et al., 1994), we have previously demonstrated both ex vivo (de boer et al., 1996) and in vivo (Schuiling et al., 1998a,1998b) that a deficiency of cNOS-derived NO contributes to the AHR after the EAR. In vivo, using the same animal model, we have also demonstrated that restoration of NO activity by induction of iNOS during the LAR may have both beneficial–bronchodilating–and detrimental–proinflammatory–effects on the AHR observed after the LAR, indicating the dualistic action of iNOS-derived NO in the airways (Schuiling et al., 1998a).
Both in guinea-pigs and in asthmatics there is evidence that at least some of the deleterious effects induced by iNOS-derived NO, including enhanced vascular permeability and epithelial damage, may proceed via peroxynitrite (ONOO−), a cytotoxic and more stable oxidant formed by the reaction between NO and superoxide anion (O2−) generated by activated inflammatory cells during the allergic reaction (Beckman & Koppenol, 1996; Sadeghi-Hashjin et al., 1996; Saleh et al., 1998; Sugiura et al., 1999). Therefore, in the present study, we investigated the roles of endogenous NO and O2− in the allergen-induced AHR after the LAR ex vivo, by examining the effects of L-NAME and the O2− scavenger superoxide dismutase (SOD) on the responsiveness to methacholine of isolated perfused tracheal tube preparations from animals at 24 h after allergen challenge.
Methods
Animals
Outbred specified pathogen free guinea-pigs (Charles River SAVO, Kiszlegg, Germany), weighing 500–700 g, were used in this study. All animals were actively IgE-sensitized to ovalbumin at 3 weeks of age as described previously (van amsterdam et al., 1989). The animals were operated on 3 weeks after sensitization and used experimentally 4–8 weeks after sensitization. The animals were housed in individual cages in climate-controlled animal quarters and were given water and food ad libitum.
All protocols described in this study were approved by the University of Groningen Committee for Animal Experimentation.
Measurement of airway function
Airway function was assessed by on-line measurement of pleural pressure (PpI) under unrestrained conditions, as described by Santing et al. (1992). Briefly, a small latex balloon (HSE, Freiburg, Germany), connected to a saline-filled canula, was surgically implanted inside the thoracic cavity. The free end of the canula was driven subcutaneously to and permanently attached in the neck of the animal. After connection via an external saline-filled canula to a pressure transducer (TC-XX, Viggo-Spectramed B.V., Bilthoven, The Netherlands), PpI was measured in centimetres of H2O, using an on-line computer system.
Provocation techniques
Ovalbumin and histamine provocations were performed by inhalation of aerosolized solutions. The provocations were performed in a specially designed 9 l animal cage, in which the guinea-pigs could move freely (Santing et al., 1992). A DeVilbiss nebulizer (type 646, DeVilbiss, Somerset, PA, U.S.A.) driven by an airflow of 8 l min−1 provided the aerosol required, with an output of 0.33 ml min−1.
Histamine provocations were performed starting with a concentration of 25 μg ml−1 in saline, followed by increasing dosage steps of 25 μg ml−1. The provocations by each concentration lasted 3 min and provocations were separated by 7-min intervals. The animals were challenged until the Ppl increased by more than 100% for at least 3 consecutive min during the 10-min period. The provocation concentration causing a 100% increase in Ppl (PC100) was derived by linear intrapolation of the concentration-Ppl response curve.
Allergen provocations were performed by inhalation of increasing aerosol concentrations of 1.0, 3.0, 5.0 and 7.0 mg ml−1 ovalbumin in saline for 3 min each, separated by 7-min intervals. Allergen inhalations were discontinued when an increase in Ppl of more than 100% was observed. No anti-histamine was needed to prevent anaphylactic shock. All provocations were preceded by a period of at least 30 min for adaptation of the animals to the cage, followed by two consecutive inhalations with saline solution, lasting 3 min each and separated by a 7-min interval.
Provocation protocol
On the first day of the experimental protocol, baseline histamine PC100 was assessed, which was repeated on the second day. Twenty-four hours later, allergen provocation was performed. At 24 h after allergen provocation (after the LAR; Santing et al., 1992; 1994) the PC100 value for histamine was re-assessed to establish the change in airway reactivity at this time point. Between allergen provocation and the measurement of histamine PC100 at 24 h, the animals were removed from the provocation cage and placed in their larger home-cage of 2500 cm2, where they could eat and drink ad libitum.
Tracheal perfusion
After the histamine PC100 determination at 24 h after ovalbumine challenge, the guinea-pigs were killed by a sharp blow on the head and exsanguinated. Non-challenged IgE-sensitized animals were used as controls. The tracheae were rapidly removed and placed in Krebs-Henseleit (KH) solution (37°C) of the following composition (mM): NaCl 117.50, KCl 5.60, MgSO4 1.18, CaCl2 2.50, NaH2PO4 1.28, NaHCO3 25.00, D-glucose 5.50; gassed with 5% CO2 and 95% O2; pH 7.4.
The tracheae were prepared free of serosal connective tissue and cut into two halves of approximately 17 mm before mounting in a perfusion setup, as described previously (de boer et al., 1996). The tracheal preparations were attached at each side to stainless steel perfusion tubes fixed in a Delrin perfusion holder. The holder with the trachea was then placed in a water-jacketed organ bath (37°C) containing 20 ml of gassed KH (the extraluminal (EL) compartment). The lumen was perfused with recirculating KH from a separate 20 ml bath (intraluminal (IL) compartment) at a constant flow rate of 17 ml min−1. Two axially centred side-hole catheters connected with pressure transducers (TC-XX, Viggo-Spectramed B.V., Bilthoven, The Netherlands) were situated at the distal and proximal ends of the trachealis to measure hydrostatic pressures at these sites. The signals were fed into a differential amplifier to obtain the difference between the two pressures (ΔP), which was plotted on a flatbed chart recorder. ΔP reflects the resistance of the tracheal segment to perfusion and is a function of the mean diameter of the trachea between the pressure taps. The transmural pressure in the trachea was set at 0 cm H2O. At the perfusion flow rate used, a baseline ΔP of 0.1 to 1.0 cm H2O was measured, depending on the diameter of the preparation.
After a 45-min equilibration period with three washes with fresh KH (both IL and EL), 1 μM isoprenaline was added to the EL compartment to assess basal tone. After three washes (30 min), the trachea was exposed to EL 40 mM KCl in KH to obtain a receptor-independent reference response. Subsequently, the preparation was washed four times with KH during 45 min until basal tone was reached and a consecutive cumulative concentration response curve (CCRC) was made with IL methacholine. In some experiments, the CCRC to IL methacholine was followed by a CCRC to EL methacholine. When used, L-NAME (100 μM) was applied in the IL reservoir 45 min prior to agonist addition. SOD (100 u ml−1) was administered to the EL reservoir 30 min prior to agonist addition (de boer et al., 1998).
Data analysis
To compensate for differences in ΔP due to variation in internal diameter of the tracheal preparations used, responses to EL and IL methacholine were expressed as a percentage of the response induced by EL 40 mM KCl. The contractile effect of 10 mM methacholine (highest concentration) was defined as Emax (de boer et al., 1996). Using this Emax the sensitivity to the agonist was evaluated as pEC50 (−log10 EC50). Changes in the in vivo airway reactivity to histamine induced by allergen provocation were expressed as the ratio of histamine PC100 values obtained 24 h before and 24 h after the allergen provocation, respectively (PC100 ratio pre/post allergen challenge).
Statistical analysis was performed using the Student's t-test for unpaired observations. Differences were considered statistically significant at P<0.05.
Chemicals
Histamine hydrochloride, ovalbumin (grade III), aluminum hydroxide, (−)-isoprenaline hydrochloride, superoxide dismutase and L-Nω-nitro arginine methyl ester (L-NAME) were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.) and methacholine chloride from Aldrich (Milwaukee, WI, U.S.A.).
Results
At 24 h after allergen challenge, the guinea-pigs displayed AHR in vivo, as indicated by a histamine PC100 ratio (pre/post allergen challenge) of 2.63±0.54 (n=12; P<0.05). Perfused tracheal tube preparations obtained from these animals showed a 1.8 fold increase in Emax to both IL and EL methacholine when compared with preparations obtained from unchallenged controls (P<0.01; Figure 1 and Table 1), without a change in the absolute reference response to KCl (ΔP=8.86±2.77 cmH2O in the challenged group vs 9.37±1.20 cmH2O in controls). No significant effects were observed on the sensitivity to methacholine (pEC50; Table 1) and on basal tone (not shown). Moreover, allergen challenge did not affect the difference in sensitivity to EL and IL methacholine, the ΔpEC50 (EL-IL) amounting to 2.01±0.13 and 1.84±0.19 log10 units for control and challenged airways, respectively.
Figure 1.
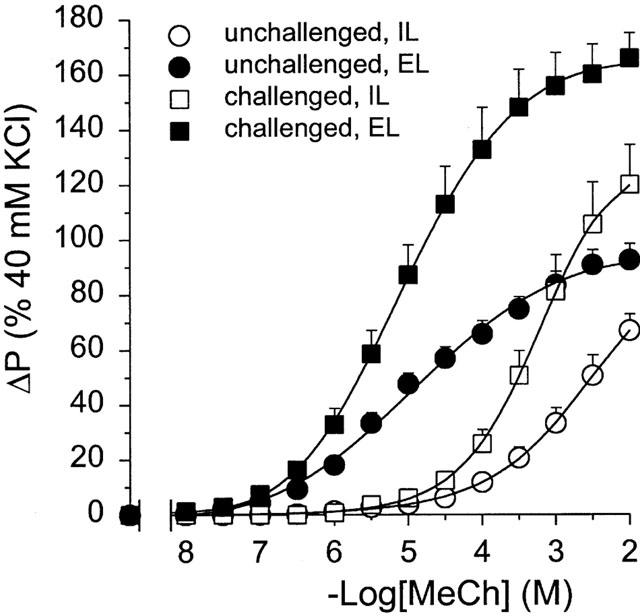
Methacholine (MeCh)-induced constriction of intact perfused tracheae obtained from unchallenged guinea-pigs and from guinea-pigs at 24 h after allergen challenge, after EL and IL administration of the agonist. Results are means±s.e.mean of nine experiments.
Table 1.
IL and EL methacholine responses in the absence or presence of L-NAME and SOD of intact perfused tracheae from unchallenged and ovalbumin-challenged guinea-pigs
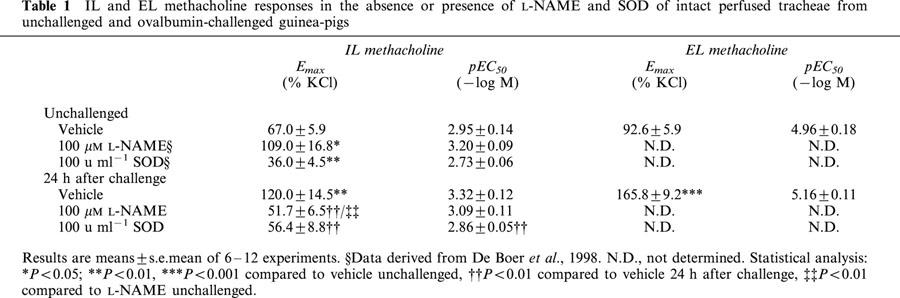
In perfused tracheal preparations from unchallenged guinea-pigs, IL administration of 100 μM L-NAME caused a significant 1.6 fold increase in the Emax of IL methacholine (P<0.05), without a significant effect on the pEC50 to the agonist (Figure 2, Table 1). By contrast, inactivation of O2− with SOD (100 u ml−1, EL) in unchallenged control airways resulted in a significant decrease in the Emax to IL methacholine to 54% of control (P<0.01), while no significant effect was observed on methacholine pEC50 (Table 1).
Figure 2.
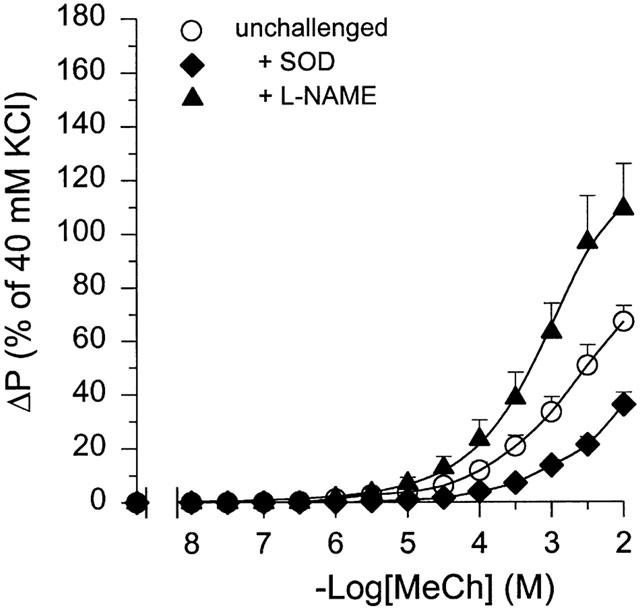
Effect of 100 μM L-NAME (IL) and 100 u ml−1 SOD (EL) on methacholine (MeCh; IL)-induced constriction of intact perfused tracheae from unchallenged guinea-pigs. Results are means±s.e.mean of 7–12 experiments.
The enhanced Emax to IL methacholine at 24 h after allergen challenge was significantly reduced in the presence of L-NAME (100 μM, IL) by 57% (P<0.01), to a level similar to that of unchallenged control preparations (Figure 3 and Table 1). No change in the pEC50 to methacholine was observed (Table 1). In the presence of SOD (100 u ml−1, EL) the enhanced responsiveness to IL methacholine was similarly attenuated (by 53%, P<0.01), while there was a small but significant decrease in the sensitivity to the agonist (Figure 3 and Table 1). Both in the control preparations and in the preparations from ovalbumin-challenged animals, L-NAME and SOD had no effect on basal tone (not shown).
Figure 3.
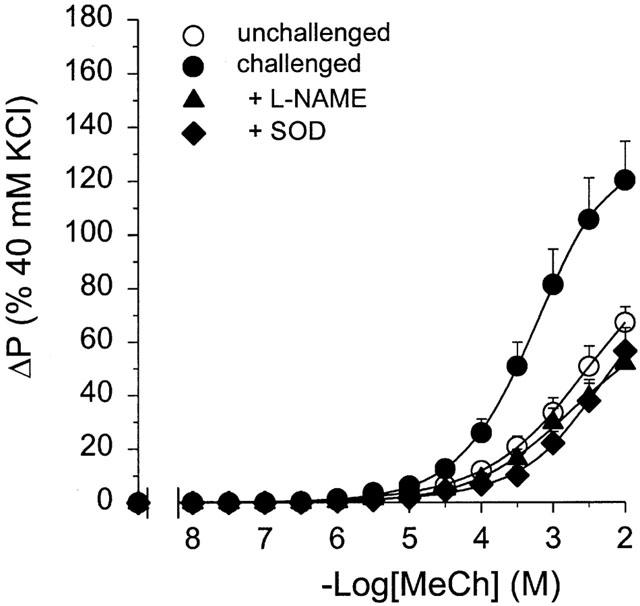
Methacholine (MeCh; IL)-induced constriction of intact perfused tracheae obtained from unchallenged guinea-pigs and from guinea-pigs at 24 h after allergen challenge, in the absence or presence of 100 μM L-NAME (IL) and 100 u ml−1 SOD (EL). Results are means±s.e.mean of 6–9 experiments.
Discussion
Using perfused tracheal tube preparations from unchallenged and ovalbumin-challenged guinea-pigs, we demonstrated that allergen-induced AHR after the LAR in vivo is associated with a significant increase in tracheal responsiveness to both IL and EL methacholine ex vivo.
Remarkably, the AHR to IL methacholine after the LAR was significantly reduced in the presence of the nonselective NOS inhibitor L-NAME, indicating a role for NO in the enhanced agonist responsiveness. In the presence of the O2− scavenger SOD a similar reduction in responsiveness to IL methacholine was observed as with L-NAME, which indicates that O2− is also involved, via a common pathway. This common pathway could involve the formation of ONOO−, the rapidly formed and highly oxidative reaction product of NO and O2− (Huie & Padmaja, 1993; Beckman & Koppenol, 1996), which has been demonstrated to induce AHR in guinea-pigs (Sadeghi-Hashjin et al., 1996). The possible involvement of ONOO− is supported by the recently observed enhanced 3-nitrotyrosine immunostaining in the airways of allergen-challenged guinea-pigs during the LAR (Sugiura et al., 1999). ONOO− leads to nitration of tyrosine residues in proteins at the 3-position adjacent to the hydroxyl group and 3-nitrotyrosine formation may hence provide evidence of local generation of ONOO−. However, tyrosine nitration may also be induced by other reactive nitrogen intermediates generated by eosinophil peroxidase and myeloperoxidase, using the O2− metabolite H2O2 and the NO metabolite NO2− as substrates (Eiserich et al., 1998; Wu et al., 1999). The presence of nitrotyrosine has recently also been demonstrated in the airways (Saleh et al., 1998; Kaminsky et al., 1999) and exhaled air (Hanazawa et al., 2000) of asthmatics, the amount of nitrotyrosine immunostaining in the epithelium and inflammatory cells showing a significant inverse correlation with the methacholine PC20 and FEV1 (Saleh et al., 1998).
The NO in the present study is presumably produced by iNOS, which is induced during the LAR both in asthmatics (Kharitonov et al., 1995) and in animal models, including guinea-pigs (Yan et al., 1995; Schuiling et al., 1998a). In asthmatic airways, iNOS may be present in the epithelium and in some inflammatory cells (Hamid et al., 1993; Renzi et al., 1997; Saleh et al., 1998), while inflammatory cells are also the likely source for the release of O2− after allergen challenge (Meltzer et al., 1989; Calhoun et al., 1992; Cerasoli et al., 1991).
The precise mechanism of ONOO− induced AHR is not known. ONOO− has cytotoxic actions, which may cause epithelial damage in the airways at relatively high concentrations of the oxidant (Sadeghi-Hashjin et al., 1996). However, no significant changes were observed for the pEC50 values of methacholine (both IL and EL) as well as for the ΔpEC50 (EL-IL), indicating that the enhanced responsiveness is not due to loss of the epithelial barrier. This corresponds to previous observations of enhanced tracheal responsiveness after the EAR, at 6 h after allergen provocation (de boer et al., 1996). Moreover, epithelial damage is unlikely to be restored after the acute inhibition of NO production and scavenging of O2− according to our protocol.
Alternatively, several observations have indicated that ONOO− may also promote smooth muscle contraction by a number of mechanisms, which could be more rapidly reversible. First, it has been demonstrated that ONOO− reversibly inhibits hyperpolarizing Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells, leading to enhanced smooth muscle contraction and vasoconstriction (Elliott et al., 1998; Brzezinska et al., 2000). In addition, in skeletal muscle ONOO− may cause reversible inhibition of sarco/endoplasmatic reticulum ATPase (SERCA) type 2 (Viner et al., 1996a,1996b), which is also present in airway smooth muscle (Grover & Kahn, 1992). In the vasculature, ONOO− has been shown to enhance prostaglandin H2 synthesis, either indirectly by inhibition of PGI2 synthase, causing enhanced PGH2 levels to act on the TxA2/PGH2 receptor before it is converted to PGE2 (Zou & Ullrich, 1996; Zou et al., 1999), or directly by activating PGH2 synthase (Upmacis et al., 1999). Evidence for PGH2 synthase activation by ONOO− has also been observed in cultured, immunostimulated macrophages (Landino et al., 1996). In line with these observations, in the dog coronary endothelium evidence was obtained for the generation of an ONOO−-dependent contractile factor after global cardiac ischaemia and reperfusion (Pearson et al., 1991).
Remarkably, L-NAME inhibited the enhanced methacholine-induced airway constriction after allergen challenge to a level that is normally observed in unchallenged animals in the absence of a NOS inhibitor, i.e. in the presence of cNOS-derived NO that counteracts methacholine-induced constriction. This indicates that the methacholine-induced airway constriction at 24 h after allergen challenge may be functionally antagonized by one or more L-NAME-insensitive relaxing factors. These relaxing factors could include stable, biologically active S-nitrosothiols, which have been demonstrated in the airways (Gaston et al., 1993), and which may be generated by NO and ONOO− (Stamler et al., 1992; Stamler, 1994, Wu et al., 1994) that are formed during the LAR. Thus, ONOO− may have both detrimental and beneficial properties in the airways, as in the cardiovascular system (Ronson et al., 1999).
As demonstrated previously (de boer et al., 1998), methacholine-induced tracheal constriction in unchallenged control airways was decreased in the presence of SOD. The SOD-induced decrease in responsiveness can be reversed by L-NAME (de boer et al., 1998), indicating that in normoreactive tracheal preparations smooth muscle relaxation by cNOS-derived NO is limited by its reaction with endogenous O2−, as has also been observed in the vasculature (Ignarro et al., 1987).
Using the same guinea-pig model of allergic asthma, we have previously demonstrated both ex vivo and in vivo that a deficiency of (cNOS-derived) NO contributes to the AHR after the EAR, at 6 h after inhalational challenge of the animals with ovalbumin aerosol (de boer et al., 1996; Schuiling et al., 1998a, 1998b). In perfused tracheal preparations of these animals, it was demonstrated that this deficiency of NO is not caused by its possible reaction with enhanced levels inflammation-induced superoxide anion (O2−) in the airways (de boer et al., 1998), but rather by limitation of L-arginine as a substrate for cNOS (de boer et al., 1999). Recent investigations have indicated that this reduced availability of substrate may be caused by reduced cellular uptake of L-arginine by cationic amino acid transporters induced by eosinophil-derived polycations (Meurs et al., 1999; Hammermann et al., 1999), which are present in the inflamed airways both at 6 and 24 h after allergen challenge (Santing et al., 1994). Another mechanism that could be involved is enhanced competition between cNOS and arginase for the substrate (Meurs et al., 2000), possibly by enhanced expression of the latter enzyme by Th2 lymphocyte-derived cytokines (Modolell et al., 1995).
In vivo, we demonstrated that the deficiency of NO after the EAR is reversed during the LAR, presumably by the induction of iNOS (Schuiling et al., 1998a). In the same study, it was shown that iNOS-derived NO has both detrimental effects on the airway reactivity, by promoting airway inflammation, and beneficial effects by causing bronchodilation. The latter observation seems to be at variance with the results obtained in the present study. However, it is important to note that in the present ex vivo study tracheal preparations were used, which may not fully reflect the balance between detrimental and beneficial effects of NO in the entire respiratory tract. This is also indicated by the observation that the AHR in vivo is partially reversed after the LAR (Schuiling et al., 1998a, 1998b), whereas in the present ex vivo study the AHR after the LAR was of similar magnitude as that observed after the EAR (1.8 fold increase in Emax, de boer et al., 1996).
In conclusion, in isolated perfused tracheal tube preparations obtained from actively IgE-sensitized guinea-pigs at 24 h after allergen provocation, the responsiveness to methacholine is increased compared to preparations obtained from non-challenged animals. Both the nonselective NOS inhibitor L-NAME and the O2− scavanger SOD significantly inhibited the observed hyperresponsiveness to a similar degree, indicating that NO and O2− are involved by a common pathway, presumably through the formation of their reaction product, ONOO−. Thus, in contrast to the airway hyperresponsiveness after the early asthmatic reaction in which deficiency of cNOS-derived NO is a major determinant, tracheal hyperresponsiveness after the late asthmatic reaction appears to involve the generation of a NO-derived pro-contractile factor.
Abbreviations
- AHR
airway hyperreactivity
- cNOS
constitutive nitric oxide synthase
- EAR
early asthmatic reaction
- EL
extraluminal
- Emax
maximal effect
- eNOS
endothelial nitric oxide synthase
- IL
intraluminal
- iNOS
inducible nitric oxide synthase
- KH
Krebs-Henseleit
- LAR
late asthmatic reaction
- L-NAME
Nω-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- nNOS
neuronal nitric oxide synthase
- ΔP
differential (hydrostatic) pressure
- Pinlet
(hydrostatic) pressure at the inlet
- Poutlet
(hydrostatic) pressure at the outlet
- pEC50
−log10 of the concentration causing 50% of the effect
- SOD
superoxide dismutase
References
- AALBERS R., KAUFFMAN H.F., VRUGT B., SMITH M., KOËTER G.H., TIMENS W., DE MONCHY J.G.R. Allergen-induced recruitment of inflammatory cells in lavage 3 and 24 hours after challenge in allergic asthmatic lungs. Chest. 1993;103:1178–1184. doi: 10.1378/chest.103.4.1178. [DOI] [PubMed] [Google Scholar]
- ASANO K., CHEE C.B.E., GASTON B., LILLY C.M., GERARD C., DRAZEN J.M., STAMLER J.S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J.Nitric Oxide Asthma: Basic Mechanisms and Clinical Management. 1998London: Academic Press Limited; 369–388.ed. Barnes, P.J., Rodger, I.W. & Thomson, N.C. pp [Google Scholar]
- BARNES P.J., BELVISI M.G. Nitric oxide and lung disease. Thorax. 1993;48:1034–1043. doi: 10.1136/thx.48.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J., LIEW F.Y. Nitric oxide and asthmatic inflammation. Immunol. Today. 1995;16:128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BROWN R.H., ZERHOUNI E.A., HIRSHMAN C.A. Reversal of bronchoconstriction by inhaled nitric oxide. Histamine versus methacholine. Am. J. Respir. Crit. Care Med. 1994;150:233–237. doi: 10.1164/ajrccm.150.1.8025755. [DOI] [PubMed] [Google Scholar]
- BRZEZINSKA A.K., GEBREMEDHIN D., CHILIAN W.M., KALYANARAMAN B., ELLIOTT S.J. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am. J. Physiol. 2000;278:H1883–H1890. doi: 10.1152/ajpheart.2000.278.6.H1883. [DOI] [PubMed] [Google Scholar]
- CALHOUN W.J., REED H.E., MOEST D.R., STEVENS C.A. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am. Rev. Respir. Dis. 1992;145:317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- CERASOLI F., TOCKER J., SELIG W.M. Airway eosinophils from actively sensitized guinea pigs exhibit enhanced superoxide anion release in response to antigen challenge. Am. J. Respir. Cell Mol. Biol. 1991;4:195–201. doi: 10.1165/ajrcmb/4.4.355. [DOI] [PubMed] [Google Scholar]
- DE BOER J., DUYVENDAK M., SCHUURMAN F.E., POUW F.M.H., ZAAGSMA J., MEURS H. Role of L-arginine in the deficiency of nitric oxide and airway hyperreactivity after the allergen-induced early asthmatic reaction in guinea pigs. Br. J. Pharmacol. 1999;128:1114–1120. doi: 10.1038/sj.bjp.0702882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BOER J., MEURS H., COERS W., KOOPAL M., BOTTONE A.E., VISSER A.C., TIMENS W., ZAAGSMA J. Deficiency of nitric oxide in allergen-induced airway hyperreactivity to contractile agonists after the early asthmatic reaction. An ex vivo study. Br. J. Pharmacol. 1996;116:1109–1116. doi: 10.1111/j.1476-5381.1996.tb16011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BOER J., POUW F.M., ZAAGSMA J., MEURS H. Effects of endogenous superoxide anion and nitric oxide on cholinergic constriction of normal and of hyperreactive guinea pig airways. Am. J. Respir. Crit. Care Med. 1998;158:1784–1789. doi: 10.1164/ajrccm.158.6.9711005. [DOI] [PubMed] [Google Scholar]
- DUPUY P.M., SHORE P.A., DRAZEN J.M., FROSTELL C., HILL W.A., ZAPOL W.M. Bronchodilator action of inhaled nitric oxide in guinea pigs. J. Clin. Invest. 1992;90:421–428. doi: 10.1172/JCI115877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURHAM S.R., CRADDOCK C.F., COOKSON W.O., BENSON M.K. Increases in airway responsiveness to histamine precede allergen-induced late asthmatic responses. J. Allergy Clin. Immunol. 1988;82:764–770. doi: 10.1016/0091-6749(88)90077-2. [DOI] [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- ELLIOTT S.J., LACEY D.J., CHILIAN W.M., BRZEZINSKA A.K. Peroxynitrite is a contractile agonist of cerebral artery smooth muscle cells. Am. J. Physiol. 1998;275:H1585–H1591. doi: 10.1152/ajpheart.1998.275.5.H1585. [DOI] [PubMed] [Google Scholar]
- FIGINI M., EMANUELI C., BERTRAND C., SICUTERI R., REGOLI D., GEPPETTI P. Differential activation of the epithelial and smooth muscle NK1 receptors by synthetic tachykinin agonists in guinea pig trachea. Br. J. Pharmacol. 1997;121:773–781. doi: 10.1038/sj.bjp.0701188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIGINI M., RICCIARDOLO F.L.M., JAVDAN P., NIJKAMP F.P., EMANUELI C., PRADELLES P., FOLKERTS G., GEPPETTI P. Evidence that epithelium-derived relaxing factor released by bradykinin in the guinea pig trachea is nitric oxide. Am. J. Respir. Crit. Care Med. 1996;153:918–923. doi: 10.1164/ajrccm.153.3.8630573. [DOI] [PubMed] [Google Scholar]
- FISCHER A., MUNDEL P., PREISSLER U., PHILIPPIN B., KUMMER W. Nitric oxide synthase in guinea pig lower airway innervation. Neurosci. Lett. 1993;149:157–160. doi: 10.1016/0304-3940(93)90760-i. [DOI] [PubMed] [Google Scholar]
- FLAK T.A., GOLDMAN W.E. Autotoxicity of nitric oxide in airway disease. Am. J. Respir. Crit. Care Med. 1996;154:S202–S206. doi: 10.1164/ajrccm/154.4_Pt_2.S202. [DOI] [PubMed] [Google Scholar]
- GASTON B., REILLY J., DRAZEN J.M., FACKLER J., RAMDEV P., ARNELLE D., MULLINS M.E., SUGARBAKER D.J., CHEE C., SINGEL D.J. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROVER A.K., KAHN I. Calcium pump isoforms: diversity, selectivity and plasticity. Cell Calcium. 1992;13:9–17. doi: 10.1016/0143-4160(92)90025-n. [DOI] [PubMed] [Google Scholar]
- GRUETTER C.A., CHILDERS C.C., BOSSERMAN M.K., LEMKE S.M., BALL J.G., VALENTOVIC M.A. Comparison of relaxation induced by glyceryl trinitrate, isosorbide dinitrate and sodium nitroprusside in bovine airways. Am. Rev. Respir. Dis. 1989;139:1192–1197. doi: 10.1164/ajrccm/139.5.1192. [DOI] [PubMed] [Google Scholar]
- HAMID Q., SPRINGALL D.R., RIVEROS-MORENO V., CHANEZ P., HOWARTH P., REDINGTON A., BOUSQUET J., GODARD P.H., HOLGATE S., POLAK J.M. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–1513. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- HAMMERMANN R., HIRSCHMANN J., HEY C., MOSSNER J., FOLKERTS G., NIJKAMP F.P., WESSLER I., RACKE K. Cationic proteins inhibit L-arginine uptake in rat alveolar macrophages and tracheal epithelial cells. Implications for nitric oxide synthesis. Am. J. Respir. Cell Mol. Biol. 1999;21:155–162. doi: 10.1165/ajrcmb.21.2.3574. [DOI] [PubMed] [Google Scholar]
- HANAZAWA T., KHARITONOV S.A., BARNES P.J. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am. J. Respir. Crit. Care Med. 2000;162:1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Rad. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BYRNS R.E., BUGA G.M., WOOD K.S., CHAUDURI G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: Use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J. Pharmacol. Exp. Ther. 1987;244:181–189. [PubMed] [Google Scholar]
- KACMAREK R.M., RIPPLE R., COCKRILL B.A., BLOCH K.J., ZAPOL W.M., JOHNSON D.C. Inhaled nitric oxide. A bronchodilator in mild asthmatics with methacholine-induced bronchospasm. Am. J. Respir. Crit. Care Med. 1996;153:128–135. doi: 10.1164/ajrccm.153.1.8542105. [DOI] [PubMed] [Google Scholar]
- KAMINSKY D., MITCHELL A., CARROLL J.N., JAMES A., SOULTANAKIS R., JANSSEN Y. Nitrotyrosine formation in the airways and lung parenchyma of patients with asthma. J. Allergy Clin. Immunol. 1999;104:747–754. doi: 10.1016/s0091-6749(99)70283-6. [DOI] [PubMed] [Google Scholar]
- KHARITONOV S.A., LUBEC G., LUBEC B., HJELM M., BARNES P.J. L-arginine increases exhaled nitric oxide in normal human subjects. Clin. Sci. 1995;88:135–139. doi: 10.1042/cs0880135. [DOI] [PubMed] [Google Scholar]
- KOBZIK L., BREDT D.S., LOWENSTEIN C.J., DRAZEN J., GASTON B., SUGARBAKER D., STAMLER J.S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am. J. Respir. Cell. Mol. Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- KUO H.P., LIU S., BARNES P.J. The effect of endogenous nitric oxide on neurogenic plasma exudation in guinea pig airways. Eur. J. Pharmacol. 1992;221:385–388. doi: 10.1016/0014-2999(92)90728-m. [DOI] [PubMed] [Google Scholar]
- LANDINO L.M., VREWS B.C., TIMMONS M.D., MORROW J.D., MARNETT L.J. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin synthesis. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELTZER S., GOLDBERG B., LAD P., EASTON J. Superoxide generation and its modulation by adenosine in the neutrophils of subjects with asthma. J. Allergy Clin. Immunol. 1989;83:960–966. doi: 10.1016/0091-6749(89)90112-7. [DOI] [PubMed] [Google Scholar]
- MEURS H., HAMER M.A.M., PETHE S., VADON-LE GOFF S., BOUCHER J.L., ZAAGSMA J. Modulation of cholinergic airway reactivity and nitric oxide production by endogenous arginase activity. Br. J. Pharmacol. 2000;130:1793–1798. doi: 10.1038/sj.bjp.0703488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEURS H., SCHUURMAN F.E., DUYVENDAK M., ZAAGSMA J. Deficiency of nitric oxide in polycation-induced airway hyperreactivity. Br. J. Pharmacol. 1999;126:559–562. doi: 10.1038/sj.bjp.0702372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODOLELL M., CORRALIZA I.M., LINK F., SOLER G., EICHMANN K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HIGGS A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- NIJKAMP F.P., VAN DER LINDE H.J., FOLKERTS G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro. Am. Rev. Respir. Dis. 1993;148:727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- PEARSON P.J., LIN P.J., SCHAFF H.V. Production of endothelium-derived contracting factor is enhanced after coronary reperfusion. Ann. Thorac. Surg. 1991;51:788–793. doi: 10.1016/0003-4975(91)90126-b. [DOI] [PubMed] [Google Scholar]
- PERSSON M.G., FRIBERG S.G., HEDQVIST P., GUSTAFSSON L.E. Endogenous nitric oxide counteracts antigen-induced bronchoconstriction. Eur. J. Pharmacol. 1993;249:R7–R8. doi: 10.1016/0014-2999(93)90532-m. [DOI] [PubMed] [Google Scholar]
- RENZI P.M., SEBASTIAO N., AL ASAAD A.S., GIAID A., HAMID Q. Inducible nitric oxide synthase mRNA and immunoreactivity in the lungs of rats eight hours after antigen challenge. Am. J. Respir. Cell Mol. Biol. 1997;17:36–40. doi: 10.1165/ajrcmb.17.1.2307. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L.M., DI MARIA G.U., MISTRETTA A., SAPIENZA M.A., GEPPETTI P. Impairment of bronchoprotection by nitric oxide in severe asthma. Lancet. 1997;350:1297–1298. doi: 10.1016/s0140-6736(05)62474-9. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L.M., GEPPETTI P., MISTRETTA A., NADEL J.A., SAPIENZA M.A., BELLOFIORE S., DI MARIA G.U. Randomised double-blind placebo-controlled study of the effect of inhibition of nitric oxide synthesis in bradykinin-induced asthma. Lancet. 1996;348:374–377. doi: 10.1016/s0140-6736(96)04450-9. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L.M., NADEL J.A., YOISHIHARA S., GEPPETTI P. Evidence for reduction of bradykinin-induced bronchoconstriction in guinea-pigs by release of nitric oxide. Br. J. Pharmacol. 1994;113:1147–1152. doi: 10.1111/j.1476-5381.1994.tb17117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICCIARDOLO F.L.M., TREVISANI M., GEPPETTI P., NADEL J.A., AMADESI S., BERTRAND C. Role of nitric oxide and septide-insensitive NK(1) receptors in bronchoconstriction induced by aerosolised neurokinin A in guinea-pigs. Br. J. Pharmacol. 2000;129:915–920. doi: 10.1038/sj.bjp.0703135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONSON R.S., NAKAMURA M., VINTEN-JOHANSEN J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc. Res. 1999;44:47–59. doi: 10.1016/s0008-6363(99)00184-4. [DOI] [PubMed] [Google Scholar]
- SADEGHI-HASHJIN G., FOLKERTS G., HENRICKS P.A., VERHEYEN A.K., VAN DER LINDE H.J., VAN ARK I., COENE A., NIJKAMP F.P. Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. Am. J. Respir. Crit. Care Med. 1996;153:1697–1701. doi: 10.1164/ajrccm.153.5.8630623. [DOI] [PubMed] [Google Scholar]
- SALEH D., ERNST P., LIM S., BARNES P.J., GIAID A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- SANTING R.E., MEURS H., VAN DER MARK T.H.W., REMIE R., OOSTEROM W.C., BROUWER F., ZAAGSMA J. A novel method to asses airway function parameters in chronically instrumented, unrestrained guinea pigs. Pulm. Pharmacol. 1992;5:265–272. doi: 10.1016/0952-0600(92)90069-s. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., OLYMULDER C.G., ZAAGSMA J., MEURS H. Relationships among allergen-induced early and late phase airway obstructions, bronchial hyperreactivity, and inflammation in conscious, unrestrained guinea pigs. J. Allergy Clin. Immunol. 1994;93:1021–1030. doi: 10.1016/s0091-6749(94)70051-6. [DOI] [PubMed] [Google Scholar]
- SCHUILING M., MEURS H., ZUIDHOF A.B., VENEMA N., ZAAGSMA J. Dual action of iNOS-derived nitric oxide in allergen-induced airway hyperreactivity in conscious, unrestrained guinea pigs. Am. J. Respir. Crit. Care Med. 1998a;158:1442–1449. doi: 10.1164/ajrccm.158.5.9803027. [DOI] [PubMed] [Google Scholar]
- SCHUILING M., ZUIDHOF A.B., BONOUVRIE A.A., VENEMA N., ZAAGSMA J., MEURS H. Role of nitric oxide in the development and partial reversal of allergen-induced airway hyperreactivity in conscious, unrestrained guinea-pigs. Br. J. Pharmacol. 1998b;123:1450–1456. doi: 10.1038/sj.bjp.0701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMLER J.S. Redox signalling: Nitrosylation and related target interactions. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., SINGEL D.J., LOSCALZO J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- SUGIURA H., ICHINOSE M., OYAKE T., MASHITO Y., OHUCHI Y., ENDOH N., MIURA M., YAMAGATA S., KOARAI A., AKAIKE T., MAEDA H., SHIRATO K. Role of peroxynitrite in airway microvascular hyperpermeability during late allergic phase in guinea pigs. Am. J. Respir. Crit. Care Med. 1999;160:663–671. doi: 10.1164/ajrccm.160.2.9807160. [DOI] [PubMed] [Google Scholar]
- TAYLOR D.A., MCGRATH J.L., ORR L.M., BARNES P.J., O'CONNOR B.J. Effect of endogenous nitric oxide inhibition on airway responsiveness to histamine in asthma. Thorax. 1998;53:483–489. doi: 10.1136/thx.53.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UPMACIS R.K., DEEB R.S., HAIJAR D.P. Regulation of prostaglandin H2 synthase activity by nitrogen oxides. Biochemistry. 1999;38:12505–12513. doi: 10.1021/bi983049e. [DOI] [PubMed] [Google Scholar]
- VAN AMSTERDAM R.G.M., BROUWER F., ZAAGSMA J. Analysis of the β-adrenoceptor mediated inhibition of IgG- and IgE-dependent guinea pig anaphylactic tracheal smooth muscle contraction. Agents Actions. 1989;26:48–51. doi: 10.1007/BF02126559. [DOI] [PubMed] [Google Scholar]
- VINER R.I., FERRINGTON D.A., HÜHMER A.F.R., BIGELOW D.J., SCHÖNEICH C. Accumulation of nitrotyrosine on the SERCA2a isoform of SR Ca-ATPase of rat skeletal muscle during aging: a peroxynitrite-mediated process. FEBS Lett. 1996a;379:286–290. doi: 10.1016/0014-5793(95)01530-2. [DOI] [PubMed] [Google Scholar]
- VINER R.I., HÜHMER A.F.R., BIGELOW D.J., SCHÖNEICH C. The oxidative inactivation of sarcoplasmic reticulum Ca2+-ATPase by peroxynitrite. Free Rad. Res. 1996b;24:243–259. doi: 10.3109/10715769609088022. [DOI] [PubMed] [Google Scholar]
- WU W., CHEN Y., HAZEN S.L. Eosinophil peroxidase nitrates protein tyrosyl residues: implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J. Biol. Chem. 1999;274:25833–25844. doi: 10.1074/jbc.274.36.25933. [DOI] [PubMed] [Google Scholar]
- WU M., PRITCHARD K.A., KAMINSKI P.M., FAYNGERSH R.P., HINTZE T.H., WOLIN M.S. Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am. J. Physiol. 1994;266:H2108–H2113. doi: 10.1152/ajpheart.1994.266.5.H2108. [DOI] [PubMed] [Google Scholar]
- YAN Z.Q., HANSSON G.K., SKOOGH B.E., LOTVALL J.O. Induction of nitric oxide synthase in a model of allergic occupational asthma. Allergy. 1995;50:760–764. doi: 10.1111/j.1398-9995.1995.tb01221.x. [DOI] [PubMed] [Google Scholar]
- ZOU M., JENDRAL M., ULLRICH V. Prostaglandin endoperoxide-dependent vasospasm in bovine coronary arteries after nitration of prostacyclin synthase. Br. J. Pharmacol. 1999;126:1283–1292. doi: 10.1038/sj.bjp.0702434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOU M., ULLRICH V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett. 1996;382:101–104. doi: 10.1016/0014-5793(96)00160-3. [DOI] [PubMed] [Google Scholar]


