Abstract
Immune response-modulating drugs such as thalidomide may be of therapeutic value in the treatment of chronic inflammatory bowel diseases including Crohn's disease (CD). In the present study, we have investigated whether thalidomide exerts this effect by impairing endothelial cell-leukocyte interaction through down-regulation of the expression of pro-inflammatory gene products in these cells.
Transient CD-like colitis was induced in male Wistar rats by single enema with trinitrobenzene sulphonic acid (TNBS) in ethanol followed by macroscopic scoring, histology, intravital microscopy, RT–PCR and immunohistochemistry (IHC) analyses. Thalidomide or its analogue supidimide were administered in olive oil by intragastric instillation 6 h prior to the induction of colitis and then daily for one week.
Both thalidomide and supidimide (200 mg kg−1 d−1) significantly attenuated TNBS-induced colitis as compared to vehicle-treated control animals (44 and 37% inhibition, respectively), and this effect persisted for 7 days post cessation of thalidomide treatment (46% inhibition).
Moreover, thalidomide significantly reduced leukocyte sticking to postcapillary venular endothelial cells in the submucosa (by 45%), improved functional capillary density and perfusion, and attenuated endothelial interleukin-8 expression, as judged by IHC analysis. According to RT–PCR analysis, both thalidomide and supidimide also significantly reduced vascular cell adhesion molecule-1 mRNA expression in the affected part of the descending colon.
These findings suggest that thalidomide and one of its derivatives impairs CD-like TNBS-induced colitis in the rat by down-regulating endothelial adhesion molecule and chemokine expression and, as a consequence, the interaction of these cells with circulating leukocytes.
Keywords: Inflammatory bowel disease, thalidomide, microvascular endothelium, gene expression, interleukin-8
Introduction
The endothelium may play a pivotal role in chronic inflammation, including inflammatory bowel diseases (IBD), as it represents the first site of interaction of circulating professional pro-inflammatory cells such as neutrophils, monocytes and TH1-type T helper cells with the affected tissue. Once initiated, an interaction between these cells and possibly with other types of cells in the vessel wall (e.g., smooth muscle cells) may lead to manifestation of the inflammatory process in a vicious cycle (Panes & Granger, 1998; Salmi et al., 1994). Recently, it was proposed that the intestinal microcirculation, and especially the microvascular endothelium, is involved in the generation of the periodic inflammatory attacks that are typical for Crohn's disease (Hodgson, 1998; Wakefield et al., 1995). This may be due to the aberrant expression of adhesion molecules and chemokines by the endothelium, leading to an enhanced recruitment and activation of circulating leukocytes (Macdermott, 1999; Vainer, 1997). Moreover, cytokine secretion, which is important for maintaining normal gastrointestinal immune function, appears to be dysregulated in Crohn's disease and thus may promote an excessive TH1 cell response to antigenic stimuli (Rogler & Andus, 1998; Sartor, 1997; Strober et al., 1998). As a consequence, the local concentration of pro-inflammatory cytokines may be increased, reinforcing the inflammatory process not only by stimulating the adherent or emigrating leukocytes but also by enhancing the recruitment of additional leukocytes through increasing endothelial adhesion molecule and chemokine production.
Immune response-modulating drugs such as thalidomide which is capable of inhibiting bacterial lipopolysaccharide (LPS)-stimulated IL-12 and tumour necrosis factor-α (TNFα) synthesis in monocytes (Corral et al., 1999; Moller et al., 1997), may affect this endothelial cell-leukocyte interaction in chronic IBD. Indeed, recent pilot trials suggest that thalidomide treatment accelerates the healing of different types of colitis including Crohn's disease (Ehrenpreis et al., 1999; Vasiliauskas et al., 1999). To verify that thalidomide exerts this beneficial therapeutic effect by interfering with endothelial cell-leukocyte interaction, we have employed a Crohn's disease-like animal model, the trinitrobenzene sulphonic acid (TNBS)-induced colitis in the rat (Morris et al., 1989).
Methods
Materials
Oligonucleotides and molecular biology reagents were from Gibco Life Technologies BRL, Paisley, U.K. Thalidomide and supidimide were provided by Grünenthal GmbH, Aachen, Germany. The polyclonal goat anti-CD62E (mouse/rat) and the rabbit anti IL-8 (human/mouse/rat) antibodies were obtained from Research Diagnostics Inc., Flanders, U.S.A. The biotinylated mouse-anti-goat/sheep, the swine-anti-rabbit antibodies, the peroxidase-conjugated avidin and 3-amino-9-ethylcarbazole were obtained from DAKO, Hamburg, Germany. The haematoxylin, eosin and Giemsa solutions were from Merck, Darmstadt, Germany. All other chemicals were from Roth, Darmstadt or Sigma-Aldrich, Deisenhofen, Germany.
Animals
Non-fasted male Wistar rats (Winkelmann, Borchen/Westfalen, Germany) weighing 200–250 g were used for the experiments. All animals were fed standard rat chow and had free access to water and food. The animals were kept according to the German legislation on the protection of animals with a 12 h day and night rhythm.
Induction of colitis
Colitis was induced by a single intracolonic application of 20 mg TNBS dissolved in 35% ethanol (total volume of 136 μl) into the descending colon. Rats were placed under light ether anaesthesia and an 8 cm long catheter fitted onto a 1 ml syringe was inserted through the anal canal into the descending colon. The total volume was expelled with additional air and the catheter removed.
Experimental groups
The study protocol depicted in Figure 1 was used with a follow-up of 7 or 14 days. Each treatment group consisted of 9–15 animals. Thalidomide (200 mg kg−1 d−1) and its analogue, supidimide (200 mg kg−1 d−1), were suspended in 0.5 ml olive oil and administered once daily via intragastric instillation. Olive oil alone served as a control. The concentration of both drugs was chosen in analogy with their therapeutic effects in a rat model of collagen-induced arthritis (Oliver et al., 1998).
Figure 1.
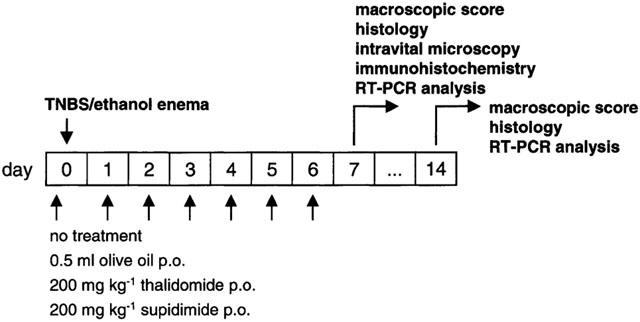
Scheme of the study protocol.
Assessment of colonic inflammation and damage
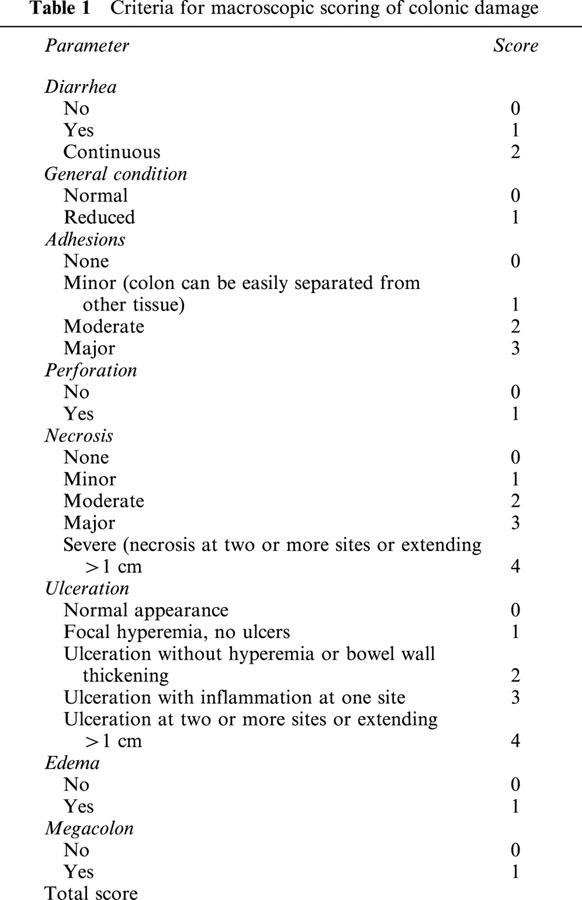
The animals were sacrificed 7 or 14 days after induction of colitis according to the study protocol (Figure 1) in deep ether anaesthesia. The colon was removed and opened longitudinally. Damage to the colon was assessed both macroscopically and histochemically (see below). Macroscopic evaluation was done using a score originally described by McCafferty et al. (1994) with some modifications (Table 1). In addition, samples from the affected part of the descending colon as well as from unaffected sites of the ascending colon were taken for immunohistochemistry and semi-quantitative RT–PCR analysis of selected gene products (see below).
Table 1.
Criteria for macroscopic scoring of colonic damage
Immuno(histo)chemistry
Tissue samples were fixed in 4% formaldehyde and embedded in paraffin. Five to 10 μm thick sections were mounted on silanized slides (Dako), and after deparaffinization stained both histochemically (haematoxylin/eosin, Giemsa) and immunohistochemically. For immunohistochemistry, haematoxylin-stained sections were incubated six times for 5 min in 0.01 M citrate buffer (pH 6.0) in a microwave oven set to high power (800 W). Thereafter they were incubated in 3% hydrogen peroxide for 15 min to block unspecific endogenous peroxidase activity followed by an overnight incubation at 4°C with a polyclonal goat anti-CD62E or rabbit anti-IL-8 antibody, respectively, at a working dilution of 1 : 10. To visualise the bound primary antibody, the following detector components were applied at the recommended working dilutions of the manufacturer (Dako): biotinylated secondary mouse-anti-goat/sheep or swine-anti-rabbit antibody as a linker, peroxidase-conjugated avidin as a label and 3-amino-9-ethylcarbazole as a chromogen.
Intravital microscopy
In two additional series of experiments, rats (minimum of three animals per treatment group) were subjected to intravital microscopy 7 days after induction of colitis. Under ether anaesthesia they were placed on a heated table to keep the body temperature constant. The trachea was cannulated to facilitate breathing. For continuous monitoring of blood pressure and application of dyes, a fine polyurethane catheter was inserted into the right carotid artery. The abdomen was then opened through a midline incision and the abdominal cavity inspected for adhesions or perforation. The descending colon was freed from adhesions and exteriorized on a specially designed stage attached to the microscope. The tissue was superfused with warmed (37°C) Ringer lactate solution to avoid drying. Special care was taken to avoid tension on the mesentery because this would have caused alterations in perfusion of the mucosa. To study the mucosal microcirculation, a longitudinal incision was placed at the antimesenteric border of the bowel wall followed by intravital microscopy of the mucosa on the opposite side of the incision.
Rather than determining colonic blood flow only (Foitzik et al., 1999), the microcirculatory analysis method described for the small bowel was adopted (Heuser et al., 2000; Ruh et al., 2000) to analyse the perfusion status of the colon. Intravital microscopy was performed with an Axiotech Vario 100 microscope for epifluorescence measurements (Zeiss) fitted with a HBO 100 mercury lamp. By using 10×(long distance), 20× and 40×(water immersion) objectives, a magnification of 243×, 476× and 933× was reached. Changes in fluorescence intensity were monitored with a CCD video camera (CF 8/1, Kappa) attached to a video system for off-line evaluation.
The intestinal microcirculation was visualized after injection of 0.8 ml 0.5% fluoresceine isothyocyanate (FITC)-labeled dextran (Sigma). A minimum of 10 randomly chosen regions of the mucosa were analysed for 40–60 s to obtain representative values for the perfusion index (PI) and the functional capillary density (FCD). PI was defined as per cent of perfused capillaries per mucosal area plus 0.5x% of all irregularly perfused capillaries per mucosal area at 243× magnification, and FCD as length of perfused nutritive capillaries per mucosal area at 243× magnification (Heuser et al., 2000). A minimum of 10 capillaries per region (total 100 capillaries) were analysed to measure red blood cell velocity (RBCV in mm s−1 at 933× magnification). Functional capillary density was assessed by computer-assisted image analysis and RBCV by line to shift analysis with the CAPIMAGE software package (Zeintl). To study leukocyte-endothelial cell interactions, submucosal postcapillary venules were monitored without opening the bowel. For leukocyte visualization, 0.2 ml 0.1% rhodamine-6 G (Sigma) was injected intraarterially. Adherent leukocytes (stickers) were defined in each vessel segment (100 μm) as cells that did not move or detach from the endothelium within 20 s, and calculated as number of cells per mm2 of endothelial surface which by assuming a cylindrical geometry was inferred from the diameter and length of the venule.
RT–PCR analysis
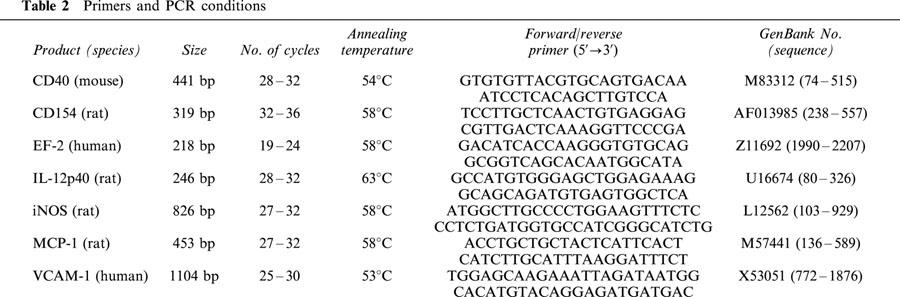
The frozen intestinal segments were minced under liquid N2 with the aid of a mortar and pestle. Total RNA was isolated with the Qiagen RNeasy kit (Qiagen, Hilden, Germany) followed by semi-quantitative RT–PCR analysis for CD40, CD40 ligand (CD154), CD106 (VCAM-1), elongation factor-2 (EF-2) as an internal standard, inducible nitric oxide synthase (iNOS), interleukin-12 p40 (IL-12), and monocyte chemoattractant protein-1 (MCP-1), essentially as described previously (Krzesz et al., 1999; Lauth et al., 2000). Individual PCR conditions are provided in Table 2. To be within the exponential phase of the PCR reaction, the appropriate number of cycles was newly established for each set of samples. The identity of the amplification products was verified by cloning and sequencing (Krzesz et al., 1999; Lauth et al., 2000). EF-2 cDNA amplification was essentially the same irrespective of whether the extracted mRNA was derived from inflamed or non-inflamed tissue.
Table 2.
Primers and PCR conditions
Statistical analysis
Unless indicated otherwise, all data in the figures and text are expressed as means±s.e.mean of n independent observations (i.e., samples from different animals). Statistical evaluation was performed either by one-way analysis of variance followed by Bonferroni multiple comparisons test (comparison of three or more groups) or unpaired two-tailed Students t-test (comparison of two groups) where appropriate with the Instat for Windows™ statistics software package (GraphPad Software) and a P value <0.05 considered statistically significant.
Results
Characteristics of the colitis model
After testing various combinations of TNBS (5–50 mg) and ethanol (20–50%), a single enema consisting of 20 mg TNBS in 35% ethanol was found to reproducibly induce a transient Crohn's disease-like colitis with a maximum inflammatory response at 3–5 days and spontaneous healing after approximately 4 weeks.
Seven days after the administration of TNBS/ethanol, significant oedema formation together with focal ulcerations, necrosis and adhesions was observed in almost all control animals (total score 8.7±0.7 of a maximum of 17, cf. Table 1, n=11). Histologically, damage to the intestinal wall appeared to be discontinuous with areas of normal mucosa next to severely necrotic ones (Figure 2a,b) or with one side of the mucosa only being affected. Moderate fibrosis was frequently apparent as well as neovascularization (Figures 2d and 5b) and, above all, a prominent infiltration of leukocytes, namely neutrophils but also monocytes and lymphocytes which tended to accumulate in or around venule-like structures in the submucosa (Figure 2c,d). This enhanced leukocyte infiltration could also be inferred from the intravital microscopy data which revealed a more than 5 fold increase in the number of leukocytes sticking to the endothelium in the postcapillary venules of the submucosa (Figure 3a). In addition, functional capillary density and mucosal perfusion were clearly reduced in animals treated with TNBS/ethanol (Figure 3b–e).
Figure 2.
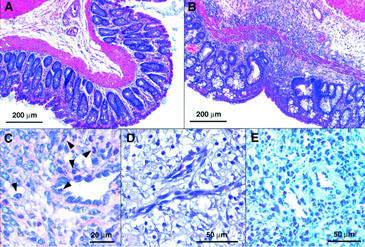
Morphology of TNBS/ethanol-induced colitis 7 days after the enema (representative histology data of at least six animals in each group). Whereas no pathological disorder was present in the descending colon of control rats (A), affected areas of the descending colon of TNBS-treated animals revealed ulcers (B), leukocyte infiltrates (B–E) and neovascularisation (D). Staining: Haematoxylin/eosin (A,B,D), Giemsa (C), haematoxylin plus secondary swine-anti-rabbit antibody (negative control for IL-8 immunoreactivity; E). Arrows in C indicate the presence of polymorphonuclear neutrophils.
Figure 5.
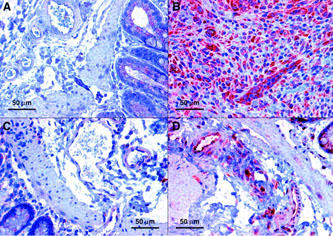
Abundance and localization of IL-8 protein in TNBS/ethanol-induced colitis 7 days after the enema. Representative sections showing weak IL-8 immunoreactivity in and around submucosal blood vessels of the descending colon from (A) control rats and (C) TNBS-treated animals receiving thalidomide, but moderate to strong IL-8 immunoreactivity in animals receiving (D) supidimide or (B) olive oil only. Comparable results were obtained with at least five additional specimens in each group of animals.
Figure 3.
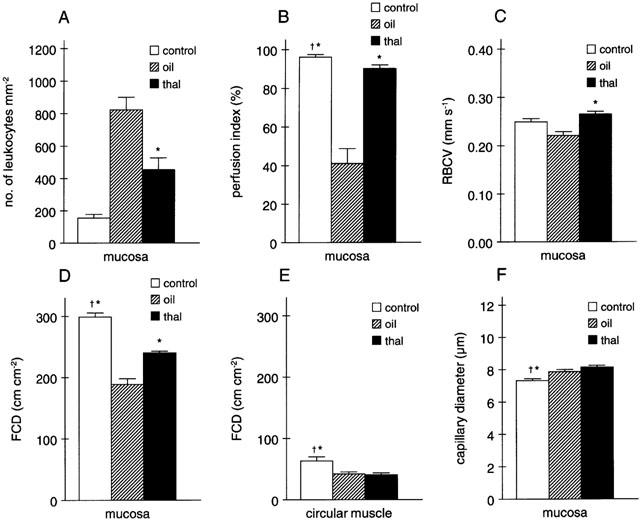
Effects of treatment with thalidomide (thal) or vehicle (oil) on (A) leukocyte adhesion to the submucosal endothelium in the descending colon, (B) perfusion index in the mucosa, (C) red blood cell velocity (RBCV) in the mucosa, (D,E) functional capillary density (FCD) in the mucosa and circular muscle, respectively, and (F) capillary diameter in rats 7 days after the TNBS/ethanol enema as compared to the situation in control animals not receiving TNBS (n=3 for each group with 10 randomly chosen regions analysed per animal; *P<0.05 vs olive oil, †P<0.05 vs thalidomide).
According to RT–PCR analysis, expression of CD154, iNOS and VCAM-1 mRNA was significantly increased in the affected intestinal wall (cf. Figure 4a–c), indicative of the ongoing inflammatory response. There was also a trend towards an increased expression of MCP-1 mRNA while expression of CD40 and IL-12 were essentially unchanged (not shown, cf. Table 5). Basal expression of the aforementioned gene products in the non-affected proximal colon was not different from that in control animals not receiving TNBS/ethanol (not shown). Moreover, immunohistochemistry revealed a prominent increase in expression of IL-8 by the microvascular endothelium (cf. Figure 5a,b) and a diffuse increase in E-selectin abundance in the affected distal colon, presumably representing soluble E-selectin (not shown).
Figure 4.
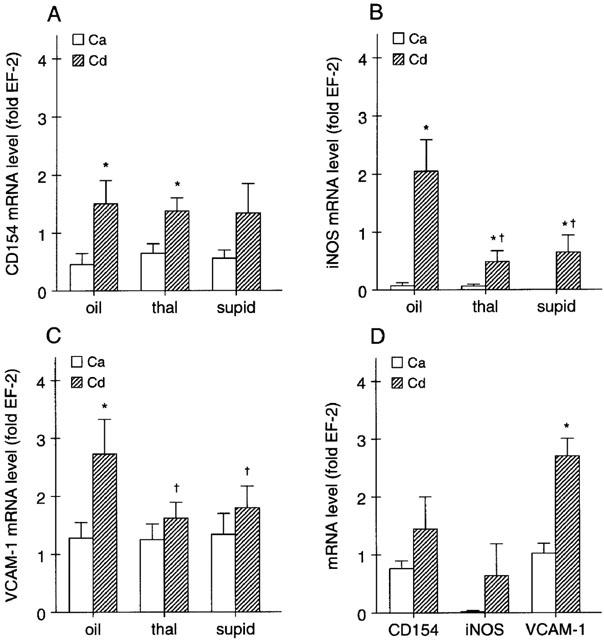
(A-C) Effects of daily treatment with thalidomide (thal), supidimide (supid) or vehicle on (A) CD154, (B) iNOS and (C) VCAM-1 mRNA abundance (expressed relative to the mRNA level of the house-keeping gene, EF-2) in the ascending colon (Ca; i.e., unaffected region) and descending colon (Cd; i.e., affected region) of rats 7 days after the TNBS/ethanol enema (n=7–9, *P<0.05 vs Ca, †P<0.05 vs olive oil). (D) Abundance of CD154, iNOS and VCAM mRNA in the Ca and Cd of control rats 14 days after the TNBS/ethanol enema (n=5–6, *P<0.05 vs Ca).
Table 5.
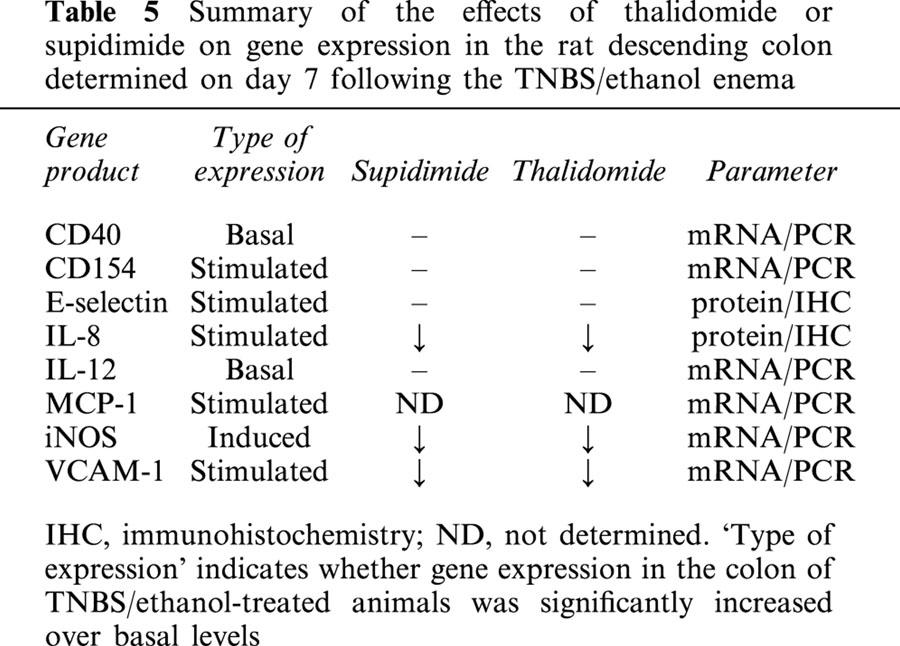
Summary of the effects of thalidomide or supidimide on gene expression in the rat descending colon determined on day 7 following the TNBS/ethanol enema
Fourteen days after the enema, damage to the colon was still apparent but clearly reduced as compared to the situation at day 7 (cf. Tables 3 and 4). Of the eight gene products analysed, only expression of VCAM-1 remained significantly elevated at this point in time (Figure 4d).
Table 3.
Effects of daily treatment with thalidomide or supidimide (200 mg kg−1 d−1) on macroscopic appearance of the descending colon 7 days after the TNBS/ethanol enema
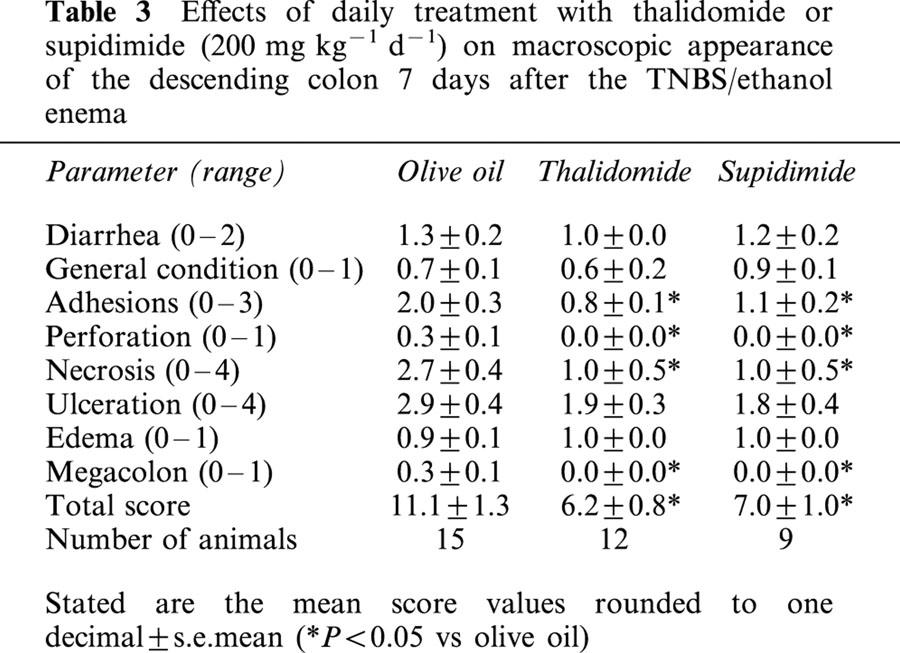
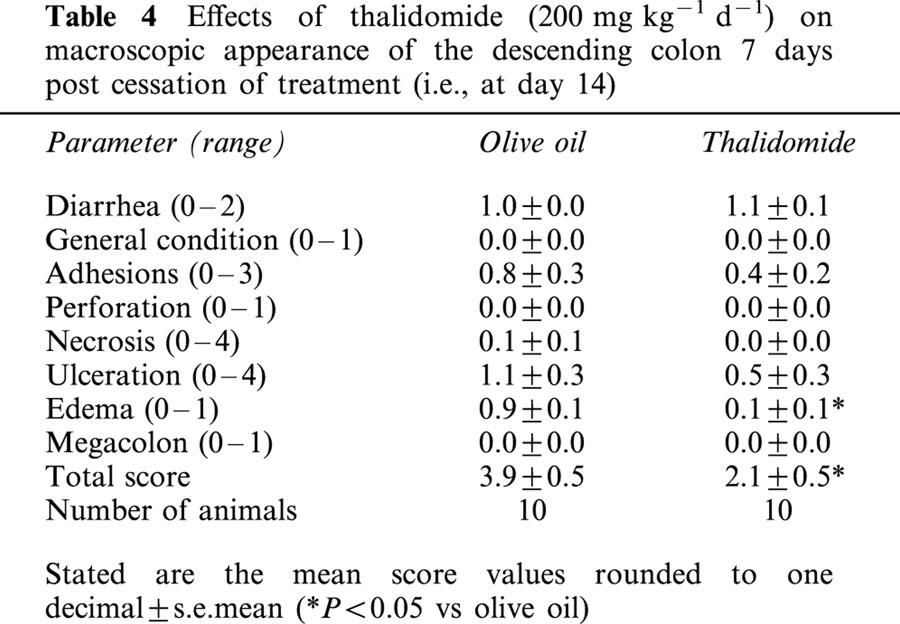
Table 4.
Effects of thalidomide (200 mg kg−1 d−1) on macroscopic appearance of the descending colon 7 days post cessation of treatment (i.e., at day 14)
Effects of thalidomide
After having established the colitis model, we asked the question whether thalidomide or its analogue is capable of attenuating colonic inflammation. There was a significant reduction in overall damage (macroscopic score) both in the thalidomide (44% reduction) and in the supidimide group (37% reduction) as compared to the vehicle-treated control animals (Table 3). Of note was that the most severe type of damage, necrosis, was reduced both in the thalidomide and in the supidimide group to about one-third of that in the olive oil-treated control group. There were also significant reductions in the severity of adhesions, perforation and megacolon formation in both treatment groups (Table 3). This anti-inflammatory effect of thalidomide was long lasting for evaluation of colonic damage 7 days post cessation of thalidomide treatment (i.e., at day 14) still revealed a significant protective effect (46% reduction in the total score as compared to the olive oil-treated control group, Table 4).
Both thalidomide and supidimide treatment significantly reduced the increase in iNOS and VCAM mRNA abundance, but not that of CD154 at day 7 (Figure 4a–c). CD40 and IL-12 mRNA expression as well as E-selectin immunoreactivity, on the other hand were not affected by both agents (cf. Table 5, not shown). Immunohistochemistry analysis, on the other hand, revealed a strong reduction in IL-8 expression in the microvascular endothelium of rats treated with thalidomide, and to a lesser extent also in the supidimide group (Figure 5c,d). This result was in good agreement with the inhibitory effect of thalidomide (45% inhibition) on endothelial cell-leukocyte interaction in the postcapillary venules of the submucosa (Figure 3a) and overall leukocyte infiltration (Figure 5c). Moreover, thalidomide treatment improved perfusion index (Figure 3b), blood flow velocity (Figure 3c), and functional capillary density in the mucosa (Figure 3d) but not in the circular muscle (Figure 3e). Treatment of control rats with thalidomide (200 mg kg−1 d−1) only for 7 days, on the other hand, had no significant effect on these parameters (Figure 6). Similarly, short-term treatment with 200 mg kg−1 thalidomide 1 day before and on the day of analysis had no effect on leukocyte sticking to the endothelium in the submucosal venules of the small intestine (229±36 leukocytes/mm2 vs 209±30 leukocytes/mm2, n=3).
Figure 6.
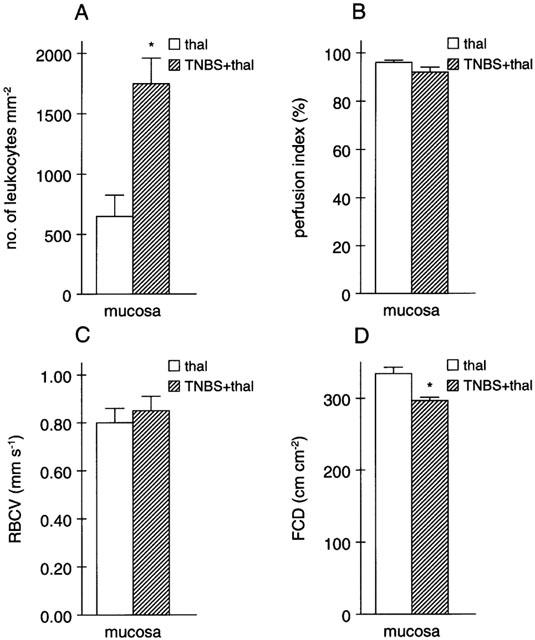
Effects of thalidomide treatment alone for 7 days (thal), as compared to daily thalidomide treatment after the TNBS/ethanol enema (TNBS+thal), on (A) endothelial cell-leukocyte interaction, (B) perfusion index, (C) red blood cell velocity (RBCV) and (D) functional capillary density (FCD) in the mucosa of the descending colon (n=3 for each group with 10 randomly chosen regions analysed per animal; *P<0.05 vs thalidomide). Note that due to the larger size of the venules analysed in these animals, the absolute number of leukocytes sticking to the endothelium as well as absolute RBCV differ from the values shown in Figure 3.
Discussion
The present findings demonstrate that thalidomide attenuates TNBS-induced colitis in the rat, an established model for human Crohn's disease (Morris et al., 1989), and this effect is maintained for several days post cessation of treatment. In a separate series of experiments with six animals per treatment group, administration of thalidomide for 1 week also seemed to accelerate the spontaneous healing process at 2 weeks post TNBS/ethanol administration, hence acting in a therapeutic manner (not shown).
Thalidomide was effective at a dose of 200 mg kg−1 d−1 which is almost two orders of magnitude greater than the clinically effective dose in man (Ehrenpreis et al., 1999; Vasiliauskas et al., 1999) but typical for rodents where the half-life of this type of drug is much shorter (2–3 h as compared to 6–8 h in humans) with an almost complete excretion (Becker et al., 1982). Thus, relatively high doses are required in most animal experiments to observe the immunomodulatory effects of thalidomide. In the rat arthritis model, thalidomide was administered orally at 200 mg kg−1 once or twice daily (Oliver et al., 1998). In a hamster cheek pouch model for studying endothelial cell-leukocyte interaction, thalidomide was given i.p. in the dose range of 20–200 mg kg−1 with a clear dose-dependent effect (Schneider et al., 1997). Furthermore, the anti-angiogenic activity of thalidomide was evident at oral doses of 200 mg kg−1 twice daily but not at 50 mg kg−1 in a rabbit model (Joussen et al., 1999). At the experimental animal level, the present findings thus confirm the recently documented therapeutic effect of thalidomide in patients with Crohn's disease (Ehrenpreis et al., 1999; Vasiliauskas et al., 1999).
The protective effect of thalidomide in the rat model, which is mimicked by its analogue supidimide, appears to be brought about by two mechanisms: (i) a more general antiinflammatory effect as suggested by the inhibition of iNOS mRNA expression, but not that of CD40, CD154, IL-12 or E-selectin (Table 5) in the inflamed intestinal wall; and (ii) a more specific attenuation of endothelial cell-leukocyte interaction in postcapillary venules of the submucosa, presumably brought about by inhibiting the release of IL-8 from the microvascular endothelium and, in addition, by limiting the enhanced expression of VCAM-1 by these cells. Interestingly, chronic IBD in humans also presents with an increased expression of iNOS and IL-8 (McLaughlan et al., 1997). IL-8 is not only a potent chemokine for polymorphonuclear neutrophils, but also activates leukocytes at sites of inflammation by up-regulating expression of the β2 integrin CD11b/CD18 (Mac-1) in these cells (Detmers et al., 1991). CD11b/CD18 interacts with ICAM-1 or E-selectin on the endothelial cells, and therefore IL-8 release may represent a crucial step in the effector phase of colonic inflammation (Strober et al., 1998). Indeed, antibodies blocking CD11b/CD18 have been shown to reduce inflammation in TNBS-induced colitis in the rat (Palmen et al., 1995). Moreover, a monoclonal antibody against IL-8 suppressed both endothelial cell-leukocyte interaction and the signs of colonic inflammation in a related rat model of chronic IBD (Arndt et al., 1996). In line with these findings is a recent report stating that thalidomide is capable of attenuating IL-8 synthesis in endothelial cells stimulated with LPS but not TNFα (Dunzendorfer et al., 1999). As endothelial cell-leukocyte interaction was still markedly elevated 7 days after the initiation of colitis, an effect that was significantly attenuated by thalidomide treatment, it would appear, and this can also be inferred from the IHC and RT–PCR data, that IL-8 release and VCAM-1 expression by the microvascular endothelium is still elevated at this point in time, thus perpetuating the inflammatory response. That thalidomide can directly interfere with endothelial cell-leukocyte interaction has also been demonstrated in the hamster cheek pouch model mentioned before where systemic thalidomide inhibited the numbers of rolling, firmly adherent and migrating leukocytes irrespective of the stimulus used, i.e. topical LPS or TNFα (McLaughlan et al., 1997).
In contrast to IL-8, the role of iNOS in TNBS-induced colitis is less clear (McLaughlan et al., 1997). Enhanced production of nitric oxide in the colon may have both beneficial and deleterious effects. It would appear, however, that the deleterious effects prevail such as the induction of a toxic megacolon (Guslandi, 1998), an increase in macrophage cytokine production including IL-8 (Southey et al., 1997), and an increased cytotoxicity due to peroxynitrite formation (Dijkstra et al., 1998). This assumption is also supported by a recent study showing that genetic disruption of the iNOS gene significantly attenuates TNBS-induced colitis in mice (Zingarelli et al., 1999). The inhibitory effect of both thalidomide and supidimide on iNOS mRNA expression may thus have contributed to their anti-inflammatory effect.
In mice, TNBS-induced colitis appears to be induced by a polarized TH1 response in the lamina propria that is not counteracted by transforming growth factor-β producing T cells, and involves the release of IL-12 from antigen-presenting cells. The resulting unfettered interferon-γ production leads to the activation of macrophages, release of pro-inflammatory cytokines, and development of colitis (Elson et al., 1995; Strober et al., 1998). This classical delayed-type hypersensitivity response to a contact allergen (which bypasses the normal oral tolerance mechanisms) is thought to play an important role also in Crohn's disease (Battaglia et al., 1999; Liu et al., 1999; Sartor, 1997; Strober et al., 1998). If this sequence of events was also true for TNBS-induced colitis in the rat, one would expect an increased expression of IL-12 (e.g., derived from antigen-presenting cells) and CD154 (as a marker for the activated TH1 cells) in the inflamed colon. While there was no appreciable increase in IL-12 mRNA abundance, CD154 expression was indeed significantly enhanced 7 days after the TNBS/ethanol enema. However, thalidomide or supidimide treatment did not affect CD154 expression, suggesting that their beneficial effect is unrelated to a suppression of T cell infiltration and/or activation. Moreover, contrary to what may be inferred from its inhibitory effect on IL-12 synthesis in human peripheral blood monocytes (Moller et al., 1997), thalidomide did not affect basal IL-12 mRNA expression in the rat colitis model. However, interpretation of these RT–PCR data should be done with some caution. Despite being based on monitoring expression of a house-keeping gene as an internal standard, this method only allows for a relative comparison of mRNA abundance between samples from the ascending and descending colon of the same animal. Moreover, RT–PCR analysis can only provide a gross overview of the changes in gene expression in the intestinal wall rather than ascribing these to individual populations of cells.
The aforementioned results nonetheless suggest that thalidomide specifically rather than uniformly attenuates the expression of pro-inflammatory gene products in the rat colitis model. On the other hand, thalidomide treatment significantly ameliorated perfusion index, functional capillary density and blood flow velocity in the mucosa 7 days after the TNBS/ethanol enema, suggesting that the drug may have an additional effect on the synthesis or mechanism of action of an endogenous vasoconstrictor substance. Preliminary evidence from this laboratory suggest that there is indeed an increase in endothelin-2 mRNA expression in the intestinal wall and in particular in the terminal branches of the mesenteric artery of TNBS/ethanol-treated rats 7 days after the enema which may contribute both to the microcirculatory disturbances (Miura et al., 1996) and the increased endothelial cell-leukocyte interaction (Boros et al., 1998). Clearly this hypothesis warrants further investigation. What also remains to be tested is whether the anti-inflammatory effect of thalidomide is perhaps even more pronounced if the drug is administered as a suppository rather than a ‘tablet'.
In summary, the aforementioned findings suggest that thalidomide impairs Crohn's disease-like TNBS-induced colitis in the rat, and that this effect is mediated at least in part by downregulating endothelial chemokine and adhesion molecule expression and, as a consequence, the recruitment of circulating leukocytes, namely polymorphonuclear neutrophils, which may represent a crucial step in the effector phase of colonic inflammation. Moreover, these findings may help to explain the therapeutic effect of thalidomide in patients with Crohn's disease.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 402, project C9). The authors are indebted to Renate Dohrmann and Annette Bennemann for expert technical assistance.
Abbreviations
- FCD
functional capillary density
- IBD
inflammatory bowel disease
- IL
interleukin
- iNOS
inducible isoform of NO synthase
- LPS
bacterial lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- RBCV
red blood cell velocity
- TNBS
trinitrobenzene sulphonic acid
- TNFα
tumor necrosis factor α
References
- ARNDT H., BOLANOWSKI M.A., GRANGER D.N. Role of interleukin 8 on leucocyte-endothelial cell adhesion in intestinal inflammation. Gut. 1996;38:911–915. doi: 10.1136/gut.38.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTAGLIA E., BIANCONE L., RESEGOTTI A., EMANUELLI G., FRONDA G.R., CAMUSSI G. Expression of CD40 and its ligand, CD40L, in intestinal lesions of Crohn's disease. Am. J. Gastroenterol. 1999;94:3279–3284. doi: 10.1111/j.1572-0241.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- BECKER R., FRANKUS E., GRAUDUMS I., GUNZLER W.A., HELM F.C., FLOHE L. The metabolic fate of supidimide in the rat. Arzneimittelforsch. 1982;32:1101–1111. [PubMed] [Google Scholar]
- BOROS M., MASSBERG S., BARANYI L., OKADA H., MESSMER K. Endothelin-1 induced leukocyte adhesion in submucosal venules of the rat small intestine. Gastroenterology. 1998;114:103–114. doi: 10.1016/s0016-5085(98)70638-9. [DOI] [PubMed] [Google Scholar]
- CORRAL L.G., HASLETT P.A., MULLER G.W., CHEN R., WONG L.M., OCAMPO C.J., PATTERSON R.T., STIRLING D.I., KAPLAN G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J. Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- DETMERS P.A., POWELL D.E., WALZ A., CLARK-LEWIS I., BAGGIOLINI M., COHN Z.A. Differential effects of neutrophil-activating peptide 1/IL-8 and its homologues on leukocyte adhesion and phagocytosis. J. Immunol. 1991;147:4211–4217. [PubMed] [Google Scholar]
- DIJKSTRA G., MOSHAGE H., VAN DULLEMEN H.M., DE JAGER-KRIKKEN A., TIEBOSCH A.T., KLEIBEUKER J.H., JANSEN P.L., VAN GOOR H. Expression of nitric oxide synthases and formation of nitrotyrosine and reactive oxygen species in inflammatory bowel disease. J. Pathol. 1998;186:416–421. doi: 10.1002/(SICI)1096-9896(199812)186:4<416::AID-PATH201>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- DUNZENDORFER S., HEROLD M., WIEDERMANN C.J. Inducer-specific bidirectional regulation of endothelial interleukin-8 production by thalidomide. Immunopharmacology. 1999;43:59–64. doi: 10.1016/s0162-3109(99)00041-7. [DOI] [PubMed] [Google Scholar]
- EHRENPREIS E.D., KANE S.V., COHEN L.B., COHEN R.D., HANAUER S.B. Thalidomide therapy for patients with refractory Crohn's disease: an open-label trial. Gastroenterology. 1999;117:1271–1277. doi: 10.1016/s0016-5085(99)70276-3. [DOI] [PubMed] [Google Scholar]
- ELSON C.O., SARTOR R.B., TENNYSON G.S., RIDDEL R.H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- FOITZIK T., KRUSCHEWSKI M., KROESEN A., BUHR H.J. Does microcirculation play a role in the pathogenesis of inflammatory bowel diseases? Answers from intravital microscopic studies in animal models. Int. J. Colorectal Dis. 1999;14:29–34. doi: 10.1007/s003840050179. [DOI] [PubMed] [Google Scholar]
- GUSLANDI M. Nitric oxide and inflammatory bowel diseases. Eur. J. Clin. Invest. 1998;28:904–907. doi: 10.1046/j.1365-2362.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- HEUSER M., POPKEN O., KLEIMAN I., POST S. Detrimental effects of octreotide on intestinal microcirculation. J. Surg. Res. 2000;92:186–192. doi: 10.1006/jsre.2000.5898. [DOI] [PubMed] [Google Scholar]
- HODGSON H.J. Pathogenesis of Crohn's disease. Baillieres Clin. Gastroenterol. 1998;12:1–17. doi: 10.1016/s0950-3528(98)90083-5. [DOI] [PubMed] [Google Scholar]
- JOUSSEN A.M., GERMANN T., KIRCHHOF B. Effect of thalidomide and structurally related compounds on corneal angiogenesis is comparable to their teratological potency. Graefes Arch. Clin. Exp. Ophthalmol. 1999;237:952–961. doi: 10.1007/s004170050330. [DOI] [PubMed] [Google Scholar]
- KRZESZ R., WAGNER A.H., CATTARUZZA M., HECKER M. Cytokine-inducible CD40 expression in vascular smooth muscle cells is mediated by nuclear factor κB and signal transducer and activator of transcription 1. FEBS Lett. 1999;453:191–196. doi: 10.1016/s0014-5793(99)00683-3. [DOI] [PubMed] [Google Scholar]
- LAUTH M., BERGER M.M., CATTARUZZA M., HECKER M. Pressure-induced upregulation of preproendothelin-1 and endothelin B receptor expression in rabbit jugular vein in situ - implications for vein graft failure? Arterioscler. Thromb. Vasc. Biol. 2000;20:96–103. doi: 10.1161/01.atv.20.1.96. [DOI] [PubMed] [Google Scholar]
- LIU Z., COLPAERT S., D'HAENS G.R., KASRAN A., DE BOER M., RUTGEERTS P., GEBOES K., CEUPPENS J.L. Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J. Immunol. 1999;163:4049–4057. [PubMed] [Google Scholar]
- MACDERMOTT R.P. Chemokines in the inflammatory bowel diseases. J. Clin. Immunol. 1999;19:266–272. doi: 10.1023/a:1020583306627. [DOI] [PubMed] [Google Scholar]
- MCCAFFERTY D.M., SHARKEY K.A., WALLACE J.L. Beneficial effects of local or systemic lidocaine in experimental colitis. Am. J. Physiol. 1994;266:G560–G567. doi: 10.1152/ajpgi.1994.266.4.G560. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLAN J.M., SETH R., VAUTIER G., ROBINS R.A., SCOTT B.B., HAWKEY C.J., JENKINS D. Interleukin-8 and inducible nitric oxide synthase mRNA levels in inflammatory bowel disease at first presentation. J. Pathol. 1997;181:87–92. doi: 10.1002/(SICI)1096-9896(199701)181:1<87::AID-PATH736>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- MIURA S., FUKUMURA D., KUROSE I., HIGUCHI H., KIMURA H., TSUZUKI Y., SHIGEMATSU T., HAN J.Y., TSUCHIYA M., ISHII H. Roles of ET-1 in endotoxin-induced microcirculatory disturbance in rat small intestine. Am. J. Physiol. 1996;271:G461–G469. doi: 10.1152/ajpgi.1996.271.3.G461. [DOI] [PubMed] [Google Scholar]
- MOLLER D.R., WYSOCKA M., GREENLEE B.M., MA X., WAHL L., FLOCKHART D.A., TRINCHIERI G., KARP C.L. Inhibition of IL-12 production by thalidomide. J. Immunol. 1997;159:5157–5161. [PubMed] [Google Scholar]
- MORRIS G.P., BECK P.L., HERRIDGE M.S., DEPEW W.T., SZEWCZUK M.R., WALLACE J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterol. 1989;96:795–803. [PubMed] [Google Scholar]
- OLIVER S.J., CHENG T.P., BANQUERIGO M.L., BRAHN E. The effect of thalidomide and 2 analogs on collagen induced arthritis. J. Rheumatol. 1998;25:964–969. [PubMed] [Google Scholar]
- PALMEN M.J., DIJKSTRA C.D., VAN DER ENDE M.B., PENA A.S., VAN REES E.P. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin. Exp. Immunol. 1995;101:351–356. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANES J., GRANGER D.N. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- ROGLER G., ANDUS T. Cytokines in inflammatory bowel disease. World J. Surg. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- RUH J., VOGEL F., SCHMIDT E., WERNER M., KLAR E., SECCHI A., GEBHARD M.M., GLASER F., HERFARTH C. Effects of hydrogen peroxide scavenger catalase on villous microcirculation in the rat small intestine in a model of inflammatory bowel disease. Microvasc. Res. 2000;59:329–337. doi: 10.1006/mvre.1999.2201. [DOI] [PubMed] [Google Scholar]
- SALMI M., GRANFORS K., MACDERMOTT R., JALKANEN S. Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases. Gastroenterology. 1994;106:596–605. doi: 10.1016/0016-5085(94)90691-2. [DOI] [PubMed] [Google Scholar]
- SARTOR R.B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am. J. Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- SCHNEIDER J., BRUCKMANN W., ZWINGENBERGER K. Extravasation of leukocytes assessed by intravital microscopy: Effect of thalidomide. Inflamm. Res. 1997;46:392–397. doi: 10.1007/s000110050209. [DOI] [PubMed] [Google Scholar]
- SOUTHEY A., TANAKA S., MURAKAMI T., MIYOSHI H., ISHIZUKA T., SUGIURA M., KAWASHIMA K., SUGITA T. Pathophysiological role of nitric oxide in rat experimental colitis. Int. J. Immunopharmacol. 1997;19:669–676. doi: 10.1016/s0192-0561(97)00107-0. [DOI] [PubMed] [Google Scholar]
- STROBER W., LÚDVÍKSSON B.R., FUSS I.J. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann. Intern. Med. 1998;128:848–856. doi: 10.7326/0003-4819-128-10-199805150-00009. [DOI] [PubMed] [Google Scholar]
- VAINER B. Role of cell adhesion molecules in inflammatory bowel diseases. Scand. J. Gastroenterol. 1997;32:401–410. doi: 10.3109/00365529709025072. [DOI] [PubMed] [Google Scholar]
- VASILIAUSKAS E.A., KAM L.Y., ABREU-MARTIN M.T., HASSARD P.V., PAPADAKIS K.A., YANG H., ZELDIS J.B., TARGAN S.R. An open-label pilot study of low-dose thalidomide in chronically active, steroid-dependent Crohn's disease. Gastroenterology. 1999;117:1278–1287. doi: 10.1016/s0016-5085(99)70277-5. [DOI] [PubMed] [Google Scholar]
- WAKEFIELD A.J., EKBOM A., DHILLON A.P., PITTILO R.M., POUNDER R.E. Crohn's disease: pathogenesis and persistent measles virus infection. Gastroenterology. 1995;108:911–916. doi: 10.1016/0016-5085(95)90467-0. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., SZABO C., SALZMAN A.L. Reduced oxidative and nitrosative damage in murine experimental colitis in the absence of inducible nitric oxide synthase. Gut. 1999;45:199–209. doi: 10.1136/gut.45.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]


