Abstract
This in vitro study was designed to determine the potential use of the NK1 antagonist, SR140333 as an anti-diarrhoeal treatment for food allergy or inflammatory bowel disease. The effect of various immune and neuronal stimuli on human colonic substance P (SP) release and the effect of SR140333 on subsequently stimulated mucosal ion transport was investigated.
Submucosal and sensory nerve fibre stimulation using electrical field stimulation (1 ms/7 Hz/7 V) and capsaicin (50 μM) respectively, mast cell activation by anti-IgE (1/250 dilution) and granulocyte stimulation using fMLP (50 μM) each released SP and evoked a secretory response.
SP and the NK1 selective agonist, Sar-SP (0.1–1000 nM) stimulated an increase in colonic secretion which was antagonized by SR140333 (pD′2=6.7 and 7.25 versus SP and Sar-SP respectively).
SR140333, at a concentration that blocked NK1-mediated secretion (500 nM), also reduced the secretory response to both αIgE and capsaicin. This suggests a pathophysiologic role for NK1 receptors.
Capsaicin evoked SP release was increased in tissue taken from Crohn's disease but not ulcerative colitis patients. The response to SP was however reduced by 70 and 89% respectively.
Mast cells and sensory afferents contribute to allergic diarrhoea. Since SR140333 reduced the secretory response to mast cell and afferent stimulation this compound may be particularly useful in reducing the symptoms of food allergy.
Keywords: Afferent, allergy, granulocyte, IBD, mast cell
Introduction
The tachykinins belong to a family of peptides including the products of two genes, the preprotachykinin (PPT) I gene, which produces substance P (SP) and neurokinin (NK) A (Nawa et al., 1983), and the PPT II gene, which produces neurokinin B (Kotani et al., 1986). These tachykinins preferentially bind to NK1, NK2 and NK3 receptors respectively. A wide range of synthetic agonists and antagonists exists for these receptor subtypes. Of these, Sar-SP (NK1; Drapeau et al., 1987) βAla-NKA (NK1/NK2; Rovero et al., 1989) and senktide (NK3; Laufer et al., 1988) are the most frequently used agonists, whilst on the other hand, the antagonists, SR140333 (NK1; Emonds-Alt et al., 1993a) and SR48968 (NK2, Emonds-Alt et al., 1993b) have been well characterized.
SP and NKA are found in the mucosa and muscle layer of the intestinal tract (Maggi et al., 1992) and tachykinin agonists have been shown to contract the circular muscle of human small and large intestine through NK2 receptors (Croci et al., 1998; Giuliani et al., 1991). In addition to possessing powerful contractile activity we have also recently described the secretory activity of the tachykinins in the guinea-pig (Goldhill & Angel, 1998). In this species, neural NK1 and NK3 receptors both induce colonic secretion, however little is known regarding the tachykinergic control of human colonic epithelium. One very recent paper (Riegler et al., 1999) has reported that SP induces non-cholinergic nerve-mediated secretion. This was inhibited by the NK1 antagonist, CP96345. Consistent with this observation is the visualization of NK1 receptors on sub-mucosal nerves.
During certain T helper cell type 2-mediated conditions such as allergy and parasitic infection, mast cells come into close apposition with tachykinergic fibres and hence it has been suggested that neural release of SP may mediate functional changes in mastocytic disorders (Stead et al., 1987). Further supportive data from humans is however required to establish a role for tachykinin antagonists in allergic diarrhoea. The observation that immunocytes may be a rich source of tachykinins (Aliakbari et al., 1987) suggests that during inflammation, SP levels may be increased as a result of infiltrating leukocytes. As well as being released by immunocytes, SP has numerous immune regulatory properties including chemotaxis and adhesion (Schratzberger et al., 1997; Brunelleschi et al., 1990; Perretti et al., 1993; Baluk et al., 1995). Furthermore, SP levels are increased (Goldin et al., 1989) and its receptor is over-expressed in IBD, a chronic inflammatory condition characterized by diarrhoea (Mantyh et al., 1988). As a result of these findings tachykinin antagonists such as SR140333 have been proposed as potential anti-inflammatory compounds (Mazelin et al., 1998). These authors demonstrated that SR140333 is able to reduce inflammation in animal models. Although this provides evidence for anti-inflammatory activity it is unclear whether the anti-secretory activity of SR140333 could prevent the diarrhoea associated with chronic diseases such as IBD. The aims of the present study were to determine the therapeutic potential of SR140333 in both allergic disorders and inflammatory bowel disease.
Methods
Human tissue
Tissue was taken immediately after excision and prepared for the study of epithelial transport or SP release. Control tissue was taken from patients undergoing surgery for colon cancer. This tissue was taken at a site distant to the tumour and was histologically normal. Inflamed tissue was obtained from ulcerative colitis or Crohn's disease patients. Tissue from Crohn's patients was divided into two groups designated involved or uninvolved depending on the presence of overt inflammation. This study was approved by the University Hospital's ethical committee.
Animal studies
A comparative study was performed to assess species differences with respect to the colonic activity of SR140333. Thus in addition to obtaining human tissue, male Hartley guinea-pigs (450–800 g; Charles River, France) and male Wistar rats (250–500 g; Charles River, France) were sacrificed using a stunning blow or an overdose of sodium pentobarbital respectively followed by desanguination. The distal colon was then removed. Animal protocols followed the guidelines set out by the Declaration of Helsinki
Colonic epithelial preparation
The colonic epithelial preparation has previously been well documented (Bunce et al., 1991). Briefly, the outer muscle layer was separated from the colonic mucosa and the resultant epithelial preparation mounted as a flat sheet between two Ussing chambers. Both the mucosal and serosal surfaces were circulated with Krebs buffer using a gas-lift (95%O2/5%CO2; pre-humidified by bubbling through distilled water), and maintained at 37±1°C. Short-circuit current (SCC) generated by the epithelium was continuously monitored using an EVC4000 voltage clamp (WPI, U.S.A.). In order to do this, one voltage sensing and one current passing electrode was inserted into each half chamber, and the electrodes connected to the EVC4000 via a pre-amplifier. The voltage generated by the epithelium was continuously short-circuited by passing current across the tissue using the current passing electrodes. Following a 30 min stabilization period, the mucosa was stimulated electrically (1 ms/7 Hz/7 V) or by capsaicin (50 μM) to activate enteric nerves and fMLP (50 μM) or αlgE (1/250 dilution) to activate granulocytes or mast cells respectively. All compounds were administered to the serosal solution for 10 min. In addition to these stimuli, cumulative concentration response curves were constructed for tachykinin agonists with successive concentrations being administered at 10 min intervals. Under these circumstances it was noted that the maximal responses of human colon to SP and NKB were lower than single concentrations of equimolar tachykinin. This did not present a problem for the calculation of potency, however for the remainder of the study we altered the protocol since antagonists used have been shown to be non-competitive. Thus for studies investigating the effect of antagonists, semi-cumulative curves were determined from responses to the addition of three agonist concentrations (0.1, 10 and 1000 nM). The time of exposure to antagonists or their solvents was 30 min. Antagonists used were TTX (1 μM), the NK1 selective antagonist, SR140333 (10–500 nM) and the NK2 selective antagonist, SR48968 (10–1000 nM). In order to determine non-specific antagonist activity, their effect on forskolin (10 μM) was determined. Studies using guinea-pig and rat were performed in an identical fashion with the exception that cumulative concentration response curves were constructed over the same concentration range as used for human studies. Agonists were administered at half-log increments, with 2 min contact times allowed, until maximum responses were observed. A 2 min contact time was sufficient to allow the response to plateau but it was too short to produce significant desensitization.
Measurement of SP release
Distal colon was striped of its smooth muscle layers by blunt dissection leaving a mucosal sheet consisting of epithelium and underlying lamina propria. Segments of mucosa (approx. 1.5 cm×0.5 cm) were allowed to equilibrate for 20 min in oxygenated Krebs buffer at 37°C. Colonic mucosa were stimulated for 10 min with tachykinin agonists, αlgE, fMLP or capsaicin. After 10 min incubation, tissue bathing fluid solution was retrieved and snap frozen in liquid nitrogen for storage at −70°C. Colonic tissues were stored for protein determination. SP levels in tissue supernatants were determined by a solid phase ELISA (Caymen Chemicals). This kit is 100% specific for SP with only trace cross-reactivity with NKA or NKB. Protein levels were determined by the method of Bradford (1976). Concentrations of SP were expressed as pg mg−1 protein.
Data handling
SCC data was continuously collected by an acquisition package which automatically determined the effect of agonist on SCC. Agonist responses were then plotted against log [agonist] and for full response curves, fitted to a sigmoid curve and pD2 (logEC50) values calculated. Where possible pKb values were calculated using the equation pKb=−log ([antagonist]/dose ratio) where the dose ratio is the EC50 for agonist in presence of antagonist: EC50 for agonist. For partial response curves the Emax was measured in the presence and absence of antagonist and the difference assessed using a paired t-test. Significance was taken as P<0.05. If Emax values were altered the pD′2 values were calculated according to van rossum (1963) by performing linear regression analysis of log [antagonist] plotted against log ((Emax(control)/Emax(antagonist))−1). The effect of inflammation on tachykinin release was assessed statistically using Students t-test. Significance was taken as P<0.05.
Drugs and solutions
The Krebs solution used was of the following composition (mM): NaCl, 118; KCl, 4.7; MgSO4. 7H2O, 1.64; KH2PO4, 1.18; glucose, 11.5; NaHCO3, 24.88; CaCl2.2H2O, 2.52. Drugs used were purchased from Sigma unless specified and were as follows: anti human IgE (Nordic); Capsaicin; fMLP; neurokinin A (NKA) TFA salt (RBI); [Sar9, Met(O2)11]-SP; βAla8-NKA4-10, TFA salt (RBI); senktide (succinyl-[Asp6, N-Me-Phe8]-SP fragment 6–11); SP acetate. The NK1 antagonist SR140333 ((S)[1-(2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl)piperidin-3-yl]ethyl)-4-phenyl-1-azoniabicyclo[2.2.2]octane) and the NK2 antagonist SR48968 ((S)-N-methyl-N[4-(4-acetylamino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)-butyl]benzamide) were synthesized in house. Peptides were stored as a stock solution in 0.1 N acetic acid at −20°C. Stock concentrations of tachykinin antagonists and fMLP were dissolved in DMSO (100%). Capsaicin was dissolved in ethanol (100%) and αIgE was reconstituted in distilled water.
Results
Epithelial response to tachykinin agonists
The synthetic ligands, Sar-SP, βAla-NKA and senktide all increased SCC in a concentration dependent fashion yielding pD2 values of 7.72, 6.74 and 7.92 and Emax values of 34±3, 28±11 and 15±3 μAmps cm−2 respectively (n=5). Partial response curves revealed that SP, NKA and NKB all evoked concentration dependent responses (Figure 1) yielding Emax values of 60±16, 27±6 and 29±7 (n=5), however the number of concentrations tested precluded the calculation of pD2 values. The secretory activity of the tachykinins was significantly reduced by TTX (Figure 1) demonstrating that neural pathways mediate this response. This inhibition ranged from 62 to 82% depending on the agonist and therefore the sub-mucosal nerves represent the predominant site of action of the tachykinins.
Figure 1.
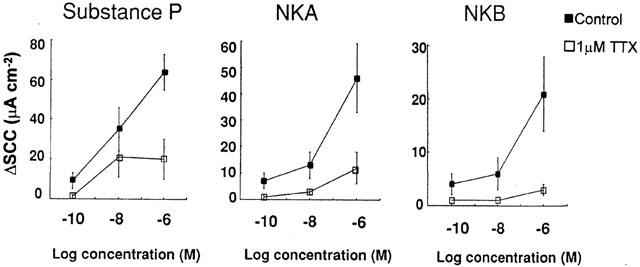
Epithelial transport response of human colonic epithelium to the natural agonists, SP; NKA and NKB. Agonists were administered in a semi-cumulative fashion in the presence or absence of 1 μM TTX. To investigate the effect of TTX paired preparations were used. Data represents the mean and standard error of 5–6 replicants. Statistical evaluation of the effect of TTX on the maximal tachykinin response was performed using Students paired t-test with *indicating a significance of P<0.05.
Antagonist potency in human tissue
The responses to SP and NKA were blocked by SR140333 and SR48968 (Figure 2). As described earlier, full concentration response curves could not be calculated and therefore it could not be determined whether antagonism was competitive. To assess antagonist potencies, pD′2 values were therefore calculated. Values obtained were respectively 6.7 and 5.9. At higher concentrations (0.5–10 μM) neither of these compounds demonstrated non-specific activity as they had no effect on forskolin-induced secretion. Due to the low potency of SR48968 no further studies using this antagonist were performed. Since there is considerable redundancy in the binding of endogenous tachykinins to their receptor subtypes the potency of SR140333 was reassessed using the specific agonist Sar-SP. This yielded a pD′2 value of 7.25.
Figure 2.
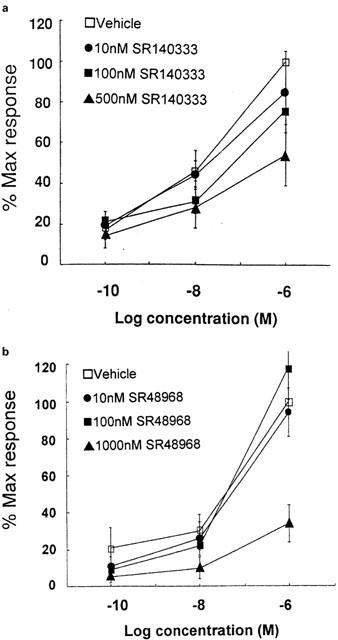
Effect of tachykinin antagonists on the epithelial transport response of human colonic epithelium to tachykinin stimulation. Responses shown are (a) SP in the presence of vehicle or SR140333 (n=5) and (b) NKA in the presence of vehicle or SR48968 (n=5). Data represents the mean and standard error of five replicants.
Anti-secretory potency of SR140333 in rat and guinea-pig
SR140333 shifted the concentration response curve to SP to the right in both rat and guinea-pig colon yielding pKb values of 9.2 and 8.53 respectively.
Tachykinin release
On the basis of agonist and antagonist studies NK1 and possibly NK3 receptors are implicated in the control of human ion transport. To add further evidence for a (patho)physiological role of these receptors we attempted to determine the stimuli for SP release. SP was chosen because it is present in the gut and because it has considerable selectivity for the NK1 receptor (Pritchard & Boden, 1995), which as discussed above appears to play a role in secretion. Under control conditions, human colonic mucosa released 86±15 pg SP mg−1 protein. A number of different stimuli were shown to cause a large release of SP including electrical field stimulation of enteric nerves, specific stimulation of sensory afferent fibres by capsaicin, αIgE activation of mast cells and granulocyte stimulation by fMLP (Figure 3). These stimuli also increased SCC (Figure 4).
Figure 3.
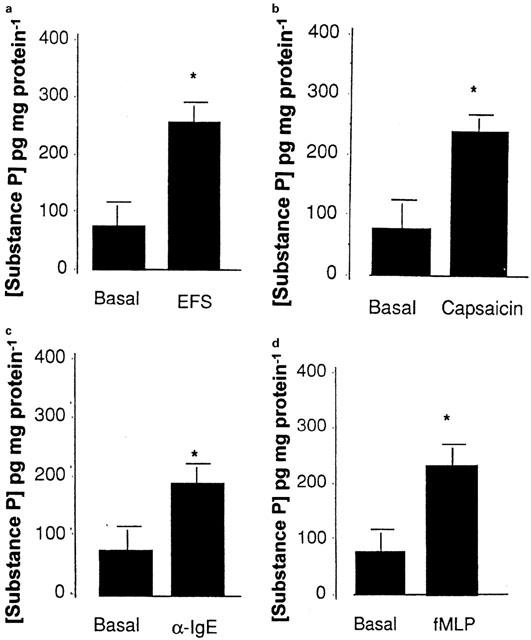
SP liberation from human colonic mucosa and epithelial transport in response to (a) electrical field stimulation of enteric nerves (7 volts/7 Hz/min) (b) specific stimulation of the sensory afferent fibres by capsaicin (50 μM), (c) αIgE activation of mast cells (1/250 dilution) and (d) granulocyte stimulation by fMLP (50 μM). SP release was measured using ELISA under basal conditions and following a 10 min period of stimulation. Data represents the mean and standard error of eight replicants. Statistical evaluation of the effect of the stimuli on SP release was performed using Students paired t-test with *indicating a significance of P<0.05.
Figure 4.
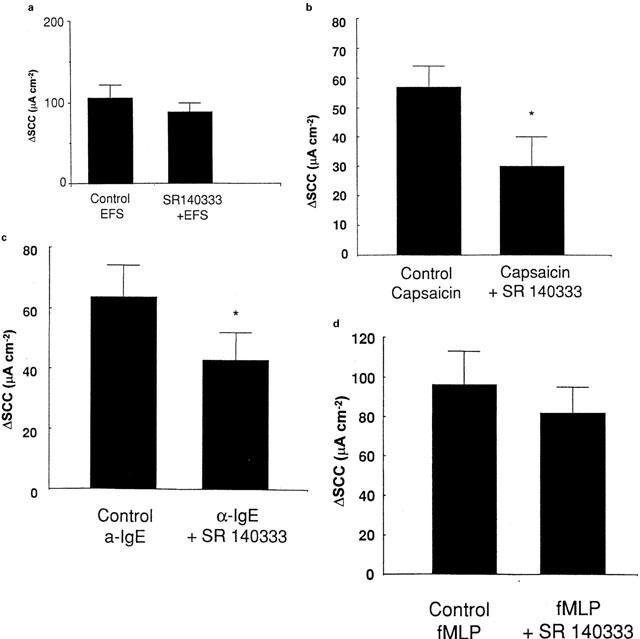
Effect of SR140333 (500 nM) on the human colonic epithelial response to (a) electrical field stimulation of enteric nerves (7 volts/7 Hz/min; n=4), (b) specific stimulation of the sensory afferent fibres by capsaicin (50 μM; n=6), (c) αIgE activation of mast cells (1/250 dilution; n=5) and (d) granulocyte stimulation by fMLP (50 μM; n=5). Statistical evaluation of the effect of the stimuli on SP release or epithelial transport was performed using Students paired t-test with *indicating a significance of P<0.05.
Possible therapeutic role of SR140333 in diseases associated with increased mast cell:nerve interaction
Since SP is released by αIgE, capsaicin and fMLP and SP evokes an NK1-mediated increase in SCC, we determined the effect of SR140333 on the ion transport responses to these stimuli. αIgE, capsaicin and fMLP each stimulated epithelial transport (Figure 3). One of the major findings of the present study was that SR140333 was able to significantly reduce the response to capsaicin and αIgE, but not to fMLP or electrical field stimulation (Figure 4).
Tachykinin release from diseased tissue
Inflamed tissue was sampled from patients with Crohn's disease or ulcerative colitis. Figure 5a,b show that SP release in response to capsaicin and electrical field stimulation was dramatically increased in tissue from Crohn's disease but not ulcerative colitis patients. Although there were quantitative changes with respect to the response to αIgE (Figure 5c) and fMLP (Figure 5d) significance was not reached. Thus not only is IBD related to increased total tissue SP content (Goldin et al., 1989) but perhaps more importantly under certain conditions SP release is also increased.
Figure 5.
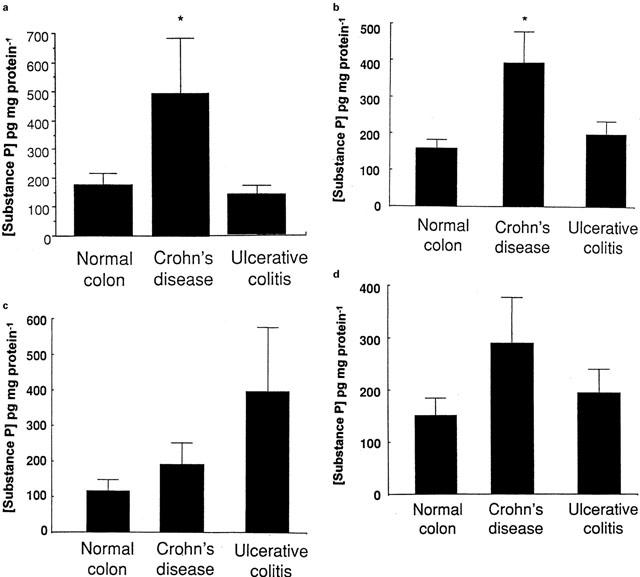
SP liberation from human colonic mucosa preparation from control (n=8), Crohn's disease (n=10) and ulcerative colitis (n=5) patients in response to (a) electrical field stimulation of enteric nerves (7 volts/7 Hz/min), (b) specific stimulation of the sensory afferent fibres by capsaicin (50 μM), (c) αIgE activation of mast cells (1/250 dilution) and (d) granulocyte stimulation by fMLP (50 μM). SP release was measured using ELISA under basal conditions and following a 10 min period of stimulation. Epithelial transport data is given in the insets of each panel. Data represents the mean and standard error of eight replicants. Statistical evaluation of the effect of the stimuli on SP release or epithelial transport was performed using Students t-test with *indicating a significance of P<0.05.
Secretory response of inflamed tissue to SP
Considering the large increase in SP release in inflamed tissue and the secretory activity of this peptide, we determined whether basal secretory activity was also increased. This is not the case, instead under basal conditions control tissue generates a SCC of 73±8 μAmps cm−2 (n=22) while inflamed tissue exhibits significantly (P<0.05) less activity with tissue from ulcerative colitis (n=6) and Crohn's disease (n=4) patients generating 16±7 and 17±6 μAmps cm−2 respectively. In addition to basal SCC being reduced the response to SP was also diminished. The maximal response of control, ulcerative colitis and Crohn's disease tissue to SP was 36±6 (n=22), 11±3 (n=6) and 4±1 (n=4) μAmps cm−2 respectively. Likewise the response to forskolin (10 μM) was also reduced from 71±11 (n=22) in controls to 11±4 (n=6) and 4±1 (n=4) μAmps cm−2 in ulcerative colitis and Crohn's disease respectively. Baseline resistance was also reduced from 161±22 (n=22) in controls to 79±6 (n=6) and 79±9 (n=4) ohms cm2 in ulcerative colitis and Crohn's disease respectively.
Discussion
Based on data obtained from animal models of diarrhoea (Mazelin et al., 1998) NK1 receptor antagonists such as SR140333 have been suggested as potential therapies for IBD and also allergic diarrhoea. The present study addressed this suggestion using human surgical specimens to characterize the functional role of NK1 receptors in mediating colonic secretion and to determine the ability of SR140333 to reduce the response to (patho)physiologic stimuli.
Sar-SP is one of the most selective NK1 agonists in general use (Drapeau et al., 1987) activating NK1 receptors with a pD2 of 8.38 (Goldhill & Angel, 1998). Sar-SP stimulated human colonic epithelial transport with a similar potency suggesting a role for NK1 receptors. Our findings are in agreement with Riegler et al. (1999) who showed that the NK1 antagonist CP96345 blocks the response to SP. In the present study, we showed for the first time that the NK1 antagonist SR140333 (Emonds-Alt et al., 1993a) was also able to reduce the secretory response to SP. This not only supports the presence of NK1 receptors but also shows that SR140333 is able to block tachykinin-mediated secretion in humans underlining its potential role as an anti-diarrhoeal treatment. It was of note that SR140333 acted as a surmountable antagonist reducing the response to SP as demonstrated in the guinea-pig longitudinal muscle (Goldhill & Angel, 1998). This contrasts with its activity in the guinea-pig and rat colonic epithelium where antagonism was non-surmountable. Furthermore the potency of SR140333 was considerably higher in the colon of the rat and guinea-pig than in the human. The reason for these differences in activity is unclear, however receptor heterogeneity, receptor redundancy and methodological considerations related to the calculation of pD′2 values were considered as possible contributory factors. SR140333 antagonized the response of human colonic epithelium to Sar-SP, however antagonism was still surmountable and also less potent (pD′2=7.25) than expected from its affinity range for the human NK1 receptor (0.019 nM to 0.7 nM depending on the tissue under investigation; Emonds-Alt et al., 1993a). The reason for the inter-species and inter-tissue variability of SR140333 remains unclear, however, NK1 subtypes cannot be excluded.
βAla-NKA had a pD2 value of 6.72, 10 fold less than its potency in the human colonic circular muscle (Giuliani et al., 1991) and closer to its potency at the NK1 receptor (Rovero et al., 1989). Furthermore the highly potent NK2 antagonist, SR48968 had little effect on the response to NKA. Thus typical NK2 receptors appear to have little role in the human colonic mucosal secretion. In contrast, senktide stimulates secretion with a pD2 value of 7.92 similar to that in the rat portal vein, a classic NK3 assay (Pritchard & Boden, 1995). This suggests that the human colonic epithelium expresses NK3 as well as NK1 receptors.
The response to each of the natural agonists, SP, NKA and NKB, was reduced by TTX, suggesting that enteric nerves play a major role in mediating the secretory response to the tachykinins.
Having demonstrated that SR140333 is active at the human colonic epithelium we next determined whether it may be able to play a role in various diarrhoeal states, including allergy and chronic inflammation. For this to be the case it is necessary to show that SP released under these conditions, causes an increase in mucosal ion transport and that this can be blocked by SR140333. Electrical field stimulation of the submucosal nerves was a powerful stimulus of SP release. The stimulus parameters employed in the present study were supra-maximal. Under these conditions, if SP were released from fibres that activate secretomotor nerves, SR140333 would not be expected to modify the response to electrical field stimulation as the secretomotor fibres would already be fully activated. This was indeed the case and furthermore the response to SP is blocked by tetrodotoxin. Neuronal release of SP is supported by immunohistochemical data directly visualizing SP in neurones (Goldin et al., 1989). Capsaicin stimulation of sensory fibres and electrical field stimulation liberated similar levels of SP. Moreover, the levels of SP released by these stimuli are nearly identical to the total SP content in non-inflamed human colon (Goldin et al., 1989). This suggests that sensory nerves are the major SP containing structures in this tissue. A third stimulus that is able to release SP is αIgE which activates mast cells directly. The most likely explanation for this is that mast cell products activate sensory fibres thereby releasing SP. This is supported by data from the rat showing that mast cells come into close apposition with nerve fibres (Stead et al., 1987). On the other hand Riegler et al. (1999) demonstrated that the response to SP is mediated by mast cells suggesting a positive feed-back loop. Thus we can conclude that there is strong neuro-immune interaction in the human colonic mucosa.
αIgE and capsaicin are both able to provoke mucosal secretion. At concentrations that reduced the response to SP, SR140333 was able to reduce the response to both of these stimuli. Since Th2 immune reactions are characterized by an increased interaction between mast cells and sensory fibres the fact that SR140333 is able to block the response to both of these stimuli strongly suggests a role for this compound in the treatment of diarrhoea associated with conditions such as food allergy.
Capsaicin- and electrically stimulated-SP release were both enhanced in Crohn's disease biopsies and this may result from increased nerve density, SP content, or sensitivity to their stimulation. Increased liberation of SP during inflammation may be expected to heighten mucosal SCC; contrary to expectation however, basal SCC was dramatically reduced in both Crohn's disease and ulcerative colitis. This could represent tissue damage and subsequent cell death, increased leakiness, or a reduction in active ion transport. Published electrophysiological data for inflamed tissue are inconsistent (e.g. Hawker et al., 1980; Sandle et al., 1990), however most studies have reported reduced sodium absorption, probably due to a reduced amiloride-sensitive pump activity, a finding in agreement with the observed reduction in basal SCC reported in the present study. To our knowledge our study is the first to investigate secretory responsiveness during IBD. The observation that SP has no secretory effect in inflamed tissue could simply represent auto-desensitization following prolonged exposure to endogenous SP. This does not appear to be the case since the response to forskolin, which increases cyclic AMP by stimulating adenylate cyclase, is also abolished suggesting that the colon loses its secretory capacity during inflammation. This is similar in some respects to animal models in which the response to acetylcholine and prostaglandin E2 is lost (Goldhill et al., 1993). Since diarrhoea associated with IBD does not therefore appear to be due to increased active ion transport, any therapeutic activity of SR140333 would be more likely due to anti-inflammatory properties (Mazelin et al., 1998).
In conclusion the major finding of the present study is that NK1 receptors play a major physiological role in the control of human colonic secretion. Blockade of these receptors by the NK1 antagonist SR140333 reduces the response to mast cell stimulation by αIgE or sensory activation by capsaicin. SR140333 and related compounds may therefore offer an excellent therapeutic option for the treatment of allergic diarrhoea.
Abbreviations
- IBD
inflammatory bowel disease
- NK
neurokinin
- PPT
preprotachykinin
- SCC
short-circuit current
- SP
substance P
- TTX
tetrodotoxin
References
- ALIAKBARI J., SREEDHARAN S.P., TURCK C.W., GOETZL E.J. Selective localization of vasoactive intestinal peptide and substance P in human eosinophils. Biochem. Biophys. Res. Comm. 1987;148:1440–1445. doi: 10.1016/s0006-291x(87)80293-0. [DOI] [PubMed] [Google Scholar]
- BALUK P., BERTRAND C., GEPPETTI P., MCDONALD D.M., NADEL J.A. NK1 receptors mediate leukocyte adhesion in neurogenic inflammation in the rat trachea. Am. J. Physiol. 1995;268:L263–L269. doi: 10.1152/ajplung.1995.268.2.L263. [DOI] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., VANNI L., LEDDA F., GIOTTI A., MAGGI C.A., FANTOZZI R. Tachykinins activate guinea-pig alveolar macrophages: involvement of NK2 and NK1 receptors. Br. J. Pharmacol. 1990;100:417–420. doi: 10.1111/j.1476-5381.1990.tb15821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUNCE K.T., ELSWOOD C.J., BALL M.T. Investigation of the 5-hydroxytryptamine receptor mechanism mediating the short-circuit current response in rat colon. Br. J. Pharmacol. 1991;102:811–816. doi: 10.1111/j.1476-5381.1991.tb12257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCI T., AUREGGI G., MANARA L., EMONDS-ALT X., LE FUR G., MAFFRAND J., MUKENGE S., FERLA G. In vitro characterization of tachykinin NK2-receptors modulating motor responses of human colonic muscle strips. Br. J. Pharmacol. 1998;124:1321–1327. doi: 10.1038/sj.bjp.0701960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAPEAU G., DEORLEANS-JUSTE P., DION S., RHALEB N.E., ROUISSI N., REGOLI D. Selective agonists for substance P and neurokinin receptors. Neuropeptides. 1987;10:43–54. doi: 10.1016/0143-4179(87)90088-6. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., ADVENIER C., CROCI T., MANARA L., NELIAT G., PONCELET M., PROIETTO V., SANTUCCI V., SOUBRIE P., VAN BROECK D., VILAIN P., LE FUR G., BRELIERE J.C. SR48968, a neurokinin A (NK2) receptor antagonist. Reg. Pept. 1993b;46:31–36. doi: 10.1016/0167-0115(93)90008-v. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO V., VAN BROECK D., SOUBRIE P., LE FUR G., BRELIERE J.C. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993a;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- GIULIANI S., BARBANTI G., TURINI D., QUARTARA L., ROVERO P., GIACHETTI A., MAGGI C.A. NK2 tachykinin receptors and contraction of circular muscle of the human colon: characterization of the NK2 receptor subtype. Eur. J. Pharmacol. 1991;203:365–370. doi: 10.1016/0014-2999(91)90892-t. [DOI] [PubMed] [Google Scholar]
- GOLDHILL J., ANGEL I. Mechanism of tachykinin NK3 receptor-mediated colonic ion transport in the guinea pig. Eur. J. Pharmacol. 1998;363:161–168. doi: 10.1016/s0014-2999(98)00797-3. [DOI] [PubMed] [Google Scholar]
- GOLDHILL J., BURAKOFF R., DONOVAN V., ROSE K., PERCY W.H. Defective modulation of colonic secretomotor neurons in a rabbit model of colitis. Am. J. Physiol. 1993;264:G671–G677. doi: 10.1152/ajpgi.1993.264.4.G671. [DOI] [PubMed] [Google Scholar]
- GOLDIN E., KARMELI F., SELINGER S., RACHMILEWITZ S. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig. Dis. Sci. 1989;34:754–757. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- HAWKER P.C., MCKAY J.S., TURNBERG L.A. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterol. 1980;79:508–511. [PubMed] [Google Scholar]
- KOTANI H., HOSHIMARU M., NAWA H., NAKANISHI S. Structure and gene organization of bovine neuromedin K precursor. Proc. Natl. Acad. Sci. USA. 1986;83:7074–7078. doi: 10.1073/pnas.83.18.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUFER R., GILON C., CHOREV M., SELINGER Z. Desensitization with a selective agonist discriminates between multiple tachykinin receptors. J. Pharmacol. Exp. Ther. 1988;245:639–643. [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., PATACCHINI R., SANTICIOLI P., THEODORSSON E., BARBANTI G., TURINI D., GIACHETTI A. Tachykinin antagonists inhibit nerve-mediated contractions in the circular muscle of the human ileum. Gastroenterology. 1992;102:88–96. doi: 10.1016/0016-5085(92)91787-5. [DOI] [PubMed] [Google Scholar]
- MANTYH C.R., GATES T.S., ZIMMERMAN R.P., WELTON M.L., PASSARO E.P., VIGNA S.R., MAGGIO J.E., KRUGER L., MANTYH P.W. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc. Natl. Acad. Sci. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZELIN L., THEODOROU V., MORE J., EMONDS-ALT X., FIORAMONTI J., BUENO L. Comparative effects of nonpeptide tachykinin receptor antagonists on experimental gut inflammation in rats and guinea-pigs. Life Sci. 1998;63:293–304. doi: 10.1016/s0024-3205(98)00271-9. [DOI] [PubMed] [Google Scholar]
- NAWA H., HIROSE T., TAKASHIMA H., INAYAMA S., NAKANISHI S. Nucleotide sequences of cloned cDNAs for two types of bovine substance P precursor. Nature. 1983;306:32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., AHLUWALIA A., FLOWER R.J., MANZINI S. Endogenous tachykinins play a role in IL-1-induced neutrophil accumulation: involvement of NK-1 receptors. Immunology. 1993;80:73–77. [PMC free article] [PubMed] [Google Scholar]
- PRITCHARD M.C., BODEN P. Tachykinin NK3 receptors: biology and development of selective peptide and nonpeptide ligands. Drugs of the Future. 1995;20:1163–1173. [Google Scholar]
- RIEGLER M., CASTAGLIUOLO I., SO P.T.C., LOTZ M., WANG C., WLK M., SOGUKOGLU T., COSENTINI E., BISCHOF G., HAMILTON G., TELEKY B., WENZL E., MATTHEWS J.B., POUTHOULAKIS C. Effects of substance P on human colonic mucosa in vitro. Am. J. Physiol. 1999;276:G1473–G1488. doi: 10.1152/ajpgi.1999.276.6.G1473. [DOI] [PubMed] [Google Scholar]
- ROVERO P., PESTELLINI V., PATACCHINI R., GIULIANI S., SANTICIOLI P., MAGGI C.A., MELI A., GIACHETTI A. A potent and selective agonist for NK-2 tachykinin receptor. Peptides. 1989;10:593–595. doi: 10.1016/0196-9781(89)90148-4. [DOI] [PubMed] [Google Scholar]
- SANDLE G.I., HIGGS N., CROWE P., MARSH M.N., VENKATESAN S., PETERS T.J. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- SCHRATZBERGER P., REINISCH N., PRODINGER W.M., KAHLER C.M., SITTE B.A., BELLMANN R., FISCHER-COLBRIE R., WINKLER H., WIEDERMANN C.J. Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J. Immunol. 1997;158:3895–3901. [PubMed] [Google Scholar]
- STEAD R.H., TOMIOKA M., QUINONEZ G., SIMON G.T., FELTEN S.Y., BIENENSTOCK J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc. Natl. Acad. Sci. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ROSSUM J.M. Cumulative dose-response curves. Arch. Int. Pharmacodyn. 1963;143:299–330. [PubMed] [Google Scholar]


