Abstract
The effect of bradykinin on the Na+-K+ pump of airway smooth muscle was investigated by measuring ouabain-sensitive 86Rb+ uptake in cultured guinea-pig tracheal smooth muscle cells.
Bradykinin induced a concentration-dependent increase in ouabain-sensitive 86Rb+ uptake, with an EC50 of 3 nM (pD2=8.50±0.10). Stimulation was not affected by indomethacin (1 μM) suggesting that it is not mediated by cycloxygenase products of arachidonic acid.
The B1 receptor agonists Lys-des-Arg9-bradykinin and des-Arg9-bradykinin had no effect on ouabain-sensitive 86Rb+ uptake. In contrast, the B1 and B2 receptor agonist Lys-bradykinin induced a concentration-dependent increase in ouabain-sensitive 86Rb+ uptake with an EC50 of 6 nM (pD2=8.21±0.20).
The B1 receptor antagonist des-Arg10-HOE 140 (1 μM) had no effect on bradykinin-stimulated ouabain-sensitive 86Rb+ uptake. The B2 receptor antagonists HOE 140 and WIN 64338 antagonized bradykinin-stimulated ouabain-sensitive 86Rb+ uptake with pKB values (−log M) of 8.20±0.08 and 8.11±0.20 respectively.
Reducing extracellular Na+ from 146 mM to 11 mM caused a 53.5% decrease in basal ouabain-sensitive 86Rb+ uptake and abolished bradykinin-induced uptake. Two inhibitors of the Na+-H+ exchanger, methylisobutyl-amiloride (MIA; 1–100 μM) and ethylisopropyl-amiloride (EIPA; 0.1–10 μM), inhibited bradykinin-stimulated ouabain-sensitive 86Rb+ uptake without affecting basal uptake.
These results suggest that bradykinin increases Na+-K+ pump activity of guinea-pig tracheal smooth muscle via stimulation of B2 receptors and activation of the Na+-H+ exchanger.
Keywords: Airway smooth muscle, bradykinin, B2 receptor, Na+-H+ exchanger, Na+-K+ pump, ouabain, rubidium-86, trachea
Introduction
Bradykinin is an endogenous inflammatory peptide that is generated by plasma and tissue kallikreins from high and low molecular weight kininogens. A role for bradykinin in the pathogenesis of asthma has been suggested since increased levels of bradykinin are found in airways of asthmatic subjects (Christiansen et al., 1987) and inhalation of bradykinin by asthmatic subjects induces bronchoconstriction in part through cholinergic mechanisms (Fuller et al., 1987). In vitro, bradykinin induces contraction and/or relaxation of isolated airways through a number of direct and indirect mechanisms. Contraction results from activation of bradykinin receptors on airway smooth muscle (ASM), release of tachykinins from sensory nerve terminals and generation of thromboxane A2 (Inoue et al., 1992; Hulsmann et al., 1994). On the other hand, bradykinin induces an epithelial-dependent relaxation of isolated airways through generation of inhibitory prostanoids and nitric oxide (Bramley et al., 1990; Schlemper & Calixto, 1994).
Bradykinin receptors are subdivided into two major subtypes, B1 and B2, which can be distinguished pharmacologically with selective agonists and antagonists (Regoli et al., 1993; 1994). Airway smooth muscle contraction is generally thought to be mediated by B2 receptors (Trifilieff et al., 1992; Pruneau et al., 1995), although the existence of a novel B3 receptor on guinea-pig tracheal smooth muscle has also been proposed (Farmer et al., 1989; Farmer & Desiato, 1994). Bradykinin receptors on airway smooth muscle are coupled to activation of phospholipases A2, C and D (Pyne & Pyne, 1993; Pyne et al., 1997), protein kinase C translocation (Pyne et al., 1994), phosphoinositide hydrolysis (Marsh & Hill, 1992), Ca2+ mobilization from intracellular and extracellular sources (Marsh & Hill, 1993), Ca2+ efflux (Farmer et al., 1991) and activation of mitogen-activated protein kinase (Pyne et al., 1997).
Airway smooth muscle possesses a Na+-K+ pump that contributes to the resting membrane potential and modulates tone. Activation of the pump with K+ (in a K+-free medium) results in hyperpolarization and relaxation of airway smooth muscle, whereas inhibition of the pump with ouabain causes depolarization and contraction (Souhrada et al., 1981; Chideckel et al., 1987; Gunst & Stropp, 1988). Na+-K+ pump activity is thought to alter smooth muscle tone as a result of changes in Ca2+ influx through voltage-dependent Ca2+ channels and Ca2+ efflux via the Na+-Ca2+ exchanger. There is increasing evidence that the Na+-K+ pump is in turn influenced by bronchoactive mediators. Vasoactive intestinal peptide and 5-hydroxytryptamine both activate the Na+-K+ pump of airway smooth muscle as measured by increases in ouabain-sensitive 86Rb+ uptake (Morrison & Vanhoutte, 1996; Rhoden et al., 2000).
The aim of the present study was to investigate the effect of bradykinin on the Na+-K+ pump of airway smooth muscle through measurements of ouabain-sensitive 86Rb+ uptake in cultured guinea-pig tracheal smooth muscle cells. The results suggest that bradykinin stimulates the Na+-K+ pump via activation of B2 receptors, and that stimulation is secondary to Na+ influx through the Na+-H+-exchanger.
Methods
Cell culture
Cultured airway smooth muscle cells were derived from male Dunkin-Hartley guinea-pigs (Covance, Denver, PA, U.S.A.) weighing 250–500 g. Animals were euthanized with an overdose of sodium pentobarbitone (150 mg kg−1 i.p.) and the trachea harvested. The trachea was cut longitudinally through the cartilage and the epithelial layer was removed by rubbing the lumenal surface with a sterile cotton-wool probe. The trachealis muscle was dissected free from the cartilage, minced with scalpel blades into ∼1 mm2 pieces and placed in culture dishes. Tissue explants were maintained in Dulbecco's Modified Eagle Medium supplemented with 10% foetal bovine serum, 100 u ml−1 penicillin and 100 μg ml−1 streptomycin in humidified air containing 5% CO2 at 37°C. The culture medium was changed 1 week after initial set up, and twice per week thereafter. Cells migrated from tissue explants and proliferated till confluence over a 2–3 week period. Cells were passaged with 0.05% trypsin and 0.53 mM EDTA followed by centrifugation at 1500 r.p.m. and resuspension in culture medium. Cells were maintained in a culture for up to five passages. For 86Rb+ uptake studies, cells were plated onto 24-well plates at a density of 2–4×104 cells well−1. Cells grown in Lab-Tek chamber slides were characterized as smooth muscle cells by immunohistochemistry using a labelled streptavidin-biotin-peroxidase kit (Zymed Laboratories, San Francisco, CA, U.S.A.). Positive staining was obtained with primary antibodies for smooth muscle-specific α-actin (Sigma, clone 1A4) and smooth muscle-specific myosin (Sigma, clone hSM-V).
Ouabain-sensitive 86Rb+ uptake
Na+-K+ pump activity was measured in confluent tracheal smooth muscle cells as ouabain-sensitive 86Rb+ uptake, as previously described (Rhoden et al., 2000). Uptake was measured in a balanced salt solution (BSS) containing (mM): NaCl 140, KCl 5, MgCl2 1, CaCl2 2, HEPES 10 and glucose 10 (pH 7.4) at 37°C. Cells were incubated for 5 min in BSS containing 10 μM bumetanide±100 μM ouabain, followed by 0.5−1 μCi ml−1 86Rb+ and bradykinin (10 pM–10 μM) for 10 min. Bumetanide was included in order to prevent uptake by the Na+-K+-2Cl− cotransporter and thereby reduce background. At the end of the uptake period, cells were washed six times in 1 ml ice-cold wash buffer. The wash buffer consisted of BSS containing RbCl instead of KCl in order to displace 86Rb+ from extracellular sites. Cells were solubilized in Lowry reagent and total cellular protein measured by the Lowry method (Sigma protein assay kit P5656). Radioactivity was measured by liquid scintillation counting (3000–6000 DPM per sample counted for 10 min with a counting efficiency of 63%). Ouabain-sensitive uptake was calculated as the difference in uptake in the presence and absence of ouabain. The effect of extracellular Na+ on Na+-K+ pump activity was investigated by replacing NaCl in BSS with equimolar choline chloride. Since the pH of BSS was corrected with NaOH, the final concentration of Na+ in BSS was 146 mM (normal BSS) or 11 mM (low Na+ BSS). Pretreatment with antagonists and inhibitors was carried out 20 min before the addition of 86Rb+.
Drugs
Bradykinin, bumetanide, des-Arg9-bradykinin, des-Arg10-HOE 140, Lys-bradykinin, Lys-des-Arg9-bradykinin, 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), 5-(N-methyl-N-isobutyl)-amiloride (MIA) and ouabain were purchased from Sigma (St. Louis, MO, U.S.A.). HOE 140 was purchased from Peninsula Laboratories (San Carlos, CA, U.S.A.) and WIN 64338 from Tocris (Ballwin, MO, U.S.A.). 86RbCl was purchased from NEN Life Science Products (Boston, MA, U.S.A.).
Data analysis
Results are expressed as mean values±s.e.mean of n experiments performed on duplicate or triplicate wells. Each n represents cells cultured from a different animal and/or passage. EC50 and pD2 (−log EC50) values for agonists were determined by non-linear regression curve fitting of concentration-response data fitted to the equation Y=Ymin+(Ymax−Ymin)/(1+[EC50/X]n), where Ymin and Ymax are the minimum and maximum responses respectively, X is the agonist concentration and n is the Hill slope (GraphPad Prism, San Diego, CA, U.S.A.). The pKB value (−log M) for HOE 140 was derived from a Schild plot of log(DR−1) versus log[B] where DR is the agonist dose-ratio in the presence and absence of antagonist B. WIN 64338 appeared to cause insurmountable antagonism, and the pKB was calculated from the equation KB=[B]/slope−1, the slope corresponding to the double reciprocal plot of equi-effective concentrations of agonist in the absence (1/A) and in the presence (1/A′) of antagonist B (Kenakin, 1984). Differences between groups were analysed by the Student's t-test or by ANOVA with the Bonferroni post-test for multiple comparisons (StatView, SAS Institute Inc., Cary, NC, U.S.A.). Statistical significance was assumed at a probability (P) value <0.05.
Results
Effect of bradykinin on 86Rb+ uptake
Bradykinin (0.1 nM–10 μM) induced a concentration-dependent increase in 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells (Figure 1A). Ouabain decreased 86Rb+ uptake by 86% and abolished the stimulation of uptake induced by bradykinin. Bradykinin increased ouabain-sensitive uptake with an EC50 of 3 nM (pD2=8.50±0.10, n=17) (Figure 1B). At a maximally effective concentration of 1 μM, bradykinin increased ouabain-sensitive 86Rb+ uptake from 12.05±1.07 to 24.90±1.46 pmol μg−1 protein min−1 suggesting a doubling of Na+-K+ pump activity. The ability of bradykinin to increase ouabain-sensitive 86Rb+ uptake was not affected by 1 μM indomethacin, with similar pD2 values in the absence (8.42±0.14, n=6) and presence (8.44±0.11, n=6) of indomethacin.
Figure 1.
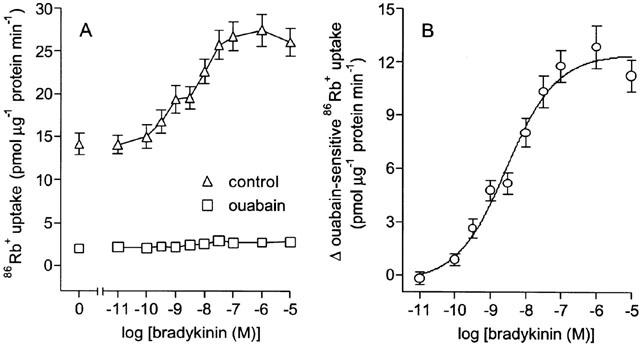
Effect of bradykinin on 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells. 86Rb+ uptake is expressed as total uptake in the absence or presence of 100 μM ouabain (A) or as the increase in ouabain-sensitive uptake induced by bradykinin (B). Points represent mean±s.e.mean (n=17).
Receptor agonists and antagonists
The receptor mediating the bradykinin-induced increase in Na+-K+ pump activity was investigated with selective agonists and antagonists. The B1 receptor agonists des-Arg9-bradykinin (0.1 nM–10 μM) and Lys-des-Arg9-bradykinin (0.1 nM–10 μM) had no effect on ouabain-sensitive 86Rb+ uptake (Figure 2). In contrast, the B1 and B2 receptor agonist Lys-bradykinin induced a concentration-dependent increase in ouabain-sensitive 86Rb+ uptake, with an EC50 of 6 nM (pD2=8.21±0.20, n=8) (Figure 2). Maximal uptake induced by Lys-bradykinin was similar to that induced by bradykinin.
Figure 2.
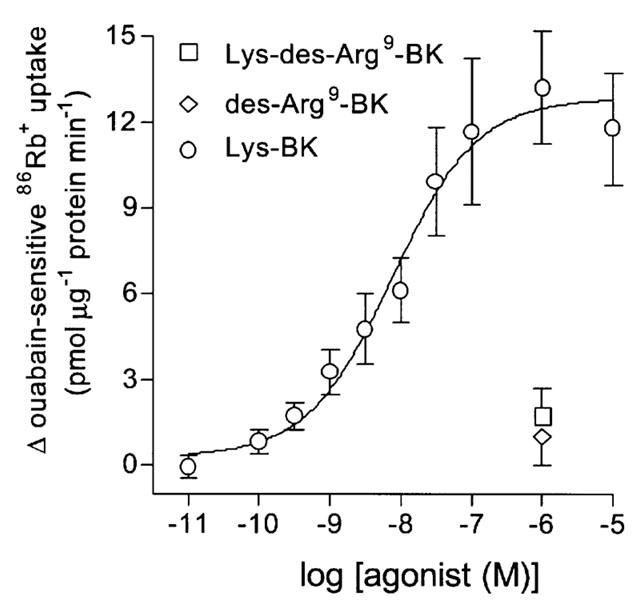
Effect of the bradykinin receptor agonists des-Arg9-bradykinin, Lys-des-Arg9-bradykinin and Lys-bradykinin on ouabain-sensitive 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells. Points represent mean±s.e.mean (n=6–8).
The B1 receptor antagonist des-Arg10-HOE 140 (1 μM) had no significant effect on bradykinin-induced ouabain-sensitive 86Rb+ uptake (Figure 3), with pD2 values for bradykinin of 8.65±0.25 (n=6) in the absence and 8.35±0.13 (n=6) in the presence of antagonist. The B2 receptor antagonists HOE 140 and WIN 64338 inhibited bradykinin-induced ouabain-sensitive 86Rb+ uptake (Figure 4). The Schild plot for HOE 140 yielded a straight line (R=0.90) with a slope (1.09±0.10) that was not significantly different from unity. The pKB value (−log M) for HOE 140 derived from the Schild plot was 8.20±0.08 (n=8). WIN 64338 caused a decrease in the maximal response to bradykinin suggesting insurmountable antagonism. The pKB value (−log M) for WIN 64338, derived from the double-reciprocal plot of equi-effective concentrations of agonist in the presence and absence of antagonist, was 8.11±0.20 (n=9). HOE 140 (10–300 nM) and WIN 64338 (10–300 nM) had no effect on resting ouabain-sensitive 86Rb+ uptake in the absence of bradykinin.
Figure 3.
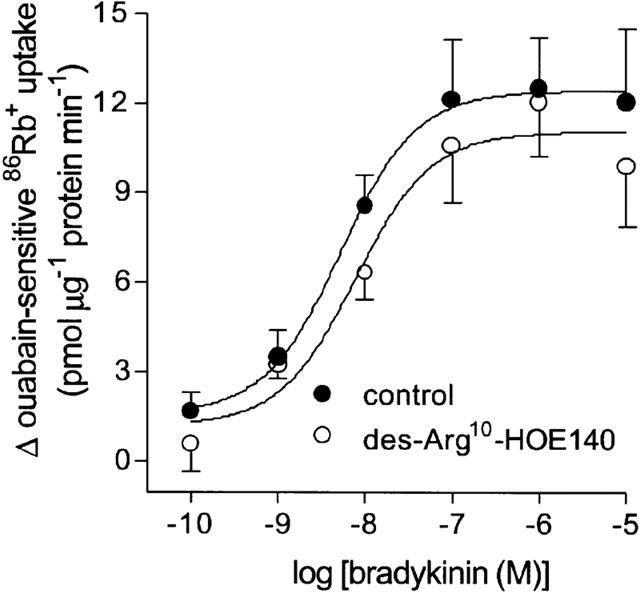
Effect of the bradykinin B1 receptor antagonist des-Arg10-HOE 140 (1 μM) on bradykinin-stimulated ouabain-sensitive 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells. Points represent mean±s.e.mean (n=8).
Figure 4.
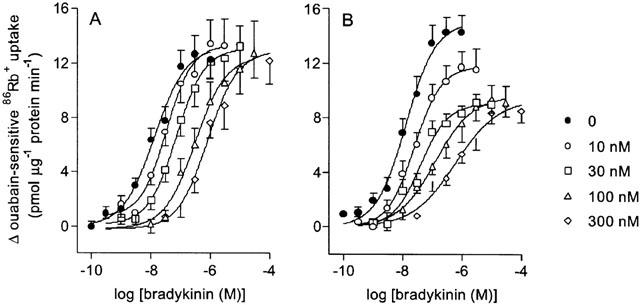
Effect of the bradykinin B2 antagonists HOE 140 (A) and WIN 64338 (B) on bradykinin-stimulated ouabain-sensitive 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells. Uptake was measured in the presence of 0, 10, 30, 100 or 300 nM antagonist. Points represent mean±s.e.mean (n=8 for HOE 140 and n=9 for WIN 64338).
Role of Na+ influx and the Na+-H+ exchanger
The role of Na+ influx in the stimulation of 86Rb+ uptake by bradykinin was investigated by first examining the effect of Na+ influx per se on ouabain-sensitive uptake. The Na+ ionophore monensin (10 nM–10 μM) induced a concentration-dependent increase in ouabain-sensitive 86Rb+ uptake (Figure 5) confirming that an increase in Na+ influx stimulates the Na+-K+ pump. In contrast, reducing Na+ influx by decreasing the extracellular concentration of Na+ from 146 mM to 11 mM caused a 53.5±3.1% reduction in ouabain-sensitive 86Rb+ uptake (P<0.001, n=8) (Figure 6). The effect of reducing the concentration of extracellular Na+ was also investigated on the ability of bradykinin (1 μM) and an equi-effective concentration of monensin (0.3 μM) to increase Na+-K+ pump activity. In the presence of 11 mM Na+, bradykinin and monensin both failed to induce a significant increase in ouabain-sensitive 86Rb+ uptake (Figure 6), suggesting that Na+ influx is necessary for bradykinin to increase Na+-K+ pump activity.
Figure 5.
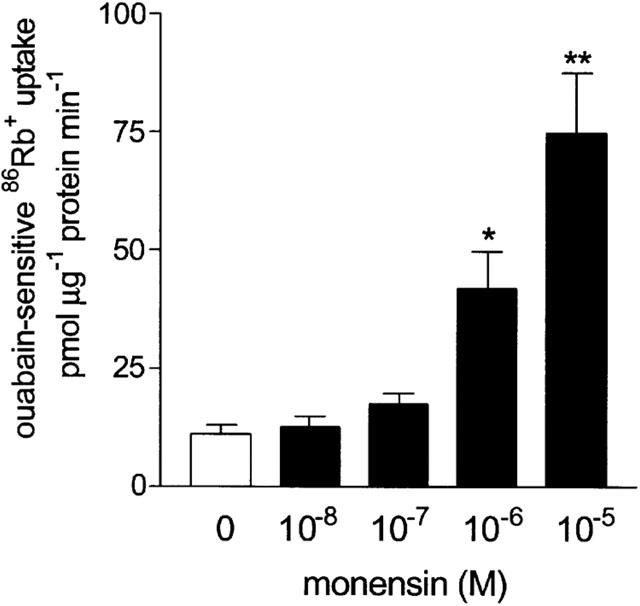
Effect of monensin on ouabain-sensitive 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells. *P<0.05, **P<0.01 vs no monensin, ANOVA with Bonferroni post-test. Points represent mean±s.e.mean (n=8–10).
Figure 6.
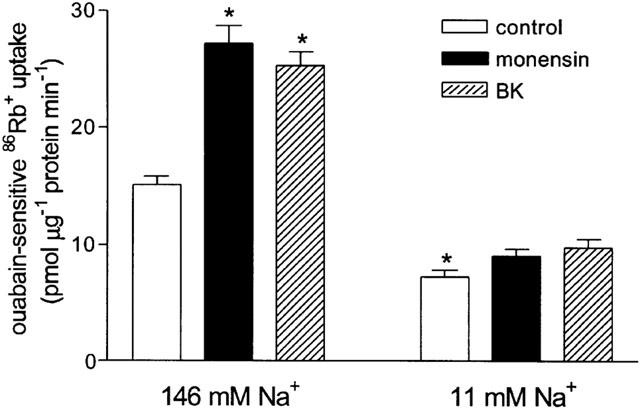
Effect of extracellular Na+ concentration on the stimulation of ouabain-sensitive 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells by monensin (0.3 μM) and bradykinin (1 μM). Uptake was measured in BSS containing 146 mM Na+ or 11 mM Na+. *P<0.05 vs control in 146 mM Na+, ANOVA with Bonferroni post-test. Points represent mean±s.e.mean (n=8).
The role of the Na+-H+ exchanger in bradykinin-stimulated Na+-K+ pump activity was assessed with MIA and EIPA, two selective inhibitors of the Na+-H+ exchanger. MIA (1–100 μM) and EIPA 0.1–10 μM) both inhibited bradykinin-stimulated 86Rb+ uptake in a concentration-dependent manner without affecting basal uptake in the absence of bradykinin (Figure 7).
Figure 7.
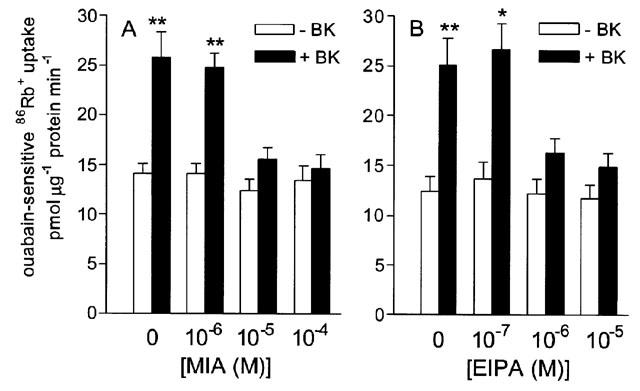
Effect of the Na+-H+ exchanger inhibitors MIA (A) and EIPA (B) on the stimulation of ouabain-sensitive 86Rb+ uptake into cultured guinea-pig tracheal smooth muscle cells by bradykinin (1 μM). Uptake was measured in the presence of 1–100 μM MIA or 0.1–10 μM EIPA. **P<0.001, *P<0.005 vs no bradykinin, unpaired Student's t-test. Points represent mean±s.e.mean (n=8).
Since amiloride analogues also inhibit voltage-dependent Ca2+ channels (Kleyman & Cragoe, 1988), the effect of nifedipine on the ability of bradykinin to stimulate ouabain-sensitive 86Rb+ uptake was examined. Bradykinin (1 μM) increased ouabain-sensitive 86Rb+ uptake by 13.3±1.4 pmol μg−1 protein min−1 in the absence of nifedipine and 13.3±1.8 pmol μg−1 protein min−1 (n=6) in the presence of 1 μM nifedipine, demonstrating that inhibition of L-type Ca2+ channels has no effect on the stimulation of the Na+-K+ pump activity by bradykinin.
Discussion
In many cells, the Na+-K+ pump is regulated by hormones, neurotransmitters and growth factors. Regulation occurs as (i) acute changes in the activation kinetics of the pump due to changes in the concentration and/or affinity of substrates (usually Na+), or (ii) through longer lasting changes in the density of active pumps on the plasma membrane due to translocation from intracellular stores or de novo synthesis (Therien & Blostein, 2000). In airway smooth muscle, VIP and 5-hydroxytryptamine produce an acute increase in Na+-K+ pump activity, measured as ouabain-sensitive 86Rb+ uptake, and this effect is mediated by an increase in Na+ influx into cells (Morrison & Vanhoutte, 1996; Rhoden et al., 2000). The present study demonstrates that bradykinin also increases ouabain-sensitive 86Rb+ uptake in cultured guinea-pig tracheal cells, and stimulation is comparable in magnitude to that produced in the same cell type by 5-hydroxytryptamine (Rhoden et al., 2000). Stimulation is also dependent upon extracellular Na+ suggesting that it is secondary to an increase in Na+ influx. The Na+ ionophore monensin increased ouabain-sensitive 86Rb+ uptake confirming that an increase in Na+ influx per se can stimulate the Na+-K+ pump in airway smooth muscle.
Na+ influx into cells occurs through Na+ channels and through a number of Na+-coupled transporters. The increase in Na+-K+ pump activity induced by bradykinin was inhibited by the amiloride analogues EIPA and MIA suggesting that it may be mediated by an increase in Na+ influx via the Na+-H+ exchanger. The Na+-H+ exchanger exists in multiple isoforms that differ in their sensitivity to amiloride analogues (Yun et al., 1995). NHE1 is the ubiquitous amiloride-sensitive isoform that is inhibited by submicromolar EIPA and MIA (Kleyman & Cragoe, 1988). Other tissue-specific isoforms exhibit a 50–400 fold lower sensitivity to EIPA (Yun et al., 1995). The NHE isoform present in airway smooth muscle has not been determined, but the concentration-dependence for inhibition of bradykinin-induced 86Rb+ uptake by EIPA and MIA may suggest the presence of alternate isoforms with a lower amiloride-sensitivity. High concentrations of EIPA and MIA (10–1000 μM) also inhibit other ion transport systems including the Na+-K+ pump, Na+ channels, the Na+-Ca2+ exchanger and voltage-dependent Ca2+ channels (Kleyman & Cragoe, 1988). EIPA and MIA had no effect on basal ouabain-sensitive 86Rb+ uptake suggesting that these agents do not have a direct effect on the Na+-K+ pump in airway smooth muscle. Furthermore, resting Na+-K+ pump activity is maintained by Na+ influx through amiloride-insensitive pathways. Voltage-dependent Ca2+ channels are unlikely to be involved in the effects of amiloride analogues since nifedipine, a more selective inhibitor of L-type Ca2+ channels, had no effect on bradykinin-stimulated 86Rb+ uptake.
Bradykinin receptors can be classified pharmacologically into B1 and B2 subtypes according to the order of potency of agonists and antagonists (Regoli et al., 1993; 1994). The results of this study suggest that increase in Na+-K+ pump activity induced by bradykinin is mediated by B2 receptors. Lys-des-Arg9-bradykinin and des-Arg9-bradykinin are B1-selective agonists, and neither affected ouabain-sensitive 86Rb+ uptake. In contrast, bradykinin and Lys-bradykinin stimulated 86Rb+ uptake with similar pD2 values of 8.50 and 8.21 respectively, and this is in agreement with their potency on B2 receptor systems (Regoli et al., 1993).
Stimulation of ouabain-sensitive 86Rb+ uptake by bradykinin was antagonized by HOE 140 and WIN 64338 with pKB values of 8.20 and 8.11 respectively. HOE 140 is a long-acting B2 receptor antagonist with an apparent affinity (pA2 value) of 8–9 in various bioassays (Hock et al., 1991; Regoli et al., 1993; 1994). HOE 140 displaces bradykinin binding from guinea-pig tracheal receptors and inhibits bradykinin-induced contraction of the guinea-pig trachea with a pKB of 8.13 (Trifilieff et al., 1992; Pruneau et al., 1995). WIN 64338 is a nonpeptide B2 receptor antagonist with pA2 values of 7.1–8.2 on guinea-pig B2 receptor systems (Regoli et al., 1994). WIN 64338 inhibited bradykinin-induced contraction of the guinea-pig trachea with pKB values of 7.19 (Scherrer et al., 1995) and 7.36 (Pruneau et al., 1995). In contrast, Farmer et al. (1989) and Farmer & Desiato (1994) failed to demonstrate any inhibitory effect of WIN 64338 and NPC 567, another B2 antagonist, on bradykinin-induced contractions of the guinea-pig trachea leading the authors to propose the existence of a novel B3 receptor in this preparation. Both HOE 140 and WIN 64338 have been shown to displace bradykinin binding from cultured guinea-pig tracheal smooth muscle cells confirming the presence of B2 receptors on cultured as well as freshly-isolated smooth muscle (Scherrer et al., 1998). Cultured guinea-pig tracheal smooth cells are also reported to contain B3 receptors (Farmer et al., 1991), but we have no evidence for their involvement in the regulation of the Na+-K+ pump by bradykinin.
WIN 64338 produced insurmountable antagonism of bradykinin-stimulated 86Rb+ uptake. Although WIN 64338 acts as a competitive antagonist in most systems (Regoli et al., 1994), it causes insurmountable antagonism of guinea-pig tracheal contractions (Pruneau et al., 1995; Scherrer et al., 1995). Potential causes of insurmountable antagonism include (i) non-specific inhibition of post-receptor events; (ii) irreversible or noncompetitive antagonism; (iii) pseudo-irreversible or slowly-reversing antagonism due to slow dissociation of the antagonist from the receptor (Robertson et al., 1994); (iv) allosteric modulation of the receptor (Kaumann & Frenken, 1985); and (v) competitive antagonism against an indirect agonist (Kenakin, 1993).
HOE 140 acted as a competitive antagonist against bradykinin-stimulated 86Rb+ uptake, with no change in the maximal response to bradykinin and a Schild plot slope of unity. In the guinea-pig isolated trachea, HOE 140 produces both surmountable and insurmountable antagonism of bradykinin-induced contractions (Pruneau et al., 1995; Trifilieff et al., 1992).
Both forms of antagonism also occur in other bioassays in a tissue and species-dependent manner (Rhaleb et al., 1992; Feletou et al., 1994), and have been confirmed through the controlled expression of recombinant B2 receptors from different species (Bachvarov et al., 1995). In some systems, HOE 140 also acts as a partial agonist (Feletou et al., 1994) and an inverse agonist (Leeb-Lundberg et al., 1994). In our experiments, HOE 140 had no effect (stimulatory or inhibitory) on resting ouabain-sensitive 86Rb+ uptake in the absence of bradykinin, suggesting that the last two properties of HOE 140 are not evident in this preparation.
The physiological relevance of bradykinin-induced stimulation of the Na+-H+ exchanger and the consequent increase in Na+-K+ pump activity is uncertain. Contraction of airway smooth muscle is associated with a transient decrease in intracellular pH (pHi), followed by a slow recovery that it is mediated by the Na+-H+ exchanger (Bose et al., 1990). Thus, activation of the Na+-H+ exchanger by bradykinin may reflect the need to extrude excess protons produced during contraction in order to restore pHi. An increase in Na+ influx through the Na+-H+ exchanger would increase Na+-K+ pump activity in an attempt to restore resting [Na+i]. Since an increase in Na+-K+ pump activity is associated with hyperpolarization, this phenomenon may also represent a negative feedback mechanism that tends to oppose contraction in response to stimulation of B2 receptors on airway smooth muscle. Such an autoregulatory mechanism has been proposed in other smooth muscles. Phorbol esters inhibit agonist-induced contractions of ileal smooth muscle through activation of the Na+-K+ pump, and cause a 24% increase in ouabain-sensitive 86Rb+ uptake (Sasaguri & Watson, 1990). Similarly, serotonin attenuates contractions of isolated arteries by increasing Na+-K+ pump activity (Moreland et al., 1985), and causes a 287% increase in ouabain-sensitive 86Rb+ uptake in cultured vascular smooth muscle cells (Navran et al., 1991). Activation of the Na+-K+ pump is also thought to mediate the after-hyperpolarization seen in ileal smooth muscle following acetylcholine application (Bolton, 1973). Clearly, measurements of membrane potential, [Na+i], pHi and tone are needed to establish the physiological role of bradykinin-induced changes in ion transport observed in the present study.
In conclusion, bradykinin increases Na+-K+ pump activity of cultured airway smooth muscle via activation of B2 receptors and stimulation of Na+ influx through the Na+-H+ exchanger. Further studies are needed to determine the mechanism by which bradykinin activates the Na+-H+ exchanger and the functional implication for the regulation of airway smooth muscle tone by bradykinin.
Acknowledgments
This study was supported, in part, by grant HL54114 from the National Heart, Lung and Blood Institute (U.S.A.).
Abbreviations
- BSS
balanced salt solution
- EIPA
5-(N-ethyl-N-isopropyl)-amiloride
- MIA
5-(N-methyl-N-isobutyl)-amiloride
References
- BACHVAROV D.R., SAINT-JACQUES E., LARRIVEE J.-F., LEVESQUE L., RIOUX F., DRAPEAU G., MARCEAU F. Cloning and pharmacological characterization of the rabbit bradykinin B2 receptor. J. Pharmacol. Exp. Ther. 1995;275:1623–1630. [PubMed] [Google Scholar]
- BOLTON T.B. The role of the electronic sodium pumping in the response of smooth muscle to acetylcholine. J. Physiol. 1973;228:713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSE R., YU J., CRAGOE E.J., DELAIVE J. Cytosolic pH changes in contracting canine trachealis smooth muscle. Progr. Clin. Biol. Res. 1990;327:695–702. [PubMed] [Google Scholar]
- BRAMLEY A.M., SAMHOUN M.N., PIPER P.J. The role of the epithelium in modulating the responses of guinea-pig trachea induced by bradykinin in vitro. Br. J. Pharmacol. 1990;99:762–766. doi: 10.1111/j.1476-5381.1990.tb13003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIDECKEL E.W., FROST J.L., MIKE P., FEDAN J.S. The effect of ouabain on tension in isolated respiratory tract smooth muscle of humans and other species. Br. J. Pharmacol. 1987;92:609–614. doi: 10.1111/j.1476-5381.1987.tb11363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIANSEN S.C., PROUD D., COCHRANE C.G. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J. Clin. Invest. 1987;79:188–197. doi: 10.1172/JCI112782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER S.G., BURCH R.M., MEEKER S.A., WILKINS D.E. Evidence for a pulmonary B3 bradykinin receptor. Mol. Pharmacol. 1989;36:1–8. [PubMed] [Google Scholar]
- FARMER S.G., DESIATO M.A. Effects of a novel nonpeptide bradykinin B2 receptor antagonist on intestinal and airway smooth muscle: further evidence for the tracheal B3 receptor. Br. J. Pharmacol. 1994;112:461–464. doi: 10.1111/j.1476-5381.1994.tb13095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER S.G., ENSOR J.E., BURCH R.M. Evidence that cultured airway smooth muscle cells contain bradykinin B2 and B3 receptors. Am. J. Respir. Cell. Mol. Biol. 1991;4:273–277. doi: 10.1165/ajrcmb/4.3.273. [DOI] [PubMed] [Google Scholar]
- FELETOU M., GERMAIN M., THURIEAU C., FAUCHERE J.-L., CANET E. Agonistic and antagonistic properties of the bradykinin B2 receptor antagonist, Hoe 140, in isolated blood vessels from different species. Br. J. Pharmacol. 1994;112:683–689. doi: 10.1111/j.1476-5381.1994.tb13130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULLER R.W., DIXON C.M., CUSS F.M., BARNES P.J. Bradykinin-induced bronchoconstriction in humans. Mode of action. Am. Rev. Respir. Dis. 1987;135:176–180. doi: 10.1164/arrd.1987.135.1.176. [DOI] [PubMed] [Google Scholar]
- GUNST S.J., STROPP J.Q. Effect of Na-K adenosinetriphosphatase activity on relaxation of canine tracheal smooth muscle. J. Appl. Physiol. 1988;64:635–641. doi: 10.1152/jappl.1988.64.2.635. [DOI] [PubMed] [Google Scholar]
- HOCK F.J., WIRTH K., ALBUS U., LINZ W., GERHARDS H.J., WIEMER G., HENKE S., BREIPOHL G., KONIG W., KNOLLE J., SCHOLKENS B.A. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br. J. Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULSMANN A.R., RAATGEEP H.R., SAXENA P.R., KERREBIJN K.F., DE JONGSTE J.C. Bradykinin-induced contraction of human peripheral airways mediated by both bradykinin B2 and thromboxane prostanoid receptors. Am. J. Respir. Crit. Care Med. 1994;150:1012–1018. doi: 10.1164/ajrccm.150.4.7921430. [DOI] [PubMed] [Google Scholar]
- INOUE H., KOTO H., TAKATA S., AIZAWA H., IKEDA T. Excitatory role of axon reflex in bradykinin-induced contraction of guinea pig tracheal smooth muscle. Am. Rev. Respir. Dis. 1992;146:1548–1552. doi: 10.1164/ajrccm/146.6.1548. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., FRENKEN M. A paradox: the 5-HT2-receptor antagonist ketanserin restores the 5-HT-induced contraction depressed by methysergide in large coronary arteries of calf. Allosteric regulation of 5-HT2-receptors. Naunyn-Schmiedebergs Arch. Pharmacol. 1985;328:295–300. doi: 10.1007/BF00515556. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P. The classification of drugs and drug receptors in isolated tissues. Pharmacol. Rev. 1984;36:165–222. [PubMed] [Google Scholar]
- KENAKIN T.P.Antagonism of indirect agonists Pharmacological analysis of drug-receptor interaction, 2nd edition. 1993New York: Raven Press; 318–321.ed. Kenakin T.P. pp [Google Scholar]
- KLEYMAN T.R., CRAGOE E.J., JR Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- LEEB-LUNDBERG L.M.F., MATHIS S.A., HERZIG M.C.S. Antagonists of bradykinin that stabilize a G-protein-uncoupled state of the B2 receptor act as inverse agonists in rat myometrial cells. J. Biol. Chem. 1994;269:25970–25973. [PubMed] [Google Scholar]
- MARSH K.A., HILL S.J. Bradykinin B2 receptor-mediated phosphoinositide hydrolysis in bovine cultured tracheal smooth muscle cells. Br. J. Pharmacol. 1992;107:443–447. doi: 10.1111/j.1476-5381.1992.tb12765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSH K.A., HILL S.J. Characteristics of the bradykinin-induced changes in intracellular calcium ion concentration of single bovine tracheal smooth muscle cells. Br. J. Pharmacol. 1993;110:29–35. doi: 10.1111/j.1476-5381.1993.tb13767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELAND R.S., VAN BREEMEN C., BOHR D.F. Mechanism by which serotonin attenuates contractile response of canine mesenteric arterial smooth muscle. J. Pharmacol. Exp. Ther. 1985;232:322–329. [PubMed] [Google Scholar]
- MORRISON K.J., VANHOUTTE P.M. Stimulation of sodium pump by vasoactive intestinal peptide in guinea-pig isolated trachea: potential contribution to mechanisms underlying relaxation of smooth muscle. Br. J. Pharmacol. 1996;118:557–562. doi: 10.1111/j.1476-5381.1996.tb15438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVRAN S.S., ALLAIN G., GARCIA H.F., ALLEN J.C., SEIDEL C.L. Serotonin-induced Na+/K+ pump stimulation in vascular smooth muscle cells. Evidence for coupling to multiple receptor mechanisms. J. Pharmacol. Exp. Ther. 1991;256:297–303. [PubMed] [Google Scholar]
- PRUNEAU D., LUCCARINI J.M., DEFRENE E., PAQUET J.L., BELICHARD P. Pharmacological evidence for a single bradykinin B2 receptor in the guinea-pig. Br. J. Pharmacol. 1995;116:2106–2112. doi: 10.1111/j.1476-5381.1995.tb16418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYNE N.J., MOUGHAL N., STEVENS P.A., TOLAN D., PYNE S. Protein kinase C-dependent cyclic AMP formation in airway smooth muscle: the role of type II adenylate cyclase and the blockade of extracellular-signal-regulated kinase-2 (ERK-2) activation. Biochem. J. 1994;304:611–616. doi: 10.1042/bj3040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYNE S., PYNE N.J. Differential effects of B2 receptor antagonists upon bradykinin-stimulated phospholipase C and D in guinea-pig cultured tracheal smooth muscle. Br. J. Pharmacol. 1993;110:477–481. doi: 10.1111/j.1476-5381.1993.tb13835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYNE N.J., TOLAN D., PYNE S. Bradykinin stimulates cAMP synthesis via mitogen-activated protein kinase-dependent regulation of cytosolic phospholipase A2 and prostaglandin E2 release in airway smooth muscle. Biochem. J. 1997;328:689–694. doi: 10.1042/bj3280689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGOLI D., GOBEIL F., NGUYEN Q.T., JUKIC D., SEOANE P.R., SALVINO J.M., SAWUTZ D.G. Bradykinin receptor types and B2 subtypes. Life Sci. 1994;55:735–749. doi: 10.1016/0024-3205(94)00557-5. [DOI] [PubMed] [Google Scholar]
- REGOLI D., JUKIC D., GOBEIL F., RHALEB N.E. Receptors for bradykinin and related kinins: a critical analysis. Can. J. Physiol. Pharmacol. 1993;71:556–567. doi: 10.1139/y93-079. [DOI] [PubMed] [Google Scholar]
- RHALEB N.E., ROUISSI N., JUKIC D., REGOLI D., HENKE S., BREIPOHL G., KNOLLE J. Pharmacological characterization of a new highly potent B2 receptor antagonist (HOE 140: D-Arg-[Hyp3,Thi5,D-Tic7,Qic8]bradykinin) Eur. J. Pharmacol. 1992;210:115–120. doi: 10.1016/0014-2999(92)90661-m. [DOI] [PubMed] [Google Scholar]
- RHODEN K.J., DODSON A.M., KY B. Stimulation of the Na+-K+ pump in cultured guinea pig airway smooth muscle cells by serotonin. J. Pharmacol. Exp. Ther. 2000;293:107–112. [PubMed] [Google Scholar]
- ROBERTSON M.J., DOUGALL I.G., HARPER D., MCKECHNIE K.C., LEFF P. Agonist-antagonist interactions at angiotensin receptors: application of a two-state receptor model. Trends in Pharmacological Sciences. 1994;15:364–369. doi: 10.1016/0165-6147(94)90156-2. [DOI] [PubMed] [Google Scholar]
- SASAGURI T., WATSON S.P. Phorbol esters inhibit smooth muscle contractions through activation of Na+-K+ ATPase. Br. J. Pharmacol. 1990;99:237–242. doi: 10.1111/j.1476-5381.1990.tb14687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER D., DAEFFLER L., TRIFILIEFF A., GIES J.P. Effects of WIN 64338, a nonpeptide bradykinin B2 receptor antagonist, on guinea-pig trachea. Br. J. Pharmacol. 1995;115:1127–1128. doi: 10.1111/j.1476-5381.1995.tb15013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER D., SCHMIDLIN F., LACH E., DA SILVA A., LANDRY Y., GIES J.P. Effect of WIN 64338, a B2 bradykinin receptor antagonist on guinea-pig tracheal smooth muscle cells in culture. Fund. Clin. Pharmacol. 1998;12:188–193. doi: 10.1111/j.1472-8206.1998.tb00940.x. [DOI] [PubMed] [Google Scholar]
- SCHLEMPER V., CALIXTO J.B. Nitric oxide pathway-mediated relaxant effect of bradykinin in the guinea-pig isolated trachea. Br. J. Pharmacol. 1994;111:83–88. doi: 10.1111/j.1476-5381.1994.tb14027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUHRADA M., SOUHRADA J.F., CHERNIACK R.M. Evidence for a sodium electrogenic pump in airway smooth muscle. J. Appl. Physiol. 1981;51:346–352. doi: 10.1152/jappl.1981.51.2.346. [DOI] [PubMed] [Google Scholar]
- THERIEN A.G., BLOSTEIN R. Mechanisms of sodium pump regulation. Am. J. Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- TRIFILIEFF A., DA SILVA A., LANDRY Y., GIES J.P. Effect of Hoe 140, a new B2 noncompetitive antagonist, on guinea pig tracheal bradykinin receptors. J. Pharmacol. Exp. Ther. 1992;263:1377–1382. [PubMed] [Google Scholar]
- YUN C.H., TSE C.M., NATH S.K., LEVINE S.A., BRANT S.R., DONOWITZ M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am. J. Physiol. 1995;269:G1–G11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]


