Abstract
Previous studies in this laboratory have shown that diazepam behaves as a phosphodiesterase 4 (PDE 4) inhibitor. It has been reported that PDE-4 inhibitors activate the hypothalamic-pituitary-adrenocortical (HPA) axis in the rat. In the present study we have examined whether activation of the cyclic AMP-dependent protein kinase (PKA) is involved in the effect of diazepam on basal HPA axis activity.
Acute systemic administration of diazepam (10 mg kg−1 i.p.) was found to increase the basal HPA axis activity, increasing the plasma concentrations of corticotrophin (ACTH) and corticosterone 30 min post injection. Diazepam also elevated cyclic AMP content of the hypothalamus.
Pretreatment of the animals with dexamethasone (1 mg kg−1 s.c.) for 3 days completely abolished the effect of diazepam on HPA axis activity.
The antagonists of central and peripheral benzodiazepine receptors, flumazenil (10 mg kg−1 i.p.) and PK 11195 (5 mg kg−1 i.p.) did not affect the diazepam induced increase of HPA axis activity nor did they have an effect per se.
The increase in ACTH and corticosterone levels was significantly reduced by the cyclic AMP-dependent protein kinase (PKA) inhibitor, H-89, given either subcutaneously (5 mg kg−1 s.c.) or intracerebroventricularly (i.c.v.; 28 μg in 10 μl).
The results indicate that diazepam can stimulate basal HPA axis activity in the rat by a cyclic AMP-dependent PKA mediated pathway.
Keywords: Corticosterone; corticotrophin; 3′,5′ cyclic AMP; diazepam; H 89; hyphothalamic-pituitary-adrenal axis; phosphodiesterase inhibitors; protein kinase A inhibitors; rolipram
Introduction
Corticotrophin-releasing hormone (CRH-41) which is produced by parvocellular neurons in the hypothalamic paraventricular nucleus and released from terminals in the median eminence, is recognized as a major regulator of corticotrophin release from the pituitary gland (Vale et al., 1981). It is now well established that cyclic AMP plays a fundamental role in the signal transducing mechanisms that control the secretory activity of the hypothalamus, the pituitary gland and the adrenal cortex (Sette et al., 1994). Indeed the secretion of CRH-41 from the hypothalamus is positively influenced by cyclic AMP (Suda et al., 1985, Widmaier et al., 1989; Hu et al., 1992), and cyclic AMP response elements are known to be contained in the promotor region of the gene that encodes this peptide (Emanuel et al., 1990).
The actions of cyclic AMP are terminated by cyclic nucleotide phosphodiesterase (PDE) enzymes which catalyse the intracellular hydrolysis of the nucleotide to 5′-adenosine monophosphate (Butcher & Sutherland, 1962). PDE enzymes play a critical role in regulating the cellular responses driven by cyclic AMP. The PDEs comprise a heterogeneous family of enzymes and as many as 10 phosphodiesterase gene families have been identified (Borger et al., 1993, Soderling & Beavo, 2000). PDE-4 is widely distributed, being particularly abundant in the central nervous system, immune/inflammatory cells and the reproductive system (Nicholson et al., 1991). Recent in vitro studies point to a role for PDE-4 in the release of CRH and ACTH from the rat hypothalamus and anterior pituitary gland respectively (Hadley et al., 1993, Kumari et al., 1997). Initial studies using non-selective phosphodiesterase inhibitors and cyclic AMP analogues have demonstrated that the release of ACTH from pituitary cells appears to be stimulated through a cyclic AMP-dependent PKA-mediated pathway (Hadley et al., 1993; 1996). In other studies it has been shown that selective PDE-4 inhibitors such as rolipram and denbufylline can increase plasma levels of ACTH and corticosterone in the rat (Kumari et al., 1997).
We have demonstrated that diazepam has a selective PDE-4 inhibitory activity and this has functional and biochemical repercussion since it potentiates the positive inotropic effect as well as the enhancement of intracellular cyclic AMP levels induced by cyclic AMP related inotropic agents (Martinez et al., 1995a, 1995b; Collado et al., 1998; Janvier et al., 1999). Diazepam can affect ACTH and glucocorticoid release in rodents and primates but the mechanism of action by which this agent produce their effects on neuroendocrine functions are complex (see de souza, 1990 for review). Indeed, previous studies have shown that diazepam decreases HPA axis activity in stressful contexts, although the influences on basal release are less consistent (Lakic et al., 1986; Pohorecky et al., 1988; de boer et al., 1990; 1991; Pivac & Pericic, 1993). The effect of acute administration of diazepam on basal corticosterone levels is related to several factors which include the dose administered and gender (Pivac & Pericic, 1993; Wilson et al., 1996). For example, in unstressed rodents low doses of diazepam either diminish or fail to alter glucocorticoid levels whereas high doses of this agent increase corticosterone levels by an unknown mechanism (Lakic et al., 1986; Kalman et al., 1997).
The present study is designed to determine whether the PDE-4 inhibitory action of diazepam, and hence a cyclic AMP-dependent PKA-mediated pathway is involved in the stimulatory effect of diazepam on basal HPA axis activity. To this end we have studied whether diazepam mimics a typical PDE-4 inhibitor, rolipram, in its effects on ACTH and corticosterone release as well as whether the effects are reduced by blocking the cyclic AMP-dependent PKA pathway with H-89 (Hidaka & Kobayashi, 1992). In addition we have investigated the possible involvement of central and peripheral benzodiazepine receptors examining the effects of antagonists of these receptors.
Methods
Animals
Male Sprague-Dawley rats (200–300 g) were housed four to five per cage under a 12 h light/dark cycle (L:0800–2000 h) in a room with controlled temperature (22±1°C) and humidity (50±10%) and given free access to food and water.
Because stress can affect the activity of the HPA axis, the experimental design included efforts to reduce the potential effects of stress. The rats were accustomed to handling over a period of 7 days before the beginning of each experiment. On the day of the experiment, the animals were placed in a quiet room and left undisturbed for 1 h. All experiments were carried out between 1000 and 1200 h to avoid the influence of diurnal rhythm on plasma ACTH and corticosterone concentrations.
In some groups of animals a cannula was implanted intracerebroventricularly (i.c.v.) in the right lateral ventricle under sodium pentobarbitone anaesthesia (30 mg kg−1 i.p.). A stainless steel cannula was inserted using the following stereotactic coordinates: 1.5 mm posterior to bregma, 1.5 lateral to midline and 4 mm below the interaural line. After surgery, animals were allowed to recover for at least 7 days before drugs were administered. At the end of each experiment, the correct positions of the cannulae were verified post mortem.
Drugs
The following drugs were used: Pre-dissolved diazepam (5 mg ml−1) or vehicle (16% ethanol v v−1 and 40% propylene glycol v v−1 in phosphate-buffered saline, (pH 6.8-7) and flumazenil were from Roche (Madrid, Spain). PK 11195 [1-(2-chlorophenyl)-N-methyl-N-(1-methyl-propyl)-3-isoquinoline carboxamide] was from RBI (Natick, U.S.A.); H-89 (N-[2-(p-bromo-cinnamylamino, ethyl-5-isoquinolinesulfonamide) was from ICN (U.S.A.); rolipram and dexamethasone were from Sigma Chemical Co (Madrid, Spain).
The drugs were prepared freshly each day. PK 11195 and flumazenil were suspended in tween 80 (2 drops in 10 ml saline). H-89, rolipram and dexamethasone were dissolved in saline (0.9% sodium chloride).
Experimental procedure
To test the influence of diazepam on HPA axis activity, plasma concentrations of ACTH and corticosterone as well as the tissue levels of cyclic AMP in the hypothalamus and pituitary gland were determined in rats treated with diazepam (10 mg kg−1 i.p.) or vehicle.
After the treatments, the animals were killed by decapitation 30 min later. Trunk blood was collected in prechilled tubes containing EDTA (1 mg ml−1 of blood), centrifuged for 15 min at 4°C and plasma was stored at −30°C until assayed. The samples from each experiment were analysed in the same assays.
The hypothalamus and the pituitary glands were dissected and freshly frozen and stored at −80°C until assayed.
To determine the possible involvement of central and peripheral benzodiazepine receptors in the adrenocortical response to diazepam, animals were pretreated with the antagonists of these receptors, flumazenil (10 mg kg−1 i.p.) and PK 11195 (5 mg kg−1 i.p.) respectively or a corresponding volume of vehicle, 5 min before diazepam (10 mg kg−1 i.p.). Animals were sacrificed 30 min after diazepam administration.
In order to compare the effect of diazepam with the effects of a typical PDE-4 inhibitor, rolipram was included in this study. In a separate group of animals, rolipram (0.2 mg kg−1 i.p.) or a corresponding volume of saline, was administered 20 min prior to death.
To establish whether diazepam exerts its effects at the hypothalamo/pituitary or adrenocortical level, we also studied its effects in dexamethasone pretreated rats. Animals were pretreated with dexamethasone (1 mg kg−1 s.c.) or saline during 3 days with the last dose 12 h before the experiment, and then they were injected with diazepam (10 mg kg−1 i.p.) and sacrificed 30 min later.
The possible involvement of PKA in the effect produced by diazepam on HPA axis activity, was assessed by treating animals with the selective cyclic AMP-dependent protein kinase inhibitor H-89. Animals were injected with H-89 or saline (controls) either subcutaneously (5 mg kg−1) or intracerebroventricularly (28 μg in 10 μl) 30 min before diazepam. Animals were sacrificed 30 min after diazepam administration. The doses of H89 were chosen according to previous studies (Cunha et al., 1999). It is known that H89 on its own is devoid of effect on basal ACTH release from pituitary cells (Asaba et al., 1998). The plasma levels of diazepam were determined in a separate group of rats at 5, 15 and 30 min after diazepam injection by gas liquid chromatography.
Hormone assays
Corticosterone
Plasma corticosterone concentrations were determined by radioimmunoassay (RIA) using a commercially available kit for rats (125I-corticosterone RIA ICN Biomedicals, U.S.A.). The sensitivity of the assay was 0.39 ng ml. The antibody cross-reacted 100% with corticosterone and <0.1% with other steroids.
Corticotrophin (ACTH)
Plasma ACTH was measured without prior extraction, by RIA using a commercially available RIA kit (125-I h ACTH ICN Biomedicals, Inc, U.S.A.). Human ACTH 1–39 prepared by synthesis was used in this kit. The antibody cross-reacted 100% with ACTH 1–39 and 100% with ACTH 1–24 and less than 0.1% with β-endorphin, α-MSH and γ-MSH. The sensitivity of the assay was 10 pg ml−1. The intra and inter-assay coefficients of variation were 6.0 and 10.7% respectively.
Estimation of cyclic AMP in the hypothalamus and pituitary gland
Levels of cyclic AMP were determined by radioimmunoassay (125I-TME-S-cAMP, Diagnostic Pasteur, France) After decapitation, the brains and the pituitary glands were removed rapidly, fresh-frozen, and stored immediately at −80°C until use. The dissected hypothalamus and the pituitary glands were weighed and then homogenized in 0.6 ml cold perchloric acid (0.3 M) with a Polytron homogenizer (setting 4 for 30 s) and centrifuged (12,000 r.p.m., 4°C, 15 min). The supernatants were treated with potassium hydroxide until pH 6.2 was reached. The sensitivity of the assay was 2 pmol ml−1. The intra and inter-coefficients of variations were 7.7 and 8.2%, respectively. The antibody cross-reacted 100% with 3′5′-cyclic AMP and less than 0.3% with other nucleotides.
Estimation of plama diazepam concentrations
Diazepam concentrations in plasma were determined by gas-liquid chromatography (GLC) as previously described (Friedman et al., 1986). In brief, fixed amounts of appropriate internal standards were added to the unknown plasma sample. Calibration standard were prepared by addition of known amounts of diazepam together with the same amount of internal standard to drug-free plasma. Unknown and calibration samples of plasma were then extracted into an organic solvent (benzene: isoamyl alcohol 98, 5 : 1,5), separated, evaporated to dryness, reconstituted and subjected to GLC with electron-capture detection.
Statistical analysis
Statistical evaluation of the data was performed using one-way analysis of variance (ANOVA), followed by Student-Newman-Keuls for individual comparisons. The results were considered significant when P values were <0.05.
Results
Effects of diazepam and rolipram on plasma levels of ACTH and corticosterone
Acute systemic administration of diazepam (10 mg kg−1 i.p.) significantly increased both plasma ACTH and corticosterone levels when compared to the respective control group (Figure 1). This dose of diazepam produced serum concentrations of 3.7±0.25, 1.4±0.1 and 1.08±0.06 μg ml−1 at 5, 15 and 30 min post injection. The effect of diazepam is not due to the solvent, since no significant difference was found in ACTH and corticosterone levels between the group that received the solvent of diazepam and that which was injected with saline. In order to compare the effect of diazepam to that of a typical PDE-4 inhibitor, rolipram was included in this study. The effect of the acute administration of rolipram (0.2 mg kg−1 i.p.) on the plasma ACTH as well as corticosterone levels is shown in Figure 1. As can be seen, rolipram significantly increased the plasma ACTH as well as the corticosterone levels compared to the respective saline injected control group. No difference was found between the effect of diazepam and that produced by rolipram on the secretion of ACTH and corticosterone.
Figure 1.
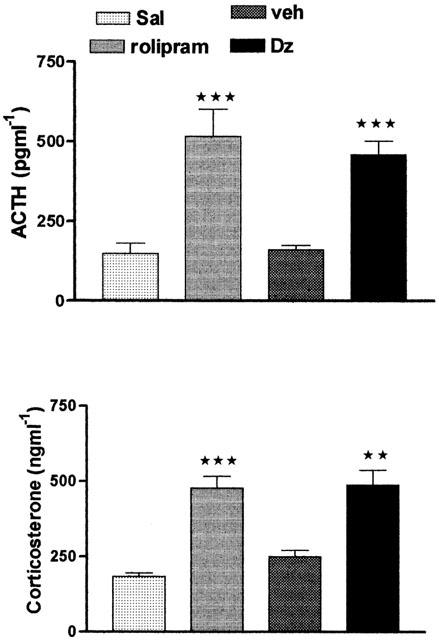
Plasma concentrations of ACTH and corticosterone after acute administration of diazepam (10 mg kg−1 i.p.) or rolipram (0.2 mg kg−1 i.p.). Control animals were injected with the respective vehicle. Testing occurred 30 or 20 min after diazepam or rolipram injections respectively. Values are the mean±s.e.mean of at least five experiments. Significance was determined by one-way ANOVA followed by the Student-Newman-Keuls test. ***P<0.001 vs control; **P<0.01 vs control.
Figure 2 depicts plasma concentrations of ACTH and corticosterone of rats pretreated with flumazenil (10 mg kg−1 i.p.) or PK 11195 (5 mg kg−1 i.p.) 5 min prior to diazepam. Neither flumazenil nor PK 11195 modified the increase in plasma ACTH and corticosterone concentrations induced by diazepam. These doses of flumazenil and PK 11195 did not have an effect per se, on plasma levels of corticosterone (307±49 ng ml−1 and 259±43 ng ml−1 respectively) when compared to those in the control group treated with vehicle (284±19 ng ml−1) (P>0.05). In order to learn whether the effect of diazepam was produced at hypothalamo/pituitary or adrenal level, we administered diazepam to rats pretreated with dexamethasone for 3 days. In these animals, the plasma levels of corticosterone after diazepam administration were drastically reduced (40±0.1 ng ml−1) when compared to the effect of diazepam in the vehicle pretreated group (420±25 ng ml−1) (P<0.001).
Figure 2.
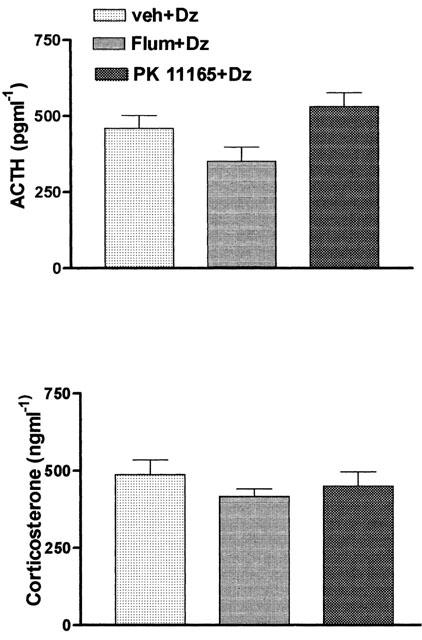
Plasma concentrations of ACTH and corticosterone 30 min after acute injection of diazepam (10 mg kg−1 i.p.) in rats pretreated 5 min before with flumazenil (10 mg kg−1 i.p.), PK 11165 (5 mg kg−1 i.p.) or vehicle. Significance was determined by one-way ANOVA followed by the Student-Newman-Keuls test. Values are the mean±s.e.mean of at least five experiments.
Effect of diazepam on the tissue levels of cyclic AMP in the hypothalamus and pituitary gland
The tissue levels of cyclic AMP were significantly elevated in the hypothalamus 30 min after diazepam injection when compared to those in the vehicle-injected control group. However the cyclic AMP content in the pituitary gland was not significantly modified after diazepam administration (Figure 3).
Figure 3.
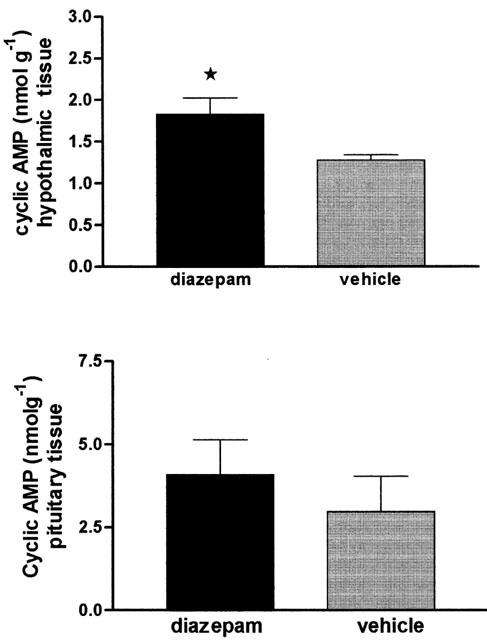
Tissue levels of cyclic AMP in the hypothalamus and pituitary gland, 30 min after acute injection of diazepam (10 mg kg−1) or vehicle (control). Significance was determined by one-way ANOVA followed by Student-Newman-Keuls test. *P<0.05 vs control.
Effects of H-89 on the pituitary-adrenocortical response to diazepam
To study the involvement of PKA in the diazepam-induced increase of HPA axis activity, animals were treated with the selective inhibitor of cyclic AMP-dependent PKA, H-89 (28 μg in 10 μl i.c.v.) or (5 mg kg−1 s.c.) 30 min prior to diazepam administration. Figure 4 depicts the plasma levels of ACTH and corticosterone of rats acutely treated with H-89, 30 min prior to diazepam. This drug administered either subcutaneously or i.c.v., significantly reduced the diazepam-induced elevations of plasma ACTH and corticosterone when compared to their respective control group that received saline either i.c.v. or s.c. prior to diazepam.
Figure 4.
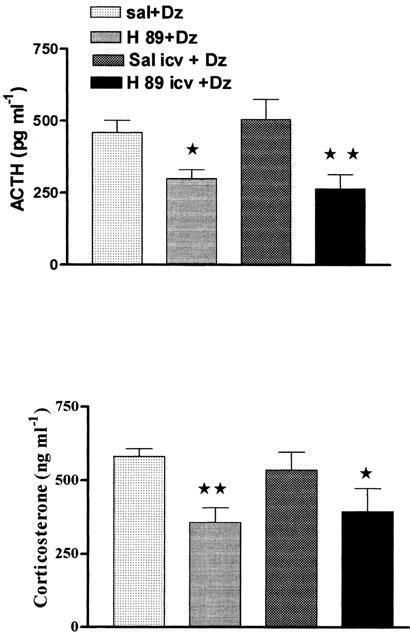
Plasma concentrations of ACTH and corticosterone 30 min after acute injection of diazepam (10 mg kg−1 i.p.) in rats pretreated 30 min before diazepam with H-89 or saline (control), administered s.c. (5 mg kg−1) or i.c.v. (28 μg in 10 μl). Values are the mean±s.e.mean of at least five experiments. Significance was determined by one-way ANOVA followed by the Student-Newman-Keuls test. **P<0.01 vs respective control; * P<0.05 vs respective control.
Discussion
The results of this study indicate that acute systemic administration of diazepam (at 10 mg kg−1) to rats elicits an elevation in basal plasma ACTH as well as in corticosterone levels and the effects are significantly reduced by H-89, an inhibitor of the cyclic AMP-dependent PKA pathway. Futhermore the effect of diazepam on ACTH and corticosterone secretion is similar to that produced by rolipram and occurs concomitantly with an increase in the cyclic AMP content of the hypothalamus but not of the pituitary gland.
We have recently demonstrated that diazepam inhibits the PDE 4 isoenzyme (Collado et al., 1998) which is particularly abundant in the central nervous system and seems to be involved in the regulation of HPA axis activity (Hadley et al., 1993; Kumari et al., 1997). The possibility that diazepam facilitates the secretion of ACTH and corticosterone by increasing cyclic AMP production was therefore investigated in this study by examining the interaction between H-89, a potent cyclic AMP-dependent protein kinase inhibitor and diazepam on basal HPA axis activity. Our results show that either s.c. or i.c.v. administration of H-89 significantly reduces the effects of diazepam on plasma ACTH and corticosterone secretion, what indicates that part of the diazepam effect on stimulation of the HPA axis is mediated through a cyclic AMP-dependent mechanism. The present study also demonstrates that diazepam produces an increase in the cyclic AMP content of the hypothalamus but not the pituitary gland. These results are similar to those reported for rolipram, which also enhances the cyclic AMP content of the hypothalamus but not of the pituitary gland (Kumari et al., 1997). The lack of effect at the pituitary level has been hypothesized to be due to the low turnover of cyclic AMP in resting pituitary cells and accordingly blockade of the PDE has little effect on nucleotide levels (Kumari et al., 1997). It would be of interest to measure cyclic AMP levels in the paraventricular nucleus of the hypothalamus and corticotroph cells in the pituitary gland but the fact that diazepam increases the levels of cyclic AMP in the hypothalamus in parallel with the levels of ACTH and corticosterone in the circulation demonstrates that diazepam acts in the hypothalamic nucleus where CRH synthesis and secretion take place. In addition diazepam may also act in the corticotrophes of the anterior pituitary but from our study we cannot define the precise site where the effect of diazepam occurs.
Previous studies have demonstrated that diazepam at concentrations around 10 μM can stimulate the production of cyclic AMP in slices of guinea-pig cerebral cortex (York & Davies, 1981). This effect has been explained partially by an inhibition of adenosine uptake by diazepam which results in an increase in extracellular adenosine and concomitant activation of the adenosine receptor coupled to adenylate cyclase (York & Davies, 1981). However the diazepam induced increase in ACTH release observed in our experiments cannot be explained by enhancement of endogenous adenosine since it is known that this agent exerts a tonic inhibitory influence on ACTH release (Anand-Srivastava et al., 1989).
Our results demonstrate that diazepam, like rolipram, behaves as a typical PDE 4 inhibitor and support the view that PDE 4 inhibitors exert a stimulant effect on HPA axis activity in the rat (Kumari et al., 1997). The stimulatory effect of diazepam on corticosterone secretion does not seem to take place at the adrenal level since it was abolished by pretreatment with dexamethasone, a steroid which suppresses the centrally mediated HPA response to stressful stimuli but does not modify the adrenal response to exogenous ACTH (Hadley et al., 1996). It has been previously suggested that the effects of diazepam on the HPA axis are mediated by actions at the central benzodiazepine receptor site associated with the GABA A receptor complex (de boer et al., 1990; Pivac & Pericic, 1993). However, in our experiments we found that the effect of diazepam was not mediated by the benzodiazepine/GABA/channel chloride receptor complex since it was not antagonized by flumazenil which is an effective inhibitor of the effects mediated by this complex (Haefely et al., 1985). Because of its relatively short half life, flumazenil was administered 35 min before sacrifice, ensuring that it would exert its maximal effect (Brogden & Goa, 1991). Thus the effect of diazepam on basal HPA axis activity seems to involve cellular mechanisms different from GABA-mediated chloride conductance. This is consistent with other non GABA-related actions produced by concentrations of benzodiazepines in the therapeutic range, such as the previously reported effects of diazepam in the heart (Collado et al., 1998; Janvier et al., 1999) as other tissues (Baldessarini, 1996).
Alteration in HPA responses may also involve diazepam induced changes in peripheral benzodiazepine receptors (PBR). These receptors have been identified in a number of peripheral tissues including the adrenal cortex as well as in the central nervous system (Gavish et al., 1992a, 1992b). The agonist of PBR, Ro 5-4864 has been reported to increase ACTH and corticosterone release by directly stimulating hypothalamic CRH release (Calogero et al., 1990). Since diazepam can also bind to PBR, we examined the effects of diazepam in the presence of an antagonist of PBR, PK 11195. In these experiments the plasma concentrations of ACTH and corticosterone did not differ significantly from those in the group that received vehicle plus diazepam. Our results therefore indicate that the stimulatory effect of diazepam on basal HPA axis activity is not mediated by peripheral benzodiazepine receptor activation. This finding is consistent with previous studies showing that PK 11195, even at high doses did not antagonize the effect of the agonist of PBR 4-chlorodiazepam on plasma corticosterone but on the contrary this compound has been shown to stimulate the HPA axis in the rat (Calogero et al., 1990).
The IC50 of diazepam as an inhibitor of PDE 4 isoenzyme is 8 μM (Collado et al., 1998) which corresponds to a serum concentration of 2.5 μg ml−1. In the present study the serum concentrations at 5 min post-injection were 3.7 μg ml−1 which clearly produces an inhibitory effect on PDE 4 isoenzyme. The dose of diazepam used in this study is greater than the doses that are used for chronic treatment of anxiety (2–30 mg day−1) which produce plasma levels of the drug in the range of 0.02–1.01 μg ml−1 (Greenblatt et al., 1981). However, diazepam at high doses is also used for other clinical application such as the control of status epilepticus (up to 3 mg/kg infused over 24 h) or the induction of anaesthesia (Parfitt, 1999), which could lead to serum concentrations of 6.01±1.21 μg ml−1 (Samuelson et al., 1981; Reynolds, 1996). This could be an important consideration, especially in the central nervous system where PDE 4 is the main isoenzyme involved in the hydrolysis of cyclic AMP (Nicholson et al., 1991).
In conclusion, our results indicate that under certain experimental conditions diazepam stimulates basal HPA axis activity in the rat. The effect appears to be at least partially mediated by the secondary messenger cyclic AMP through blockade of PDE 4 in the hypothalamus and possibly also in the anterior pituitary, leading to increased secretion of ACTH and corticosterone. The effect does not appear to be mediated by the benzodiazepine/GABA channel chloride receptor complex or by peripheral benzodiazepine receptors. Whether diazepam exerts similar effects on the HPA axis in other species is not known and further investigation will be necessary to establish the clinical relevance of these findings.
Acknowledgments
This work was supported by Ministerio de Educación y Cultura, BFI 2000-1013 (Spain).
Abbreviations
- ACTH
corticotrophin
- cyclic AMP
3′5′-cyclic adenosine monophosphate
- HPA
Hypothalamic-pituitary-adrenal
- i.p.
intraperitoneal
- i.c.v.
intracerebroventicular
- PKA
Protein kinase A
- s.c.
subcutaneous
References
- ANAND-SRIVASTAVA M.B., CANTIN M., GUTKOWSKA J. Adenosine regulates the release of adrenocorticotropic hormone (ACTH) from cultured anterior pituitary cells. Mol. Cell. Biochem. 1989;15:21–28. doi: 10.1007/BF00228276. [DOI] [PubMed] [Google Scholar]
- ASABA K., MAKINO S., HASHIMOTO K. Effect of urocortin on ACTH secretion from rat anterior pituitary in vitro and in vivo: comparison with corticotropin-releasing hormone. Brain Res. 1998;806:95–103. doi: 10.1016/s0006-8993(98)00747-1. [DOI] [PubMed] [Google Scholar]
- BALDESSARINI J.Drugs and the treatment of psychiatric disorders: Psychosis and anxiety Goodman & Gilman's The Pharmacological basis of therapeutics. 1996New York, Mac Graw-Hill; 399–430.Harman J.B, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG eds [Google Scholar]
- BORGER G., MICHAELI T., MARTINS T., JOHN T.S., STEINER B., RODGERS L., RIGGS S., WIGGER M., FERGUSON K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential target for antidepressant drugs. Mol. Cell. Biol. 1993;13:6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROGDEN R.N., GOA K.L. Flumazenil. A reappraisal of its pharmacological properties and therapeutics efficacy as a benzodiazepine antagonist. Drugs. 1991;42:1061–1089. doi: 10.2165/00003495-199142060-00010. [DOI] [PubMed] [Google Scholar]
- BUTCHER R.W., SUTHERLAND E.W. Adenosine 3′5′-phosphate in biological materials 1. Purification and properties of cyclic 3′5′-nucleotide phosphodiesterase and use of this enzyme to characterise adenosine 3′-5′ phosphate in human orine. J. Biol. Chem. 1962;237:1244–1250. [PubMed] [Google Scholar]
- CALOGERO A.E., KAMILARIS T.C., BERNARDINI R., JOHNSON E.O., CHROUSOS G.P., GOLD P.W. Effects of peripheral benzodiazepine receptor ligands on hypothalamic-pituitary-adrenal axis function in the rat. J. Pharmacol. Exp. Ther. 1990;253:729–737. [PubMed] [Google Scholar]
- COLLADO M.C., BELETA J., MARTINEZ E., MIRALPEIX M., DOMENECH T., PALACIOS J.M., HERNANDEZ J. Functional and biochemical evidence for diazepam as a cyclic nucleotide phosphodiesterase type 4 inhibitor. Br. J. Pharmacol. 1998;123:1047–1054. doi: 10.1038/sj.bjp.0701698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., TEIXEIRA M.M., FERREIRA S.H. Pharmacological modulation of secondary mediator system–cyclic AMP and cyclic GMP–on inflammatory hyperalgesia. Br. J. Pharmacol. 1999;127:671–678. doi: 10.1038/sj.bjp.0702601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BOER S.F., DER GUGTEN J.V., SLANGEN J.L. Brain benzodiazepine receptor-mediated effects on plasma catecholamine and corticosterone concentrations in rats. Brain Res. Bull. 1990;24:843–847. doi: 10.1016/0361-9230(90)90149-t. [DOI] [PubMed] [Google Scholar]
- DE BOER S.F., DER GUGTEN J.V., SLANGEN J.L. Effects of chlordiazepoxide, flumazenil and DMCM on plasma catecholamine and corticosterone concentrations in rats. Pharmacol. Biochem. Behav. 1991;38:13–19. doi: 10.1016/0091-3057(91)90583-n. [DOI] [PubMed] [Google Scholar]
- DE SOUZA E.B. Neuroendocrine effects of benzodiazepines. J. Psychiat. Res. 1990;24:111–119. doi: 10.1016/0022-3956(90)90042-o. [DOI] [PubMed] [Google Scholar]
- EMANUEL R.L., GIRARD D.M., THULL D.C., MAJZOUB J.A. Second messengers involved in the regulation of corticotropin-releasing hormone mRNA and peptide in cultured rat fetal hypothalamic primari cultures. Endocrinology. 1990;126:3016–3021. doi: 10.1210/endo-126-6-3016. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN H., ABERNETHY D.R., GREENBLATT D.J., SHADER R.I. The pharmacokinetics of diazepam and desmethyldiazepam in rat brain and plasma. Psychopharmacology. 1986;88:267–270. doi: 10.1007/BF00180822. [DOI] [PubMed] [Google Scholar]
- GAVISH M., BAR-AMI S., WEIZMAN R. The endocrine system and mitochondrial benzodiazepine receptors. Mol. Cell. Endocrinol. 1992a;88:1–13. doi: 10.1016/0303-7207(92)90003-o. [DOI] [PubMed] [Google Scholar]
- GAVISH M., KATZ Y., BAR-AMI S., WEIZMAN R. Biochemical, physiological and pathological aspects of the peripheral benzodiazepine receptor. J. Neurochem. 1992b;58:1589–1601. doi: 10.1111/j.1471-4159.1992.tb10030.x. [DOI] [PubMed] [Google Scholar]
- GREENBLATT D.J., LAUGHREN T.P., ALLEN M.D., HARMATZ J.S., SHADER R.I. Plasma diazepam and desmethyldiazepam concentration during long-term diazepam therapy. Br. J. Clin. Pharmacol. 1981;11:35–40. doi: 10.1111/j.1365-2125.1981.tb01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADLEY A.J., FLACK J.D., BUCKINGHAM J.C. Effects of selective phosphodiesterase inhibitors on the release of ACTH and LH from the rat anterior pituitary gland in vitro. Pharmacol. Commun. 1993;3:283–295. [Google Scholar]
- HADLEY A.J., KUMARI M., COVER P.O., OSBORNE J., FLACK J.D., BUCKINGHAM J.C. Stimulation of the hypothalamo-pituitary-adrenal axis in the rat by the type 4-phosphodiestersae (PDE-4) inhibitor denbufylline. Br. J. Pharmacol. 1996;119:463–470. doi: 10.1111/j.1476-5381.1996.tb15695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAEFELY W.E., KYBURZ E., GERECKE E., MOHLER H. Recent advances in the molecular pharmacology of benzodiazepine receptors and in the structure activity relationships of their agonists and antagonists. Adv. Drugs Res. 1985;14:165–322. [Google Scholar]
- HIDAKA H., KOBAYASHI R. Pharmacology of protein kinase inhibitors. Ann. Rev. Pharmacol. Toxicol. 1992;32:377–379. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- HU S.-B., TANNAHILL L.A., BISWAS S., LIGHTMAN S.L. Release of corticotropin-releasing factor-41, arginine vasopressin and oxitocin from fetal hypothalamic cells in culture: response to activation of intracellular second messenger and corticosteroids. J. Endocrinol. 1992;132:57–65. doi: 10.1677/joe.0.1320057. [DOI] [PubMed] [Google Scholar]
- JANVIER J.J., GARCIA-ESTAÑ J., HERNANDEZ J. Diazepam reduces action potential duration in guinea-pig papillary muscle by a cAMP-dependent mechanism. Life Sci. 1999;64:2383–2389. doi: 10.1016/s0024-3205(99)00192-7. [DOI] [PubMed] [Google Scholar]
- KALMAN B.A., KIM P.J., COLE M.A., CHI M.S., SPENCER R.L. Diazepam attenuation of restraint stress-induced corticosterone levels is enhanced by prior exposure to repeated restraint. Psychoneuroendocrinology. 1997;22:349–360. doi: 10.1016/s0306-4530(97)00026-7. [DOI] [PubMed] [Google Scholar]
- KUMARI M., COVER P.O., POYSER R.H., BUCKINGHAM J.C. Stimulation of the hypothalamo-Pituitary-adrenal axis in the rat by three selective type-4 phosphodiesterase inhibitors: in vitro and in vivo studies. Br. J. Pharmacol. 1997;121:459–468. doi: 10.1038/sj.bjp.0701158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAKIC N., PERICIC D., MANEY H. Mechanism by which picrotoxin and a high dose of diazepam elevate plasma corticosterone level. Neuroendocrinology. 1986;43:331–335. doi: 10.1159/000124547. [DOI] [PubMed] [Google Scholar]
- MARTINEZ E., COLLADO M.C., MARIN J., HERNANDEZ J. Mechanism involved in the positive inotropic effect of diazepam. Br. J. Pharmacol. 1995a;114:248. [Google Scholar]
- MARTINEZ E., PEÑAFIEL R., COLLADO M.C., HERNANDEZ J. Diazepam potentiates the positive inotropic effect of isoprenaline in rat ventricle strips: role of cyclic AMP. Eur. J. Pharmacol. 1995b;228:169–175. doi: 10.1016/0014-2999(95)00325-f. [DOI] [PubMed] [Google Scholar]
- NICHOLSON C.D., CHALLISS R.A.J., SHAHID M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol. Sci. 1991;12:19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- PARFITT K. Martindale Extra Pharmacopeia. London: The Pharmaceutical Press; 1999. [Google Scholar]
- PIVAC N., PERICIC D. Inhibitory effect of diazepam on the activity of the hypothalamic-pituitary-adrenal axis in female rats. J. Neural Transmission. 1993;92:173–186. doi: 10.1007/BF01244876. [DOI] [PubMed] [Google Scholar]
- POHORECKY L.A., COTLER S., CARBONE J.J., ROBERT P. Factor modifying the effect of diazepam on plasma corticosterone levels in rats. Life Sci. 1988;43:2159–2167. doi: 10.1016/0024-3205(88)90367-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS J.E.F. Martindale Extra Pharmacopoeia. London: The Pharmaceutical Press; 1996. [Google Scholar]
- SETTE C., VICINI E., CONT M. Modulation of cellular responses by hormones: a role of cAMP specific, rolipram sensitive phosphodiesterases. Mol. Cell. Endocrinol. 1994;100:75–79. doi: 10.1016/0303-7207(94)90282-8. [DOI] [PubMed] [Google Scholar]
- SAMUELSON P.N., REVES J.G., KOUCHOUKOS N.T., SMITH L.R., DOLE K.M. Hemodynamic responses to anaesthetic induction with midazolam or diazepam in patients with ischemic heart disease. Anaesth. Anal. 1981;60:802–809. [PubMed] [Google Scholar]
- SODERLING S.H., BEAVO J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Current Opin. Cell. Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- SUDA T., YAJIMOTO F., TOMORI N., DEMURA H., SHIZUME K. In vitro study of immunoreactive corticotropin-releasing factor release from the rat hypothalamus. Life Sci. 1985;37:1499–1505. doi: 10.1016/0024-3205(85)90181-x. [DOI] [PubMed] [Google Scholar]
- VALE W., SPIESS J., RIVIER C., RIVIER J. Characterization of a residue hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- WIDMAIER E.P., LIM A.T., VALLE W. Secretion of corticotropin-releasing factor from cultured rat hypothalamic cells. Effects of catecholamines. Endocrinology. 1989;124:583–590. doi: 10.1210/endo-124-2-583. [DOI] [PubMed] [Google Scholar]
- WILSON M.A., BISCARDI R., SMITH M.D., WILSON S.P. Effects of benzodiazepine agonist exposure on cortocotropin-releasing factor content and hormonal stress responses: divergent responses in male and ovaridectomized female rats. J. Pharmacol. Exp. Ther. 1996;278:1073–1082. [PubMed] [Google Scholar]
- YORK M.J., DAVIES L.P. The effect of diazepam on adenosine uptake and adenosine-stimulated adenylate cyclase in guinea-pig brain. Can. J. Physiol. Pharmacol. 1981;60:302–307. doi: 10.1139/y82-041. [DOI] [PubMed] [Google Scholar]


