Abstract
Nonsteroidal anti-inflammatory drugs have been reported to exacerbate hypertension and to interfere with the effectiveness of some anti-hypertensive therapies. In this study, we tested the effects of a gastric-sparing, nitric oxide-releasing derivative of aspirin (NCX-4016) on hypertension in rats.
Hypertension was induced by administering L-NAME in the drinking water (400 mg l−1). Groups of rats were treated daily with aspirin, NCX-4016 or vehicle.
NCX-4016 significantly reduced blood pressure relative to the aspirin-treated group over the 2-week period of treatment. Aspirin and, to a lesser extent, NCX-4016 suppressed whole blood thromboxane synthesis.
In anaesthetized rats, acute intravenous administration of NCX-4016 caused a significant fall in mean arterial pressure in hypertensive rats, but was devoid of such effects in normotensive controls.
In vitro, NCX-4016 relaxed phenylephrine-pre-contracted aortic rings obtained from both normotensive and hypertensive rats, and significantly reduced their responsiveness to the contractile effects of phenylephrine.
These results suggest that NCX-4016 reduces blood pressure in hypertensive rats, not simply through the direct vasodilatory actions of the nitric oxide released by this compound, but also through possible interference with the effects of endogenous pressor agents. These properties, added to its anti-thrombotic effects, suggest that NCX-4016 may be a safer alternative to aspirin for use by hypertensive patients.
Keywords: Nitric oxide, blood pressure, prostaglandin, anti-inflammatory, norepinephrine
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) exert their beneficial effects in large part by suppressing prostaglandin synthesis through the inhibition of cyclo-oxygenase (COX) enzymes. On the other hand, this inhibition is also largely responsible for the well known deleterious effects of these compounds in the upper gastrointestinal tract (Wallace, 1997). In recent years, in an attempt to reduce NSAID-induced gastrointestinal toxicity, a novel approach was developed involving the addition of a nitric oxide (NO)-releasing moiety to traditional NSAID molecules (Wallace et al., 1994a,1994b; 1995; Davies et al., 1997). This derivatization does not compromise the anti-inflammatory or analgesic activities of the parent compounds, and the gastrointestinal tolerability of these ‘NO-NSAIDs' seems to be attributable to the small amounts of NO released, which counteract the vasoconstriction and leukocyte adherence caused by traditional NSAIDs within the gastric microcirculation (Wallace et al., 1994a,1994b; 1995; Davies et al., 1997).
NSAIDs can interfere with the efficacy of some anti-hypertensive drugs and, in some groups of patients, they can potentiate a pre-existing hypertensive status (Houston, 1991; Whelton, 1999). Celecoxib, a selective inhibitor of COX-2, has also been shown to increase blood pressure (BP) in the rat and to exacerbate pre-existing hypertension (Muscará et al., 2000a). In previous studies, we showed that the chronic administration of an NO-releasing derivative of naproxen to rats significantly attenuated the increase in BP induced by either the addition to the drinking water of a nitric oxide synthase inhibitor (Muscará et al., 1998) or partial unilateral renal artery stenosis (Muscará et al., 2000b). In contrast, equimolar doses of naproxen significantly potentiated the hypertensive status in both models.
The anti-inflammatory, analgesic and anti-pyretic properties of aspirin are well established. Increasingly, aspirin is also used chronically at low doses for prophylaxis of stroke and myocardial infarction (Patrono, 1994). In common with other NO-NSAIDs, an NO-releasing aspirin derivative (NCX-4016) has been shown to share with the parent compound the analgesic, anti-inflammatory (Al-Swayeh et al., 2000) and anti-thrombotic properties (Wallace et al., 1995; 1999), but to produce markedly less gastric ulceration (Wallace et al., 1995; 1999; Takeuchi et al., 1998a,1998b). Furthermore, it has been shown that treatment with NCX-4016 in vivo leads to an effective prevention of both hemorrhagic shock-induced gastric damage (Wallace et al., 1997) and azoxymethane-induced colonic adenocarcinoma in the rat (Bak et al., 1998).
In the present study, we examined the effects of daily treatment for 2 weeks with aspirin or NCX-4016 on the hypertension induced in rats by pharmacological inhibition of nitric oxide synthesis. In addition, the in vitro effects of NCX-4016 on the contractility of aortic rings obtained from either normotensive or hypertensive rats were examined.
Methods
Animals
Male, Wistar rats weighing 200–250 g were obtained from Charles River Breeding Farms (Montreal, PQ, Canada). The rats were housed in polypropylene cages in groups of two or three rats per cage, and received laboratory chow ad libitum. All experimental procedures were approved by the Animal Care Committee of the University of Calgary and were carried out in accordance with the guidelines of the Canadian Council on Animal Care.
Effects of NCX-4016 versus aspirin in hypertensive rats
Four groups of 6–7 rats each were studied. In three of the groups, hypertension was induced by addition of a nitric oxide synthase inhibitor, Nω-nitro-L-arginine methylester (L-NAME), to the drinking water at a concentration of 400 mg l−1. The fourth group received normal tap water. Two weeks after starting the study, BP was measured using the tail cuff method (Muscará et al., 2000a). The rats were conscious and only mildly restrained during the measurements, which were always made between 13:00 and 15:00. The BP measurements were considered valid only when three consecutive readings did not differ by more than 5 mmHg. The mean of the three measured values was then recorded. The individual performing the measurements was unaware of the treatments the rats had received. The rats with L-NAME-induced hypertension continued to receive the supplemented drinking water for the following 2 weeks. During that time, the groups were treated each day (between 15:00 and 16:00) with vehicle or with either aspirin (ASA; 10 mg kg−1) or an equimolar dose of NCX-4016 (18.6 mg kg−1), all given orally. The vehicle for both drugs was 1% carboxymethylcellulose (1 ml kg−1). The fourth group of rats received normal drinking water and received daily oral treatment with vehicle. BP measurements were made weekly, 16–20 h after administration of ASA, NCX-4016 or vehicle.
Thromboxane synthesis
At the end of the 2-week period of drug administration, and 2 h after the final administration of vehicle, ASA or NCX-4016, the rats were anaesthetized with sodium pentobarbitone (65 mg kg−1 i.p.) and blood samples were taken from the descending abdominal aorta. Thromboxane B2 synthesis by whole blood was determined as previously described (Wallace et al., 1995).
Serum nitrite and nitrate concentrations
Blood sample aliquots (2 ml) were allowed to clot at room temperature, the sera were separated by centrifugation (10 min at 2000×g) and stored at −20°C until analysed for their nitrite (NO2−) and nitrate (NO3−) concentrations by high performance liquid chromatography (Muscará & de Nucci, 1996).
Plasma renin activity (PRA)
Blood sample aliquots (1 ml) were collected into pre-cooled tubes containing 0.8 mg of EDTA. The plasma was immediately separated by centrifugation (2000×g for 10 min at 4°C) and stored at −20°C until analysed. PRA was estimated by the generation of angiotensin I from endogenous angiotensinogen at pH 6.5 and 37°C over a 4-h period. Angiotensin I concentrations were measured by radioimmunoassay using a commercially available kit.
In vivo response to sodium nitroprusside
Groups of rats (n=6 each) received L-NAME-supplemented or normal drinking water for 2 weeks. The rats were anaesthetised by an intraperitoneal injection of sodium pentobarbitone (65 mg kg−1) and placed on a heating blanket to maintain their body temperature at 37°C. The left carotid artery was cannulated with PE-10 tubing and connected to a pressure transducer for continuous monitoring of systolic and diastolic arterial pressure. The right femoral vein was cannulated with PE-50 tubing through which increasing doses of sodium nitroprusside (0.8–16 μg kg−1) were injected. The effects on mean arterial pressure (MAP) were recorded over a period of 4 h. For comparison, groups of normal and hypertensive rats, prepared as described above, received NCX-4016 (18.6 mg kg−1) intravenously and BP responses were monitored for 4 h.
Pharmacokinetics
Normal rats were treated orally with either NCX-4016 (93 mg kg−1) or isosorbide dinatrate (ISDN; 37 mg kg−1), the doses of these drugs being equivalent on a molar basis in terms of the number of NO-releasing moieties. Blood samples were obtained at regular time intervals up to 24 h after dosing (n=4 rats at each time-point) and serum nitrite and nitrate concentrations were measured as described above. Serum samples obtained from rats that received NCX-4016 were also analysed for their salicylate concentrations, which were measured using a spectrophotometric assay (Wallace et al., 1999).
In vitro vascular response of aortic rings
Groups of rats (n=5 each) received L-NAME-supplemented or normal drinking water for 2 weeks and were then anaesthetized with sodium pentobarbitone (65 mg kg−1 i.p.). Thoracic aortae were obtained and cleaned of all connective tissue, then cut into rings of approximately 3–4 mm in length. Each ring was mounted under 2 g passive tension in 25 ml organ baths containing a physiological salt solution maintained at 37°C and bubbled with 95% O2-5% CO2. Isometric tension was recorded with a force displacement transducer (Grass FT .03) coupled to a Grass polygraph model 7D. The bathing solution had the following composition (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 12.5 and glucose 11.1. Tissues (endothelium-intact preparations) were allowed to equilibrate for 1 h before starting the experiments. After the equilibration period, cumulative concentration-response curves to phenylephrine were generated. Then, glyceryl trinitrate (GTN) or NCX-4016 was added cumulatively to elicit relaxations and the concentration-response curves were constructed. After a 15 min incubation period with either GTN or NCX-4016 (each at 10 μM), the responses to a range of concentrations of phenylephrine were again examined. In all of the experiments, each ring preparation was exposed to a single NO donor and from each aorta, one ring served as the control by being exposed only to phenylephrine. Some experiments were performed in which the tissues were exposed to the soluble guanylate cyclase ODQ (1H-[1,2,4]- oxadiazol-(4,3-a) quinoxalin-1-one; 10 μM).
Another series of in vitro experiments, similar to those outlined above, were performed using aortic rings obtained from 2-kidney 1-clip (2K1C) renovascular hypertensive rats. Briefly, after anaesthetizing the rats by exposure to Halothane vapour (5% in oxygen), a 3 cm retroperitoneal flank incision was performed under sterile conditions. The left kidney was exposed, the renal artery was then carefully dissected free of the renal vein and its partial occlusion was achieved by placement on the vessel of a silver clip with an internal diameter of 0.2 mm. The wound was closed with a running 3-0 silk suture and the rats were then randomly assigned to one of the three treatment groups (n=8 per group), as described below. Sham-operated rats underwent identical surgical procedures, except that a clip was not applied to the renal artery. Two weeks after the surgical procedure, the thoracic aortae were obtained from the rats and prepared as described above.
Statistical analysis
All data are shown as mean±s.e.mean. Differences among groups were analysed using a one-way analysis of variance followed by the Student-Neuman-Keul's test for multiple comparisons. Values of probability less than 5% (P<0.05) were considered significant.
Materials
L-NAME, aspirin, sodium nitroprusside, isosorbide dinitrate, norepinephrine, ODQ and the kits for determining serum salicylate concentrations were obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.). NCX-4016 (2-acetoxybenzoate 2-[1-nitroxymethyl]-phenyl ester) was provided by NicOx S.A. (Sophia Antipolis, France). GTN was obtained from Omega (Montreal, PQ, Canada). Kits for measurement of thromboxane were obtained from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). The radioimmunoassay kit for angiotensin I was obtained from Peninsula Laboratories (San Carlos, CA, U.S.A.). The kit for measurement of serum salicylate concentrations was obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). All other supplies and reagents were obtained from Fisher Scientific (Edmonton, AB, Canada).
Results
In vivo studies in conscious rats
Rats drinking water supplemented with L-NAME exhibited a sustained increase in BP throughout the 4-week study period. Rats treated daily with aspirin exhibited significantly higher BP values than the rats treated with either vehicle or NCX-4016. The BP increase due to L-NAME was attenuated by daily treatment with NCX-4016 during the first week. While there was no significant difference between the vehicle and NCX-4016-treated groups at the end of 2 weeks of treatment, the BP values in the latter were significantly reduced (P<0.001) relative to the group treated with aspirin (Figure 1).
Figure 1.
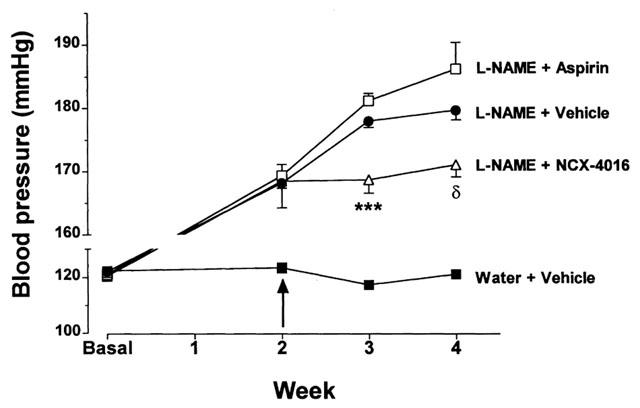
Effects of aspirin and NCX-4016 (NCX) on systolic blood pressure in L-NAME treated rats. The arrow indicates the point at which once-daily treatment with the test compounds was initiated. δP<0.05 versus the L-NAME+ASA-treated group; ***P<0.001 versus the L-NAME+vehicle or L-NAME+ASA-treated groups.
As shown in Table 1, addition of L-NAME to the drinking water resulted in a marked (∼70%) reduction in serum nitrate levels, with no effect on serum nitrite levels. In rats treated daily with aspirin, there were similarly low levels of serum nitrate. However, in rats receiving L-NAME in the drinking water and treated daily with NCX-4016, serum nitrate levels were significantly elevated relative to the control group.
Table 1.
Effects of aspirin and NCX-4016 on thromboxane synthesis, serum NOx− levels and plasma renin activity in L-NAME-induced hypertension

TXB2 production in both the aspirin- and NCX-4016-treated rats was significantly inhibited relative to that observed in the vehicle-treated groups (Table 1). However, the degree of inhibition of whole blood TXB2 synthesis was more pronounced in the ASA-treated rats than in the NCX-4016 group.
All of the groups receiving L-NAME in the drinking water exhibited decreased plasma renin activity in comparison with the control group. Neither aspirin nor NCX-4016 treatment caused additional changes in plasma renin activity.
In vivo studies in anaesthetized rats
The anti-hypertensive effects of NCX-4016 were further evaluated in anaesthetized rats. Initially, we characterized the responsiveness of normal rats and rats with L-NAME induced hypertension to a standard NO donor. Figure 2A shows that the fall in MAP caused by the intravenous administration of sodium nitroprusside was greater in the hypertensive rats than in controls at all doses tested. The decrease in BP was evident almost immediately after injection of sodium nitroprusside, but the BP had returned to normal levels within 5–10 min. Intravenous administration of NCX-4016 induced a significant fall in MAP in hypertensive rats, while in the normotensive rats, it had no effect (Figure 2B). The maximal decrease in BP was observed 40–60 min after administration of NCX-4016, and a significant lowering of BP was still evident at the end of the 4 h recording period.
Figure 2.
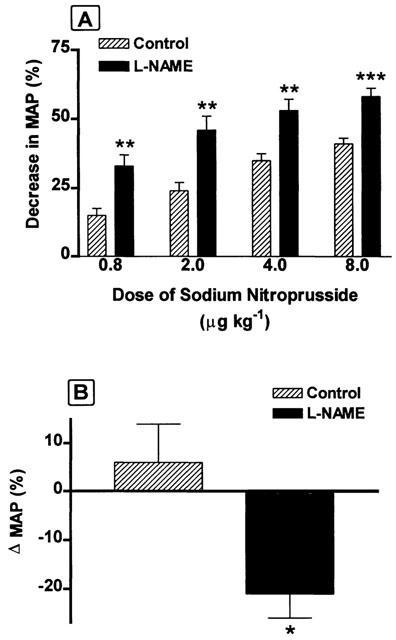
(A) Hypotensive effects of increasing intravenous doses of sodium nitroprusside (SNP) obtained from either control or L-NAME-treated rats expressed as percentage of the initial mean arterial pressure (MAP) values. (B) Effects of a single intravenous dose of NCX-4016 (18.6 mg kg−1) on blood pressure in control and L-NAME-treated rats, expressed as percentage of the initial MAP values.
Pharmacokinetics
The kinetic profiles of in vivo nitric oxide-release from NCX-4016 and ISDN are shown in Figure 3A. Following the administration of ISDN, there was a rapid increase in serum NOx− concentrations, which peaked at 1.5 h. In the case of NCX-4016, maximum serum nitrate concentrations were observed 6 h after administration, without any significant change in serum nitrite levels compared to the basal values. From 6 to 24 h after the administration of either NO-donor, the circulating NOx (nitrate+nitrite) concentrations were identical. Figure 3B shows that serum salicylate concentrations followed the same kinetic profile as that observed for nitrate anion following the oral administration of NCX-4016.
Figure 3.
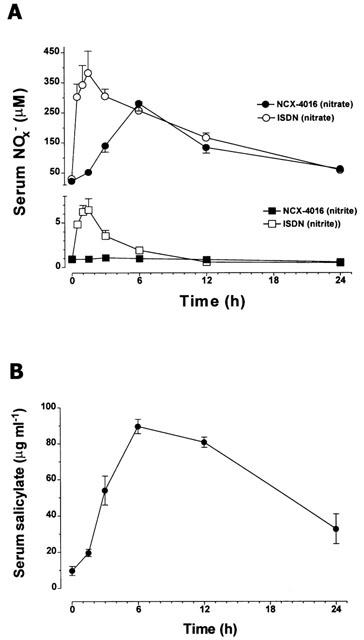
(A) Serum levels of nitrite (squares) and nitrate (circles) as a function of time, following the oral administration of single equimolar doses of either isosorbide dinitrate (37 mg kg−1) or NCX-4016 (93 mg kg−1). (B) Salicylate concentrations measured in serum samples collected after NCX-4016 administration as a function of time.
In vitro studies
Figure 4A shows the in vitro vasorelaxant activities of NCX-4016 and GTN in phenylephrine pre-contracted aortic rings obtained from L-NAME-treated and control rats (phenylephrine was used at concentrations ranging from 1 to 3 μM to produce 70% of maximal contraction). Vessels from rats with L-NAME-induced hypertension were more sensitive to the relaxant activity of GTN than those obtained from controls (pEC50: 8.17±0.06 vs 7.76±0.03, respectively; P<0.01), with no significant alteration in the maximal response. In the case of NCX-4016, phenylephrine pre-contracted aortic rings from L-NAME-treated rats showed an identical pattern of response to this compound when compared to that observed with tissues obtained from control rats (pEC50: 7.12±0.04 vs 7.11±0.07, respectively). As shown in Figure 4B, vessels from 2K1C hypertensive rats did not differ significantly from those from the sham-operated controls in terms of the relaxation response to either GTN (pEC50 8.37±0.09 and 8.33±0.06, respectively) or NCX-4016 (pEC50 7.12±0.09 and 7.15±0.06, respectively). The vasorelaxant activities of both GTN and NCX-4016 were completely abolished when the soluble guanylate cyclase inhibitor ODQ was present in the tissue bath (data not shown).
Figure 4.
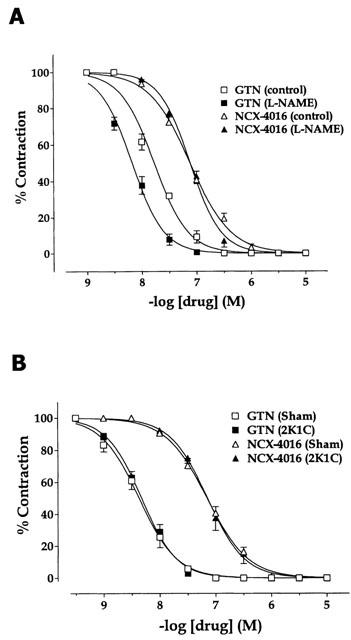
In vitro dose-dependent relaxation to glyceryl trinitrate (GTN) or NCX-4016 of phenylephrine pre-contracted aortic rings obtained from either hypertensive or the corresponding normotensive controls. Hypertension was induced either by the administration of L-NAME in the drinking water (A) or by partial unilateral occlusion of the renal artery (2K1C model; B).
Figure 5 shows the phenylephrine-induced contraction curves obtained with aortic rings from either control (Figure 5A) or L-NAME treated rats (Figure 5B). The respective pEC50 values were 6.49±0.04 and 7.19±0.03 (P<0.001). Pre-incubation of aortic rings obtained from control rats with 10−5 M of either GTN or NCX-4016 for 15 min induced significant decreases of the pEC50 value for phenylephrine-induced contraction (GTN: 6.17±0.06, P<0.05; NCX-4016: 5.74±0.11, P<0.001). However, while the pre-incubation of the aortic rings with GTN did not cause any significant alteration of the maximal phenylephrine-induced contraction (Emax: 100% and 97.5±2.5% for control and GTN, respectively), pre-incubation with NCX-4016 led to a decrease of the maximal contractile response to phenylephrine (Emax: 63.3±6.3%, P<0.001; Figure 5A). In the case of vascular tissue obtained from L-NAME treated rats, the pre-incubation with either of the NO-donors led to a significant loss in the efficacy of phenylephrine-induced vasoconstriction (Emax: 61.3±4.3% for GTN and 51.1±5.0% for NCX-4016; P<0.001 vs control) and the dose-response curves to phenylephrine were significantly shifted to the right (pEC50: 6.36±0.09 for GTN and 6.24±0.10 for NCX-4016; P<0.001 vs control).
Figure 5.
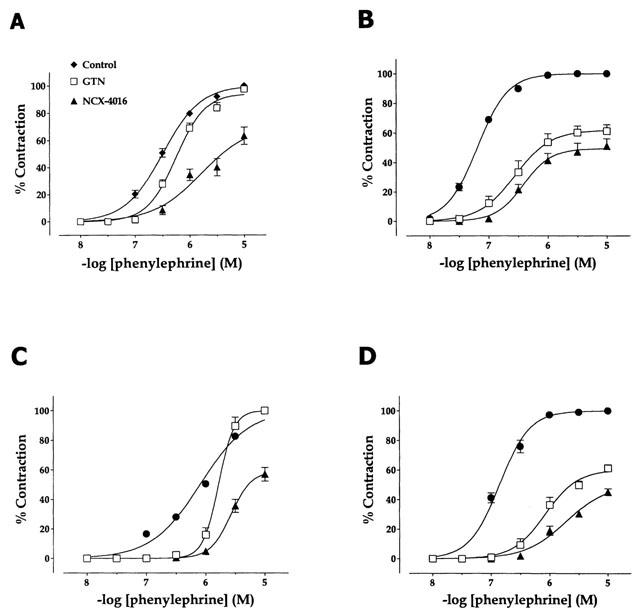
In vitro dose-dependent contraction to phenylephrine of aortic rings obtained from rats drinking either water (A) or water supplemented with L-NAME (B), and sham-operated rats (C) or 2K1C-renovascular hypertensive rats (D) before (control) and after a 15-min incubation period with 10 μM of either glyceryl trinitrate or NCX-4016.
Phenylephrine contracted aortic rings from sham-operated and 2K1C hypertensive rats in a dose-dependent manner, with pEC50 values of 6.10±0.03 and 6.82±0.05, respectively (P<0.001; Figure 5C,D). Pre-incubation of the aortic rings from sham-operated rats with either GTN or NCX-4016 (Figure 5C) induced a rightward shift of the phenylephrine contraction curves (pEC50: 5.78±0.04 and 5.13±0.06 for GTN and NCX-4016, respectively; P<0.001), the effect of pre-incubation with NCX-4016 being significantly more pronounced than that with GTN (P<0.001). However, while the pre-incubation of the aortic rings with 10−5 M GTN did not cause any alteration of the maximal phenylephrine-induced contraction, pre-incubation with NCX-4016 led to a significant decrease of the maximal contraction to phenylephrine (Emax: 57.0±4.6%, P<0.001). On the other hand, in the case of the vascular tissue from 2K1C rats (Figure 5D), pre-incubation with each of the NO donors led to a significant loss of efficacy (Emax: 61.3±2.4% for GTN and 45.3±2.3% for NCX-4016; P<0.001 vs control), which was more pronounced in the case of NCX-4016 (P<0.001 vs GTN). In addition, phenylephrine contraction potency was also decreased by both compounds (pEC50: 5.96±0.10 for GTN and 5.62±0.07 for NCX-4016; P<0.001 vs control), but in a significantly more effective manner by NCX-4016 (P<0.01 vs GTN).
Discussion
Chronic administration of NO synthase inhibitors leads to the development of a hypertensive state that is characterized by endothelial dysfunction (Ribeiro et al., 1992; Baylis et al., 1992). In human hypertension, endothelial dysfunction can occur secondary to a number of disorders, including hyperlipidemia (Shimokawa & Vanhoutte, 1989a), ischaemia-reperfusion (Ku, 1982) and atherosclerosis (Shimokawa & Vanhoutte, 1989b). On the other hand, the inter-play between NO and vasodilator prostaglandins at the renal level has a fundamental role in the maintenance of renal homeostasis and BP control (Patrono & Dunn, 1987; Oates et al., 1988). In this way, inhibition of renal cyclo-oxygenase by NSAIDs can lead to an impairment of BP control, especially in hypertensive patients (Houston, 1991).
The results of the present study show that daily administration of aspirin significantly exacerbates the hypertensive state induced by addition of L-NAME to the drinking water. In contrast, daily administration of an NO-releasing derivative of aspirin, NCX-4016, significantly attenuated BP relative to the parent drug. Delivery of NO in this way therefore partially compensates for the endothelial dysfunction induced by chronic L-NAME administration. Moreover, it would appear that NCX-4016 significantly altered the responsiveness of the vascular smooth muscle to pressor agents such as phenylephrine, which would contribute further to an attenuation of the hypertensive state.
The decrease in nitric oxide synthesis, particularly by the vascular endothelium, in L-NAME-treated rats is likely to be the most important factor contributing to the observed hypertension. The mechanism underlying the further increase in BP with aspirin administration is less clear. There was no further reduction of NOx− levels with aspirin administration over that seen in L-NAME-treated rats receiving vehicle. Aspirin caused a profound suppression of thromboxane synthesis, but that would not contribute to an increase in BP (if anything, the contrary). Moreover, aspirin administration did not significantly affect plasma renin activity. It is possible that marked suppression of endothelial and renal prostaglandin synthesis (particularly prostacyclin synthesis) by aspirin may have been a key factor in the exacerbation of hypertension by this drug. In a setting of diminished NO production, there would likely be a greater role for PGs in the regulation of BP. On the other hand, given the short half-life of aspirin, it would seem highly unlikely that suppressed PG synthesis would persist for as long as 21–24 h after administration of this drug, which is when the BP measurements were performed. Nevertheless, we cannot rule out the possibility that suppression of COX activity by aspirin contributes to its ability to exacerbate hypertension in the rat, and that the relatively weaker inhibition of COX by NCX-4016 contributes to the lack of exacerbation of hypertension by that drug.
Previous studies have documented that NCX-4016 releases NO (Fiorucci et al., 2000; Carini et al., 2001). In the present study, the elevated plasma NOx− levels and the reduction of mean arterial pressure confirmed that release of NO occurs over several hours after oral administration of NCX-4016. The peak levels of NOx− achieved following administration of NCX-4016 were substantially below that observed following administration of isosorbide dinitrate at a dose equimolar to that of NCX-4016 in terms of the number of NO-releasing moieties. Consistent with this observation was the finding that NCX-4016 produced substantially lower reductions in mean arterial pressure than isosorbide dinitrate. Thus, NO release from NCX-4016 appears to occur in smaller amounts than from a conventional organic nitrate, and at a slower rate. This profile of release of NO from NCX-4016 may be important in terms of the ability of this drug to produce long-lasting reductions of mean arterial pressure in hypertensive rats. The slow release of NO from NCX-4016 has recently been confirmed in a study in which NO release was measured, as nitrosylhemoglobin, by electron spin resonance spectroscopy (Carini et al., 2001). In that study, intact NCX-4016 was not detected in blood of rats following oral administration. From the present study, we know that one of the metabolites of this compound is salicylate, and that there is negligible conversion of NCX-4016 to aspirin, as evident by the modest inhibition of thromboxane synthesis. It is therefore likely that NCX-4016 is rapidly de-acetylated, and then esterases cleave the salicylate moiety from the NO-releasing moiety. While it has not yet been demonstrated, we would speculate that the NO-releasing moiety survives in the blood for prolonged periods of time, thereby giving rise to the prolonged release of NO. The complete nature of the metabolism of NCX-4016 remains unknown, but it is clearly important that this be more fully investigated.
L-NAME-treated rats exhibited a marked increase in the sensitivity to the vasodilator effects of sodium nitroprusside. Similarly, NCX-4016 caused a significant reduction in mean arterial pressure in hypertensive rats at a dose that did not alter BP in normotensive rats, and in previous studies, daily administration of an NO-releasing derivative of naproxen for 4 weeks failed to significantly reduce systemic BP in normal rats (Muscará et al., 1998). These observations are consistent with previous reports that the hypersensitivity of vascular smooth muscle to exogenous NO develops when NO synthase is chronically suppressed (Moncada et al., 1991; Henrion et al., 1996). The in vitro studies of GTN demonstrated that this increase in the sensitivity of vascular smooth muscle occurred specifically in the model of hypertension induced by chronic NO suppression (L-NAME), since no shift in the dose-response curve was observed with aortic rings harvested from rats with 2K1C-induced hypertension. With respect to the L-NAME model, however, the change in reactivity of vascular smooth muscle appears to vary with the NO donor. While there was a significant leftward shift of the dose-response curve for GTN-induced relaxation of aortic strips from rats chronically treated with L-NAME, there was no shift in the dose-response curve for relaxation induced by NCX-4016. The reasons for this difference between NCX-4016 and GTN are not clear, but may relate to the relative amounts of NO generated by these compounds and/or the rate of release of NO.
That NCX-4016 produced vasorelaxation via a cyclic GMP-mediated mechanism is supported by the observation that the relaxation of aortic strips in vitro was completely abolished by the soluble guanylate cyclase inhibitor, ODQ. Nevertheless, the results of the present study, and in particular the in vitro studies, suggest that NCX-4016 exerted beneficial effects in terms of blood pressure regulation through mechanisms other than just NO-mediated relaxation of vascular smooth muscle. Both NCX-4016 and GTN caused hyporesponsiveness to phenylephrine of rat aortic rings in vitro after a 15-min pre-incubation with 10 μM of either of these NO-donors. GTN attenuated the efficacy of phenylephrine to contract aorta rings from hypertensive rats, irrespective of the method used to induce hypertension. In contrast, however, preincubation of aortic strips with NCX-4016 led to a loss of the efficacy of phenylephrine irrespective of whether the tissues were taken from normotensive or hypertensive rats. Given the fact that NCX-4016 releases small amounts of NO relative to traditional organic nitrates such as GTN, these results lead us to postulate that apart from the NO/cyclic GMP-mediated effects of these NO-donors, NCX-4016 appears to alter vascular reactivity through other, as yet unidentified, NO-independent mechanisms. While the exact nature of such mechanisms are unclear, it is noteworthy that NCX-4016 has recently been shown to exert effects independent of its actions on soluble guanylate cyclase, namely the inhibition of interleukin-1β synthesis as a consequence of inactivation of one or more caspases (Fiorucci et al., 2000).
In summary, NCX-4016, an NO-releasing aspirin derivative, is able to significantly reduce systemic BP in L-NAME-induced hypertensive rats. The beneficial effects of this compound on hypertension may be mediated, at least in part, through attenuation of the sympathetic-mediated blood vessel contraction and by a direct vasodilator action of the NO released from this compound. NCX-4016 has been shown not to cause gastric damage (Takeuchi et al., 1998b; Wallace et al., 1999; Fiorucci et al., 2000), but to exert more potent anti-thrombotic actions than aspirin in a model of pulmonary thromboembolism (Momi et al., 2000). These characteristics suggest that NCX-4016 is a safer alternative to aspirin for the treatment of ongoing inflammatory processes and prevention of myocardial infarction in patients with hypertension or increased sensitivity to the gastric damaging actions of traditional NSAIDs.
Acknowledgments
This work was supported by a grant from the Heart and Stroke Foundation of Canada. Dr Wallace is a Canadian Institutes of Health Research Senior Scientist and an Alberta Heritage Foundation for Medical Research Scientist. Dr Muscará supported by a fellowship from the National Council of Research of Brazil (CNPq number 301437/95-5).
Abbreviations
- BP
blood pressure
- COX
cyclo-oxygenase
- GTN
glyceryl trinitrate
- ISDN
isosorbide dinitrate
- 2K1C
2 kidney, 1 clip
- L-NAME
Nω-nitro-L-arginine methylester
- MAP
mean arterial pressure
- NO
nitric oxide
- NSAID
nonsteroidal anti-inflammatory drug
- ODQ
1H-[1,2,4]-oxadiazol-(4,3-a) quinoxalin-1-one
- PRA
plasma rennin activity
References
- AL-SWAYEH O.A., CLIFFORD R.H., DEL SOLDATO P., MOORE P.K. A comparison of the anti-inflammatory and anti-nociceptive activity of nitroaspirin and aspirin. Br. J. Pharmacol. 2000;129:343–350. doi: 10.1038/sj.bjp.0703064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAK A.W., MCKNIGHT W., LI P., DEL SOLDATO P., CALIGNANO A., CIRINO G., WALLACE J.L. Cyclooxygenase-independent chemoprevention with an aspirin derivative in a rat model of colonic adenocarcinoma. Life Sci. 1998;62:PL367–PL373. doi: 10.1016/s0024-3205(98)00191-x. [DOI] [PubMed] [Google Scholar]
- BAYLIS C., MITRUKA B., DENG A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J. Clin. Invest. 1992;90:278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARINI M., ALDINI G., STEFANI R., ORIOLI M., FACINO R.M.Nitrosylhemoglobin, an unequivocal index of nitric oxide release from nitroaspirin: in vitro and in vivo studies in the rat by electron spin resonance (ESR) spectroscopy J. Pharmaceut. Biom. Analysis 2001(in press) [DOI] [PubMed]
- DAVIES N.M., ROSETH A.G., APPLEYARD C.B., MCKNIGHT W., DEL SOLDATO P., CALIGNANO A., CIRINO G., WALLACE J.L. NO-naproxen vs. naproxen: ulcerogenic, analgesic and anti-inflammatory effects. Aliment. Pharmacol. Ther. 1997;11:69–79. doi: 10.1046/j.1365-2036.1997.115286000.x. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., SANTUCCI L., CIRINO G., MENCARELLI A., FAMILIARI L., DEL SOLDATO P., MORELLI A. IL-1β converting enzyme is a target for nitric oxide-releasing aspirin: new insights in the antiinflammatory mechanism of nitric oxide-releasing nonsteroidal anti-inflammatory drugs. J. Immunol. 2000;165:5245–5254. doi: 10.4049/jimmunol.165.9.5245. [DOI] [PubMed] [Google Scholar]
- HENRION D., DOWELL F.J., LEVY B.I., MICHEL J.B. In vitro alteration of aortic vascular reactivity in hypertension induced by chronic NG-nitro-L-arginine methyl ester. Hypertension. 1996;28:361–366. doi: 10.1161/01.hyp.28.3.361. [DOI] [PubMed] [Google Scholar]
- HOUSTON M.C. Nonsteroidal anti-inflammatory drugs and antihypertensives. Am. J. Med. 1991;90:42S–47S. doi: 10.1016/0002-9343(91)90485-g. [DOI] [PubMed] [Google Scholar]
- KU D.D. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982;218:576–578. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- MOMI S., EMERSON M., PAUL W., LEONE M., MEZZASOMA A.M., DEL SOLDATO P., PAGE C.P., GRESELE P. Prevention of pulmonary thromboembolism by NCX 4016, a nitric oxide-releasing aspirin. Eur. J. Pharmacol. 2000;397:177–185. doi: 10.1016/s0014-2999(00)00223-5. [DOI] [PubMed] [Google Scholar]
- MONCADA S., REES D.D., SCHULZ R., PALMER R.M.J. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCARÁ M.N., DE NUCCI G. Simultaneous determination of nitrite and nitrate anions in plasma, urine and cell culture supernatants by high-performance liquid chromatography with post column reactions. J. Chromatography B Biomed. Appl. 1996;686:157–164. doi: 10.1016/s0378-4347(96)00229-0. [DOI] [PubMed] [Google Scholar]
- MUSCARÁ M.N., LOVREN F., MCKNIGHT W., TRIGGLE C.R., CIRINO G., WALLACE J.L. Anti-hypertensive properties of a nitric oxide-releasing naproxen derivative in 2-kidney, 1-clip rats. Am. J. Physiol. 2000a;279:H528–H535. doi: 10.1152/ajpheart.2000.279.2.H528. [DOI] [PubMed] [Google Scholar]
- MUSCARÁ M.N., MCKNIGHT W., WALLACE J.L. Effect of a nitric oxide-releasing naproxen derivative on hypertension and gastric damage induced by chronic nitric oxide inhibition in the rat. Life Sci. 1998;62:PL235–PL240. doi: 10.1016/s0024-3205(98)00072-1. [DOI] [PubMed] [Google Scholar]
- MUSCARÁ M.N., VERGNOLLE N., LOVREN F., TRIGGLE D.R., ELLIOTT S.N., ASFAHA S., WALLACE J.L. Selective cyclooxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br. J. Pharmacol. 2000b;129:1423–1430. doi: 10.1038/sj.bjp.0703232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OATES J.A., FITZGERALD G.A., BRANCH R.A., JACKSON E.K., KNAPP H.R., ROBERTS L.J. Clinical implications of prostaglandin and thromboxane A2 formation. New Engl. J. Med. 1988;319:761–767. doi: 10.1056/NEJM198809223191206. [DOI] [PubMed] [Google Scholar]
- PATRONO C. Aspirin as an anti-platelet drug. New Engl. J. Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- PATRONO C., DUNN M.J. The clinical significance of inhibition of renal prostaglandin synthesis. Kidney Int. 1987;32:1–12. doi: 10.1038/ki.1987.164. [DOI] [PubMed] [Google Scholar]
- RIBEIRO M.O., ANTUNES E., DE NUCCI G., LOVISOLO S.M., ZATZ R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992;20:298–303. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., VANHOUTTE P.M. Hypercholesterolemia causes generalized impairment of endothelium-dependent relaxation to aggregating platelets in porcine arteries. J. Am. Coll. Cardiol. 1989a;13:1402–1408. doi: 10.1016/0735-1097(89)90318-5. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., VANHOUTTE P.M. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ. Res. 1989b;64:900–914. doi: 10.1161/01.res.64.5.900. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI K., SUZUKI K., YAMAMOTO H., ARAKI H., MIZOGUCHI H., UKAWA H. Cyclooxygenase-2 selective and nitric oxide-releasing nonsteroidal anti-inflammatory drugs and gastric mucosal responses. J. Physiol. Pharmacol. 1998a;49:501–513. [PubMed] [Google Scholar]
- TAKEUCHI K., UKAWA H., KONAKA A., KITAMURA M., SUGAWA Y. Effect of nitric oxide-releasing aspirin derivative on gastric functional and ulcerogenic responses in rats: comparison with plain aspirin. J. Pharmacol. Exp. Ther. 1998b;286:115–121. [PubMed] [Google Scholar]
- WALLACE J.L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., DEL SOLDATO P., BAYDOUN A.R., CIRINO G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., WILSON T.L., DEL SOLDATO P., CIRINO G. Reduction of shock-induced gastric damage by a nitric oxide-releasing aspirin derivative: role of neutrophils. Am. J. Physiol. 1997;273:G1246–G1251. doi: 10.1152/ajpgi.1997.273.6.G1246. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MUSCARÁ M.N., MCKNIGHT W., DICAY M., DEL SOLDATO P., CIRINO G. In vivo antithrombotic effects of a nitric oxide-releasing aspirin derivative, NCX-4016. Thromb. Res. 1999;93:43–50. doi: 10.1016/s0049-3848(98)00134-0. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M.B., CIRINO G. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994a;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M.B., CIRINO G. A diclofenac derivative without ulcerogenic properties. Eur. J. Pharmacol. 1994b;23:249–255. doi: 10.1016/0014-2999(94)90136-8. [DOI] [PubMed] [Google Scholar]
- WHELTON A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am. J. Med. 1999;106:13S–24S. doi: 10.1016/s0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]


