Abstract
Troglitazone, an insulin sensitizing agent, has a direct positive inotropic effect. However, the mechanism of this effect remains unclear. Thus, we examined the inotropic effect of troglitazone while focusing on intracellular Ca2+ handling.
Troglitazone significantly increased peak isovolumic left ventricular pressure (LVPmax), peak rate of rise of LVP (dP/dtmax), peak rate of fall of LVP (dP/dtmin) in isolated rat hearts perfused at a constant coronary flow and heart rate. This inotropic effect of troglitazone was not inhibited by pretreatment with carbachol (muscarine receptor agonist), H89 (protein kinase A inhibitor), U73122 (phospholipase C inhibitor), H7 (protein kinase C inhibitor), verapamil (L-type Ca2+ channel antagonist), thapsigargin (Ca2+-adenosine triphosphatase inhibitor) or ryanodine (ryanodine receptor opener).
Radioimmunoassay showed that the cyclic adenosine monophosphate concentration in the left ventricle was not increased by troglitazone.
Whole-cell patch clamp analysis revealed that troglitazone had no effect on inward Ca2+ currents in cardiomyocytes.
In fura-2 loaded perfused rat hearts, troglitazone exerted its positive inotropic effect without increasing Ca2+ concentration.
These results suggest that neither the inward Ca2+ currents nor Ca2+ handling in the sarcoplasmic reticulum was involved in the inotropic effect of troglitazone. Furthermore, troglitazone exerted its positive inotropic effect without affecting the intracellular concentration of Ca2+.
In conclusion, the positive inotropic effect of troglitazone is mediated by a sensitization of Ca2+.
Keywords: Troglitazone, thiazolidinedione, positive inotropic effect, Ca2+-sensitizing effect, cardiac function, heart
Introduction
Troglitazone was the first thiazolidinedione developed for the treatment of type 2 diabetes mellitus and other insulin-resistant diseases (Iwamoto et al., 1991; Suter et al., 1992; Nolan et al., 1994). It has been reported that troglitazone has various cardiovascular effects besides its insulin-sensitizing effect. Troglitazone has a vasodilatory effect and decreases blood pressure both in animals (Yoshioka et al., 1993; Kaufman et al., 1995) and in humans (Ogihara et al., 1995). Regarding its effects on the heart, Ghazzi et al. (1997) showed that troglitazone increased cardiac output and stroke volume, as a result of decreased peripheral resistance. We also demonstrated that troglitazone has direct positive inotropic and negative chronotropic effects as well as a coronary dilatory effect in isolated perfused rat hearts (Shimoyama et al., 1999). From these results, troglitazone seems beneficial for patients with heart failure. However, the mechanisms of the inotropic effects of troglitazone in the heart, still remain to be elucidated. Therefore, it is of great importance to clarify the mechanisms of the positive inotropic effect of troglitazone not only for treating diabetic patients with heart failure, but also for developing new unique agents which would exert positive inotropic and vasorelaxant effects without increasing the heart rate.
In a previous study, we demonstrated that the inotropic effect of troglitazone was not mediated by α-, β-adrenergic receptors or L-type Ca2+ channels (Shimoyama et al., 1999). In the present study, therefore, we examined the inotropic effect of troglitazone focused on intracellular Ca2+ handling.
Methods
Isolated heart preparation
Male Wistar rats (300 to 350 g, 12 weeks old) were used for isolated isovolumic heart preparations as described previously (Ogino et al., 1995). All animals were handled in strict accordance with the Tottori University Guide for the Care and Use of Laboratory Animals. In brief, after rats were heavily anaesthetized by intraperitoneal injection of a cocktail of ketamine HCl (40 mg) and xylazine (2 mg) dissolved in heparin (1000 i.u.), the heart was rapidly excised, and the aorta was cannulated for retrograde perfusion with a 14-gauge needle connected to a modified Langendorff perfusion system that consisted of a warmed storage vat and a condenser at 37°C, and an adjustable-speed rotary pump (model 7523-40, Masterflex, Barrington, IL, U.S.A.). The coronary perfusion pressure (CPP) was held at 80 mmHg by maintaining a constant flow of modified Tyrode's solution (in mM: NaCl 144, KCl 5, CaCl2 1.5, MgCl2 0.9, N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulphonic acid] (HEPES) 6, and glucose 5, pH 7.4) equilibrated with 100% oxygen. Perfusate was not recirculated. Ventricular function was assessed by measuring the left ventricular pressure (LVP) with a fluid-filled latex balloon inserted into the left ventricle through the mitral valves and inflated to give an end-diastolic pressure of 5 mmHg. The transducer was connected to a Mac Lab System (model Power Lab 8sp, AD Instruments, Castle Hill, Australia) and the LVP and CPP were measured. After being attached to the Langendorff perfusion system, the heart was allowed to stabilize for at least 30 min, during which time LVP and CPP (constant coronary pressure at 80 mmHg) were monitored. Troglitazone (0.2, 0.5 and 1 μmol) dissolved in 0.1 ml of the medium [5% dimethyl sulphoxide (DMSO) and 19% bovine albumin] was administered as a bolus injection directly into the condenser, which contained 20 ml of perfusate (the final concentration of troglitazone in the perfusate was estimated to be 10, 25 and 50 μM, respectively). The same volume of medium (5% DMSO and 19% bovine albumin) but without troglitazone was used as control. In preliminary studies, we confirmed that neither DMSO nor albumin affected any haemodynamic parameters in the isolated hearts (data not shown).
To eliminate the effect of changes in coronary vascular resistance and heart rate, the coronary bed was maximally dilated by adding sodium nitroprusside (5 μM) to the perfusate and the hearts were paced at a constant rate (240 beats per minute) using a pacing generator (model SEN3201, Nihon Kohden, Tokyo, Japan) as previously described (Shimoyama et al., 1999). To inhibit several different putative Ca2+ signalling pathways, we added carbachol (muscarine receptor agonist, 3 μM), H89 [cyclic adenosine monophosphate (cyclic AMP)-dependent protein kinase [protein kinase A (PKA)] inhibitor, 300 nM], verapamil (L-type Ca2+ channel antagonist, 3 nM), ryanodine (ryanodine receptor opener, 10 nM), thapsigargin [Ca2+-adenosine triphosphatase (Ca2+-ATPase) inhibitor, 300 nM], U73122: 1-[6-[[17(-3-methoxyestra-1,3,5 (10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione [phospholipase C (PLC) inhibitor, 1 μM] or H7 [protein kinase C (PKC) inhibitor, 60 nM] to the perfusate 20 min before the administration of troglitazone (n=5, respectively). In our preliminary studies, based on previous reports (Endoh, 1995; Inui et al., 1988; Bleasdale et al., 1990; Thastrup et al., 1990; Ward & Moffat, 1992), we tried various concentrations of these inhibitors and determined the effective concentrations which minimally affected baseline haemodynamic parameters (Table 1).
Table 1.
Baseline values of coronary flow, LVPmax, dP/dtmax and dP/dtmin
Measurement of cyclic AMP concentration in the left ventricle
In isolated perfused rat hearts, the left ventricle was removed 1 min after injection of troglitazone (1 μmol) or vehicle (the same amount of the medium but without troglitazone) and immediately frozen in liquid nitrogen (n=7, respectively). Frozen tissues were homogenized and extracted with 6% trichloroacetic acid. The extracts were centrifuged for 15 min at 3000 r.p.m. and 4°C. Supernatants were subjected to radioimmunoassay (cyclic AMP kit Eiken, Eikenkagaku Co., Tokyo, Japan), to determine the cyclic AMP content.
Whole-cell patch clamp method
Ventricular myocytes of male Wistar rats (300 to 350 g, 12 weeks old, n=5) were isolated by conventional enzymatic dissociation as described previously (Tanaka et al., 1999). We only used myocytes derived from the left ventricle. Whole-cell currents were recorded using the conventional whole-cell patch clamp technique. The external solution was modified Tyrode's solution (in mM: NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, CsCl 5, HEPES 5, and glucose 5.5, pH 7.4). The internal pipette solution contained (mM) Cs-glutamate 110, CsCl 20, tetraethylammonium chloride 20, MgCl2 1, ethylene glycol-bis (b-amino ethyl ether) tetraacetic acid 5, HEPES 5 and Mg- adenosine triphosphate 5 (pH 7.2 with CsOH). Two mM 4-aminopyridine was added to the external solution to eliminate transient outward currents. L-type Ca2+ currents were elicited every 6 s from a holding potential of −40 mV by applying square pulses to each test potential (−30 to 60 mV) for 200 ms. To quantify L-type Ca2+ current, currents were measured as the difference between the peak and steady-state current during the pulses. The same medium (5% DMSO and 19% bovine albumin) but without troglitazone was used as control.
Measurement of intracellular Ca2+ concentration
Perfused rat hearts were loaded by fura-2 acetoxymethyl ester (fura-2 AM) (4.0 mM) for 30 min. After loading, the hearts were excited by ultraviolet light at a wavelength of 340 or 380 nm, then the emitted fluorescence intensity was measured with CAF-110 (JASCO Co., Tokyo, Japan). The fluorescence ratio before and after administration of isoproterenol (bolus injection of 5 pmol) or troglitazone (bolus injection of 1 μmol) was calculated using Mac Lab System. The same amount of medium but without isoproterenol or troglitazone was used as control.
Materials
Troglitazone was kindly donated by Sankyo Co. (Tokyo, Japan). Carbachol, verapamil, ryanodine, thapsigargin and H7 were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). H89, U73122, and fura-2 AM were obtained from Seikagaku Co. (Tokyo, Japan), Cayman Chemical Company (Ann Arbor, MI, U.S.A.), and Dojindo Laboratories (Kumamoto, Japan), respectively.
Statistical analysis
Two-way analysis of variance and Fisher's exact test for post hoc analysis were carried out for multiple comparisons among groups. All data are expressed as the mean values±s.e.mean. P<0.05 was considered statistically significant.
Results
The baseline values of coronary flow, peak isovolumic LVP (LVPmax), peak rate of rise of LVP (dP/dtmax), peak rate of fall of LVP (dP/dtmin) are summarized in Table 1 (n=5, respectively). There were no statistically significant differences in most parameters among the groups, except for the effect of verapamil+troglitazone on LVPmax and of ryanodine+troglitazone on LVPmax, dP/dtmax and dP/dtmin; however, the magnitude of these differences was very small and would not be expected to cause any differences in the response to troglitazone.
The positive inotropic effect of troglitazone is not mediated via Ca2+ influx through L-type Ca2+ channel or Ca2+ handling in the sarcoplasmic reticulum
At first, we investigated the effect of troglitazone on Ca2+ influx via the L-type Ca2+ channel induced by activation of PKA or PKC. LVPmax, dP/dtmax and dP/dtmin reached their maximum value 1 min after the administration of troglitazone (bolus injection of 1 μmol), and troglitazone exerted these effects in a dose-dependent manner as we had previously described (data not shown) (Shimoyama et al., 1999). Thus, the impact of the pretreatment drugs on LVPmax, dP/dtmax and dP/dtmin was evaluated 1 min after the administration of troglitazone.
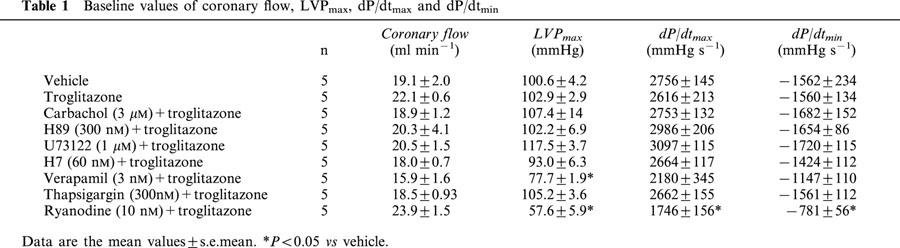
Pretreatment with carbachol did not have any effect on the positive inotropic effect of troglitazone (bolus injection of 1 μmol), whereas those of isoproterenol (bolus injection of 5 pmol) were completely inhibited by pretreatment with carbachol (Figure 1A,B). In addition, pretreatment with H89, U73122 or H7 did not inhibit the inotropic effect of troglitazone (Figure 1C). To confirm these results, we administered different concentrations of troglitazone (bolus injection of 0.2 and 0.5 μmol). The inotropic effect of low doses of troglitazone was not inhibited by any of these pharmacological interventions (data not shown). Moreover, the cyclic AMP concentration in the left ventricle was not increased by troglitazone (Figure 1D). These data suggest that the positive inotropic effect of troglitazone is not mediated via the cyclic AMP-PKA or the PLC-PKC pathway.
Figure 1.
The positive inotropic effect of troglitazone is not mediated via the cyclic AMP-PKA pathway or the PLC-PKC pathway. (A) Representative tracings of LVP before and after administration of isoproterenol (bolus injection of 5 pmol) or troglitazone (bolus injection of 1 μmol) after treatment with carbachol (3 μM) in isolated rat hearts. (B) The summarized data for the effect of vehicle, isoproterenol or troglitazone on LVPmax, dP/dtmax and dP/dtmin (n=5, respectively). While the positive inotropic effect of isoproterenol was completely inhibited by carbachol, the inotropic effect of troglitazone was not. (C) The positive inotropic effect of troglitazone was not inhibited by H89, U73122 or H7, either (n=5, respectively). (D) The cyclic AMP concentration in the left ventricle, as measured by radioimmunoassay (n=7, respectively), did not differ significantly between hearts perfused with vehicle and those perfused with troglitazone. Changes of parameters are expressed as per cent changes from the baseline values (B and C). *P<0.05 vs vehicle.
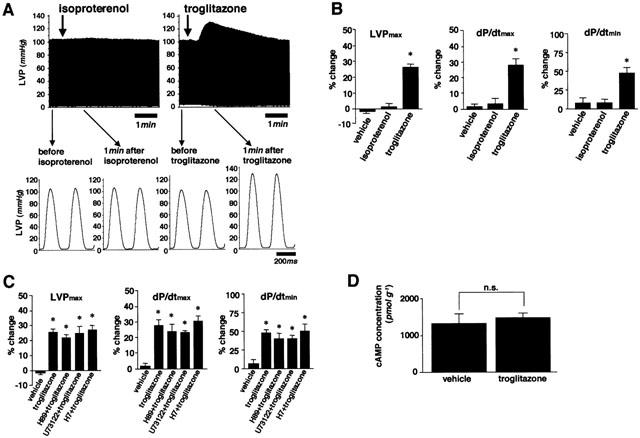
Next, we evaluated the effect of troglitazone on the inward Ca2+ influx through L-type Ca2+ channels. The inotropic effect of troglitazone was not inhibited by pretreatment with verapamil in isolated perfused hearts (Figure 2A). Additionally, the effect of troglitazone on the inward L-type Ca2+ current in isolated rat ventricular myocytes was investigated using whole-cell patch clamp method. A representative time course of peak inward L-type Ca2+ current before and during perfusion of troglitazone (50 μM) is shown in Figure 2B. Although the peak current gradually decreased due to a run down phenomenon under the whole-cell patch clamp condition, troglitazone had little effect on the inward Ca2+ current. As shown in Figure 2C,D, there was no significant difference in the amplitude and kinetics of L-type Ca2+ current. These results suggest that the inotropic effect of troglitazone is not mediated via the increase in Ca2+ influx through L-type Ca2+ channels.
Figure 2.
Ca2+ influx through L-type Ca2+ channels was not involved in the positive inotropic effect of troglitazone. (A) The positive inotropic effect of troglitazone was not inhibited by pretreatment with verapamil in perfused rat hearts (n=5, respectively). (B) Representative time course of peak inward L-type Ca2+ current in ventricular myocytes before and during perfusion of troglitazone (50 μM). (C) Original current traces recorded at −20, 0, and 20 mV in the absence and presence of troglitazone. (D) The summarized data for I–V relationships of the inward L-type Ca2+ current before and during in the presence of troglitazone (n=6, respectively). Note that troglitazone did not have any effect on the Ca2+ current in left ventricular myocytes. Changes of parameters are expressed as per cent changes from the baseline values (A). *P<0.05 vs vehicle.
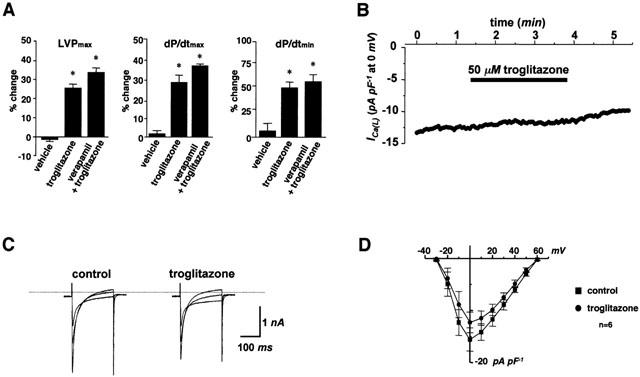
Moreover, to clarify the mechanism of the inotropic effect of troglitazone, we examined Ca2+ handling in the sarcoplasmic reticulum. Pretreatment with thapsigargin or ryanodine did not inhibit the inotropic effect of troglitazone (bolus injection of 1 μmol) (Figure 3). Furthermore, we confirmed that neither thapsigargin nor ryanodine inhibited the inotropic effect of low doses of troglitazone (bolus injection of 0.2 and 0.5 μmol) (data not shown). These data suggest that the positive inotropic effect of troglitazone is not mediated via Ca2+ handling in the sarcoplasmic reticulum.
Figure 3.
The positive inotropic effect of troglitazone is not mediated via Ca2+ handling in the sarcoplasmic reticulum. Pretreatment with thapsigargin or ryanodine did not inhibit the inotropic effect of troglitazone (bolus injection of 1 μmol, n=5, respectively). Changes of parameters are expressed as per cent changes from the baseline values. *P<0.05 vs vehicle.
Troglitazone exerts its inotropic effect without increasing intracellular Ca2+ concentration
Finally, we examined the effects of troglitazone on the intracellular concentration of Ca2+ using fura-2. Representative tracings of LVP and fura-2 fluorescence ratio before and during treatment with isoproterenol (bolus injection of 5 pmol) or troglitazone (bolus injection of 1 μmol) are shown in Figure 4A,B, respectively. Although both isoproterenol and troglitazone exerted positive inotropic effects as evidenced by increases in LVPmax, isoproterenol, but not troglitazone, increased the fura-2 fluorescence ratio (Figure 4C). This result suggests that troglitazone exerts its positive inotropic effect without increasing the intracellular concentrations of Ca2+.
Figure 4.
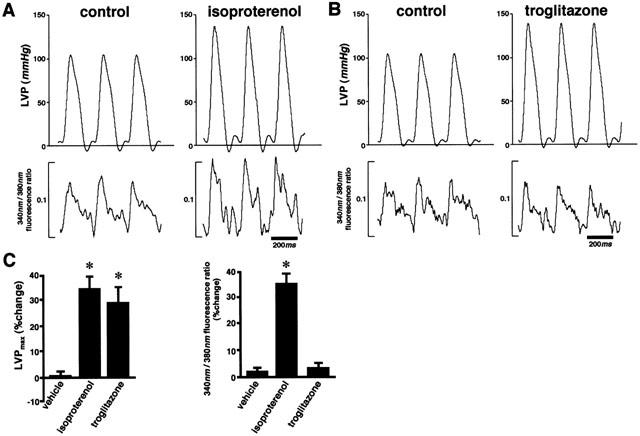
Troglitazone exerts its positive inotropic effect without increasing Ca2+ concentration in perfused hearts. (A and B) Representative tracings of LVP and fura-2 fluorescence ratio in an isovolumic-perfused rat heart before and after treatment with isoproterenol (A), bolus injection of 5 pmol) or troglitazone (B), bolus injection of 1 μmol). (C) The average effect of isoproterenol and troglitazone on LVPmax and Ca2+ concentration (n=5, respectively). Although both isoproterenol and troglitazone increased LVPmax, isoproterenol but not troglitazone increased the fura-2 fluorescence ratio. Changes of parameters are expressed as per cent changes from the baseline values (C). *P<0.05 vs vehicle.
Discussion
In this study, we demonstrated that the positive inotropic effect of troglitazone was independent of Ca2+ influx and Ca2+ handling in the sarcoplasmic reticulum. In addition, troglitazone induced a positive inotropic effect without affecting the intracellular Ca2+ concentration. These results suggest that the Ca2+-sensitizing effect is involved in the positive inotropic effect of troglitazone.
The exact mechanism of the inotropic effect of troglitazone remains unclear. Recently, new inotropic agents that increase Ca2+ sensitivity have been produced and have attracted much attention. Such drugs are called ‘Ca2+-sensitizers', and they are considered useful because no fear of Ca2+ overload which induces ventricular arrhythmia, tachycardia and increasing myocardial oxygen consumption leading to myocardial damage (Endoh, 1995). In the present study, we demonstrated that troglitazone exerts its inotropic effect without affecting intracellular Ca2+ handling or increasing intracellular Ca2+ concentration. These observations suggest that troglitazone may exert its positive inotropic effect through a Ca2+-sensitizing effect. It has been reported that the Ca2+-sensitizing effect is mediated by (1) an enhancement of the affinity between Ca2+ and troponin C; (2) an acceleration of the interaction among troponin, tropomyosin and actin; or (3) facilitation of the formation of crossbridges (Endoh, 1995). Regarding the haemodynamic effects of troglitazone, however, there are some differences between troglitazone and Ca2+-sensitizers. For example, Ca2+-sensitizers reduce the diastolic function by increasing Ca2+ sensitivity even in the diastolic phase (Gwathmey & Hajjar, 1990). In contrast, troglitazone increased not only LVPmax and dP/dtmax but also dP/dtmin, which means that troglitazone improves the diastolic function as well. In addition, the results of previous studies and ours have shown that troglitazone exerts negative chronotropic effects rather than positive chronotropic ones on the heart (Ogihara et al., 1995; Saku et al., 1997; Shimoyama et al., 1999), in contrast with the effect of Ca2+-sensitizing agents. These results indicate that troglitazone might have other effects besides its Ca2+-sensitizing effect. Moreover, this implies that the augmentation in sympathetic nervous system activity in response to peripheral vasodilation was not observed after administration of troglitazone. Taken together, these haemodynamic effects of troglitazone seem to be beneficial for patients with heart failure since troglitazone might suppress sympathetic nervous system activity as well as decrease cardiac oxygen consumption by reducing heart rate and afterload.
In the present study, troglitazone had no effect on L-type Ca2+ currents in rat ventricular myocytes, while it has been reported that troglitazone inhibits L-type Ca2+ currents in atrial myocytes of guinea-pigs (Nakajima et al., 1999), or in rabbit ventricular myocytes (Ikeda & Watanabe, 1998). The reasons for the discrepancy between our results and those of previous reports are not clear, however, one explanation may be the different species used in these studies. Generally, inhibition of L-type Ca2+ currents is known to induce a negative inotropic effect in the heart. Therefore, it is likely that troglitazone induced a positive inotropic effect without increasing the Ca2+ influx through L-type Ca2+ channels. Further investigations will be needed to clarify the exact mechanism of the Ca2+-sensitizing effect of troglitazone.
At present, troglitazone cannot be clinically used in patients because of its deleterious effects on the liver, however, there are several thiazolidinediones, such as rosiglitazone and pioglitazone, that have proved effective and useful in diabetic patients. Interestingly, the unique haemodynamic effects of troglitazone are not induced by other insulin-sensitizing agents (Uchida et al., 2000). While pioglitazone does not exert any significant haemodynamic effect on the heart, rosiglitazone has haemodynamic effects similar to those of troglitazone. Thus, rosiglitazone may also be a Ca2+- and insulin-sensitizing agent which could prove beneficial for diabetic patients with heart failure. In addition, it is possible that the evaluation of the precise mechanism and chemical structure of these unique drugs will lead to the development of new Ca2+-sensitizing drugs for patients with heart failure.
There are no available data on the mechanism of the inotropic effects of troglitazone. Although troglitazone cannot be used clinically at present, clarification of the mechanism of its unique beneficial effects on the cardiovascular system might lead to the development of new inotropic agents and a novel pharmacological approach to treat heart failure.
Acknowledgments
We thank Dr Chiaki Shigemasa, Dr Kazuhiko Uchida and Dr Kazuhiko Sonoyama, Division of Cardiology, Department of Medicine, Faculty of Medicine, Tottori University, for their useful suggestions and critical reading of the manuscript.
Abbreviations
- AM
acetoxymethyl ester
- cyclic AMP
cyclic adenosine monophosphate
- Ca2+-ATPase
Ca2+-adenosine triphosphatase
- CPP
coronary perfusion pressure
- DMSO
dimethyl sulphoxide
- HEPES
N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulphonic acid]
- LVP
left ventricular pressure
- LVPmax
peak isovolumic LVP
- dP/dtmax
peak rate of rise of LVP
- dP/dtmin
peak rate of fall of LVP
- PKA
cyclic AMP-dependent protein kinase (protein kinase A)
- PKC
protein kinase C
- PLC
phospholipase C
References
- BLEASDALE J.E., THAKUR N.R., GREMBAN R.S., BUNDY G.L., FITZPATRICK F.A., SMITH R.J., BUNTING S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- ENDOH M. The effects of various drugs on the myocardial inotropic response. Gen. Pharmacol. 1995;26:1–31. doi: 10.1016/0306-3623(94)00144-c. [DOI] [PubMed] [Google Scholar]
- GHAZZI M.N., PEREZ J.E., ANTONUCCI T.K., DRISCOLL J.H., HUANG S.M., FAJA B.W., THE TROGLITAZONE STUDY GROUP. WHITCOMB R.W. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. Diabetes. 1997;46:433–439. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- GWATHMEY J.K., HAJJAR R.J. Effect of protein kinase C activation on sarcoplasmic reticulum function and apparent myofibrillar Ca2+ sensitivity in intact and skinned muscles from normal and diseased human myocardium. Circ. Res. 1990;67:744–752. doi: 10.1161/01.res.67.3.744. [DOI] [PubMed] [Google Scholar]
- IKEDA S., WATANABE T. Effects of troglitazone and pioglitazone on the action potentials and membrane currents of rabbit ventricular myocytes. Eur. J. Pharmacol. 1998;357:243–250. doi: 10.1016/s0014-2999(98)00557-3. [DOI] [PubMed] [Google Scholar]
- INUI M., WANG S., SAITO A., FLEISCHER S. Characterization of junctional and longitudinal sarcoplasmic reticulum from heart muscle. J. Biol. Chem. 1988;263:10843–10850. [PubMed] [Google Scholar]
- IWAMOTO Y., KUZUYA T., MATSUDA A., AWATA T., KUMAKURA S., INOOKA G., SHIRAISHI I. Effect of new oral antidiabetic agent CS-045 on glucose tolerance and insulin secretion in patients with NIDDM. Diabetes Care. 1991;14:1083–1086. doi: 10.2337/diacare.14.11.1083. [DOI] [PubMed] [Google Scholar]
- KAUFMAN L.N., PETERSON M.M., DE GRANGE L.M. Pioglitazone attenuates diet-induced hypertension in rats. Metabolism. 1995;44:1105–1109. doi: 10.1016/0026-0495(95)90000-4. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA T., IWASAWA K., OONUMA H., IMUTA H., HAZAMA H., ASANO M., MORITA T., NAKAMURA F., SUZUKI J., SUZUKI S., KAWAKAMI Y., OMATA M., OKUDA Y. Troglitazone inhibits voltage-dependent calcium currents in guinea pig cardiac myocytes. Circulation. 1999;99:2942–2950. doi: 10.1161/01.cir.99.22.2942. [DOI] [PubMed] [Google Scholar]
- NOLAN J.J., LUDVIK B., BEERDSEN P., JOYCE M., OLEFSKY J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N. Engl. J. Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- OGIHARA T., RAKUGI H., IKEGAMI H., MIKAMI H., MASUO K. Enhancement of insulin sensitivity by troglitazone lowers blood pressure in diabetic hypertensives. Am. J. Hypertens. 1995;8:316–320. doi: 10.1016/0895-7061(95)96214-5. [DOI] [PubMed] [Google Scholar]
- OGINO K., BURKHOFF D., BILEZIKIAN J.P. The hemodynamic basis for the cardiac effects of parathyroid hormone (PTH) and PTH-related protein. Endocrinology. 1995;136:3024–3030. doi: 10.1210/endo.136.7.7789328. [DOI] [PubMed] [Google Scholar]
- SAKU K., ZHANG B., OHTA T., ARAKAWA K. Troglitazone lowers blood pressure and enhances insulin sensitivity in Watanabe heritable hyperlipidemic rabbits. Am. J. Hypertens. 1997;10:1027–1033. doi: 10.1016/s0895-7061(97)00160-x. [DOI] [PubMed] [Google Scholar]
- SHIMOYAMA M., OGINO K., TANAKA Y., IKEDA T., HISATOME I. Hemodynamic basis for the acute cardiac effects of troglitazone in isolated perfused rat hearts. Diabetes. 1999;48:609–615. doi: 10.2337/diabetes.48.3.609. [DOI] [PubMed] [Google Scholar]
- SUTER S.L., NOLAN J.J., WALLANCE P., GUMBINER B., OLEFSKY J.M. Metabolic effects of a new oral hypoglycemic agent, CS-045, in non-insulin dependent diabetic subjects. Diabetes Care. 1992;15:193–203. doi: 10.2337/diacare.15.2.193. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., HISATOME I., MIYAMOTO J., URASHIMA T., IKEDA K., YAMANOUCHI Y., SASAKI N., KINUGAWA T., OGINO K., IGAWA O., YOSHIDA A., SHIGEMASA C., KURATA Y., SATO R. Enhancing effects of salicylate on tonic and phasic block of Na+ channels by class 1 antiarrhythmic agents in the ventricular myocytes and the guinea pig papillary muscle. Biochim. Biophys. Acta. 1999;1418:320–334. doi: 10.1016/s0005-2736(99)00043-7. [DOI] [PubMed] [Google Scholar]
- THASTRUP O., CULLEN P.J., DRΦBAK B.K., HANLEY M.R., DAWSON A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCHIDA K., OGINO K., SHIMOYAMA M., HISATOME I., SHIGEMASA C. Acute hemodynamic effects of insulin-sensitizing agents in isolated perfused rat hearts. Eur. J. Pharmacol. 2000;400:113–119. doi: 10.1016/s0014-2999(00)00359-9. [DOI] [PubMed] [Google Scholar]
- WARD C.A., MOFFAT M.P. Positive and negative inotropic effects of phorbol 12-myristate 13-acetate: relationship to PKC-dependence and changes in [Ca2+]i. J. Mol. Cell. Cardiol. 1992;24:937–948. doi: 10.1016/0022-2828(92)91861-x. [DOI] [PubMed] [Google Scholar]
- YOSHIOKA S., NISHINO H., SHIRAKI T., IKEDA K., KOIKE H., OKUNO A., WADA M., FUJIWARA T., HORIKOSHI H. Antihypertensive effects of CS-045 treatment in obese Zucker rats. Metabolism. 1993;42:75–80. doi: 10.1016/0026-0495(93)90175-n. [DOI] [PubMed] [Google Scholar]


