Abstract
The spasmolytic and anti-spasmogenic activity of β-adrenoceptor agonists on airways smooth muscle is thought to involve activation of the cyclic AMP/cyclic AMP-dependent protein kinase (PKA) cascade. Here we have tested the hypothesis that PKA mediates the anti-spasmogenic activity of isoprenaline and other cyclic AMP-elevating agents in guinea-pig isolated trachea by utilizing a number of cell permeant cyclic AMP analogues that act as competitive ‘antagonists' of PKA.
Anion-exchange chromatography of guinea-pig tracheae resolved two peaks of PKA activity that corresponded to the type I (∼5%) and type II (∼93%) isoenzymes.
Pre-treatment of tracheae with zardaverine (30 μM), vasoactive intestinal peptide (VIP) (1 μM) and the non-selective activator of PKA, Sp-8-CPT-cAMPS (10 μM), produced a non-parallel rightwards shift in the concentration-response curves that described acetylcholine (ACh)-induced tension generation. The type II-selective PKA inhibitor, Rp-8-CPT-cAMPS (300 μM), abolished this effect.
Pre-treatment of tracheae with Sp-8-Br-PET-cGMPS (30 μM) produced a non-parallel rightwards shift of the concentration-response curves that described ACh-induced tension generation. The selective cyclic GMP-dependent protein kinase (PKG) inhibitor, Rp-8-pCPT-cGMPS (300 μM), abolished this effect.
Pre-treatment of tracheae with isoprenaline (1 μM) produced a 10 fold shift to the right of the ACh concentration-response curve by a mechanism that was unaffected by Rp-8-Br-cAMPS (300 μM, selective inhibitor of type I PKA), Rp-8-CPT-cAMPS (300 μM) and Rp-8-pCPT-cGMPS (300 μM).
We conclude that the anti-spasmogenic activity of Sp-8-CPT-cAMPS, zardaverine and VIP in guinea-pig trachea is attributable to activation of the cyclic AMP/PKA cascade whereas isoprenaline suppresses ACh-induced contractions by a mechanism(s) that is independent of PKA and PKG.
Keywords: β-Adrenoceptor agonist, airways tone, cyclic AMP, protein kinase A, isoprenaline
Introduction
β2-Adrenoceptor agonists are the most effective and safest sympathomimetic bronchodilators currently available for the treatment of asthma (Dennis et al., 2000) and act as functional antagonists as they relax airways smooth muscle (ASM) and prevent contraction regardless of the constrictor stimulus. Nevertheless, an increased understanding of the molecular and biochemical basis underlying these beneficial effects could lead to the development of improved therapies. According to the classical view, β2-adrenoceptor agonists reduce ASM tone by increasing adenylyl cyclase activity, cyclic AMP mass and, subsequently, the activation state of cyclic AMP-dependent protein kinase (PKA). PKA, in turn, phosphorylates several target proteins that regulate the contractile machinery, changing its function such that relaxation or an anti-spasmogenic influence is effected (for reviews see Giembycz & Raeburn, 1991; Torphy, 1994). However, several investigations have also led to the view that β2-adrenoceptor agonists relax ASM via a cyclic AMP-independent mechanism. Thus, salbutamol has been shown to directly activate high conductance Ca2+-activated K+-channels (BKCa) via the α-subunit of the stimulatory G-protein, Gs (Kume et al., 1994). Further complexity is provided by the finding that cyclic AMP can cross-activate cyclic GMP-dependent protein kinase (PKG) in smooth muscle (Jiang et al., 1992a, 1992b; Murthy & Makhlouf, 1995; Ruiz-Velasco et al., 1998; White et al., 2000), which raises the possibility that β-adrenoceptor agonists could also affect ASM tone via the activation of PKG.
In the present study we investigated the role of PKA in the anti-spasmogenic activity of isoprenaline on guinea-pig isolated trachea and have made a comparison with other agents that are also believed to act via a cyclic AMP/PKA-dependent mechanism. To this end a pharmacological approach was adopted using diastereoisomers of cyclic AMP and cyclic GMP analogues that selectively inhibit PKA and PKG. These compounds specifically compete with the endogenously synthesized cyclic nucleotide and so block the activation of their respective protein kinase. Moreover, their high cell permeance and resistance to cyclic nucleotide phosphodiesterases (PDE), renders these tools particularly suitable for use in intact cells and tissues (Dostmann et al., 1990).
Methods
Preparation of guinea-pig tracheae
Male Dunkin-Hartley guinea-pigs (Harlan-Olac Ltd, Bicester, Oxfordshire, U.K.), ranging in weight from 300 to 500 g, were killed by cervical dislocation. The lungs, with trachea and bronchi attached, were rapidly removed and placed in oxygenated Krebs-Henseleit (KH) solution (composition in mM): NaCl 118, KCl 5.9, MgSO4 1.2, CaCl2 2.5, NaH2PO4 1.2, NaHCO3 25.5, glucose 5.6, at room temperature and trimmed free of adherent fat and connective tissue. Indomethacin (10 μM) was present in the KH solution throughout the experiment to prevent the formation of bioactive prostanoids. The trachea was dissected away from the lungs and main bronchi, and opened longitudinally by cutting through the cartilage. After removing the epithelium by gentle rubbing of the mucosal surface with a cotton wool-coated probe, eight transverse segments of trachea were prepared and each suspended by steel hooks in 5 ml tissue baths containing KH solution at 37°C. Tissues were placed under a resting tension of 1 g, which is optimal for measuring changes in contractile force in this species, and allowed to equilibrate for at least 60 min before commencing the experiment. Changes in force were measured isometrically with force-displacement transducers (model FT-03c, Grass Instrument, Quincy, MA, U.S.A.) and recorded on a Grass 7D Polygraph. In some experiments, epithelium-denuded tracheae were frozen in liquid nitrogen and used subsequently for determining the amount of type I and type II PKA in this tissue.
Protocol for contractile studies
After the equilibration period, cumulative concentration-response curves were constructed to ACh (10 nM–10 mM) according to the method of van rossum (1963). The basal tone of each tissue was then re-established, by thorough washing of the tissues over a period of at least 30 min, and preparations were then studied in pairs. One tissue was pre-treated for 30 min with a PKA and/or PKG inhibitor, as indicated, and the other tissue received the relevant vehicle. Isoprenaline, vasoactive intestinal peptide (VIP), zardaverine, Sp-8-CPT-cAMPS, Sp-8-Br-PET-cGMPS or their respective vehicle was added to each tissue for the times indicated in the text, and cumulative concentration-response curves were reconstructed to ACh.
Quantification of PKA Isoenzymes
The type I and type II isoenzymes of PKA in guinea-pig trachea were separated by anion-exchange chromatography as described previously (Giembycz & Diamond, 1990) using Q-Sepharose as the exchange matrix in 10 mM MOPS (pH 7.2). PKA activity was determined by measuring the cyclic AMP-dependent phosphorylation of Kemptide (Giembycz & Diamond, 1990).
Drugs, chemicals and analytical reagents
The following reagents were obtained from the Sigma Chemical Company (Poole, Dorset, U.K.): indomethacin, acetylcholine chloride, isoprenaline, L-ascorbic acid and VIP. Sp-8-CPT-cAMPS, Rp-8-CPT-cAMPS, Rp-8-Br-cAMPS, Sp-8-Br-PET-cGMPS and Rp-8-pCPT-cGMPS were purchased from Biolog Life Science Institute (Bremen, Germany) and the PKA inhibitor peptide, TYADFIASGRTGRRNAI-NH2, was from Calbiochem (Nottingham). Zardaverine was kindly provided by Byk-Gulden (Konstanz, Germany).
Indomethacin was made up as a 1 mg ml−1 solution in phosphate buffer (in mM): KH2PO4 20, Na2HPO4 120 (pH 7.8); isoprenaline was dissolved KH solution containing 10 mg ml−1 ascorbic acid and zardaverine and Sp-8-Br-PET-cGMPS were made up in 100% DMSO. All other drugs were dissolved in distilled water.
Statistical analysis
Data are expressed as mean±standard error of the mean (s.e.mean) of n independent observations. Concentration-response curves were analysed by non-linear iterative regression with the ‘PRISM' curve-fitting program (GraphPad software, San Diego, CA, U.S.A.) from which pD2 values were subsequently derived from curves of best-fit. Statistical evaluation was carried out by a Student's t-test for paired data. The null hypothesis was rejected when P<0.05.
Results
PKA isoenzymes in guinea-pig trachea
At least two generic isoforms of PKA co-exist in many cells and tissues that are arbitrarily denoted type I and type II based on the order from which they are eluted from anion-exchange columns. Two peaks of PKA activity were routinely resolved from homogenates of guinea-pig trachea by Q-Sepharose anion-exchange chromatography eluting at 110 and 305 mM NaCl. Neither of these activities was seen in the absence of exogenous cyclic AMP suggesting that the first and second peak of activity correspond to the type I and type II PKA isoforms respectively (Figure 1). Moreover, the peptide TYADFIASGRTGRRNAI-NH2, which corresponds to the active sequence in PKIα (Glass et al., 1989), abolished the cyclic AMP-dependent phosphorylation of Kemptide (data not shown). In four independent experiments, type I and type II PKA accounted for 4.9±3.2 and 93.1±2.3% of the total phosphotransferase activity recovered from the column respectively (Figure 1).
Figure 1.
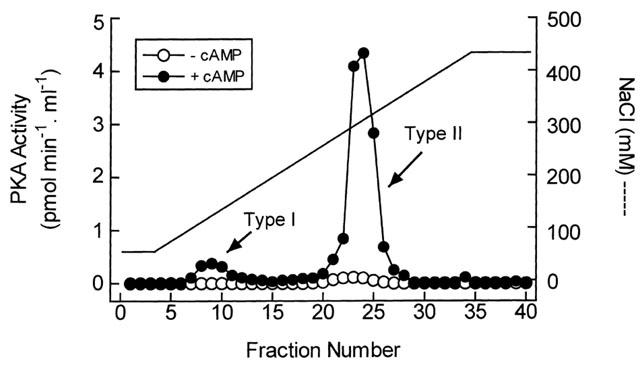
Resolution of PKA isoenzymes from guinea-pig trachea by anion-exchange chromatography. Epithelium-denuded tracheae were homogenized, centrifuged (31,000×g, 4°C) and the resulting supernatant applied to a Q-Sepharose anion-exchange column. Protein was eluted with a linear salt gradient running from 70 to 420 mM NaCl and PKA activity in each fraction was assayed for PKA activity in the absence and presence of cyclic AMP (10 μM) as described in Giembycz & Diamond (1990). The profile is representative of four independent observations.
Effect of a selective inhibitor of type II PKA on the anti-spasmogenic activity of Sp-8-CPT-cAMPS, zardaverine and VIP
Exposure of guinea-pig trachealis to Sp-8-CPT-cAMPS (10 μM; 30 min), a cell permeant and PDE-resistant activator of type I and type II PKA (Dostmann et al., 1990), produced a non-parallel, rightwards shift (approximately 16 fold at the EC50) of the mean concentration-response curve that described ACh-induced force development, and reduced the maximum response by 40% (Figure 2a). In contrast, Sp-8-CPT-cAMPS failed to exert an anti-spasmogenic effect in tissues that were pre-treated (300 μM; 30 min) with the selective inhibitor of type II PKA, Rp-8-CPT-cAMPS (Dostmann et al., 1990), under identical experimental conditions (Table 1; Figure 2). Rp-8-CPT-cAMPS (300 μM; 30 min) itself exerted no significant effect on the ACh concentration-response relationship (data not shown).
Figure 2.
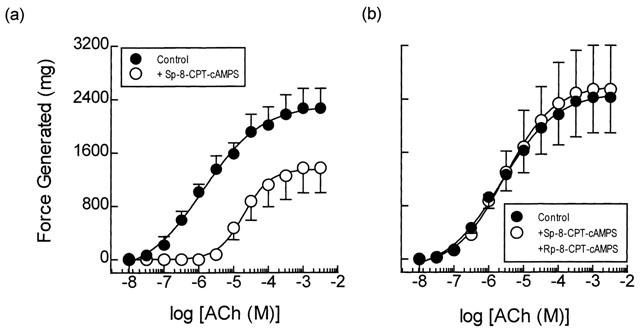
Anti-spasmogenic activity of Sp-8-CPT-cAMPS on ACh-induced tension development and the effect of the selective type II PKA inhibitor Rp-8-CPT-cAMPS. Cumulative concentration-response curves were constructed to ACh in the absence and presence of Sp-8-CPT-cAMPS (10 μM; panel a). In matched paired tissues concentration-response curves were also constructed to ACh in the absence and presence of Sp-8-CPT-cAMPS (10 μM) and Rp-8-CPT-cAMPS (300 μM; panel b) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Table 1.
Anti-spasmogenic activity of Sp-8-CPT-cAMPS and cyclic AMP-elevating drugs on ACh-induced tension development and the effect of the selective type II PKA inhibitor Rp-8-CPT-cAMPS
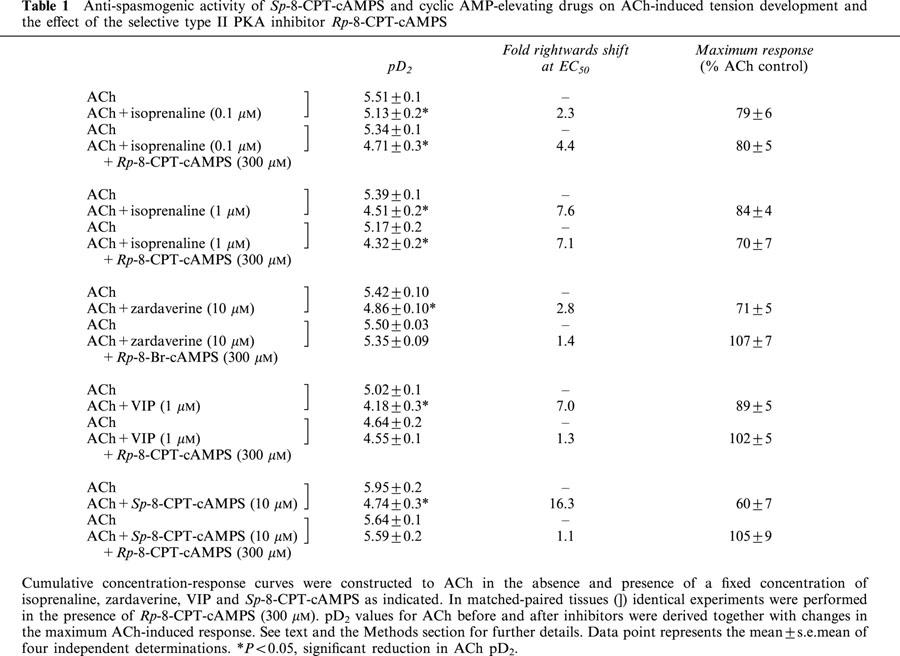
Treatment of guinea-pig trachealis with the dual PDE3/PDE4 inhibitor, zardaverine (30 μM; 30 min; Schudt et al., 1991) and VIP (1 μM; 15 min), which has been shown previously to relax ASM in many species (Said, 1995) through activation of receptors coupled positively to adenylyl cyclase (Lazarus et al., 1986; Luis & Said, 1990), also produced a non-competitive inhibition of ACh-induced force development (Figures 3a and 4a; Table 1). Thus, the mean ACh concentration-response curves were displaced 3 and 7 fold to the right for zardaverine and VIP respectively and their maxima were suppressed 10–30% (Table 1). However, neither zardaverine nor VIP antagonized ACh-induced tension generation in tracheal smooth muscle that was pre-treated with Rp-8-CPT-cAMPS (300 μM; 30 min) (Table 1; Figures 3b and 4b).
Figure 3.
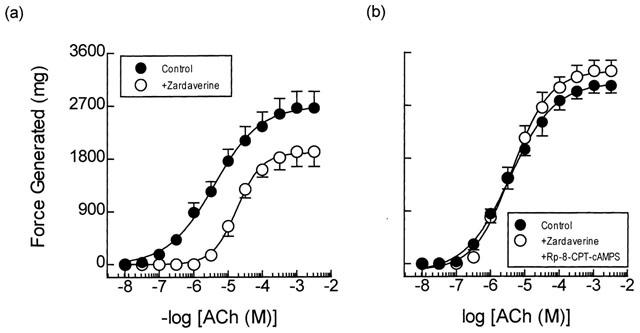
Anti-spasmogenic activity of zardaverine on ACh-induced tension development and the effect of the selective type II PKA inhibitor Rp-8-CPT-cAMPS. Cumulative concentration-response curves were constructed to ACh in the absence and presence of zardaverine (30 μM; panel a). In matched paired tissues concentration-response curves were also constructed to ACh in the absence and presence of zardaverine (30 μM) and Rp-8-CPT-cAMPS (300 μM; panel b) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Figure 4.
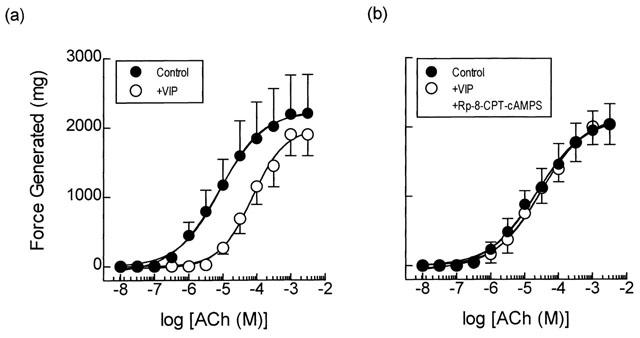
Anti-spasmogenic activity of VIP on ACh-induced tension development and the effect of the selective type II PKA inhibitor Rp-8-CPT-cAMPS. Cumulative concentration-response curves were constructed to ACh in the absence and presence of VIP (1 μM; panel a). In matched paired tissues concentration-response curves were also constructed to ACh in the absence and presence of VIP (1 μM) and Rp-8-CPT-cAMPS (300 μM; panel b) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Effect of Rp-8-CPT-cAMPS on the anti-spasmogenic activity of isoprenaline
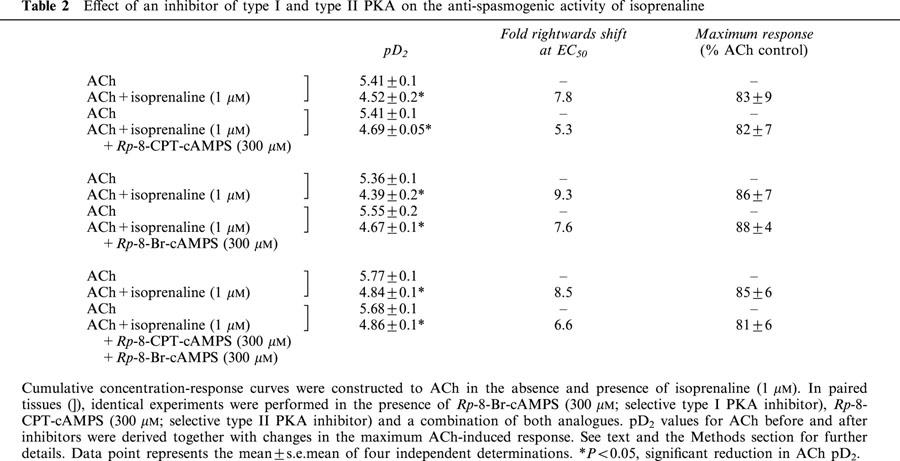
Pre-treatment of guinea-pig trachealis with isoprenaline (0.1 and 1 μM; 15 min) produced non-parallel rightwards shifts of the mean concentration-response curve that described ACh-induced force development and reduced the maximum responses by approximately 20% (Figure 5a,c). In contrast to the results obtained with Sp-8-CPT-cAMPS, zardaverine and VIP described above, the type II PKA inhibitor, Rp-8-CPT-cAMPS (300 μM; 30 min), did not antagonize the anti-spasmogenic activity of isoprenaline (Table 1; Figure 5b,d). Similarly, Rp-8-Br-cAMPS (300 μM; 30 min), a competitive ‘antagonist' of PKA with a preference for the type I isoenzyme (Gjertsen et al., 1995), alone and in combination with Rp-8-CPT-cAMPS, also failed to prevent isoprenaline from antagonizing ACh-induced contractions (Figure 6; Table 2).
Figure 5.
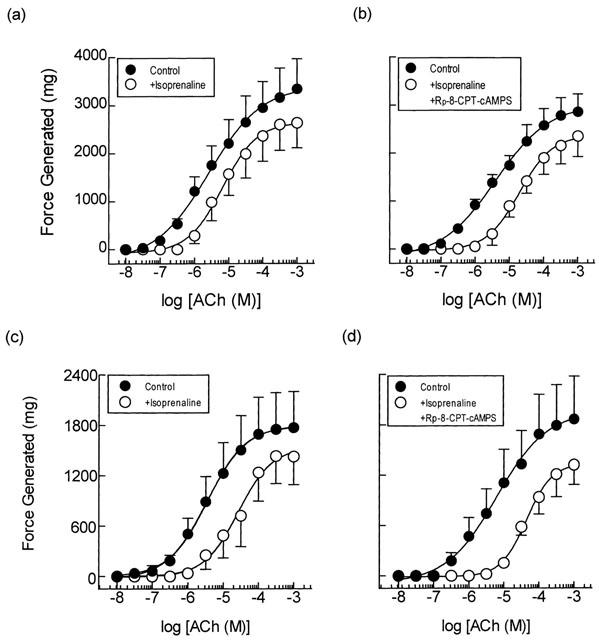
Anti-spasmogenic activity of isoprenaline on ACh-induced tension development and the effect of the selective type II PKA inhibitor Rp-8-CPT-cAMPS. Cumulative concentration-response curves were constructed to ACh in the absence and presence of isoprenaline (0.1 and 1 μM; panels a and c respectively). In matched paired tissues concentration-response curves were also constructed to ACh in the absence and presence of isoprenaline (0.1 and 1 μM) and Rp-8-CPT-cAMPS (300 μM; panels b and d respectively) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Figure 6.
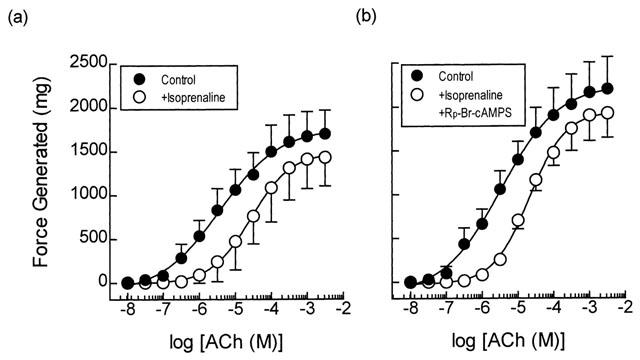
Anti-spasmogenic activity of isoprenaline on ACh-induced tension development and the effect of the selective type I PKA inhibitor Rp-8-Br-cAMPS. Cumulative concentration-response curves were constructed to ACh in the absence and presence of isoprenaline (1 μM; panel a). In matched paired tissues concentration-response curves were also constructed to ACh in the absence and presence of isoprenaline (1 μM) and Rp-8-CPT-cAMPS (300 μM; panel b) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Table 2.
Effect of an inhibitor of type I and type II PKA on the anti-spasmogenic activity of isoprenaline
Role of PKG in the anti-spasmogenic activity of isoprenaline
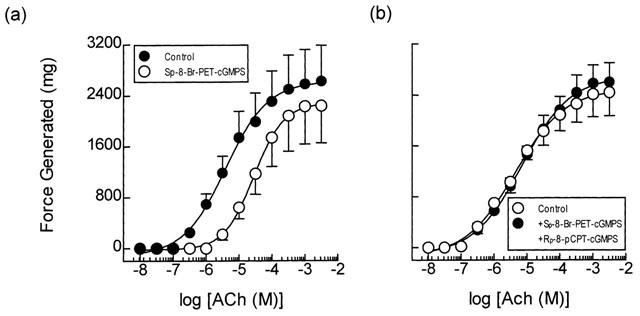
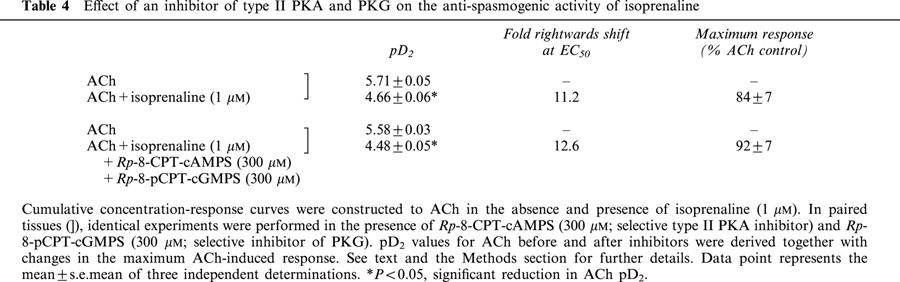
Exposure of guinea-pig trachealis to Sp-8-Br-PET-cGMPS (30 μM; 30 min), a cell permeant and PDE-resistant activator of the type Iα and Iβ PKG isoenzymes (Butt et al., 1995), produced a non-parallel, rightwards shift (approximately 7 fold at the EC50) of the mean concentration-response curve that described ACh-induced force development, and reduced the maximum response by 13% (Table 3; Figure 7a). However, Sp-8-Br-PET-cGMPS failed to exert an anti-spasmogenic effect in tissues that were pre-treated (300 μM; 30 min) with Rp-8-pCPT-cGMPS (Butt et al., 1994), an inhibitor of type Iα, Iβ and II PKG isoenzymes under identical experimental conditions (Table 3; Figure 7b). In contrast, the anti-spasmogenic activity of isoprenaline (1 μM; 15 min) in guinea-pig trachealis was unaffected by Rp-8-pCPT-cGMPS in terms of both the potency of ACh and the maximum response attained (Figure 8). The combination of Rp-8-pCPT-cGMPS and Rp-8-CPT-cAMPS also failed to reverse the anti-spasmogenic isoprenaline (Figure 9; Table 4). Rp-8-pCPT-cGMPS, and Rp-8-pCPT-cGMPS and Rp-8-CPT-cAMPS in combination exerted no significant effect on the ACh concentration-response relationship (data not shown).
Table 3.
Effect of an inhibitor of PKG on the anti-spasmogenic activity of isoprenaline and Sp-8-Br-PET-cGMPS
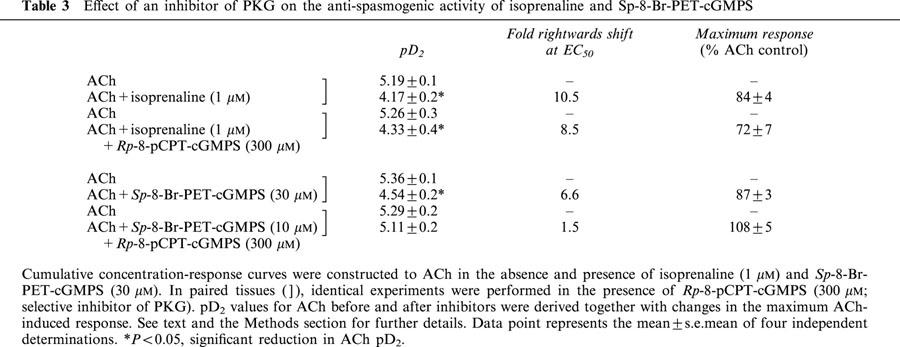
Figure 7.
Anti-spasmogenic activity of Sp-8-Br-PET-cGMPS on ACh-induced tension development and the effect of the PKG inhibitor Rp-8-pCPT-Br-cGMPS. Cumulative concentration-response curves were constructed to ACh in the absence and presence of Sp-8-Br-PET-cGMPS (30 μM; panel a). In matched paired tissues concentration-response curves were also constructed to ACh in the absence and presence of Sp-8-Br-PET-cGMPS (30 μM) and Rp-8-pCPT-Br-cGMPS (300 μM; panel b) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Figure 8.
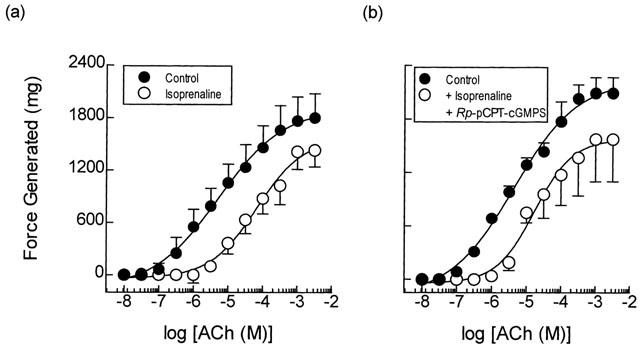
Anti-spasmogenic activity of isoprenaline on ACh-induced tension development and the effect of the PKG inhibitor Rp-8-pCPT-Br-cGMPS. Cumulative concentration-response curves were constructed to ACh in the absence and presence of isoprenaline (0.1 μM; panel a). In matched paired tissues concentration-response curves were also constructed to ACh in the absence and presence of isoprenaline (0.1 μM) and Rp-8-pCPT-Br-cGMPS (300 μM; panel b) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Figure 9.
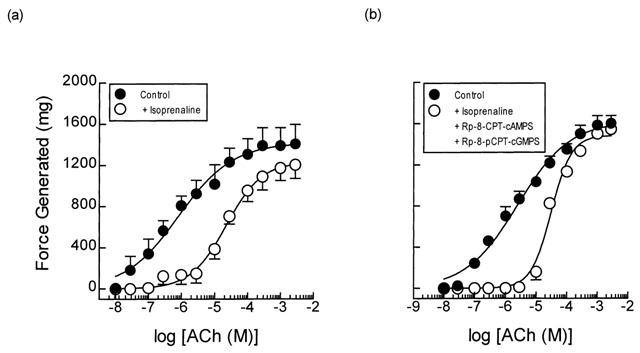
Anti-spasmogenic activity of isoprenaline on ACh-induced tension development and the effect of a PKA and PKG inhibitor in combination. Cumulative concentration-response curves were constructed to ACh in the absence and presence of isoprenaline (0.1 μM; panel a). In matched paired tissues (panel b) concentration-response curves were also constructed to ACh in the absence and presence of isoprenaline (0.1 μM), Rp-8-pCPT-Br-cGMPS (300 μM) and Rp-8-CPT-cAMPS (300 μM) in combination. See text and the Methods section for further details. Data point represents the mean±s.e.mean of four independent determinations.
Table 4.
Effect of an inhibitor of type II PKA and PKG on the anti-spasmogenic activity of isoprenaline
Discussion
A substantial body of data support the hypothesis that cyclic AMP can relax ASM and protect against contraction through the activation of PKA (see Giembycz & Raeburn, 1991). However, whether PKA is responsible for these same functional responses elicited by β2-adrenoceptor agonists is still equivocal (see Introduction for details). Thus, the present study was conducted to gain further information on the role of PKA in the anti-spasmogenic activity of isoprenaline and a number of other cyclic AMP-elevating drugs in guinea-pig isolated trachea.
A pharmacological approach was adopted by making use of cell permeant, PDE-resistant cyclic AMP analogues that act as selective inhibitors of the type I and type II isoenzymes of PKA. Our initial studies concentrated on type II PKA as it accounted for greater than 93% of the total activity in guinea-pig trachea. According to one criterion outlined by Sutherland and co-workers (Robinson et al., 1971) and Krebs (1973), a biological response evoked by a drug or receptor agonist should be mimicked by the suspected cyclic nucleotide or analogue thereof. Consistent with this criterion the effect of zardaverine and VIP, which evoked a rightward shift of the ACh concentration-response curve and depressed the maximum response, was mimicked by Sp-8-CPT-cAMPS, a cell permeant, PDE-resistant activator of type II PKA (Dostmann et al., 1990). Moreover, the type II PKA inhibitor, Rp-8-CPT-cAMPS, abolished the anti-spasmogenic effect of these drugs indicating that a common mechanism, involving PKA, accounted for this effect. A similar role for PKA in mediating the effects of VIP and PDE inhibitors in other tissues has been reported including relaxation of gastric smooth muscle cells (Gu et al., 1992) and suppression of the respiratory burst in human alveolar macrophages (Dent et al., 1994).
Isoprenaline also evoked a non-parallel rightwards shift of the ACh concentration-response curve but this effect was not prevented by Rp-8-CPT-cAMPS at a concentration that blocked the anti-spasmogenic effect of Sp-8-CPT-cAMPS, zardaverine and VIP. As guinea-pig tracheae express both isoforms of PKA, the cyclic AMP generated by isoprenaline might selectively activate type I PKA, which prompted an evaluation of a type I inhibitor (Rp-Br-cAMPS) alone, and in combination with Rp-8-CPT-cAMPS. However, the anti-spasmogenic activity of isoprenaline was preserved in Rp-Br-cAMPS-, and Rp-Br-cAMPS and Rp-8-CPT-cAMPS-treated tissues providing persuasive evidence that the inhibition of ACh-induced tension generation occurred by a PKA-independent mechanism. Insensitivity of β-adrenoceptor-mediated responses to PKA antagonists has been reported in several other smooth muscle preparations including porcine coronary artery (White et al., 2000) and rabbit portal vein (Ruiz-Velasco et al., 1998). In contrast, the ability of isoprenaline to evoke a positive inotropic effect in guinea-pig and rat isolated cardiac ventricular myocytes (Bell & McDermott, 1994; Money-Kyrle et al., 1998) and to facilitate the release of glutamate from rat cerebrocortical membranes (Herrero & Sanchez-Prieto, 1996) is markedly attenuated by Rp-cAMPS analogue inhibitors of PKA. One explanation of this discrepancy is that the signal transduction pathway recruited by a β-adrenoceptor agonist may be tissue-dependent and/or related to the functional response that is elicited.
An alternative pathway that could explain the mechanical effects of β-adrenoceptor agonists on ASM is through cross-activation of PKG by cyclic AMP. This phenomenon was first described in isoprenaline-stimulated pig coronary artery (Jiang et al., 1992a) and has since been found in other smooth muscle preparations (Ruiz-Velasco et al., 1998; Murthy & Makhlouf, 1995; White et al., 2000). In canine trachealis, cyclic AMP activates PKG with a potency that is only slightly lower than for the activation of PKA (Torphy et al., 1982) implying that a similar mechanism could also operate in intact ASM. Indeed, Francis et al. (1988) have showed that the potency of a range of cyclic nucleotide analogues to relax guinea-pig trachea was strongly correlated with their ability to activate PKG but not PKA. Moreover, this relationship held regardless of whether the compounds were analogues of cyclic AMP or cyclic GMP. In the present study the selective PKG inhibitor, Rp-8-pCPT-cGMPS, failed to antagonize the protective effect of isoprenaline under conditions where the anti-spasmogenic effect of the cyclic GMP analogue, Sp-Br-cGMPS, was abolished suggesting that PKG does not mediate the anti-spasmogenic effect of isoprenaline in guinea-pig trachea. The hypothesis that both PKA and PKG need to be activated for the full anti-spasmogenic effect of β2-adrenoceptor agonists to be elicited in this tissue was also rejected on the basis that Rp-8-pCPT-cGMPS in combination with Rp-8-CPT-cAMPS also failed to reverse the effect of isoprenaline.
Changes in ASM tone evoked by β-adrenoceptor agonists may be dependent on the opening of BKCa (Jones et al., 1990; Miura et al., 1992). Indeed, PKA and PKG, in the presence of ATP, increase the open state probability of BKCa in excised patches isolated from single tracheal myocytes (Kume et al., 1989, 1994; Alioua et al., 1995). However, activation of these channels by cyclic nucleotide-dependent phosphorylation cannot explain the results of the present study as Rp-Br-cAMPS, Rp-8-CPT-cAMPS and Rp-8-pCPT-cGMPS did not antagonize the anti-spasmogenic effect of isoprenaline.
To strengthen the hypothesis that PKA is not involved in the anti-spasmogenic activity of isoprenaline we reasoned that additional studies with PKA inhibitors that act at the ATP-binding site (e.g. H-89, KT 5720) could be instructive. It is now recognized that β2-adrenoceptors can signal through intracellular receptors other than PKA as well as by cyclic AMP-independent pathways (see below). However, of the limited pharmacological tools currently available none were suitable in this experimental setting. Thus, H-89 (Chijiwa et al., 1990) is a β-adrenoceptor antagonist at concentrations equivalent to those required to inhibit PKA (Penn et al., 1999) and clearly can not be used to probe the mechanism of action of isoprenaline. To circumvent this problem we tested the selective indolocarbazole PKA inhibitor, KT 5720 (Kase et al., 1987) but, unexpectedly, this compound potentiated the contractile activity of ACh (authors' unpublished observations). The explanation for this effect is unclear, although it was recently demonstrated that indolocarbazoles can bind to an allosteric site within certain of the muscarinic receptor subtypes and interact in a positive co-operative fashion with ACh (Lazareno et al., 2000). Caution should, therefore, be exercised in studies using KT 5720 and muscarinic agonists.
If PKA does not mediate the anti-spasmogenic activity of isoprenaline then what are the alternatives? One possibility is that isoprenaline gated BKCa independently of PKA, possibly through the direct binding of Gsα (Kume et al., 1992, 1994). Unfortunately, this mechanism could not be rigorously tested as charybdotoxin and iberiotoxin, which block BKCa, contracted guinea-pig trachealis (authors' unpublished observations), which is consistent with previously published data (Patel et al., 1998). It should be noted that isoprenaline can relax guinea-pig trachealis in the absence of membrane hyperpolarization (Cook et al., 1993), which suggests that activation of BKCa may play, at most, a supportive rather than central role.
Although not formally studied in the present investigation it has been demonstrated that cyclic AMP can bind and activate a family of small guanine nucleotide-exchange factors (GEFs), which include exchange factor activated by cyclic AMP (Epac also called cyclic AMPGEF) (de rooij et al., 1998; 2000; Kawasaki et al., 1998). In the cyclic AMP-bound form, Epac directly activates the small Ras-like GTPase, Rap1, which in some cells leads to the phosphorylation of extracellular signal-regulated kinase (ERK) (Bos, 1998). In other tissues β2-adrenoceptors can couple to src-Ras-Raf-MKK-ERK signalling cascades via cyclic AMP-independent, Gβγ-, Giα or Gsα-mediated mechanisms (Ma et al., 2000; Wenzel-Seifert & Seifert, 2000). We have previously found that procaterol, a potent and highly selective β2-adrenoceptor agonist, dephosphorylates basal and histamine-stimulated pERK in intact ASM (Koch et al., 2000), which indicates that neither of the aforementioned processes account for the protective effect of isoprenaline on guinea-pig trachealis. The mechanism of action of isoprenaline, therefore, remains unclear.
In conclusion, the results of the current investigation support the dogma that PKA-dependent processes account for the anti-spasmogenic activity of VIP, Sp-8-CPT-cAMPS and zardaverine in guinea-pig trachealis whereas neither PKA nor PKG, alone or together, mediate the protective effect of isoprenaline under identical experimental conditions. Given the implication of these findings and the absence of confirmatory data with other pharmacological tools, further studies clearly are required to probe the role of PKA in β2-adrenoceptor signalling in ASM. To this end we are currently developing techniques to infect ASM cells with the endogenous inhibitors of PKA (Zheng et al., 2000 and references therein), by protein-mediated transduction, in the form of TAT fusions (Schwarze et al., 2000), and adenovirus expression vectors (Lum et al., 1999).
Acknowledgments
The authors thank GlaxoSmithKline for financial support.
Abbreviations
- ASM
airways smooth muscle
- BKCa
large conductance Ca2+-activated K+ channel
- ERK
extracellular signal-regulated kinase
- KH
Krebs-Henseleit
- PDE
phosphodiesterase
- PKA
cyclic AMP-dependent protein kinase
- PKG
cyclic GMP-dependent protein kinase
- Rp-8-CPT-cAMPS
8-(4-chlorophenylthio) adenosine-3′,5′-cyclic monophosphorothioate–Rp diastereoisomer
- Rp-8-Br-cAMPS
8-bromoadenosine-3′,5′-cyclic monophosphorothioate–Rp diastereoisomer
- Rp-8-pCPT-cGMPS
Rp-8-(4-chlorophenylthio) guanosine-3′,5′-cyclic monophosphorothioate–Rp diastereoisomer
- Sp-8-CPT-cAMPS
8-(4-chlorophenylthio) adenosine-3′,5′-cyclic monophosphorothioate–Sp diastereoisomer
- Sp-8-Br-PET-cGMPS
β-phenyl-1-N2-etheno-8-bromoguanosine-3′,5′-cyclic monophosphorothioate–Sp diastereoisomer
- VIP
vasoactive intestinal peptide
References
- ALIOUA A., HUGGINS J.P., ROUSSEAU E. PKG-Iα phosphorylates the α-subunit and upregulates reconstituted GKCa channels from tracheal smooth muscle. Am. J. Physiol. 1995;268:L1057–L1063. doi: 10.1152/ajplung.1995.268.6.L1057. [DOI] [PubMed] [Google Scholar]
- BELL D., MCDERMOTT B.J. Use of the cyclic AMP antagonist, Rp-cAMPS, to distinguish between cyclic AMP-dependent and cyclic AMP-independent contractile responses in rat ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 1994;26:1439–1448. doi: 10.1006/jmcc.1994.1163. [DOI] [PubMed] [Google Scholar]
- BOS J.L. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 1998;17:6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTT E., EIGENTHALER M., GENIESER H.G. Rp-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. Eur. J. Pharmacol. 1994;269:265–268. doi: 10.1016/0922-4106(94)90095-7. [DOI] [PubMed] [Google Scholar]
- BUTT E., POHLER D., GENIESER H.G., HUGGINS J.P., BUCHER B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by Rp-8-bromo-PET-cyclic GMPS. Br. J. Pharmacol. 1995;116:3110–3116. doi: 10.1111/j.1476-5381.1995.tb15112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIJIWA T., MISHIMA A., HAGIWARA M., SANO M., HAYASHI K., INOUE T., NAITO K., TOSHIOKA T., HIDAKA H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- COOK S.J., SMALL R.C., BERRY J.L., CHIU P., DOWNING S.J., FOSTER R.W. β-Adrenoceptor subtypes and the opening of plasmalemmal K+-channels in trachealis muscle: electrophysiological and mechanical studies in guinea-pig tissue. Br. J. Pharmacol. 1993;109:1140–1148. doi: 10.1111/j.1476-5381.1993.tb13741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE ROOIJ J., REHMANN H., VAN TRIEST M., COOL R.H., WITTINGHOFER A., BOS J.L. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J. Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- DE ROOIJ J., ZWARTKRUIS F.J., VERHEIJEN M.H., COOL R.H., NIJMAN S.M., WITTINGHOFER A., BOS J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- DENNIS S,M., SHARP S.J., VICKERS M.R., FROST C.D., CROMPTON G.K., BARNES P.J., LEE T.H. Regular inhaled salbutamol and asthma: the TRUST randomised trial. Lancet. 2000;355:1676–1689. doi: 10.1016/s0140-6736(00)02238-8. [DOI] [PubMed] [Google Scholar]
- DENT G., GIEMBYCZ M.A., RABE K.F., WOLF B., BARNES P.J., MAGNUSSEN H. Theophylline suppresses human alveolar macrophage respiratory burst through phosphodiesterase inhibition. Am. J. Respir. Cell Mol. Biol. 1994;10:565–572. doi: 10.1165/ajrcmb.10.5.8179921. [DOI] [PubMed] [Google Scholar]
- DOSTMANN W.R.G., TAYLOR S.S., GENIESER H.G., JASTORFF B., DOSKELAND S.O., ØGREID D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinase I and II with analogs of adenosine 3,5-cyclic phosphorothioates. J. Biol. Chem. 1990;265:10484–10491. [PubMed] [Google Scholar]
- FRANCIS S.H., NOBLETT B.D., TODD B.W., WELLS J.N., CORBIN J.D. Relaxation of vascular smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP dependent protein kinase. Mol. Pharmacol. 1988;34:506–517. [PubMed] [Google Scholar]
- GIEMBYCZ M.A., DIAMOND J. Evaluation of Kemptide, a synthetic serine-containing heptapeptide, as a phosphate acceptor for the estimation of cyclic AMP-dependent protein kinase activity in respiratory tissues. Biochem. Pharmacol. 1990;39:271–273. doi: 10.1016/0006-2952(90)90026-h. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., RAEBURN D. Putative substrates for cyclic nucleotide-dependent protein kinases and the control of airway smooth muscle tone. J. Auton. Pharmacol. 1991;270:365–398. doi: 10.1111/j.1474-8673.1991.tb00260.x. [DOI] [PubMed] [Google Scholar]
- GJERTSEN B.T., MELLGREN G., OTTEN A., MARONDE E., GENIESER H.G., JASTORFF B., VINTERMYR O.K., MCKNIGHT G.S., DOSKELAND S.O. Novel Rp-cAMPS analogs as tools for inhibition of cAMP-kinase in cell culture. J. Biol. Chem. 1995;270:20599–20607. doi: 10.1074/jbc.270.35.20599. [DOI] [PubMed] [Google Scholar]
- GLASS D.B., CHENG H.C., MENDE-MUELLER L., REED J., WALSH D.A. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J. Biol. Chem. 1989;264:8802–8810. [PubMed] [Google Scholar]
- GU Z.F., JENSEN R.T., MATON P.N. A primary role for protein kinase A in smooth muscle relaxation induced by adrenergic agonists and neuropeptides. Am. J. Physiol. 1992;263:G360–G364. doi: 10.1152/ajpgi.1992.263.3.G360. [DOI] [PubMed] [Google Scholar]
- HERRERO I., SANCHEZ-PRIETO J. cAMP-dependent facilitation of glutamate release by β-adrenergic receptors in cerebrocortical nerve terminals. J. Biol. Chem. 1996;271:30554–30560. doi: 10.1074/jbc.271.48.30554. [DOI] [PubMed] [Google Scholar]
- JIANG H., COLBRAN J.L., FRANCIS J.L., CORBIN J.D. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J. Biol. Chem. 1992a;267:1015–1019. [PubMed] [Google Scholar]
- JIANG H., SHABB J.B., CORBIN J.D. Cross-activation: overriding cAMP/cGMP selectivities of protein kinases in tissues. Biochem. Cell Biol. 1992b;70:1283–1289. doi: 10.1139/o92-175. [DOI] [PubMed] [Google Scholar]
- JONES T.R., CHARETTE L., GARCIA M.L., KACZOROWSKI G.J. Selective inhibition of relaxation of guinea-pig trachea by charybdotoxin, a potent Ca++-activated K+ channel inhibitor. J. Pharmacol. Exp. Ther. 1990;255:697–706. [PubMed] [Google Scholar]
- KASE H., IWAHASHI K., NAKANISHI S., MATSUDA Y., YAMADA K., TAKAHASHI M., MURAKATA C., SATO A., KANEKO M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- KAWASAKI H., SPRINGETT G.M., MOCHIZUKI N., TOKI S., NAKAYA M., MATSUDA M., HOUSMAN D.E., GRAYBIEL A.M. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- KOCH A., NASUHARA Y., BARNES P.J., LINDSAY M.A., GIEMBYCZ M.A. Extracellular signal-regulated kinase 1/2 control Ca2+-independent force development in histamine-stimulated bovine tracheal smooth muscle. Br. J. Pharmacol. 2000;131:981–989. doi: 10.1038/sj.bjp.0703663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS E.G. Endocrinology. Proceedings of the 4th International Congress. Excerpta Medica, Amsterdam; 1973. The mechanism of hormonal regulation by cyclic AMP; pp. 17–29. [Google Scholar]
- KUME H., GRAZIANO M.P., KOTLIKOFF M.I. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide binding proteins. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUME H., HALL I.P., WASHABAU R.J., TAKAGI K., KOTLIKOFF M.I. β-adrenergic agonists regulate K channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUME H., TAKAI A., TOKUNO H., TOMITA T. Regulation of Ca2+-dependent K+-channels in tracheal myocytes by phosphorylation. Nature. 1989;341:152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., POPHAM A., BIRDSALL N.J.M. Allosteric interactions of staurosporine and other indolocarbazoles with N-[methyl-3H]scopolamine and acetylcholine at muscarinic receptor subtypes: identification of a second allosteric site. Mol. Pharmacol. 2000;58:194–207. doi: 10.1124/mol.58.1.194. [DOI] [PubMed] [Google Scholar]
- LAZARUS S.C., BASBAUM C.B., BARNES P.J., GOLD W.M. Mapping of VIP receptors by use of immunocytochemical probe for the intracellular mediator cyclic AMP. Am. J. Physiol. 1986;251:C115–C119. doi: 10.1152/ajpcell.1986.251.1.C115. [DOI] [PubMed] [Google Scholar]
- LUIS J., SAID S.I. Characterization of VIP- and helodermin-preferring receptors on human small cell carcinoma cell lines. Peptides. 1990;11:1239–1244. doi: 10.1016/0196-9781(90)90158-2. [DOI] [PubMed] [Google Scholar]
- LUM H., JAFFE H.A., SCHULZ I.T., MASOOD A., RAYCHAUDHURY A., GREEN R.D. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am. J. Physiol. 1999;277:C580–C588. doi: 10.1152/ajpcell.1999.277.3.C580. [DOI] [PubMed] [Google Scholar]
- MA Y.C., HUANG J., ALI S., LOWRY W., HUANG X.Y. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- MIURA M., BELVISI M.G., STRETTON C.D., YACOUB M.H., BARNES P.J. Role of potassium channels in bronchodilator responses in human airways. Am. Rev. Respir. Dis. 1992;146:132–136. doi: 10.1164/ajrccm/146.1.132. [DOI] [PubMed] [Google Scholar]
- MONEY-KYRLE A.R., DAVIES C.H., RANU H.K., O'GARA P., KENT N.S., POOLE-WILSON P.A., HARDING S.E. The role of cAMP in the frequency-dependent changes in contraction of guinea-pig cardiomyocytes. Cardiovasc. Res. 1998;37:532–540. doi: 10.1016/s0008-6363(97)00253-8. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am. J. Physiol. 1995;268:C171–C180. doi: 10.1152/ajpcell.1995.268.1.C171. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., GIEMBYCZ M.A., KEELING J.E., BARNES P.J., BELVISI M.G. Inhibition of cholinergic neurotransmission in guinea pig trachea by NS1619, a putative activator of large-conductance, calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 1998;286:952–958. [PubMed] [Google Scholar]
- PENN R.B., PARENT J.L., PRONIN A.N., PANETTIERI R.A., BENOVIC J.L. Pharmacological inhibition of protein kinases in intact cells: antagonism of beta-adrenergic receptor ligand binding by H-89 reveals limitations of usefulness. J. Pharmacol. Exp. Ther. 1999;288:428–437. [PubMed] [Google Scholar]
- ROBINSON G.A., BUTCHER R.W., SUTHERLAND E.W. Cyclic AMP. Academic Press: New York; 1971. pp. 72–90. [Google Scholar]
- RUIZ-VELASCO V., ZHONG J., HUME J.R., KEEF K.D. Modulation of Ca2+ channels by cyclic nucleotide cross-activation of opposing protein kinases in rabbit portal vein. Circ. Res. 1998;23:557–565. doi: 10.1161/01.res.82.5.557. [DOI] [PubMed] [Google Scholar]
- SAID S.I.Vasoactive intestinal peptide Airways Smooth Muscle: Peptide Receptors, Ion Channels and Signal Transduction. 1995Birkhauser Verlag AG: Basel; 209–259.ed. Raeburn, D. & Giembycz, M.A [Google Scholar]
- SCHUDT C., WINDER S., MULLER B., UKENA D. Zardaverine as a selective inhibitor of phosphodiesterase isozymes. Biochem. Pharmacol. 1991;42:153–162. doi: 10.1016/0006-2952(91)90694-z. [DOI] [PubMed] [Google Scholar]
- SCHWARZE S.R., HRUSKA K.A., DOWDY S.F. Protein transduction: unrestricted delivery into all cells. Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J. β-adrenoceptors, cAMP and airway smooth muscle relaxation: challenges to the dogma. Trends Pharmacol. Sci. 1994;15:370–374. doi: 10.1016/0165-6147(94)90157-0. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., FREESE W.B., RINARD G.A., BRUNTON L.L., MAYER S.E. Cyclic nucleotide-dependent protein kinase in airway smooth muscle. J. Biol. Chem. 1982;257:11609–11616. [PubMed] [Google Scholar]
- VAN ROSSUM J.M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch. Int. Pharmacodyn. Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- WENZEL-SEIFERT K., SEIFERT R. Molecular analysis of β2-adrenoceptor coupling to Gs-, Gi- and Gq-proteins. Mol. Pharmacol. 2000;58:954–966. doi: 10.1124/mol.58.5.954. [DOI] [PubMed] [Google Scholar]
- WHITE R.E., KRYMAN J.P., EL-MOWAFY A.M., CARRIER G.O. cAMP-Dependent vasodilators cross-activate the cGMP-dependent protein kinase cascade to stimulate BKCa channel activity in coronary artery smooth muscle cells. Circ. Res. 2000;28:897–905. doi: 10.1161/01.res.86.8.897. [DOI] [PubMed] [Google Scholar]
- ZHENG L., YU L., TU Q., ZHANG M., HE H., CHEN W., GAO J., YU J., WU Q., ZHAO S. Cloning and mapping of human PKIB and PKIG, and comparison of tissue expression patterns of three members of the protein kinase inhibitor family including PKIA. Biochem. J. 2000;349:403–407. doi: 10.1042/0264-6021:3490403. [DOI] [PMC free article] [PubMed] [Google Scholar]


