Abstract
Calcitonin gene-related peptide (CGRP), amylin and adrenomedullin (AM) belong to the same family of peptides. Accumulating evidence indicate that the calcitonin (CT) receptor, the CT receptor-like receptor (CRLR) and receptor-activity-modifying proteins (RAMPs) form the basis of all the receptors in this family of peptides.
Using reverse transcriptase–polymerase chain reaction the presence of mRNA sequences encoding the CRLR, RAMP1 and RAMP2 were demonstrated in porcine left anterior descending (LAD) coronary arteries, whereas porcine calcitonin (CT) receptor mRNA was not present. The partial porcine mRNA sequences shared 82–92% nucleotide identity with human sequences.
The human peptides αCGRP, βCGRP, AM and amylin induced relaxation with pEC50 values of 8.1, 8.1, 6.7 and 6.1 M respectively.
The antagonistic properties of a novel non-peptide CGRP antagonist ‘Compound 1' (WO98/11128), βCGRP8–37 and the proposed AM receptor antagonist AM22–52 were compared to the well-known CGRP1 receptor antagonist αCGRP8–37.
The αCGRP8–37 and βCGRP8–37 induced concentration-dependent (10−7–10−5 M) rightward shift of both the αCGRP and βCGRP concentration-response curves. βCGRP8–37 (10−6 M) had the same effect as αCGRP8–37 (10−6 M), but with less potent rightward shift of the concentration-response curves for αCGRP, AM and amylin.
Preincubation with ‘Compound 1' (10−7–10−5 M) and AM22–52 (10−6 M) had no significant antagonistic effect.
In conclusion, the building blocks forming CGRP and AM receptors were present in the porcine LAD, whereas those of the amylin receptor were not. αCGRP, βCGRP, AM and amylin mediated vasorelaxation via the CGRP receptors. No functional response was detected to adrenomedullin via the adrenomedullin receptor.
Keywords: CGRP, adrenomedullin, amylin, calcitonin receptor-like receptor, receptor-activity-modifying proteins, porcine coronary arteries
Introduction
Calcitonin gene-related peptide (CGRP), adrenomedullin (AM) and amylin are structurally related peptides with considerable homology and with different effects in the coronary circulation (Muff et al., 1995).
CGRP is a 37 amino acid peptide discovered in 1982 (Amara et al., 1982). The two isoforms of CGRP, α- and β-CGRP, have similar biological activities (Amara et al., 1985; Morris et al., 1984). CGRP is released from peripheral sensory nerves (Franco-Cereceda et al., 1987), and a rich supply of CGRP-immunoreactive nerve fibres has been demonstrated at the adventitial-medial border of human coronary arteries and veins (Gulbenkian et al., 1993; Saetrum et al., 1995). CGRP is a potent vasodilator causing a decrease in blood pressure and increase in heart rate when administered intravenously to healthy volunteers (Gennari & Imbimbo, 1985). CGRP potently relaxes human isolated coronary arteries (Gulbenkian et al., 1993), and has even been demonstrated to cause dilation of coronary arteries at the site of atheromatous stenoses and to delay the onset of myocardial ischaemia during treadmill exercise in patients with chronic stable angina (Uren et al., 1993). CGRP released within the coronary circulation and in the atria is believed to be a physiological defence reaction to ongoing ischaemia (Mair et al., 1990; Lechleitner et al., 1992).
Amylin is a 37 amino acid peptide, originally found in the islet β-cells of the pancreas (Cooper et al., 1987). It is co-secreted from the β-cells with insulin (Kahn et al., 1990) in response to glucose (Kanatsuka et al., 1989). The expression of amylin has been demonstrated in other tissues, but never in vascular cells. Amylin is a well-known vasodilator, although its most important effect probably is to reduce the tissue-glucose response to insulin (Feuerstein et al., 1995). Amylin is also involved in the development of islet amyloid in the β-cells and thus in the pathogenesis of type 2 diabetes mellitus (Hoppener et al., 2000).
AM is a 52 amino acid peptide originally discovered in a human pheochromocytoma in 1993 (Kitamura et al., 1993). AM is a circulating vasodilator peptide expressed in a number of cell types (Kitamura et al., 1995) including endothelial cells (Sugo et al., 1994a) and vascular smooth muscle cells (Sugo et al., 1994b). The mRNA encoding AM has been detected in porcine coronary arteries (Nishimura et al., 1997). AM has strong hypotensive properties, and has been demonstrated to induce relaxation in different vascular beds including porcine coronary arteries (Kureishi et al., 1995). AM has also been shown to increase coronary blood flow in conscious sheep (Parkes, 1995). Further, AM has important antiproliferative actions on vascular cells (Kano et al., 1996), and recently the basal production of AM in the human coronary circulation was shown to be attenuated in subjects with coronary atherosclerosis, possibly due to the atherosclerosis-induced endothelial dysfunction and thereby decreased AM production (Hojo et al., 2000).
Recent investigations indicate that the calcitonin (CT) receptor and the calcitonin receptor-like receptor (CRLR) form the basis of all the receptors for the calcitonin/CGRP/amylin/AM family of peptides (Foord & Marshall, 1999). Thus, CGRP and AM bind to the same receptor, the calcitonin receptor-like receptor (CRLR), with receptor specificity being determined by receptor-activity-modifying proteins (RAMPs). Three different RAMPs have been described in human tissue, RAMP1, RAMP2 and RAMP3. Co-expression of RAMP1 and CRLR reveals a CGRP-receptor, whereas co-expression of RAMP2 or RAMP3 and CRLR form an AM receptor (McLatchie et al., 1998). Similarly, RAMPs can interact with the CT-receptor gene product to induce expression of distinct amylin receptor phenotypes (Christopoulos et al., 1999).
Till now the lack of selective (preferably nonpeptide) agonists and antagonists has prevented thorough exploration of the receptor distribution and subtypes. The best receptor blockers availably for CGRP and adrenomedullin has been the peptide fragments αCGRP8–37, βCGRP8–37 and AM22–52 (Chiba et al., 1989; Longmore et al., 1994; Eguchi et al., 1994). For over a decade the sensitivity to the αCGRP8–37 fragment has been used to differentiate between two proposed classes of CGRP receptors termed CGRP1 and CGRP2 (Dennis et al., 1990). Thus, αCGRP8–37 demonstrates higher affinity for the CGRP1 receptor subtype (pA2=7–8) than the CGRP2 receptor (pA2=5.5–6.5) (Juaneda et al., 2000). A relatively limited number of non-peptide CGRP receptor antagonists have been described. Recently a compound named BIBN4096BS was reported to have high picomolar affinity for the CGRP receptors expressed in SK-N-MC cells (Doods et al., 2000).
In this study the expression of mRNA encoding the porcine CRLR, CT-receptor, RAMP1–3 were investigated. Furthermore, the vasodilatatory responses of αCGRP, βCGRP, amylin, AM and the potential receptor antagonists αCGRP8–37, βCGRP8–37 and AM22–52 were investigated in the porcine left ascending coronary artery (LAD) along with a novel non-peptide CGRP antagonist ‘Compound 1' (WO 98/11128) (Edvinsson et al., 2001).
Methods
Vessels
Porcine hearts were obtained fresh from an abattoir (Roskilde Slagteriskole, Roskilde, Denmark) and transported to our laboratory in ice-cold physiological salt solution (154 mM NaCl, DAK, Denmark). The left anterior descending (LAD) coronary artery was isolated near the apex of the heart and fat and connecting tissue was removed under a microscope. The artery, approximately 2 mm in diameter, was cut into ring segments, 2 mm wide.
Vasomotor responses
Each vessel segment with intact endothelium was mounted in a temperature-controlled tissue bath (37°C) containing a buffer solution (mM): NaCl 119, NaHCO3 15, KCl 4.6, CaCl2 1.5, NaH2PO4 1.2, MgCl2 1.2 and glucose 5.5. The bath was continuously bubbled with a mixture of 95% O2 and 5% CO2, giving a pH of approximately 7.4. To measure the isometric circular wall tension of the vessels, each segment was suspended between two L-shaped metal holders (0.2 mm in diameter) in a myograph (Model 610M, Danish Myo Tecnology, Denmark). The vessels were stretched to their optimal lumen diameter in order to obtain the optimal condition for active tension development as previously described (Mulvany & Halpern, 1977). After approximately 1 h, the vessels were exposed to a buffer solution containing 60 mM KCl, obtained by substituting equimolar concentrations of NaCl for KCl in the previously described buffer solution. Only vessel segments responding with a reproducible potassium-induced contraction after washout with the normal buffer solution, was used for further investigation. Peptides were added in cumulative concentrations from 10−10 to 10−7 M or 10−6 M every 5 min to vessel segments, which had been precontracted for 10 min with the thromboxane A2 agonist U46619 in a concentration of 10−7 M. The contraction induced by U46619 was set arbitrarily to 100% and used as an internal standard to which the relaxant response in the same vessel-segment was compared. When testing the effect of the potential receptor antagonists, ‘Compound 1', αCGRP8–37, βCGRP8–37 and AM22–52 were added 5 min prior to precontraction. Only one concentration-response experiment was allowed on each artery segment.
Drugs
Human αCGRP, βCGRP, AM, amylin and the fragments αCGRP8–37, βCGRP8–37 and AM22–52 were all obtained from Bachem AG, Switzerland. All peptides were dissolved in distilled water. U46619 (Sigma, St. Louis, MO, U.S.A.) was dissolved in ethanol. ‘Compound 1' (4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [1-3,5-dibromo-4-hydroxy-benzyl)-2-oxo-2-(4-phenyl-piperazin-1-yl)-ethyl]-amide, Karl Thomae GmbH, WO 98/11128) (Edvinsson et al., 2001) was synthesized by Medicinal Chemistry, Merck Research Laboratories, U.S.A. and dissolved in DMSO (dimethyl sulphoxide). Stock solutions (10−3–10−4 M) were made and stored at −20°C. Just before the experiments the solutions were diluted in buffer containing 0.2% human serum albumin.
Molecular experiments
Primer pairs were designed to detect mRNA for porcine RAMP1 (forward: 5′-GGC AGG ACC ATC AGG AGC TA-3′ and reverse: 5′-TGC TCT GCC AGA CCA CCA GT-3′) and the porcine CT receptor (forward: 5′-AGC GCC AGT GGA ACC AAT AC-3′ and reverse: 5′-ACT TCC ATG GCG ATG ACC TC-3′). The isolation of mRNA and RT–PCR assay for CRLR, RAMP2 and RAMP3 mRNA were performed using the primers and method previously described (Sams & Jansen-Olesen, 1998). The PCR amplification products of porcine CRLR, RAMP1 and RAMP2 were purified using QIAprep spin miniprep kit protocol (QIAGEN®, QIAGEN GmbH, Germany) and sequenced using an ABI Prism™ 377 DNA Sequencer (Perkin-Elmer, Sweden). Due to the sequencing technique the sequenced product are shorter than the PCR amplification products.
Data analysis and statistics
The concentration-response curves for αCGRP, βCGRP, AM and amylin were analysed by iterative nonlinear regression analysis and the sensitivity to agonists expressed as pEC50. Calculations were done using GraphPad Prism version 3.02 for Windows 95 (GraphPad Software, San Diego, CA, U.S.A., www.graphpad.com). The maximal relaxant responses of each peptide are expressed as a percentage of the contraction induced by U46619 and do not represent the Emax value. Results are given as mean±s.e.mean (n), where n is the number of vessels. The effects of agonists and antagonists were examined by comparing responses before and after antagonist treatment by means of one-way analysis of variance (ANOVA) followed by Dunnett's test. Dose-ratios (DR) were calculated and used to construct full Schild plots (Arunlakshana & Schild, 1959). The pA2-values are given with 95% confidence limits. P<0.05 was considered significant.
Results
Molecular experiments
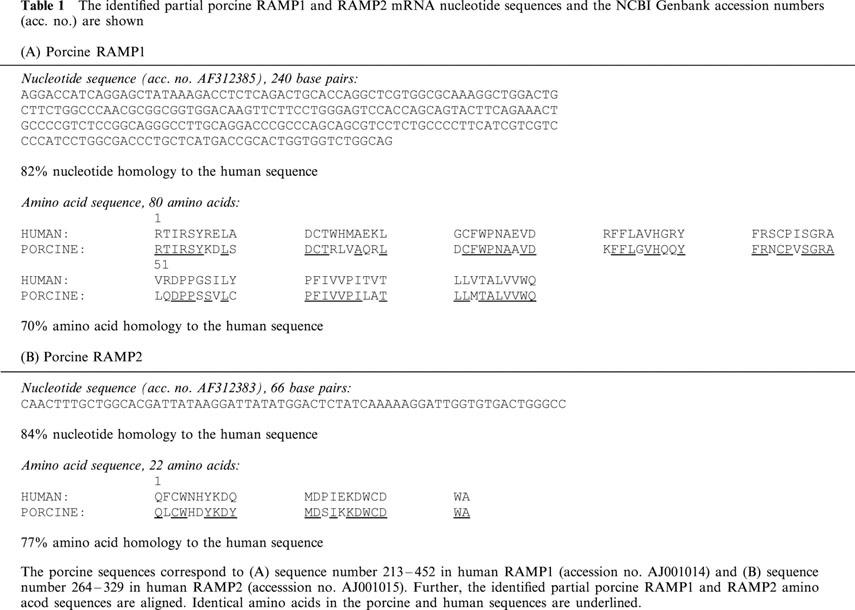
The presence of mRNA encoding CRLR, RAMP1 and RAMP2 in porcine coronary arteries demonstrated by RT–PCR (Figure 1). Using the current primers, we did not succeed in finding mRNA encoding the RAMP3 molecule. Neither did we demonstrate mRNA encoding the porcine CT-receptor in the coronary arteries. Expression of the CT-receptor in the porcine kidney was used as a positive control (data not shown). The partial CRLR sequence correspond with the porcine CGRP1 receptor (amino acids 252–375) sequenced by Elshourbagy et al. (1998) and share 98% amino acid sequence homology with the human sequence. The partial porcine sequences for RAMP1 and RAMP2 share 82–84% nucleotide sequence identity with the corresponding human sequences, whereas the amino acid sequence homology is 70–77% (Table 1).
Figure 1.
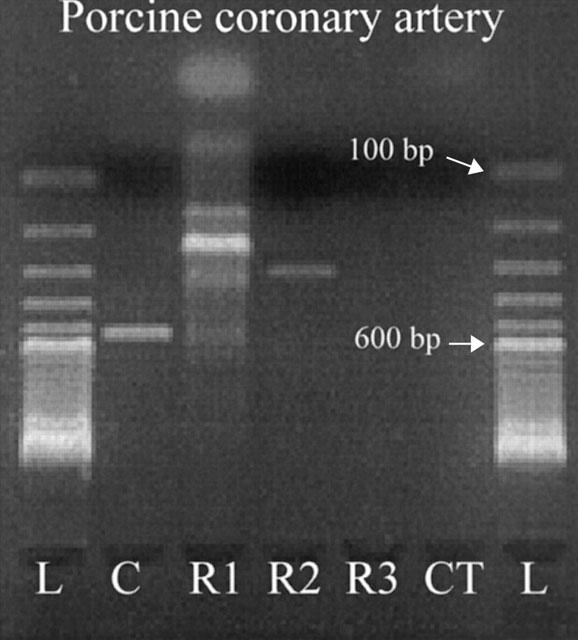
Demonstration of mRNA encoding the calcitonin receptor-like receptor (CRLR), RAMP1 and RAMP2 in the porcine left anterior descending coronary artery by RT–PCR. No bands are present in the lanes of RAMP3 (R3) and the calcitonin receptor (CT). L: 100 base pair ladder, C: CRLR, R1: RAMP1, R2: RAMP2, R3: RAMP3 and CT: calcitonin receptor.
Table 1.
The identified partial porcine RAMP1 and RAMP2 mRNA nucleotide sequences and the NCBI Genbank accession numbers (acc. no.) are shown(A) Porcine RAMP1
Vasomotor responses
The human fragments αCGRP8–37, βCGRP8–37, AM22–52 and ‘Compound 1' had no significant effect on the vasoconstriction induced by U46619 when added in doses up to 10−5 M. Data not shown.
Comparison of αCGRP, βCGRP, amylin and adrenomedullin
αCGRP and βCGRP induced similar concentration-dependent responses in the porcine coronary arteries (Figure 2, Table 2). The pEC50 values were 8.1±0.1 for both peptides and the maximal relaxations 96.6±1.4 and 95.8±2.0% respectively. The pEC50 value for AM was 6.7±0.1 with a maximal relaxation of 90.6±2.3 and 6.1±0.5% for amylin with maximal relaxation of 70.6±11.5%. AM and amylin were thus significantly different from the αCGRP and βCGRP responses. Further, the amylin response differed significantly from the AM response.
Figure 2.
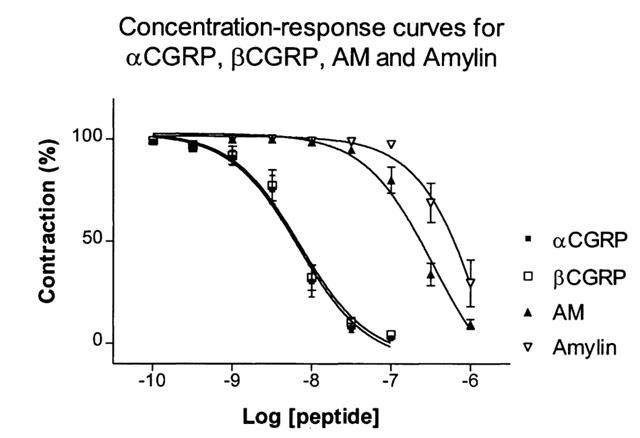
αCGRP, βCGRP, AM and amylin concentration-reponse relationship in porcine coronary arteries. Points represent mean values and vertical lines indicate s.e.mean. Relative responses are given as percentage fraction of the initial vessel response to U46619 (10−7 M) just before they were challenged with the relaxant peptides.
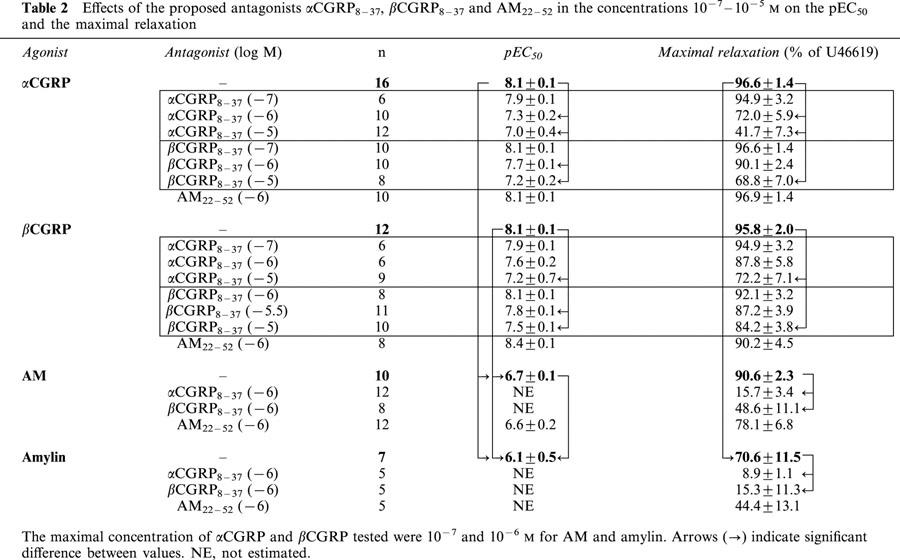
Table 2.
Effects of the proposed antagonists αCGRP8–37, βCGRP8–37 and AM22–52 in the concentrations 10−7–10−5 M on the pEC50 and the maximal relaxation
Effect of αCGRP8–37 and βCGRP8–37
The fragments αCGRP8–37 and βCGRP8–37 induced concentration-dependent (10−7–10−5 M) rightward shift of both the αCGRP and βCGRP concentration-response curves (Figure 3a–d and Table 2). βCGRP8–37 (10−6 M) had the same effect as αCGRP8–37 (10−6 M), but with less potent antagonistic properties (Figure 3c, d and Table 2). Thus, βCGRP8–37 (10−6 M) did not antagonize the relaxant effect of βCGRP and did not affect the maximal response of αCGRP. The Schild plots revealed pA2 values of 7.0 (6.4–8.6) and 6.9 (6.2–8.7) for αCGRP8–37 versus 6.3 (5.9–7.0) and 5.9 (5.7–6.5) for βCGRP8–37 when tested with αCGRP and βCGRP respectively (Figure 4). Furthermore, preincubation with αCGRP8–37 (10−6 M) and βCGRP8–37 (10−6 M) also caused a significant change in pEC50 values and the maximal responses for AM and amylin (Figures 5 and 6 and Table 2).
Figure 3.
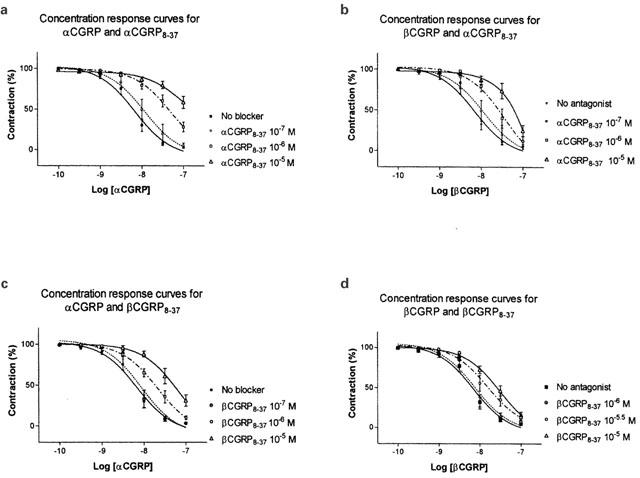
(a–d) Vasorelaxant effect of αCGRP (10−10–10−7 M) and βCGRP (10−10–10−7 M) in the porcine LAD contracted with U46619 (10−7 M). The antagonists αCGRP8–37 (10−7–10−5 M), βCGRP8–37 (10−7–10−5 M) were added 15 min and U46619 10 min prior to the αCGRP/βCGRP challenge. Points represent mean values and vertical lines indicate s.e.mean. Relative responses are given as percentage fraction of the initial vessel response to U46619 (10−7 M) just before they were challenged with αCGRP/βCGRP.
Figure 4.

Schild plots for αCGRP8–37 and βCGRP8–37 tested with human αCGRP and βCGRP as agonists in isolated porcine LAD. The Schild plot curve for αCGRP8–37 (10−7–10−5 M) tested with αCGRP was equal to 1.48× +10.3 (r=0.62; P=0.0011). The pA2 value=7.0 (6.4–8.6). The Schild plot curve for αCGRP8–37 (10−7–10−5 M) tested with βCGRP was equal to 1.23× +7.8 (r=0.63; P=0.0007). The pA2 value=6.9 (6.2–8.7). The Schild plot curve for βCGRP8–37 (10−7–10−5 M) tested with αCGRP was equal to 1.05× +7.2 (r=0.61; P=0.0031). The pA2 value=6.3 (5.9–7.0). The Schild plot curve for βCGRP8–37 (10−6–10−5 M) tested with βCGRP was equal to 1.68× +10.0 (r=0.65; P=0.0002). The pA2 value=5.9 (5.7–6.5). Each point represents mean values and vertical lines indicate s.e.mean.
Figure 5.

Vasorelaxant effect of AM in the porcine LAD contracted with U46619 (10−7 M). The proposed antagonists αCGRP8–37 (10−6 M), βCGRP8–37 (10−6 M) and AM22–52 (10−6 M) were added 15 min and U46619 10 min prior to the αCGRP (10−9–10−6 M) challenge. Points represent mean values and vertical lines indicate s.e.mean. Relative responses are given as percentage fraction of the initial vessel response to U46619 (10−7 M) just before they were challenged with AM.
Figure 6.

Vasorelaxant effect of amylin in the porcine LAD contracted with U46619 (10−7 M). The proposed antagonists αCGRP8–37 (10−6 M), βCGRP8–37 (10−6 M) and AM22–52 (10−6 M) were added 15 min and U46619 10 min prior to the αCGRP (10−9–10−6 M) challenge. Points represent mean values and vertical lines indicate s.e.mean. Relative responses are given as percentage fraction of the initial vessel response to U46619 (10−7 M) just before they were challenged with amylin.
Figure 7.
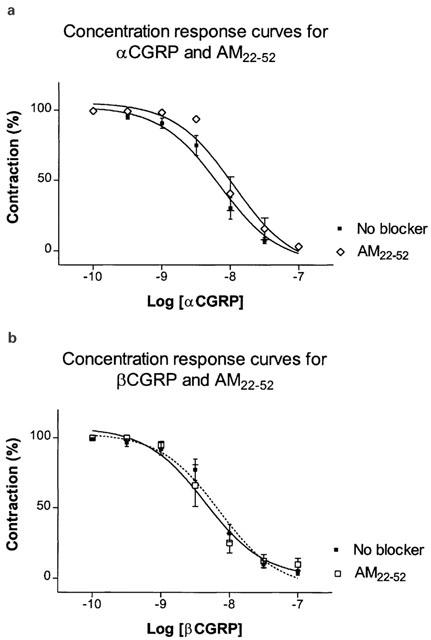
(a,b) Vasorelaxant effect of αCGRP (10−10–10−7 M) and βCGRP (10−10–10−7 M) in the porcine LAD contracted with U46619 (10−7 M). The antagonist AM22–52 (10−6 M) was added 15 min and U46619 10 min prior to the αCGRP/βCGRP challenge. Points represent mean values and vertical lines indicate s.e.mean. Relative responses are given as percentage fraction of the initial vessel response to U46619 (10−7 M) just before they were challenged with αCGRP/βCGRP.
Effect of AM22–52
Preincubation with AM22–52 (10−6 M) caused a slight but not significant rightward shift without affecting the maximal response of the concentration-response curves for AM and amylin. Neither did AM22–52 antagonize the response of α- and β-CGRP (Figures 5 and 6, 7a,b and Table 2).
Effect of ‘Compound 1'/(WO98/11128)
The effect of αCGRP was tested after preincubation with ‘Compound 1' (10−7–10−5 M). No antagonistic effect was found (Figure 8).
Figure 8.
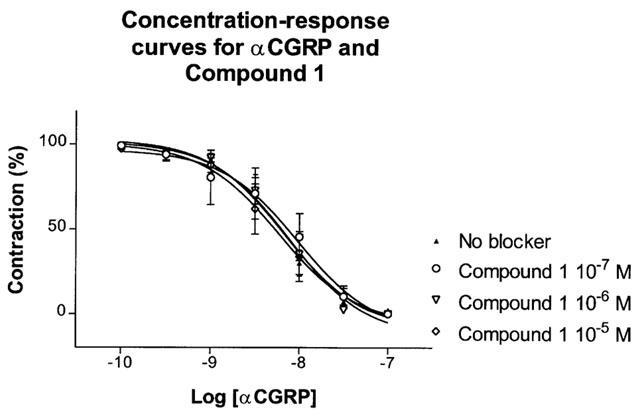
Vasorelaxant effect of αCGRP in the porcine LAD contracted with U46619 (10−7 M). The non-peptide CGRP antagonist ‘Compound 1' (10−7–10−5 M) was added 15 min and U46619 10 min prior to the αCGRP (10−10–10−7 M) challenge. Points represent mean values and vertical lines indicate s.e.mean. Relative responses are given as percentage fraction of the initial vessel response to U46619 (10−7 M) just before they were challenged with αCGRP.
Discussion
RAMPs are proteins identified within the last few years. They interact and modify the phenotype of at least two family of receptors or class II group of G-protein receptors, the CGRP and CT receptor (Sexton, 1999). Three potential consequences of RAMP interaction with its associated receptors have been described: (1) intracellular transport of the receptor to the cell surface; (2) modification of the receptor glycosylation; and (3) direct and indirect modification of the ligand binding site through association with the receptor at the cell surface (Foord & Marshall, 1999). Our results demonstrate the presence of mRNA sequences encoding the RAMPs and CRLR in the porcine LAD. We were not able to demonstrate RAMP3 using primers designed against the human sequence. But expression of RAMP1 and RAMP2 were demonstrated. Expression and formation of CGRP- and AM receptors are therefore possible (McLatchie et al., 1998). The CT-receptor was not demonstrated. Therefore, the presence of specific amylin receptors in the porcine LAD is unlikely. To our knowledge the presence of amylin receptors have only been shown in the kidney, brain and vas deferens (Chai et al., 1998; Christopoulos et al., 1995; Tomlinson & Poyner, 1996) but never in vessels. RAMPs and CRLR have also been demonstrated in human cerebral arteries (Sams & Jansen-Olesen, 1998). The identified partial mRNA sequences demonstrate a high degree of homology with the human sequences, thereby indicating that the sequences in fact represent CRLR, RAMP1 and RAMP2 mRNA.
CGRP and AM are well known vasodilators in the coronary circulation and Yoshimoto et al. (1998) has previously tested these peptides in the porcine LAD. In the present study the vasorelaxant responses of αCGRP, βCGRP and AM and the blocking effect of αCGRP8–37 (10−6 M) on αCGRP and AM were confirmed. But in contrast to the investigations by Yoshimoto et al. we found significant antagonistic effect of the αCGRP8–37 (10−6 M) fragment on the relaxation induced by βCGRP.
The presence of CRLR and RAMP1 might be expected to give a CGRP1-like receptor. However the measured pA2 for αCGRP8–37 of 6.9–7.0 is considerably below the value of around 8 found for porcine CRLR and RAMP1 in HEK293 cell expression system (Elshourbagy et al., 1998). Differences between pA2 values for αCGRP8–37 in cell lines and in real tissue are also seen with other species and one explanation could be that the receptors are better exposed in cell cultures than in real tissue with different diffusion gradient barriers. Thus, the pA2 values of 7.0 (6.4–8.6) and 6.9 (6.2–8.7) produced by the human αCGRP8–37 fragment in the present study on the porcine coronary artery tissue is in line with previous studies where pA2 or pKb values were 5.7–7 (Foulkes et al., 1991), 7.2 (Yoshimoto et al., 1998), 5.9–6.0 (neglecting data with high Schild plot slope) (Waugh et al., 1999) and 6.3–6.7 (Wisskirchen et al., 1999). Relatively little CGRP pharmacology has been carried out on the pig to say what pA2 values would be expected for a CGRP1 and CGRP2 receptor in this species. Using the common criteria for distinguishing between the CGRP receptor subtypes the antagonist affinity for αCGRP8–37 is consistent with a CGRP1 receptor, but the 95% confidence limits for the pA2 determination covers the entire range from CGRP1 to CGRP2. The pA2 values of 6.3 (5.9–7.0) and 5.9 (5.7–6.5) for βCGRP8–37 indicate lower affinity at the CGRP receptor site compared to the αCGRP8–37 fragment.
The αCGRP8–37 fragment also blocked the vasodilatatory effect of amylin, indicating that amylin can act via the CGRP receptors. A recent study by Sheykhzade & Nyborg (2000) also shows that amylin act as a non-competitive antagonist on CGRP1 receptors in rat coronary arteries.
Till now a relatively limited number of non-peptide CGRP receptor antagonists has been described. Recently a compound named BIBN4096BS was reported to have high picomolar affinity for the CGRP receptors expressed in SK-N-MC cells and was characterized as a human-selective antagonist (Doods et al., 2000). ‘Compound 1' is a novel non-peptide CGRP antagonist and was presented as a functional CGRP receptor blocker in human SK-N-MC cells with a pKi value of 7.8 and in human cerebral arteries with a pA2 values of 7.7 (Edvinsson et al., 2001). In porcine LAD, however, ‘Compound 1' demonstrated no antagonistic effect. The use of different species could explain the diverse results and perhaps ‘Compound 1' has human-selective antagonistic properties as well. Similarly, in our study the βCGRP8–37 fragment generally acted as αCGRP8–37, but with less potency. This is in contrast to studies in rat pulmonary artery, vas deferens and guinea-pig atria where these receptor antagonists antagonized the αCGRP responses with similar affinity (Wisskirchen et al., 1998; Longmore et al., 1994).
The human AM22–52 fragment has been tested as an AM receptor antagonist in many studies and in different species. Thus, in a radio-ligand binding study in porcine tissue AM22–52 acted as an AM receptor antagonist (Dang et al., 1999). AM22–52 has also been shown to block the vasodilatory effect of AM without affecting the response of CGRP in the vascular bed of rat and cat (Dogan et al., 1997; Gardiner et al., 1999; Saita et al., 1998; Takao et al., 1999), but in the cat hind limb vasculature the AM vasodilator response was unchanged after administration of AM22–52 (Champion et al., 1997). In the present study we only find a slight non-significant inhibition of the vasorelaxant effects of AM and amylin when preincubating with AM22–52. The fragment is not a very potent AM inhibitor and its specificity has been questioned (Hinson et al., 2000). This is probably why different studies show different actions of AM22–52. AM and amylin may still act via an AM receptor, but clarification of this question is not possible until a potent and specific AM receptor blocker has been identified. But CRLR has a higher affinity for RAMP1 than RAMP2 (Buhlmann et al., 1999), which might explain the failure to detect a functional response to adrenomedullin.
In conclusion, CGRP and AM are important in modulating blood flow in the coronary circulation. A CGRP receptor (CRLR+RAMP1) is present in the porcine coronary arteries, but the antagonist affinity for αCGRP8–37 is neither consistent with a CGRP1 nor a CGRP2 receptor. The βCGRP8–37 showed less potent CGRP receptor antagonism than the αCGRP8–37 fragment. The vasorelaxant effect of αCGRP, AM and amylin in the porcine coronary arteries can solely be explained by interaction with CGRP receptors. An AM receptor (CRLR+RAMP2) was also demonstrated, but no vasorelaxant effect of AM was shown via the AM receptor. This may be due to the lack of specificity and potency of the AM22–52 receptor antagonist or the fact that CRLR has a higher affinity for RAMP1 than RAMP2. No amylin receptor (CT+RAMPs) was demonstrated in the porcine LAD so the effect of amylin is probably mediated via the CGRP receptors. ‘Compound 1', a novel non-peptide CGRP receptor antagonist, had no effect on the porcine LAD.
Acknowledgments
The study was supported through the following foundations: ‘The Danish Heart Foundation', Grant No. 99-1-2-19-22679; ‘The Danish Hospital Foundation for Medical Research'; ‘Novo Nordisk Research Foundation'; ‘The Danish Medical Association Research Fund' and ‘Fonden til Lægevidenskabens fremme, A.P. Møller og Hustru Chastine Mc-Kinney Møllers Fond til almene formaal'. The cDNA sequences reported in this paper have been deposited in the NCBI Genebank Database, accession numbers AF312383 and AF312385. The authors would like to thank Betina Christensen for excellent assistance with the PCR protocols.
Abbreviations
- AM
adrenomedullin
- CGRP
calcitonin gene-related peptide
- Compound 1 (WO 98/11128)
(4-(2-Oxo-2, 3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [1-3,5-dibromo-4-hydroxy-benzyl)-2-oxo-2-(4-phenyl-piperazin-1-yl)-ethyl]-amide
- CRLR
calcitonin receptor-like receptor
- CT
Calcitonin
- DMSO
dimethyl sulphoxide
- HEK
human embryonic kidney
- LAD
left anterior descending coronary artery
- RAMP
receptor-activity-modifying proteins
- SK-N-MC
human neuroblastoma cells
References
- AMARA S.G., ARRIZA J.L., LEFF S.E., SWANSON L.W., EVANS R.M., ROSENFELD M.G. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- AMARA S.G., JONAS V., ROSENFELD M.G., ONG E.S., EVANS R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUHLMANN N., LEUTHAUSER K., MUFF R., FISCHER J.A., BORN W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- CHAI S.Y., CHRISTOPOULOS G., COOPER M.E., SEXTON P.M. Characterization of binding sites for amylin, calcitonin, and CGRP in primate kidney. Am. J. Physiol. 1998;274:F51–F62. doi: 10.1152/ajprenal.1998.274.1.F51. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., SANTIAGO J.A., MURPHY W.A., COY D.H., KADOWITZ P.J. Adrenomedullin-(22–52) antagonizes vasodilator responses to CGRP but not adrenomedullin in the cat. Am. J. Physiol. 1997;272:R234–R242. doi: 10.1152/ajpregu.1997.272.1.R234. [DOI] [PubMed] [Google Scholar]
- CHIBA T., YAMAGUCHI A., YAMATANI T., NAKAMURA A., MORISHITA T., INUI T., FUKASE M., NODA T., FUJITA T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8–37) Am. J. Physiol. 1989;256:E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- CHRISTOPOULOS G., PAXINOS G., HUANG X.F., BEAUMONT K., TOGA A.W., SEXTON P.M. Comparative distribution of receptors for amylin and the related peptides calcitonin gene related peptide and calcitonin in rat and monkey brain. Can. J. Physiol. Pharmacol. 1995;73:1037–1041. doi: 10.1139/y95-146. [DOI] [PubMed] [Google Scholar]
- CHRISTOPOULOS G., PERRY K.J., MORFIS M., TILAKARATNE N., GAO Y., FRASER N.J., MAIN M.J., FOORD S.M., SEXTON P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- COOPER G.J., WILLIS A.C., CLARK A., TURNER R.C., SIM R.B., REID K.B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl. Acad. Sci. U.S.A. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANG K., DISA J., GOUT B., AIYAR N. Comparative affinities of adrenomedullin (AM) and calcitonin gene-related peptide (CGRP) for [125I] AM and [125I] CGRP specific binding sites in porcine tissues. J. Recept. Signal. Transduct. Res. 1999;19:803–817. doi: 10.3109/10799899909042874. [DOI] [PubMed] [Google Scholar]
- DENNIS T., FOURNIER A., CADIEUX A., POMERLEAU F., JOLICOEUR F.B., ST PIERRE S., QUIRION R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J. Pharmacol. Exp. Ther. 1990;254:123–128. [PubMed] [Google Scholar]
- DOGAN A., SUZUKI Y., KOKETSU N., OSUKA K., SAITO K., TAKAYASU M., SHIBUYA M., YOSHIDA J. Intravenous infusion of adrenomedullin and increase in regional cerebral blood flow and prevention of ischemic brain injury after middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 1997;17:19–25. doi: 10.1097/00004647-199701000-00004. [DOI] [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., WU D., ENTZEROTH M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDVINSSON L., SAMS A., JANSEN-OLESEN I., TAJTI J., KANE S.A., RUTLEDGE R.Z., KOBLAN K.S., HILL R.G., LONGMORE J. Characterisation of the effects of a non-peptide CGRP receptor antagonist in SK-N-MC cells and isolated human cerebral arteries. Eur. J. Pharmacol. 2001;415:39–44. doi: 10.1016/s0014-2999(00)00934-1. [DOI] [PubMed] [Google Scholar]
- EGUCHI S., HIRATA Y., IWASAKI H., SATO K., WATANABE T.X., INUI T., NAKAJIMA K., SAKAKIBARA S., MARUMO F. Structure-activity relationship of adrenomedullin, a novel vasodilatory peptide, in cultured rat vascular smooth muscle cells. Endocrinology. 1994;135:2454–2458. doi: 10.1210/endo.135.6.7988431. [DOI] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., ADAMOU J.E., SWIFT A.M., DISA J., MAO J., GANGULY S., BERGSMA D.J., AIYAR N. Molecular cloning and characterization of the porcine calcitonin gene- related peptide receptor. Endocrinology. 1998;139:1678–1683. doi: 10.1210/endo.139.4.5860. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN G., WILLETTE R., AIYAR N. Clinical perspectives of calcitonin gene related peptide pharmacology. Can. J. Physiol. Pharmacol. 1995;73:1070–1074. doi: 10.1139/y95-152. [DOI] [PubMed] [Google Scholar]
- FOORD S.M., MARSHALL F.H. RAMPs: accessory proteins for seven transmembrane domain receptors. Trends Pharmacol. Sci. 1999;20:184–187. doi: 10.1016/s0165-6147(99)01347-4. [DOI] [PubMed] [Google Scholar]
- FOULKES R., SHAW N., BOSE C., HUGHES B. Differential vasodilator profile of calcitonin gene-related peptide in porcine large and small diameter coronary artery rings. Eur. J. Pharmacol. 1991;201:143–149. doi: 10.1016/0014-2999(91)90337-p. [DOI] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., HENKE H., LUNDBERG J.M., PETERMANN J.B., HOKFELT T., FISCHER J.A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987;8:399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. Influence of CGRP (8–37), but not adrenomedullin (22–52), on the haemodynamic responses to lipopolysaccharide in conscious rats. Br. J. Pharmacol. 1999;127:1611–1618. doi: 10.1038/sj.bjp.0702718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENNARI C., IMBIMBO B. Effects of prednisone and deflazacort on vertebral bone mass. Calcif. Tissue Int. 1985;37:592–593. doi: 10.1007/BF02554912. [DOI] [PubMed] [Google Scholar]
- GULBENKIAN S., SAETRUM O.O., EKMAN R., COSTA A.N., WHARTON J., POLAK J.M., MELO J., EDVINSSON L. Peptidergic innervation of human epicardial coronary arteries. Circ. Res. 1993;73:579–588. doi: 10.1161/01.res.73.3.579. [DOI] [PubMed] [Google Scholar]
- HINSON J.P., KAPAS S., SMITH D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- HOJO Y., IKEDA U., KATSUKI T.A., SHIMADA K. Decreased adrenomedullin production in the coronary circulation of patients with coronary artery disease. Heart. 2000;84:88. doi: 10.1136/heart.84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPENER J.W., AHREN B., LIPS C.J. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 2000;343:411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- JUANEDA C., DUMONT Y., QUIRION R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol. Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- KAHN S.E., D'ALESSIO D.A., SCHWARTZ M.W., FUJIMOTO W.Y., ENSINCK J.W., TABORSKY G.J., JR, PORTE D., JR Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- KANATSUKA A., MAKINO H., OHSAWA H., TOKUYAMA Y., YAMAGUCHI T., YOSHIDA S., ADACHI M. Secretion of islet amyloid polypeptide in response to glucose. FEBS Lett. 1989;259:199–201. doi: 10.1016/0014-5793(89)81527-3. [DOI] [PubMed] [Google Scholar]
- KANO H., KOHNO M., YASUNARI K., YOKOKAWA K., HORIO T., IKEDA M., MINAMI M., HANEHIRA T., TAKEDA T., YOSHIKAWA J. Adrenomedullin as a novel antiproliferative factor of vascular smooth muscle cells. J. Hypertens. 1996;14:209–213. doi: 10.1097/00004872-199602000-00009. [DOI] [PubMed] [Google Scholar]
- KITAMURA K., KANGAWA K., KAWAMOTO M., ICHIKI Y., NAKAMURA S., MATSUO H., ETO T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- KITAMURA K., KANGAWA K., MATSUO H., ETO T. Adrenomedullin. Implications for hypertension research. Drugs. 1995;49:485–495. doi: 10.2165/00003495-199549040-00001. [DOI] [PubMed] [Google Scholar]
- KUREISHI Y., KOBAYASHI S., NISHIMURA J., NAKANO T., KANAIDE H. Adrenomedullin decreases both cytosolic Ca2+ concentration and Ca(2+)- sensitivity in pig coronary arterial smooth muscle. Biochem. Biophys. Res. Commun. 1995;212:572–579. doi: 10.1006/bbrc.1995.2008. [DOI] [PubMed] [Google Scholar]
- LECHLEITNER P., GENSER N., MAIR J., DIENSTL A., HARING C., WIEDERMANN C.J., PUSCHENDORF B., SARIA A., DIENSTL F. Calcitonin gene-related peptide in patients with and without early reperfusion after acute myocardial infarction. Am. Heart J. 1992;124:1433–1439. doi: 10.1016/0002-8703(92)90054-y. [DOI] [PubMed] [Google Scholar]
- LONGMORE J., HOGG J.E., HUTSON P.H., HILL R.G. Effects of two truncated forms of human calcitonin-gene related peptide: implications for receptor classification. Eur. J. Pharmacol. 1994;265:53–59. doi: 10.1016/0014-2999(94)90222-4. [DOI] [PubMed] [Google Scholar]
- MAIR J., LECHLEITNER P., LANGLE T., WIEDERMANN C., DIENSTL F., SARIA A. Plasma CGRP in acute myocardial infarction. Lancet. 1990;335:168. doi: 10.1016/0140-6736(90)90040-c. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MORRIS H.R., PANICO M., ETIENNE T., TIPPINS J., GIRGIS S.I., MACINTYRE I. Isolation and characterization of human calcitonin gene-related peptide. Nature. 1984;308:746–748. doi: 10.1038/308746a0. [DOI] [PubMed] [Google Scholar]
- MUFF R., BORN W., FISCHER J.A. Calcitonin, calcitonin gene-related peptide, adrenomedullin and amylin: homologous peptides, separate receptors and overlapping biological actions. Eur. J. Endocrinol. 1995;133:17–20. doi: 10.1530/eje.0.1330017. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., SEGUCHI H., SAKIHARA C., KUREISHI Y., YOSHIMURA H., KOBAYASHI S., KANAIDE H. The relaxant effect of adrenomedullin on particular smooth muscles despite a general expression of its mRNA in smooth muscle, endothelial and epithelial cells. Br. J. Pharmacol. 1997;120:193–200. doi: 10.1038/sj.bjp.0700881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKES D.G. Cardiovascular actions of adrenomedullin in conscious sheep. Am. J. Physiol. 1995;268:H2574–H2578. doi: 10.1152/ajpheart.1995.268.6.H2574. [DOI] [PubMed] [Google Scholar]
- SAETRUM O.O., GULBENKIAN S., BERGDAHL A., BARROSO C.P., ANDRADE N.C., POLAK J.M., MELO J.Q., EDVINSSON L. Innervation of human epicardial coronary veins: immunohistochemistry and vasomotility. Cardiovasc. Res. 1995;29:463–468. doi: 10.1016/0008-6363(96)88520-8. [DOI] [PubMed] [Google Scholar]
- SAITA M., SHIMOKAWA A., KUNITAKE T., KATO K., HANAMORI T., KITAMURA K., ETO T., KANNAN H. Central actions of adrenomedullin on cardiovascular parameters and sympathetic outflow in conscious rats. Am. J. Physiol. 1998;274:R979–R984. doi: 10.1152/ajpregu.1998.274.4.R979. [DOI] [PubMed] [Google Scholar]
- SAMS A., JANSEN-OLESEN I. Expression of calcitonin receptor-like receptor and receptor-activity-modifying proteins in human cranial arteries. Neurosci. Lett. 1998;258:41–44. doi: 10.1016/s0304-3940(98)00844-1. [DOI] [PubMed] [Google Scholar]
- SEXTON P.M. Recent advances in our understanding of peptide hormone receptors and RAMPs. Curr. Opin. Drug Discov. Dev. 1999;2:440–448. [PubMed] [Google Scholar]
- SHEYKHZADE M., NYBORG N.C. Non-competitive antagonism of amylin on CGRP(1)-receptors in rat coronary small arteries. Br. J. Pharmacol. 2000;130:386–390. doi: 10.1038/sj.bjp.0703316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGO S., MINAMINO N., KANGAWA K., MIYAMOTO K., KITAMURA K., SAKATA J., ETO T., MATSUO H.Endothelial cells actively synthesize and secrete adrenomedulli Biochem. Biophys. Res. Commun. 1994a2011160–1166.[published erratum appears in Biochem Biophys Res Commun 1994 Sep 15;203(2):1363] [DOI] [PubMed] [Google Scholar]
- SUGO S., MINAMINO N., SHOJI H., KANGAWA K., KITAMURA K., ETO T., MATSUO H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem. Biophys. Res. Commun. 1994b;203:719–726. doi: 10.1006/bbrc.1994.2241. [DOI] [PubMed] [Google Scholar]
- TAKAO M., TOMITA M., TANAHASHI N., KOBARI M., FUKUUCHI Y. Transient vasodilatory effects of adrenomedullin on cerebral parenchymal microvessels in cats. Neurosci. Lett. 1999;268:147–150. doi: 10.1016/s0304-3940(99)00408-5. [DOI] [PubMed] [Google Scholar]
- TOMLINSON A.E., POYNER D.R. Multiple receptors for calcitonin gene-related peptide and amylin on guineapig ileum and vas deferens. Br. J. Pharmacol. 1996;117:1362–1368. doi: 10.1111/j.1476-5381.1996.tb16737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UREN N.G., SEYDOUX C., DAVIES G.J. Effect of intravenous calcitonin gene related peptide on ischaemia threshold and coronary stenosis severity in humans. Cardiovasc. Res. 1993;27:1477–1481. doi: 10.1093/cvr/27.8.1477. [DOI] [PubMed] [Google Scholar]
- WAUGH D.J., BOCKMAN C.S., SMITH D.D., ABEL P.W. Limitations in using peptide drugs to characterize calcitonin gene-related peptide receptors. J. Pharmacol. Exp. Ther. 1999;289:1419–1426. [PubMed] [Google Scholar]
- WISSKIRCHEN F.M., BURT R.P., MARSHALL I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br. J. Pharmacol. 1998;123:1673–1683. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSKIRCHEN F.M., GRAY D.W., MARSHALL I. Receptors mediating CGRP-induced relaxation in the rat isolated thoracic aorta and porcine isolated coronary artery differentiated by halpha CGRP8 - 37. Br. J. Pharmacol. 1999;128:283–292. doi: 10.1038/sj.bjp.0702764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMOTO R., MITSUI-SAITO M., OZAKI H., KARAKI H. Effects of adrenomedullin and calcitonin gene-related peptide on contractions of the rat aorta and porcine coronary artery. Br. J. Pharmacol. 1998;123:1645–1654. doi: 10.1038/sj.bjp.0701805. [DOI] [PMC free article] [PubMed] [Google Scholar]


