Abstract
C-terminal-binding protein/brefeldin A-ADP ribosylated substrate (CtBP/BARS) plays key roles in development and oncogenesis as a transcription co-repressor, and in intracellular traffic as a promoter of Golgi membrane fission. Co-repressor activity is regulated by NAD(H) binding to CtBP/BARS, while membrane fission is associated with its acyl-CoA-dependent acyltransferase activity. Here, we report the crystal structures of rat CtBP/BARS in a binary complex with NAD(H), and in a ternary complex with a PIDLSKK peptide mimicking the consensus motif (PXDLS) recognized in CtBP/BARS cellular partners. The structural data show CtBP/BARS in a NAD(H)-bound dimeric form; the peptide binding maps the recognition site for DNA-binding proteins and histone deacetylases to an N-terminal region of the protein. The crystal structure together with the site-directed mutagenesis data and binding experiments suggest a rationale for the molecular mechanisms underlying the two fundamental co-existing, but diverse, activities supported by CtBP/BARS in the nucleus and in Golgi membranes.
Keywords: acyl-CoA/brefeldin A/Golgi membrane/NAD/transcription co-repression
Introduction
C-terminal-binding protein/brefeldin A-ADP ribosylated substrate (CtBP/BARS) is a dual-function, 50 kDa protein involved in the two widely diverse activities of intracellular membrane trafficking and gene transcription. In the cellular trafficking context, it was first identified as the ADP ribosylation substrate of brefeldin A (a fungal toxin that disrupts the Golgi complex structure and intracellular traffic), and later shown to be an essential component of the machinery controlling Golgi tubule fission (De Matteis et al., 1994; Di Girolamo et al., 1995; Mironov et al., 1997; Spanò et al., 1999; Weigert et al., 1999). BARS fission-inducing activity in the Golgi complex is associated with the acyl-CoA-dependent conversion of lysophosphatidic acid (LPA) into phosphatidic acid (PA), a process that can lead to local PA accumulation in these membranes, hence enhancing the curvature of the phospholipid bilayer, and thus promoting Golgi tubule fission (Weigert et al., 1999; Corda et al., 2002). In vivo, BARS controls the exit of transport carriers from the Golgi complex (our unpublished data).
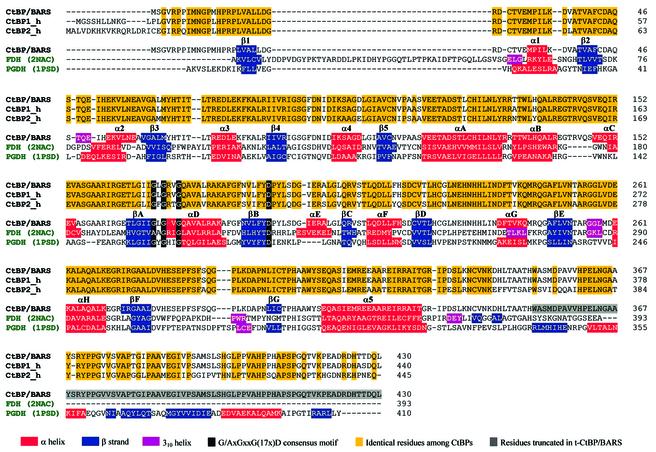
In addition, BARS has been shown to be a member of the CtBP family, as it displays very high amino acid sequence identities relative to mammalian CtBP1 and CtBP2 (98 and 80% sequence identity, respectively) (Figure 1), which both act as co-repressors of transcription regulators (Schaeper et al., 1995; Turner and Crossley, 1998; Criqui-Filipe et al., 1999). Although the precise mechanisms of their co-repressor activity in transcription are still under investigation, it is known that the CtBPs act as dimers, and that they recognize the consensus pentapeptide motif PXDLS (where X is often leucine or valine) in DNA-binding proteins (Turner and Crossley, 2001). The CtBPs also bind histone deacetylases containing the PXDLS motif, and are thus likely to act as protein bridges between histone deacetylases and DNA-bound factors (Turner and Crossley, 2001). The same recognition motif is present in viral proteins, such as E1A (Boyd et al., 1993), suggesting that CtBP binding to both cellular and viral transcription repressors is part of a widespread regulatory mechanism, which is known to be regulated by NAD(H) (Zhang et al., 2002). Moreover, very recently, CtBP has been shown to display a low but detectable dehydrogenase activity using pyruvate as a substrate (Kumar et al., 2002). On this basis, and in consideration of their structural relationship to NAD-dependent dehydrogenases, CtBPs have been proposed to be dehydrogenases that have evolved the ability to bind the PXDLS recognition motif (Kumar et al., 2002; Marmorstein, 2002).
Fig. 1. Structure-based sequence alignments. Rat CtBP/BARS (GenBank AAC79427.2) is aligned with human CtBP1 (SWISS-PROT: Q13363), human CtBP2 (SWISS-PROT: P56545), formate dehydrogenase (FDH; PDB-code 2NAC) and d-3-phosphoglycerate dehydrogenase (PGDH; PDB-code 1PSD). All sequence alignments were performed using the CLUSTALW program (Thompson et al., 1994); in the case of NAD-dependent dehydrogenases (green codes), the sequence alignments have been corrected manually based on their three-dimensional structure comparisons with t-CtBP/BARS. The different structure and homology information data are colour coded, as explained in the figure.
How CtBP/BARS acts as a bifunctional protein that can encompass two entirely different cellular functions in the nucleus and in Golgi membranes still remains unclear (Spanò et al., 1999; Weigert et al., 1999; Turner and Crossley, 2001; Corda et al., 2002; Kumar et al., 2002; Marmorstein, 2002). To delineate further the structural bases of the transcriptional co-repressor and/or acyltransferase activities displayed by CtBP/BARS, we report here the crystal structure of a truncated form of the rat protein (t-CtBP/BARS, devoid of the 80, mostly hydrophobic, C-terminal residues) (Nardini et al., 2002) in binary complex with NAD(H) at 2.3 Å resolution, and in ternary complex with NAD(H) and the PIDLSKK peptide, the latter in two different crystal forms. The data presented locate the recognition site for CtBP/BARS cellular protein partners and, together with consideration of site-specific mutants and competition experiments on NAD(H)/acyl-CoA cofactor binding, suggest the structural transitions needed for CtBP/BARS switching between its nuclear and Golgi activities.
Results and discussion
Structure determination
The structure of t-CtBP/BARS was solved on crystals belonging to the hexagonal space group P6422 (one t-CtBP/BARS molecule per asymmetric unit) by means of multiple wavelength anomalous diffraction (MAD), based on the anomalous scattering of Se atoms. The protein model was refined at 2.3 Å resolution using a native data set collected at the ESRF synchrotron source. The final t-CtBP/BARS model contains 331 residues, 125 water molecules, one NAD(H), one glycerol and two formate molecules (Rfactor = 22.2% and Rfree = 28.1%, respectively), with ideal stereochemical parameters (Table I) (Engh and Huber, 1991).
Table I. Data collection and refinement statistics on the P6422 crystal form.
| t-CtBP/BARS:NAD(H) | t-CtBP/BARS:NAD(H):peptide | |
|---|---|---|
| Data collection statisticsa | ||
| Space group | P6422 | P6422 |
| Unit cell dimensions (Å) | a = b = 88.7, c = 163.0 | a = b = 84.8, c = 159.7 |
| Resolution (Å) | 2.3 | 3.1 |
| Completeness (%) | 99.3 (100) | 97.7 (98.4) |
| Mosaicity (°) | 0.7 | 1.2 |
| Redundancy | 11.0 | 4.4 |
| Rmerge (%)b | 5.9 (30.2) | 12.5 (41.2) |
| I/σ | 25.5 (8.7) | 8.9 (3.5) |
| Refinement statistics and model quality | ||
| Rfactor (%) | 22.2 | 25.6 |
| Rfree (%)c | 28.1 | 31.5 |
| No. of residues | 331 | 331+7 (peptide) |
| No. of waters | 125 | 36 |
| No. of glycerol molecules | 1 | – |
| No. of formate anions | 2 | – |
| R.m.s.d. bond lengthsd (Å) | 0.008 | 0.004 |
| R.m.s.d. bond angles (°) | 1.6 | 1.1 |
aValues in parentheses are for the highest resolution shell.
bRmerge = ΣhΣi|Ihi – <Ih>|/ΣhΣi Ihi.
cRfree estimation is based on 10% of data withheld for cross-validation.
dThe quality of the final model was assessed using the program PROCHECK (Laskowski et al., 1993).
A full data set for the t-CtBP/BARS:NAD(H):peptide ternary complex was collected at 3.1 Å resolution, on the same crystal form, at the ESRF synchrotron source (Table I). The ternary complex structure was refined to 3.1 Å resolution using the refined t-CtBP/BARS structure as a starting model. The final model contains 331 residues (15–345), 36 water molecules, one NAD(H), and one PIDLSKK molecule (Rfactor = 25.6 % and Rfree = 31.5%, respectively), with ideal stereochemical parameters (Table I) (Engh and Huber, 1991). Additionally, crystals of the t-CtBP/BARS:NAD(H):peptide ternary complex, grown in the P3221 space group (six independent t-CtBP/BARS molecules per asymmetric unit), were also analysed and refined against a 3.5 Å resolution data set, collected at the DESY-EMBL synchrotron source.
The CtBP/BARS fold
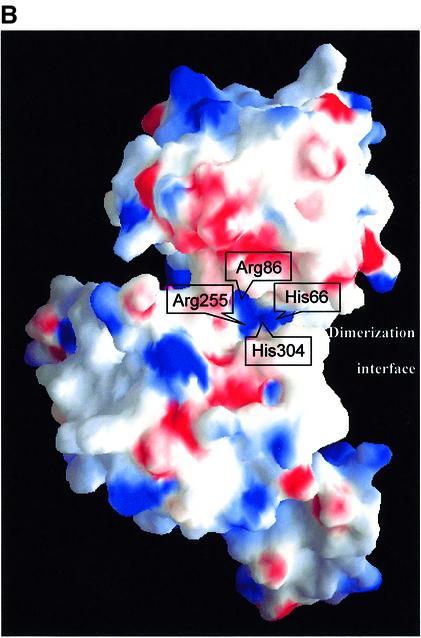
t-CtBP/BARS forms an elongated homodimer, consistent with gel filtration experiments on the expressed protein (our unpublished data), that displays a 2-fold crystallographic symmetry in the P6422 crystal form (Figure 2A; in view of the higher resolution achieved, unless specifically stated, the following data and discussion refer to this crystal form). Each t-CtBP/BARS subunit consists of two compact domains separated by a deep cleft; both of these are structural variants of the Rossmann dinucleotide-binding domain (Rao and Rossmann, 1973). In accordance with the literature on enzymes with similar structures (Schuller et al., 1995; Dengler et al., 1997; Kumar et al., 2002), the two domains are referred to as the nucleotide-binding (residues 1–112 and 309–350) and the substrate-binding (residues 113–308) domains. Pairing of two nucleotide-binding domains builds the central core of the dimer, whereas the two substrate-binding domains are at opposite poles of the assembled dimer (Figure 2A). A mainly hydrophobic region builds the dimer association interface, which covers ∼3135 Å2 on each monomer (Figure 2B). The main dimer packing interactions are based on antiparallel pairing of the αA helices in the two subunits, on swapping of the αB–loop–αC motif (Bennett et al., 1995), on contacts along the βF–βG loop and on the βG–α5 region (Figures 1 and 2A).
Fig. 2. (A) Ribbon diagram of the t-CtBP/BARS dimer. The substrate- and the nucleotide-binding domains of each subunit are shown in similar green and red shades, respectively, with the swapping residues 132–154 in blue. Subunit A is in lighter shades than subunit B. Secondary structure elements are indicated: primed elements belong to the t-CtBP/BARS B subunit. A 2-fold crystallographic axis is shown between the two monomers. The bound NAD(H) and PIDLSKK peptide molecules are shown in ball-and-stick representations (black and magenta, respectively). This figure was prepared with the programs MOLSCRIPT (Kraulis, 1991) and Raster3D (Merritt and Murphy, 1994). (B) Electrostatic surface representation of the t-CtBP/BARS subunit. Access to the tunnel lined by residues His66, Arg86, Arg255 and His304 is indicated. The protein structure is rotated by ∼70° around the vertical axis relative to (A). This figure was generated using GRASP (Nicholls et al., 1991).
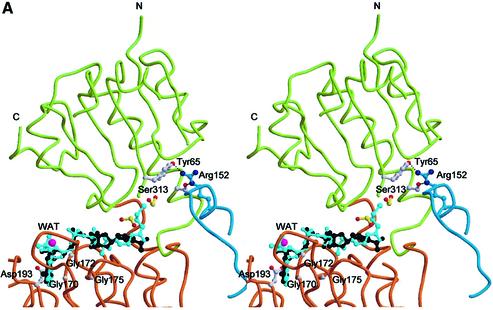
Within each nucleotide-binding domain, the typical G/AxGxxG(17x)D consensus motif is located in the Gly170–Asp193 stretch, among the βA, αD and βB secondary structure elements, where a tightly bound NAD(H) molecule is found (Figures 1 and 3A). Since dinucleotides were not present in the crystallization solutions, the bound NAD(H) is likely to be the result of specific uploading during t-CtBP/BARS expression/purification. Our structural results for the CtBP/BARS:NAD(H) binary complex are in good general agreement with those reported very recently on the CtBP minimal dehydrogenase domain (CtBP-MDD, residues 28–353, PDB code 1MX3) (Kumar et al., 2002), for which an r.m.s.d. of 0.3 Å was calculated in a structural comparison over 326 Cα pairs (t-CtBP/BARS 16–341, CtBP-MDD 27–352). Significant differences are only present in the dinucleotide-binding cavity, where the bound dinucleotide molecule shows a staggered orientation of the nicotinamide carboxamide group relative to the pyridine ring in CtBP/BARS that was not observed in CtBP-MDD.
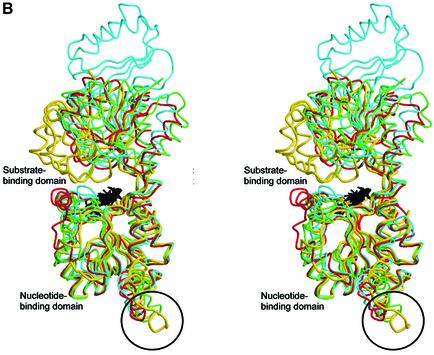
Fig. 3. (A) Stereo view of the cofactor-binding site. The substrate- and nucleotide-binding domains are colour coded as in Figure 2A. The bound NAD(H) and formate molecules are in black and yellow, respectively. The modelled CoA molecule is shown in light blue. Residues 140–154 from t-CtBP/BARS subunit B are shown in blue. Residues belonging to subunits A and B are shown in white and blue, respectively. A water molecule, close to the adenine ribose, is shown in magenta. (B) Structural comparisons of t-CtBP/BARS versus dehydrogenases. Stereo view of the structural superposition of the monomeric subunit from t-CtBP/BARS (yellow), d-lactate dehydrogenase (green, PDB-code 2DLD), d-2-hydroxyisocaproate dehydrogenase (red, PDB-code 1DXY) and d-3-phosphoglycerate dehydrogenase (light blue, PDB-code 1PSD). Bound NAD molecules are shown in black. The unique conformation of the t-CtBP/BARS dimerization residues 140–154 is highlighted by a circle.
Structural related proteins and dinucleotide binding
Overall, the tertiary and quaternary structures and the NAD(H) binding mode of t-CtBP/BARS are very similar to those of the d-stereoisomer-specific NAD-dependent dehydrogenases (Goldberg et al., 1994; Lamzin et al., 1994; Schuller et al., 1995; Stoll et al., 1996; Dengler et al., 1997) with which t-CtBP/BARS shares <20% sequence identity (Figure 1). The structural relationships extend not only to a good conservation of secondary structure elements (Figures 1 and 3B), but also to an excellent match of the dehydrogenase active site, the His/Asp/Arg triad (His304, Glu284, Arg255 in t-CtBP/BARS). Nevertheless, the dehydrogenase activity displayed by CtBP so far has only been modest (Kumar et al., 2002; Marmorstein, 2002; Zhang et al., 2002).
Protein backbone structural comparisons show that t-CtBP/BARS adopts a ‘closed’ conformation, where the substrate- and nucleotide-binding domains are in close contact relative to the overall tertiary structure of the NAD-dependent dehydrogenases (Figure 3B). This t-CtBP/BARS closed conformation, which tightly embraces the dinucleotide molecule, is triggered by specific interactions at the dimerization interface, where residues 140–154, linking the αB and αC helices of the B subunit, come in close contact with the β1–α1 and β3–α3 loops of the substrate-binding domain of the A subunit. This interaction is stabilized mainly by van der Waals contacts and by inter-subunit hydrogen bonds [residues Arg(B)152–Tyr(A)65 and Arg(B)152– Ser(A)313] (Figure 3A). These contacts are absent in the structurally homologous NAD-dependent dehydrogenases, with the exception of holo-formate dehydrogenase (FDH) (Lamzin et al., 1994), where loop 9–52, deleted in other dehydrogenases and in CtBP/BARS (Figure 1), provides an interaction at the dimer interface that is topologically different from, but structurally comparable with, that observed in t-CtBP/BARS.
In the t-CtBP/BARS:NAD(H) binary complex, the dinucleotide molecule binds in a conformation consistent with several previous observations of NAD(H) binding to dehydrogenases (Carugo and Argos, 1997) (Figure 3A). The staggered orientation (∼45°) of the nicotinamide carboxamide group relative to the pyridine ring suggests that the bound dinucleotide may be in its reduced form, different from that reported for the CtBP-MDD crystal structure, which displays the oxidized NAD+ coenzyme (Kumar et al., 2002). Binding of NAD(H) to t-CtBP/BARS and the likely occurrence of the reduced dinucleotide form are fully in keeping with recent results on NAD(H) regulation of CtBP transcription co-repression activity and binding to its cellular protein partners (Zhang et al., 2002).
Analysis of the NAD(H) binding mode in t-CtBP/BARS highlights the functional role of two specific residue sites. On one hand, residue Asp193 (Figure 3A), which is hydrogen bonded to the adenine ribose O2′ and O3′ atoms, selects against binding of NADP(H) by electrostatic repulsion exerted on the dinucleotide 2′-phosphate group. On the other, the positive charges of residues Arg255 and His304 (Figure 2B), which fall close to the nicotinamide C4 redox centre, appear to favour binding of the reduced, neutral coenzyme (NADH) over NAD+. Both structural features have functional implications, and are in support of the biochemical data showing that NADP(H) is virtually not effective on CtBP co-repressor activity, and that NADH/NAD+ selectively promotes CtBP co-repression; however, the differential roles of NADH versus NAD+ are still under debate (Kumar et al., 2002; Zhang et al., 2002).
As a first conclusion, we propose that NAD(H) binding to t-CtBP/BARS is instrumental in promoting the stabilization of the closed form of the protein observed in the crystals. Attainment of this closed form is required for the promotion of the stabilization of the tight protein dimer through the domain-swapping mechanism described above.
Peptide binding (co-repression activity)
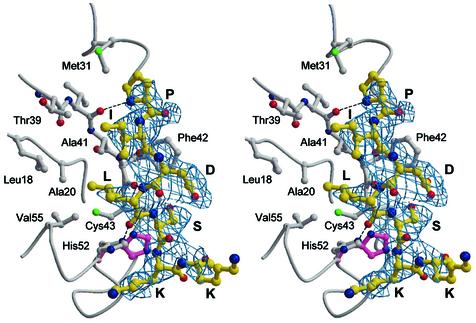
To shed more light on the structural bases of the CtBP interactions with cellular and viral transcriptional repressors (Zhang et al., 2002), crystallographic evidence was sought on the location of the PXDLS consensus peptide recognition site within t-CtBP/BARS. The crystal structure of the t-CtBP/BARS:NAD(H):PIDLSKK ternary complex (in the P6422 crystal form) reveals that the consensus peptide binds in an extended conformation at a surface cleft in the N-terminal part of the substrate-binding domain (Figure 2A). Binding of the PIDLSKK peptide is driven by docking of its isoleucine and leucine side chains into a hydrophobic surface groove, between the β1 and β2 strands, and the α2 helix. This cleft is lined by Leu18, Ala20, Met31, Thr39, Ala41, Phe42, Cys43, His52 and Val55 (Figure 4), which are also fully conserved residues in both CtBP1 and CtBP2 (Figure 1). Further stabilizing contacts involve three main chain–main chain hydrogen bonds between the peptide and the protein at residues Val40 and Phe42 (Figure 4).
Fig. 4. Consensus peptide-binding site. Stereo view of the consensus PIDLSKK peptide (yellow) bound to the N-terminal region of t-CtBP/BARS. Hydrogen bonds (dotted lines), and an induced conformational transition occurring at the His52 residue (magenta, in the absence of the consensus peptide) are highlighted. The 2Fo – Fc electron density map at 3.1 Å resolution is shown as a blue grid.
Although at a lower resolution that was imposed by crystal quality, binding of the PIDLSKK peptide was confirmed by crystallographic analysis of the t-CtBP/BARS ternary complex in the P3221 crystal form. In particular, the consensus peptide is located at the same surface cleft in the N-terminal part of the substrate-binding domain in four of the six independent asymmetric unit t-CtBP/BARS molecules; the other two t-CtBP/BARS molecules are involved in crystal contacts obstructing the peptide-binding cleft. Thus, the crystallographic analysis on the P3221 crystal form confirms that binding of the consensus peptide to the identified cleft occurs independently of crystal lattice packing considerations.
Structural comparisons between the binary and ternary t-CtBP/BARS complexes show that besides some local residue adaptations to host the peptide, binding of the consensus peptide is not associated with any significant tertiary/quaternary structure modification. Furthermore, we note that the consensus peptide-binding site has no direct contact with the NAD(H)-binding region (they are ∼25 Å apart). Our structural results are, therefore, in agreement with biochemical data that have shown that residues 1–100 of mouse CtBP are sufficient and necessary for binding Net, a transcription repressor from the Ets family (Criqui-Filipe et al., 1999), and that mutation of the CtBP nucleotide-binding site maintains the basal interaction with E1A (Zhang et al., 2002). Both Net and E1A bind CtBP through a PXDLS motif.
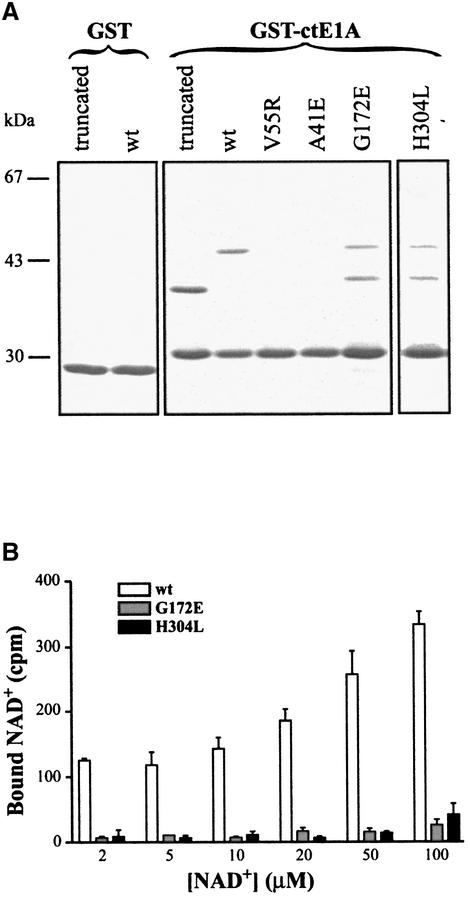
To provide independent evidence of the consensus peptide-binding site, two residues (Ala41 and Val55) building up the peptide recognition cleft identified above (Figure 4) were individually mutated. The ability of the mutated CtBP/BARS to bind the viral E1A protein was then measured in pull-down experiments using the E1A 44 C-terminal-amino acids, where the PXDLS sequence is located, fused to GST (GST–ctE1A). Both full-length and t-CtBP/BARS wild-type proteins were pulled-down efficiently by GST–ctE1A. In contrast, the full-length Ala41→Glu and Val55→Arg mutants did not bind GST–ctE1A (Figure 5A). We next examined whether abolishing NAD(H) binding would also affect the binding of the PXDLS sequence. To test this, we mutated residues in the cofactor-binding site (Gly172→Glu and His304→Leu). While preventing the NAD(H) binding (Figure 5B), these mutations did not affect CtBP/BARS binding to GST–ctE1A (Figure 5A). Therefore, collectively, the structural and mutational data reported here indicate that the consensus peptide-binding cleft and the NAD(H)-binding site are physically separated in the protein structure. It should be noted that this observation does not fully agree with the conclusions reached by Kumar et al. (2002) that were based on multiple site mutations at the dinucleotide-binding site of CtBP-MDD, where a close structural location of the two binding sites was suggested.
Fig. 5. (A) Binding to the E1A C-terminus. GST or GST–ctE1A were linked to glutathione–Sepharose beads and then incubated with t-CtBP/BARS (truncated), full-length wild-type (wt), full-length Ala41→Glu (A41E), full-length Val55→Arg (V55R), full-length Gly172→Glu (G172E) and full-length His304→Leu (H304L). After washing, proteins retained by the beads were analysed by SDS–PAGE and Coomassie blue staining. Migration of molecular weight standards is indicated on the left. G172E and H304L are partly proteolysed samples. (B) NAD binding. Wild-type (wt), Gly172→Glu (G172E) and His304→Leu (H304L) were incubated with the indicated concentrations of NAD+, as described in Materials and methods. The amounts of NAD+ bound to the proteins were quantified using an Instant Imager. Bars indicate standard deviations.
Based on evidence provided by our crystallographic investigation, by the mutational data and by previous solution studies on CtBP activity and regulation (Boyd et al., 1993; Schaeper et al., 1995; Turner and Crossley, 1998, 2001; Criqui-Filipe et al., 1999; Zhang et al., 2002), we propose that CtBP/BARS co-repression activity relies on the ability of the protein to switch to a closed conformation upon NAD(H) binding (as opposed to an open conformation in the absence of the dinucleotide, where the two domains are more separated). Such a conformational switch would yield a stable, tight, dimeric assembly where the PXDLS peptide recognition sites (at opposite poles of the dimer) are positioned/oriented properly for the promotion of multimeric protein complex formation (with DNA-binding proteins, histone deacetylases and/or other co-repressors; Turner and Crossley, 2001).
Acyl-CoA binding (acyltransferase activity)
To analyse whether the observed closed dimeric conformation is also compatible with CtBP/BARS acyltransferase activity in Golgi membranes, we modelled an acyl-CoA molecule bound to the protein interdomain cleft, based on the common ADP molecular framework shared by NAD(H) and CoA (Figure 3A). The hypothesis that NAD(H) and acyl-CoA share the same binding site is supported by our recent studies showing that CtBP/BARS binds palmitoyl-CoA and oleoyl-CoA with affinities of 5.0 and 2.5 µM, respectively, and that NADH and NAD+ are effective competitors of palmitoyl-CoA and oleoyl-CoA binding to both full-length and t-CtBP/BARS, with IC50s in the 30–100 nM range (our unpublished data). Modelling of the CoA molecule at the NAD(H) site shows an obvious good match of the common molecular structures, including the probable location of the CoA 3′-phosphate group, which would replace a water molecule that is hydrogen bonded to the NAD(H) O3′ atom (Figure 3A). Room to accommodate the acyl-CoA β-mercaptoethylamine moiety is present near the NAD(H) nicotinamide group, where an internal cavity hosts two formate anions (from the crystallization medium; Nardini et al., 2002).
According to the above model, the thioester bond connecting the aliphatic acyl tail to CoA (e.g. in a bound palmitoyl-CoA) would match one of the formate sites, at ∼10 Å from the protein surface [in the direction of Arg(B)152] (Figure 3A). However, in the closed t-CtBP/BARS conformation, there is no room available next to the thioester bond site for the accommodation of the acyl-CoA aliphatic tail due to the intervening protein segment (comprised of the αB and αC helices) contributed by the opposing dimer subunit. Conversely, the binding of an acyl-CoA molecule with the C14/C20 aliphatic tail that is needed for membrane fission activity (Weigert et al., 1999) would disrupt the domain-swapping interaction that stabilizes the t-CtBP/BARS dimer association. Thus, the above considerations suggest that binding of acyl-CoA must be coupled with, and therefore induce, a markedly different and more open conformation of CtBP/BARS. Indeed, rigid body re-orientation of the substrate-binding domain upon ligation of the cofactor is a common feature found in the structurally homologous NAD-dependent dehydrogenases (Lamzin et al., 1994).
Interestingly, an open CtBP/BARS form is required for a substrate as large as LPA to approach the bound acyl-CoA, as is needed for the onset of the acyltransferase activity. A protein matrix tunnel connecting the NAD(H)/acyl-CoA-binding site to the solvent in t-CtBP/BARS may serve this purpose. The tunnel, which is partly detectable in the closed enzyme form (Figure 2B), is lined mainly by positively charged residues (His66, Arg86, Arg255 and His304), which would provide the necessary electrostatic environment to host the negatively charged LPA head group. However, in the closed NAD(H)-bound structure, residue Arg255 shields the acyl-CoA-binding site from the solvent, in the inner part of the tunnel. Thus LPA would be able to approach the cofactor-binding site only when the substrate- and nucleotide-binding domains move apart, as suggested for the CtBP/BARS acyl-CoA-bound form.
Conclusions
In this analysis, we have combined t-CtBP/BARS in a ternary complex including both NAD(H) and the PIDLSKK consensus peptide together with data on single site mutants that map to the consensus peptide cleft. This, along with cofactor-binding data and the consequent modelling, allows a rationalization of the mosaic of biochemical data on both the co-repression and acyltransferase activities of the protein. NAD(H) binding can promote the formation of the closed dimeric form of the protein, with the PXDLS consensus peptide-binding sites located at the two N-terminal regions (Figure 2A). Such dimeric assembly is instrumental in CtBP/BARS co-repressor action since it provides a stable and bivalent aggregation nucleus for multimeric protein complexes that can bridge repressors and their targets (Turner and Crossley, 2001). On the other hand, while possibly not fully preventing formation of the CtBP/BARS dimeric species, binding of acyl-CoA at the coenzyme interdomain cleft would definitely affect the αB–loop–αC motif-swapping and subunit interactions which have been shown here to lock the substrate- and nucleotide-binding domains in the closed form of CtBP/BARS. A substantial conformational transition, that can be triggered by acyl-CoA binding and that leads to an open structure, would render acyl-CoA accessible to the LPA substrate through a protein tunnel located at the substrate- and nucleotide-binding domain interface. We thus propose that NAD(H) and acyl-CoA binding are two mutually exclusive events that are structurally crucial for switching between the alternative functional roles of CtBP/BARS in transcription co-repression and membrane fission, respectively.
Materials and methods
Crystallization, data collection and processing
t-CtBP/BARS bearing a poly-histidine tag at its N-terminus was expressed in Escherichia coli as described previously (Spanò et al., 1999; Nardini et al., 2002). Crystallization experiments on t-CtBP/BARS were performed using the hanging drop vapour diffusion method. Bipyramidal-shaped crystals, with a typical size of 0.2 × 0.1 × 0.1 mm3, grew in a few days in a crystallization solution containing 1.8–2.1 M ammonium formate, 100 mM HEPES pH 7.5 (Nardini et al., 2002). The crystals belong to the space group P6422, with unit cell parameters of a = b = 88.7 Å, c = 163.0 Å, and with one molecule in the asymmetric unit. A native data set was collected at 2.3 Å resolution using synchrotron radiation (ID14-EH1 beamline, ESRF, Grenoble, France) (Table I).
Expression and purification of the Se-Met-substituted t-CtBP/BARS were performed as described previously (Nardini et al., 2002). The crystals of the Se-Met protein are isomorphous with those of wild type t-CtBP/BARS. Three-wavelength MAD data sets were collected at the DESY-EMBL synchrotron source (BW7A beamline, Hamburg, Germany) to a maximum resolution of 3.3 Å.
The ternary complex with the PIDLSKK peptide was obtained by soaking the t-CtBP/BARS crystals for ∼30 min in a stabilizing solution containing 5 mM peptide concentration. This procedure was used for both the P6422 t-CtBP/BARS crystal form and a second crystal form belonging to the P3221 space group (unit cell parameters a = b = 143.7 Å, c = 263.5 Å, six t-CtBP/BARS molecules per asymmetric unit) grown at 1.8–2.2 M ammonium sulfate, 200 mM NaCl, 100 mM cacodylate buffer pH 6.5.
The peptide itself was synthesized manually using the standard method of solid-phase peptide synthesis which followed the 9-fluorenylmethoxycarbonyl (Fmoc) strategy with minor modifications (Wellings and Atherton, 1997). Briefly, 100 mg of Rink AM deprotected resin (Novabiochem AG, Laufelfingen, Switzerland) was treated for 40 min at 40°C with a coupling reaction mixture containing five equivalents of the appropriate Fmoc-amino acid (Advanced Biotech Italia, Italy), 4.5 equivalents of O-(benzotriazol-1-yl)-1,1,3,3-tetramethyluroniumhexafluorophosphate (Advanced Biotech Italia, Italy) and five equivalents of N,N-diisopropylethylamine (Fluka Chemie AG, Buchs, Switzerland), at a final amino acid concentration of 0.2 M in anhydrous N-methylpyrrolidone (Biosolve Ltd, The Netherlands). All the synthesized compounds were purified by reverse-phase high-performance liquid chromatography (RP-HPLC), and the molecular weights finally confirmed by electrospray ion-trap mass spectrometry. The purification of individual compounds was obtained on a Shimadzu LC-9A preparative HPLC system equipped with a Waters C18 µBondapack column (19 × 300 mm).
As a result of the peptide soaking, the t-CtBP/BARS crystals (P6422 form) often cracked; however, the resulting fragments were still able to diffract at ∼3 Å resolution. A full data set was collected at 3.1 Å resolution using synchrotron radiation (ID14-EH2 beamline, ESRF, Grenoble, France) (Table I). Peptide soaking on the P3221 crystal form allowed a maximum resolution of 3.5 Å (BW7A beamline, EMBL-DESY, Hamburg, Germany). Co-crystallization of the t-CtBP/BARS:peptide complex regularly yielded crystals under the standard crystallization conditions, but their diffraction quality was lower (4 Å resolution).
All diffraction data were processed using DENZO, SCALEPACK (Otwinoski and Minor, 1997) and programmes from the CCP4 suite (CCP4, 1994).
Structure determination and refinement
The position of four of the seven expected Se-Met sites was determined using the Shake and Bake program (Weeks and Miller, 1999). MAD phases, based on the anomalous Se atom signal, were determined at 3.3 Å resolution using SOLVE (Terwilliger and Berendzen, 1999), with a figure of merit of 0.6. The electron density map was remarkably improved by solvent flattening and phase extension to 2.5 Å resolution using the DM program (CCP4, 1994). The resulting electron density map was of good quality, clearly displaying almost all the main molecular features and residues. The protein model was traced using the O program (Jones et al., 1991). Nearly all of the complete polypeptide chain could be fitted in full agreement with the amino acid sequence. The molecular model was then refined at the maximum resolution (2.3 Å) using the CNS program (Brünger et al., 1998). The final model contains 331 residues, 125 water molecules, one NAD(H), one glycerol and two formate molecules (Rfactor = 22.2% and Rfree = 28.1%, respectively), with ideal stereochemical parameters (Table I) (Engh and Huber, 1991). No electron density was observed for residues 1–14 and 346–350. Residue 27 has been modelled as S-hydroxycysteine.
The t-CtBP/BARS:NAD(H):peptide ternary complex was refined to 3.1 Å resolution by the CNS program (Brünger et al., 1998) using the refined t-CtBP/BARS structure as starting model. After partial refinement, 2Fo – Fc electron density maps showed detailed side chain features that allowed unambiguous modelling of the peptide structure for the first five residues (PIDLS). Conversely, poor density was available for the two C-terminal lysine residues of the peptide. The final model contains 331 residues (15–345), 36 water molecules, one NAD(H) and one PIDLSKK molecule (Rfactor = 25.6% and Rfree = 31.5%, respectively), with ideal stereochemical parameters (Table I) (Engh and Huber, 1991). A very similar procedure was followed to analyse peptide binding to the P3221 crystal form.
t-CtBP/BARS coordinates and structure factors have been deposited with the Protein Data Bank (Berman et al., 2000) with accession codes 1hku and r1hkusf for the binary complex, and 1hl3 and r1hl3sf for the ternary complex, respectively.
Expression and purification of mutated proteins
GST–ctE1A DNA expressing the last 44 amino acids of E1A-243R fused to GST was a gift from Dr C.Svensson (Sollerbrant et al., 1996). GST–ctE1A was expressed and purified as described in Boyd et al. (1993). Mutations of CtBP/BARS were generated from the pET11d-His-BARS plasmid with the QuikChange Site-Directed Mutagenesis Kit (Stratagene) by using the following oligonucleotides (only forward primers are indicated): Val55→Arg, GGAGATCCATGAGAAGCGA CTGAATGAGGCAGTGG; Ala41→Glu, GGATGTGGCCACAGTAG AATTCTGTGATGCGCAGTC; Gly172→Glu, CATCATTGGACTA GAGCGTGTGGGCCAGG; His304→Leu, CATCTGCACCCCCCTTG CTGCATGGTACAG. Poly-histidine-tagged CtBP/BARS and mutants were expressed and purified as described previously (Nardini et al., 2002).
GST pull-down assay
A 10 µg aliquot of GST–ctE1A or GST was attached to glutathione–Sepharose beads by incubating for 1 h at 4°C in 50 mM HEPES pH 7.0, 250 mM NaCl, 0.5% NP-40. A 5 µg aliquot of wild-type or mutated CtBP/BARS was then added to the beads in the same buffer and incubated for 1 h at 4°C. After extensive washing, proteins were eluted by boiling in Laemmli sample buffer, and then run on a 10% SDS–polyacrylamide gel and revealed by Coomassie blue.
NAD binding experiments
A 5 µg aliquot of proteins was spotted onto nitrocellulose filters by a slot-blot apparatus (Bio-Rad). Filters were then incubated at room temperature for 2 h in 1.5 ml of 20 mM HEPES pH 7.4, 0.1% Triton X-100 (HT buffer) in the presence of different concentrations of NAD+ (specific activity: 5 µCi/µmol). Filters were washed three times for 10 min with 2 ml of HT buffer, dried and analysed using a Packard Instant Imager.
Acknowledgments
Acknowledgements
We thank the staff of the BW7A beamline at EMBL-DESY, Hamburg (Germany), and the staff of the ID14-EH1 and ID14-EH2 beamlines at ESRF, Grenoble (France) for data collection facilities and assistance. We are grateful to D.Bordo and A.Spallarossa for help during the MAD data collection. This work was supported by grants from the Italian Ministry of Education MIUR-FIRB, from the Italian Space Agency (I/R/294/02), from the Italian Association for Cancer Research (AIRC, Milan, Italy), the Italian National Research Council (CNR, Rome, Italy) Progetto Finalizzato ‘Biotecnologie’ ctr.n. 01.00027.PF49, and Progetto Speciale ‘Genetica Molecolare’. M.B. is grateful to the G.Gaslini Institute and the CBA/CINRO for continuous support.
References
- Bennett M.J., Schlunegger,M.P. and Eisenberg,D. (1995) 3D Domain swapping: a mechanism for oligomer assembly. Protein Sci., 4, 2455–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H.M., Westbrook,J., Fenz,Z., Gilliland,G., Bhat,T.N., Weissig,H., Shindyalov,I.N. and Bourne,P.E. (2000) The Protein Data Bank. Nucleic Acids Res., 28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J.M., Subramanian,T., Schaeper,U., La Regina,M., Bayley,S. and Chinnadurai,G. (1993) A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J., 12, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- Carugo O. and Argos,P. (1997) NADP-dependent enzymes. I: conserved stereochemistry of cofactor binding. Proteins, 28, 10–28. [DOI] [PubMed] [Google Scholar]
- CCP4. (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. [DOI] [PubMed] [Google Scholar]
- Corda D., Hidalgo Carcedo,C., Bonazzi,M., Luini,A. and Spanò,S. (2002) Molecular aspects of membrane fission in the secretory pathway. Cell. Mol. Life Sci., 59, 1819–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criqui-Filipe P., Ducret,C., Maira,S.M. and Wasylyk,B. (1999) Net, a negative Ras-switchable TCF, contains a second inhibition domain, the CID, that mediates repression through interactions with CtBP and de-acetylation. EMBO J., 18, 3392–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis M.A. et al. (1994) Stimulation of endogenous ADP-ribosylation by brefeldin A. Proc. Natl Acad. Sci. USA, 91, 1114–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler U., Niefind,K., Kieß,M. and Schomburg,D. (1997) Crystal structure of a ternary complex of d-2-hydroxyisocaproate dehydrogenase from Lactobacillus casei, NAD+ and 2-oxoisocaproate at 1.9 Å resolution. J. Mol. Biol., 267, 640–660. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Silletta,M.G., De Matteis,M.A., Braca,A., Colanzi,A., Pawlak,D., Luini,A. and Corda,D. (1995) Evidence that the 50 kDa substrate of brefeldin A-dependent ADP-ribosylation binds GTP and is modulated by the G-protein βγ subunit complex. Proc. Natl Acad. Sci. USA, 92, 7065–7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh R.A. and Huber,R. (1991) Accurate bond and angle parameters for X-ray protein structure refinement. Acta Crystallogr. A, 47, 392–400. [Google Scholar]
- Goldberg J.D., Yoshida,T. and Brick,P. (1994) Crystal structure of a NAD-dependent d-glycerate dehydrogenase at 2.4 Å resolution. J. Mol. Biol., 236, 1123–1140. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou,J.Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kraulis P.J. (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 946–950. [Google Scholar]
- Kumar V., Carlson,J.E, Ohgi,K.A., Edwards,T.A., Rose,D.W., Escalante,C.R., Rosenfeld,M.G. and Aggarwal,A.K. (2002) Transcription corepressor CtBP is a NAD+-regulated dehydrogenase. Mol. Cell, 10, 857–869. [DOI] [PubMed] [Google Scholar]
- Lamzin V.S., Dauter,Z., Popov,V.O., Harutyunyan,E.H. and Wilson,K.S. (1994) High resolution structure of holo and apo formate dehydrogenase. J. Mol. Biol., 236, 759–785. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., MacArthur,M.W., Moss,D.S. and Thornton,J.M. (1993) PROCHECK, a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- Marmorstein R. (2002) Dehydrogenases, NAD and transcription. What’s the connection? Structure, 10, 1465–1466. [DOI] [PubMed] [Google Scholar]
- Merritt E.A. and Murphy,M.E.P. (1994) Raster3D version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr. D, 50, 869–873. [DOI] [PubMed] [Google Scholar]
- Mironov A. et al. (1997) Role of NAD+ and ADP-ribosylation in the maintenance of the Golgi structure. J. Cell Biol., 139, 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini M., Spanò,S., Cericola,C., Pesce,A., Damonte,G., Luini,A, Corda,D. and Bolognesi,M. (2002) Crystallization and preliminary X-ray diffraction analysis of brefeldin A-ADP ribosylated substrate (BARS). Acta Crystallogr. D, 58, 1068–1070. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp,K.A. and Honig,B. (1991) Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins, 11, 281–296. [DOI] [PubMed] [Google Scholar]
- Otwinoski Z. and Minor,W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol., 276, 307–326. [DOI] [PubMed] [Google Scholar]
- Rao S.T. and Rossmann,M.G. (1973) Comparison of super-secondary structures in proteins. J. Mol. Biol., 76, 241–256. [DOI] [PubMed] [Google Scholar]
- Schaeper U., Boyd,J.M., Verma,S., Uhlmann,E., Subramanian,T. and Chinnadurai,G. (1995) Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc. Natl Acad. Sci. USA, 92, 10467–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller D.J., Grant,G.A. and Banaszak,L.J. (1995) The allosteric ligand site in the Vmax-type cooperative enzyme phosphoglycerate dehydrogenase. Nat. Struct. Biol., 2, 69–76. [DOI] [PubMed] [Google Scholar]
- Sollerbrant K., Chinnadurai,G., Svensson,C. (1996) The CtBP binding domain in the adenovirus E1A protein controls CR1-dependent transactivation. Nucleic Acids Res., 24, 2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanò S. et al. (1999) Molecular cloning and functional characterization of brefeldin A-ADP-ribosylated substrate. J. Biol. Chem., 274, 17705–17710. [DOI] [PubMed] [Google Scholar]
- Stoll V.S, Kimber,M.S. and Pai,E.F. (1996) Insights into substrate binding by d-2-ketoacid dehydrogenases from the structure of Lactobacillus pentosusd-lactate dehydrogenase. Structure, 4, 437–447. [DOI] [PubMed] [Google Scholar]
- Terwilliger T.C. and Berendzen,J. (1999) Automated structure solution for MIR and MAD. Acta Crystallogr. D, 55, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. and Crossley,M. (1998) Cloning and characterization of mCtBP2, a co-repressor that associates with basic Krüppel-like factor and other mammalian transcriptional regulators. EMBO J., 17, 5129–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. and Crossley,M. (2001) The CtBP family: enigmatic and enzymatic transcriptional co-repressors. BioEssays, 23, 683–690. [DOI] [PubMed] [Google Scholar]
- Weeks C.M. and Miller,R. (1999) Optimizing Shake-and-Bake for proteins. Acta Crystallogr. D, 55, 492–500. [DOI] [PubMed] [Google Scholar]
- Weigert R. et al. (1999) CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature, 402, 429–433. [DOI] [PubMed] [Google Scholar]
- Wellings D.A. and Atherton,E. (1997) Standard Fmoc protocols. Methods Enzymol., 289, 44–67. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Piston,D.W. and Goodman,R.H. (2002) Regulation of corepressor function by nuclear NADH. Science, 295, 1895–1897. [DOI] [PubMed] [Google Scholar]