Abstract
The adaptor molecule Disabled-2 (Dab2) has been shown to link cell surface receptors to downstream signaling pathways. Using a small-pool cDNA screening strategy, we identify that the N-terminal domain of Dab2 interacts with Dishevelled-3 (Dvl-3), a signaling mediator of the Wnt pathway. Ectopic expression of Dab2 in NIH-3T3 mouse fibroblasts attenuates canonical Wnt/β-catenin-mediated signaling, including accumulation of β-catenin, activation of β-catenin/T-cell-specific factor/lymphoid enhancer-binding factor 1-dependent reporter constructs, and endogenous cyclin D1 induction. Wnt stimulation leads to a time-dependent dissociation of endogenous Dab2–Dvl-3 and Dvl-3–axin interactions in NIH-3T3 cells, while Dab2 overexpression leads to maintenance of Dab2–Dvl-3 association and subsequent loss of Dvl-3–axin interactions. In addition, we find that Dab2 can associate with axin in vitro and stabilize axin expression in vivo. Mouse embryo fibroblasts which lack Dab2 exhibit constitutive Wnt signaling as evidenced by increased levels of nuclear β-catenin and cyclin D1 protein levels. Based on these results, we propose that Dab2 functions as a negative regulator of canonical Wnt signaling by stabilizing the β-catenin degradation complex, which may contribute to its proposed role as a tumor suppressor.
Keywords: axin/β-catenin/Disabled 2 (Dab2)/Dishevelled-3 (Dvl-3)/Wnt
Introduction
The Wnt family of secreted glycoproteins play key roles in embryonic development, regulating cell proliferation, motility and cell fate (Cadigan and Nusse, 1997; Dale, 1998) and in adult tissues where aberrant Wnt signaling has been shown to contribute to a variety of human cancers (Polakis, 2000; Bienz and Clevers, 2000). Wnt ligand binding to its cognate receptors can stimulate several distinct signaling pathways including the canonical Wnt–β-catenin and non-canonical planar cell polarity– convergent extension (PCP–CE) pathways. Required for activation of both of these pathways is the common mediator dishevelled (Dvl) which interprets signals from various receptors and transmits them to different effector molecules. In the canonical pathway, activation of the frizzled and LRP5/6 co-receptors results in recruitment of Dvl which relays the signal to a complex composed of adenomatous polyposis coli (APC), axin, glycogen synthase kinase 3β (GSK3β) and β-catenin (Polakis, 2000; Wharton, 2003). In the absence of Wnt signaling, β-catenin is phosphorylated on N-terminal serine and threonine residues, targeting it for degradation by the ubiquitin–proteasome pathway (Kitagawa et al., 1999). In the presence of Wnt, association of Dvl with axin prevents GSK3β from phosphorylating β-catenin, leading to stabilization of β-catenin and its translocation to the nucleus. In the nucleus, β-catenin complexes with members of the T-cell-specific factor/lymphoid enhancer-binding factor 1 (TCF/LEF-1) transcription factor family to regulate target genes which include c-myc and cyclin D1 (He et al., 1998; Shtutman et al., 1999). PCP signaling, responsible for proper orientation of photoreceptor cells in Drosophila, and vertebrate CE, responsible for proper cell movement during gastrulation, both involve activation of the c-Jun N-terminal kinase (JNK) pathway through Dvl (Boutros et al., 1998; Yamanaka et al., 2002).
The vertebrate Dvl family includes three homologs, Dvl-1, 2 and 3, which exhibit overlapping patterns of expression and highly conserved domain structure (Sussman et al., 1994; Klingensmith et al., 1996; Tsang et al., 1996; Semenov and Snyder, 1997). The N-terminal DIX domain of Dvl contains sequence also found in axin and mediates interactions between Dvl molecules and axin (Kishida et al., 1999). The central PDZ domain mediates the interaction of Dvl with many different proteins including axin, Frat1, Idax, Nkd, Stbm and Dapper (reviewed in Wharton, 2003). Additionally, two kinases which have been shown to phosphorylate Dvl, casein kinase Iε (CKIε) and casein kinase II (CKII), interact with Dvl through the PDZ domain (Willert et al., 1997; Peters et al., 1999; Sakanaka et al., 1999). The C-terminal DEP domain of Dvl which binds to Prickle (Tree et al., 2002) has been shown to be required for activation of the JNK pathway (Li et al., 1999b; Moriguchi et al., 1999) and for establishment of planar cell polarity in Drosophila (Boutros et al., 1998).
Disabled-2 (Dab2) or DOC-2 is a putative tumor suppressor initially identified in a screen for transcripts downregulated in ovarian carcinoma (Mok et al., 1994) and subsequently found to be decreased in breast, prostate and metastatic pancreatic carcinoma (Schwahn and Medina, 1998; Tseng et al., 1998; Huang et al., 2001). Dab2 contains an N-terminal phosphotyrosine-binding (PTB)/-interacting (PID) domain and a proline-rich C-terminal domain (PRD) suggestive of a role as an adaptor molecule (Pawson and Scott, 1997). The C-terminal PRD has been shown to bind to Grb2, potentially inhibiting mitogenic stimulation via the Ras pathway (Xu et al., 1998; Zhou and Hsieh, 2001). Dab-2 was also shown to associate directly with clathrin and the clathrin adaptor protein AP-2 and thus to localize to clathrin-coated pits (Morris and Cooper, 2001; Mishra and Traub, 2002). Dab2 also binds the non-muscle myosin, myosin VI, which is also implicated in endocytosis (Morris et al., 2002a). Indeed, genetic ablation of Dab2 reveals a requirement for protein transport in kidney cells (Morris et al., 2002b; L.Traub, personal communication). In addition, we recently have shown that Dab2 is found in association with the transforming growth factor-β (TGF-β) type I and II receptors while directly binding to the TGF-β signaling intermediates Smad2 and Smad3 through the PID domain (Hocevar et al., 2001). Dab2 thus appears to function in several receptor-mediated signaling pathways.
Using a small-pool cDNA screening strategy to identify other binding partners for Dab2, we report that the PID domain of Dab2 interacts with the DEP domain of Dvl-3. Ectopic expression of Dab2 in NIH-3T3 cells antagonized canonical Wnt/β-catenin-mediated cellular signaling while potentiating non-canonical Wnt-mediated JNK activation. Dab2 was found to associate directly with axin and stabilize the interaction of axin with β-catenin, resulting in increased β-catenin degradation. We further show that mouse embryonic fibroblasts (MEFs) which lack Dab2 exhibit elevated levels of nuclear β-catenin, cyclin D1 protein and promoter activity as compared with control fibroblasts. On the basis of these results, we propose that Dab2 serves as a negative regulator of canonical Wnt/ β-catenin-induced signaling in mammalian cells.
Results
Identification of Dvl-3 as a Dab2-interacting protein
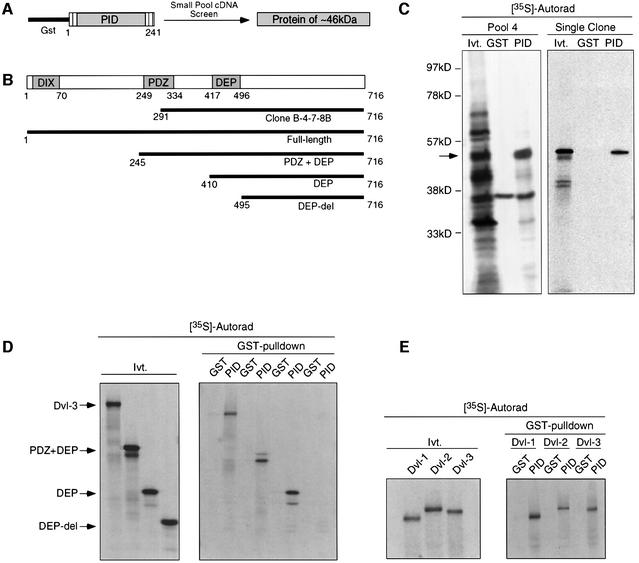
To identify proteins that interact with Dab2, we performed small-pool cDNA screening (Lustig et al., 1997) using a GST fusion protein containing the PID domain of Dab2 (Figure 1A). Using this approach, we identified an ∼46 kDa protein which specifically interacted with GST–PID and not the GST control (Figure 1C). The plasmid encoding this protein contained a 1277 bp cDNA which was found to encode the C-terminal 425 amino acids of Dvl-3 (Figure 1B). To confirm these results, binding of full-length in vitro translated Dvl-3 and various deletion constructs to GST or GST–PID beads was investigated (Figure 1B and D). While deletion of the DIX and PDZ domain of Dvl-3 did not affect binding to GST–PID, removal of the DEP domain resulted in loss of binding, demonstrating that the DEP domain is required for interaction with the PID domain of Dab2 (Figure 1D). Since the DEP domain is highly conserved among the mammalian Dvl isoforms, we investigated whether the PID domain of Dab2 could also bind to Dvl-1 and Dvl-2. Full-length in vitro translated Dvl-1, Dvl-2 and Dvl-3 proteins were subjected to GST pull-down assay with GST or GST–PID beads. The results of the binding assay reveal that the PID domain of Dab2 can interact with all three mammalian Dvl isoforms in vitro (Figure 1E).
Fig. 1. Identification of Dvl-3 as a Dab2-interacting protein. (A) Schematic representation of the small-pool cDNA screening strategy using GST–PID. (B) Schematic representation of the domain structure of Dvl-3. The amino acid residues encoded by the 46 kDa GST–PID-interacting protein as well as constructs containing various domains of Dvl-3 are as indicated. (C) Pool 4 consisting of ∼100 cDNA clones was subjected to in vitro transcription/translation in the presence of [35S]methionine, followed by incubation with control GST or GST–PID beads. Following extensive washing, GST–PID-interacting proteins were resolved by SDS–PAGE and visualized by autoradiography. An aliquot of the input in vitro transcription/translation reaction (Ivt) is included as a control. The specific GST–PID-interacting protein is marked by an arrow (left panel). Following several rounds of dilution, a single clone was obtained and analysis performed as above (right panel). (D) Full-length and various deletion constructs of Dvl-3 were subjected to in vitro transcription/translation utilizing [35S]methionine and analyzed for interaction with GST–PID as described above. The positions of the various reaction products are indicated by the arrows. (E) Constructs comprising Dvl-1, Dvl-2 and Dvl-3 were subjected to in vitro transcription/translation and analyzed for interaction with GST–PID as described above.
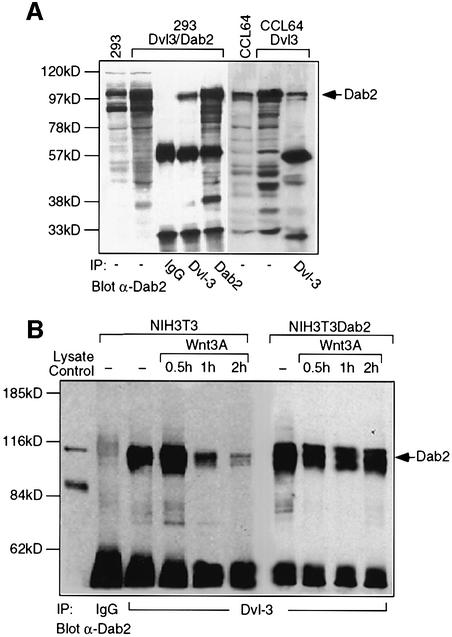
To demonstrate that Dab2 and Dvl-3 interact in vivo, Dab2 and Dvl-3 were overexpressed in HEK 293 cells followed by immunoprecipitation with Dvl-3 or Dab2 antibodies. Western analysis with an α-Dab2 antibody demonstrates that Dab2 can be co-immunoprecipitated by the Dvl-3 antibody but not by control IgG (Figure 2A). Similarly, when overexpressed Dvl-3 is immunoprecipitated from Mv1Lu mink lung epithelial (CCL64) cells, Dab2 can be found in the Dvl-3 immunocomplex (Figure 2A). We next investigated whether endogenous Dvl-3 and Dab2 could interact in NIH-3T3 cells. In unstimulated NIH-3T3 cells, endogenous Dvl-3 and Dab2 interact, as demonstrated by the ability of an α-Dvl-3 antibody to co-immunoprecipitate Dab2 (Figure 2B). Wnt-3A treatment promoted a transient increase in association of Dvl-3 and Dab2, followed by dissociation of the Dab2–Dvl-3 complex by 2 h (Figure 2B). In NIH-3T3 cells which stably overexpress Dab2 (NIH-3T3Dab2), the initial Dab2–Dvl-3 interaction is higher and is maintained following Wnt-3A stimulation (Figure 2B). Due to the ability of the PID domain of Dab2 to interact with Dvl-1 and Dvl-2 in vitro, the endogenous interaction of Dab2 with these isoforms was also investigated. While endogenous levels of Dvl-1 could not be detected in these cells, a weak interaction between Dvl-2 and Dab2 was observed in NIH-3T3 cells, while overexpression of Dab2 stimulated an increase and maintenance of this association (Figure 2C). Western analysis reveals that both NIH-3T3 and NIH-3T3Dab2 cells express comparable levels of Dvl-2 and Dvl-3 and demonstrates that Wnt-stimulated phosphorylation of Dvl-2 and Dvl-3 (Yanagawa et al., 1995) occurs normally in cells which overexpress Dab2 (Figure 2D). Together, these results show that in NIH-3T3 cells, Dab2 binds predominantly to the Dvl-3 isoform, while overexpression of Dab2 prevents Wnt-induced dissociation of the Dvl-3–Dab2 complex. We therefore focused our studies on the interaction of Dvl-3 with Dab2.
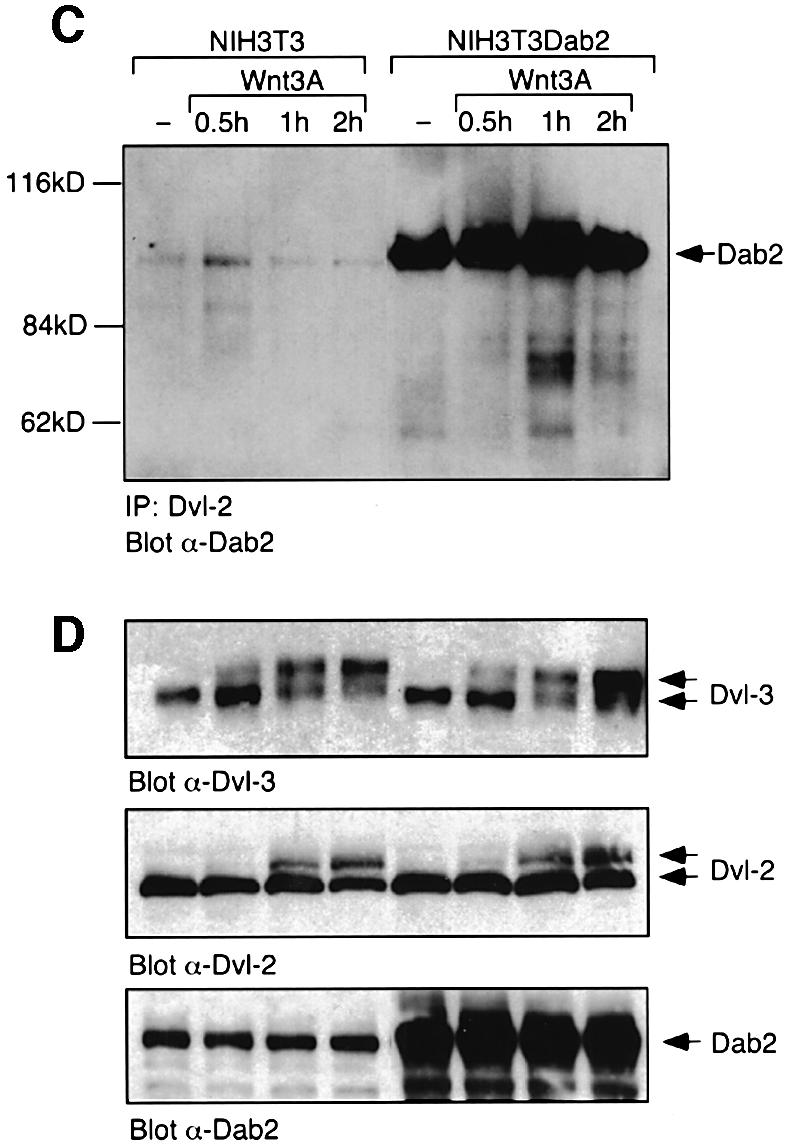
Fig. 2. Dab2 interacts with Dvl-3 in vivo. (A) Cellular lysates from HEK 293 cells co-transfected with Dab2 and Dvl-3 or Mv1Lu mink lung epithelial (CCL64) cells transfected with Dvl-3 were subjected to immunoprecipitation with control IgG, α-Dvl-3 or α-Dab2 antisera. Immunocomplexes were resolved by SDS–PAGE and subjected to western analysis with α-Dab2 antibody. The position of Dab2 is indicated by the arrow. (B and C) NIH-3T3 and NIH-3T3Dab2 cells were stimulated with control (–) or Wnt-3A-conditioned medium for the indicated times. Cell lysates were prepared and subjected to immunoprecipitation with control IgG, α-Dvl-3 or α-Dvl-2 antisera as indicated. Immunocomplexes were resolved by SDS–PAGE and the presence of Dab2 in the immunocomplex was determined by western blotting using α-Dab2 antibody. (D) The cellular levels of Dvl-3, Dvl-2 and Dab2 in NIH-3T3 and NIH3-T3Dab2 cells following Wnt-3A stimulation were determined by western analysis. The positions of unphosphorylated and phosphorylated forms of Dvl-2 and Dvl3 (top panel) and cellular Dab-2 (bottom panel) are indicated by the arrows.
Dab2 inhibitsWnt-3A-mediated signaling
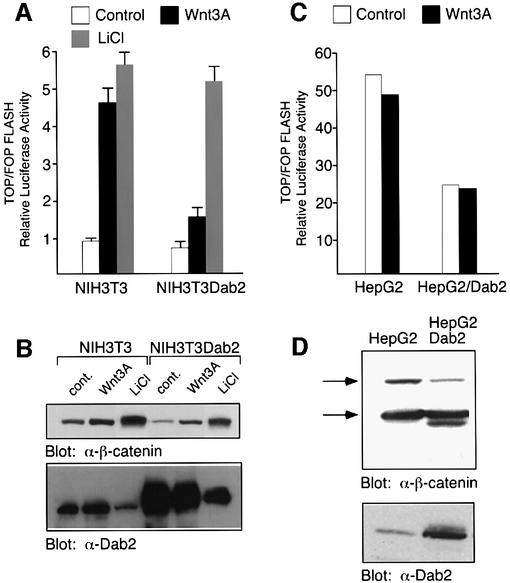
To assess the functional significance of the Dab2–Dvl interaction, we investigated whether overexpression of Dab2 affects induction of the Wnt-stimulated reporter construct TOPFLASH (Korinek et al., 1997). As shown in Figure 3A, Wnt-3A treatment of NIH-3T3 cells results in a 4- to 5-fold stimulation of TOPFLASH activity which is greatly abrogated in NIH-3T3Dab2 cells. Treatment of the cells with LiCl, a pharmacological inhibitor of GSK3β, however, results in equal transactivation of the TOPFLASH promoter in both cell lines. Western analysis of NIH-3T3 and NIH-3T3Dab2 lysates revealed that while both Wnt-3A and LiCl treatment increased expression of β-catenin, LiCl treatment led to a loss of Dab2 expression in both cell lines (Figure 3B). These results thus suggest that the ability of LiCl to stimulate TOPFLASH activity in NIH-3T3Dab2 cells is due to downregulation of Dab2 levels. We next wished to investigate the effects of Dab2 overexpression in a cell line which exhibits constitutive β-catenin/LEF-1-mediated activity. For this purpose, we chose the HepG2 cell line which expresses full-length as well as a truncated form of β-catenin lacking the phosphorylation sites for GSK3β, leading to its nuclear accumulation (de la Coste et al., 1998). We find that stable overexpression of Dab2 in HepG2 cells decreases the constitutive TOPFLASH activity displayed by these cells by ∼50% (Figure 3C). Western analysis of cell lysates derived from these cell lines demonstrates that Dab2 overexpression (Figure 3D, lower panel) leads to a decrease in expression of the full-length form of β-catenin (Figure 3D, upper panel).
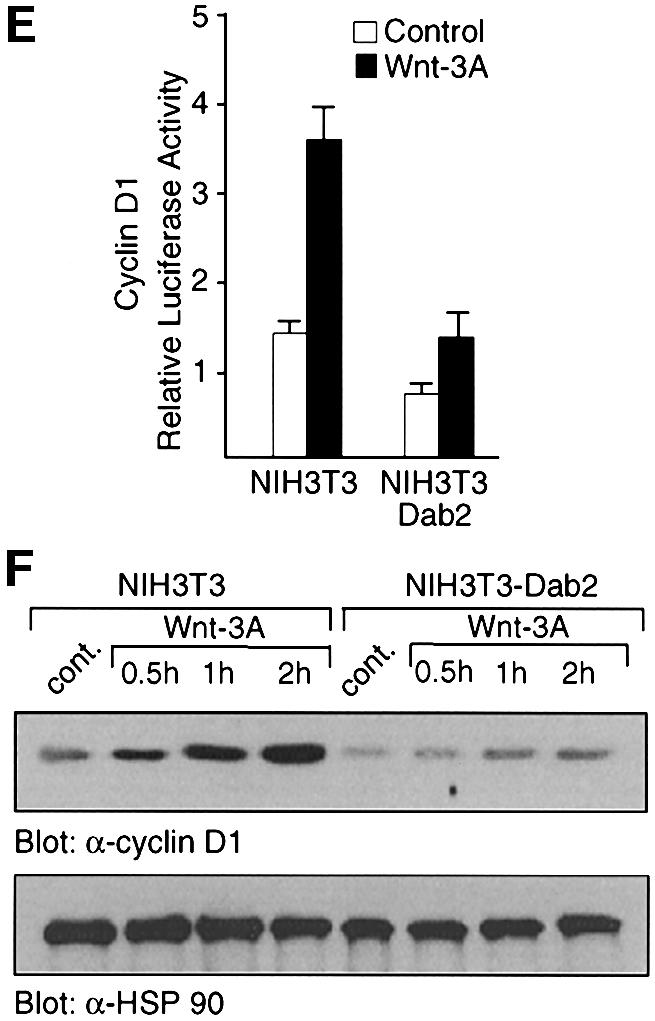
Fig. 3. Dab2 inhibits Wnt-3A-stimulated responses. (A and C) Activation of TOPFLASH and FOPFLASH luciferase reporter constructs was assessed in NIH-3T3, NIH-3T3Dab2, HepG2 and HepG2Dab2 cells following treatment with control, Wnt-3A-conditioned medium or 60 mM LiCl as indicated. Relative luciferase activity is expressed as the ratio of TOP/FOPFLASH activity in control, Wnt-3A-treated or LiCl-treated cells as indicated. (B and D) Cell lysates derived from NIH-3T3, NIH-3T3Dab2, HepG2 and HepG2Dab2 cells as stimulated above were assayed for expression levels of β-catenin and Dab2 by western analysis. The arrows mark the position of full-length and truncated forms of β-catenin expressed in HepG2 and HepG2Dab2 cells (D). (E) Activation of the cyclin D1 promoter-luciferase construct by control and Wnt-3A-conditioned medium was analyzed in NIH-3T3 and NIH-3T3Dab2 cells. Shown is the mean ± SD of duplicates from a representative experiment. (F) Cell lysates from NIH-3T3 and NIH-3T3Dab2 cells treated with control or Wnt-3A-conditioned medium for the indicated times were subjected to western blot analysis using a monoclonal antibody to cyclin D1 (top panel). Equal protein loading is demonstrated by probing the same blot with a polyclonal antibody to HSP90 (bottom panel).
To investigate further the inhibitory effects of Dab2 on Wnt-3A/β-catenin-mediated signaling, we examined whether Wnt-stimulated cyclin D1 induction is attenuated by Dab2 expression. The cyclin D1 promoter contains a TCF/LEF-1 site (Shtutman et al., 1999) and, as shown in Figure 3E, is transactivated upon Wnt-3A treatment of NIH-3T3 cells. Overexpression of Dab2 in NIH-3T3 cells reduces Wnt-3A-stimulated activation of the cyclin D1 promoter (Figure 3E). Similarly, examination of cyclin D1 protein levels reveals that overexpression of Dab2 abrogates Wnt-3A-induced induction (Figure 3F, upper panel). Western blot analysis of the same lysates for HSP90 demonstrates that Dab2 overexpression does not lead to global decreases in cellular proteins (Figure 3F, lower panel). Together, these results demonstrate that Dab2 acts as a negative regulator of canonical β-catenin/LEF-1/TCF-mediated signaling.
Dab2 potentiates Wnt-5A-mediated signaling
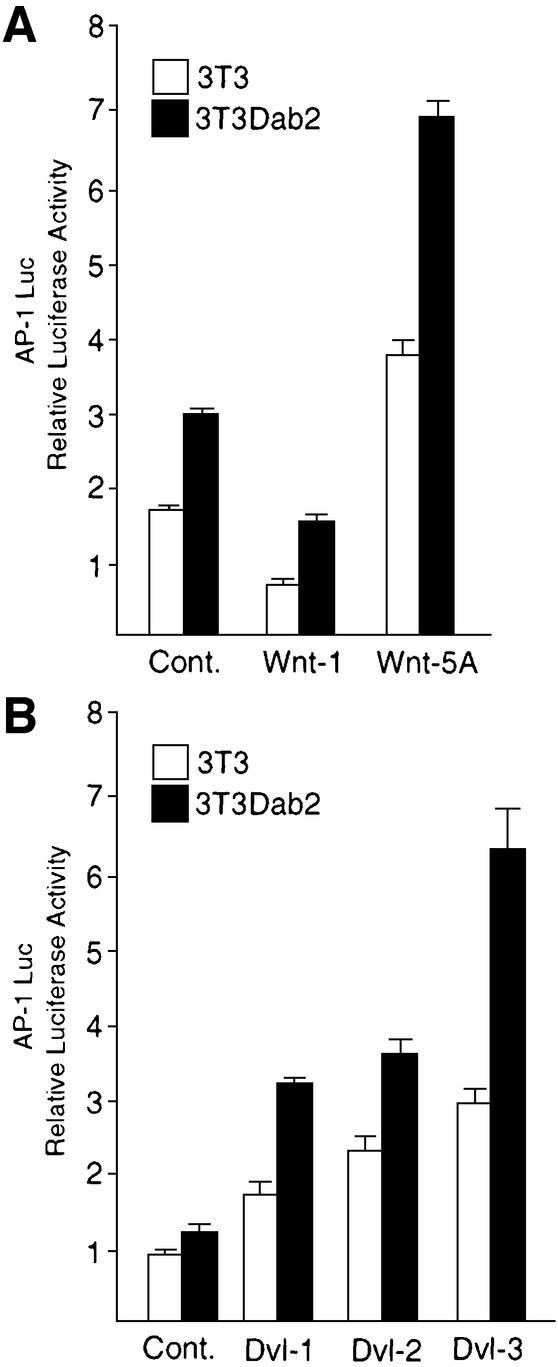
Through genetic analysis, the requirement for the Dvl proteins in the PCP–CE pathways has been demonstrated (Boutros et al., 1998). In both invertebrates and vertebrates, stimulation of this pathway by the Wnt-5A ligand subclass has been shown to be mediated through JNK (Boutros et al., 1998; Yamanaka et al., 2002). We therefore wished to determine if overexpression of Dab2 in NIH-3T3 cells altered the cellular response to Wnt-5A, using a reporter construct containing multiple AP-1 elements to monitor JNK activation. As shown in Figure 4A, Wnt-5A, but not Wnt-1, was able to stimulate AP-1-luciferase induction in NIH-3T3 and NIH-3T3Dab2 cells; however, NIH-3T3Dab2 cells exhibit higher basal as well as Wnt-5A-stimulated levels (Figure 4A). Since expression of the Dvl proteins previously has been shown to activate JNK (Li et al., 1999b; Moriguchi et al., 1999), we assessed whether overexpression of Dab2 could block the ability of transfected Dvl proteins to activate JNK. While expression of all three Dvl isoforms stimulates AP-1-luciferase induction in NIH-3T3 cells, the activation is potentiated in cells which overexpress Dab2 (Figure 4B). Together, these results demonstrate that while overexpression of Dab2 blocks canonical Wnt/β-catenin-mediated signaling, Dab2 overexpression accentuates JNK activation stimulated by the non-canonical PCP–CE pathway.
Fig. 4. Dab2 potentiates Wnt-5A-stimulated JNK activation. (A) Stimulation of an AP-1-luciferase reporter construct by co-transfection of Wnt-1 or Wnt-5A expression plasmids was analyzed in NIH-3T3 and NIH-3T3Dab2 cells. Shown is the mean ± SD of duplicates from a representative experiment. (B) NIH-3T3 and NIH-3T3Dab2 cells were co-transfected with AP-1 Luc and Dvl-1, Dvl-2 or Dvl-3 plasmids as indicated. Luciferase activity was determined after 48 h transfection. Shown is the mean ± SD of duplicates from a representative experiment.
Dab2 overexpression inhibits cytosolic and nuclear accumulation of β-catenin
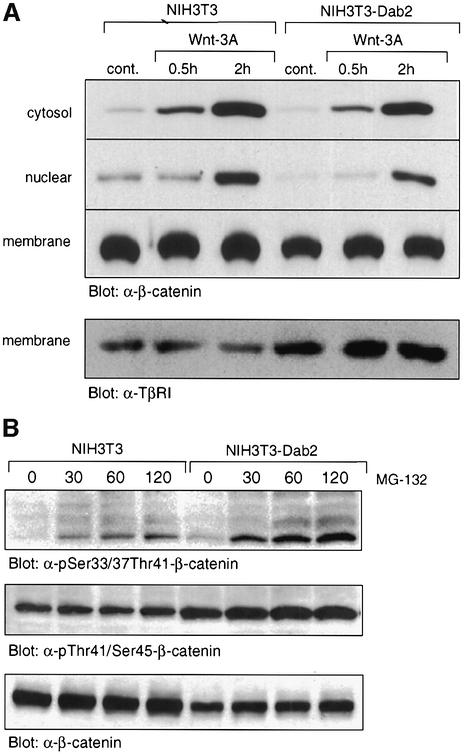
Activation of the Wnt-3A/β-catenin pathway leads to cytosolic accumulation of β-catenin, which then translocates to the nucleus to participate in transcription of Wnt target genes. Since Dab2 overexpression inhibited TCF/LEF-1-stimulated promoter transactivation, we postulated that this response might be mediated through an attenuation of Wnt-3A-stimulated β-catenin cytosolic and nuclear accumulation. As shown in Figure 5A, Wnt-3A treatment of NIH-3T3 cells leads to cytosolic and nuclear accumulation of β-catenin, while membrane-bound β-catenin levels remain the same. In NIH-3T3Dab2 cells, however, basal cytosolic, nuclear and membrane-associated β-catenin levels are reduced while Wnt-3A-induced nuclear accumulation of β-catenin is abrogated (Figure 5A).
Fig. 5. Overexpression of Dab2 inhibits Wnt-3A-mediated cytosolic and nuclear accumulation of β-catenin. (A) NIH-3T3 and NIH-3T3Dab2 cells treated with control or Wnt-3A-conditioned medium for the indicated times were subjected to subcellular fractionation as detailed in Materials and methods. The presence of β-catenin in cytosolic, nuclear and membrane fractions was determined by western analysis utilizing a monoclonal antibody to β-catenin. An equal amount of protein was loaded for nuclear and cytosolic fractions, while protein loading for membrane fractions is shown by western analysis for membrane-associated TGF-β type I receptor (TβRI). (B) Increased phosphorylation of β-catenin is observed in cells which overexpress Dab2. NIH-3T3 and NIH-3T3Dab2 cells were treated with MG-132 (25 µM) for various times as indicated. Cell lysates were subjected to western analysis to detect β-catenin phosphorylated at Ser33/37/Thr41, Thr41/Ser45 and for total β-catenin levels.
Phosphorylation of β-catenin on Ser45 by CKI followed by subsequent phosphorylation of Thr41, Ser37 and Ser33 by GSK3β targets β-catenin for proteasomal degradation (Amit et al., 2002; Liu et al., 2002). To investigate whether the decreased overall levels of β-catenin in NIH-3T3Dab2 cells could be attributed to increased β-catenin phosphorylation and subsequent degradation, we assessed the effect of the proteasomal inhibitor MG-132 on β-catenin accumulation in NIH-3T3 and NIH-3T3Dab2 cells. We find that while treatment with MG-132 results in accumulation of both phosphorylated species of β-catenin in both cell lines, more phosphorylated β-catenin is present in cells which overexpress Dab2 (Figure 5B). These results thus suggest that Dab2 overexpression promotes increased phosphorylation and thus subsequent degradation of β-catenin, leading to overall decreased Wnt-3A-stimulated accumulation of cytosolic and nuclear β-catenin.
Dab2 binds to axin and alters Dvl-3–axin interactions
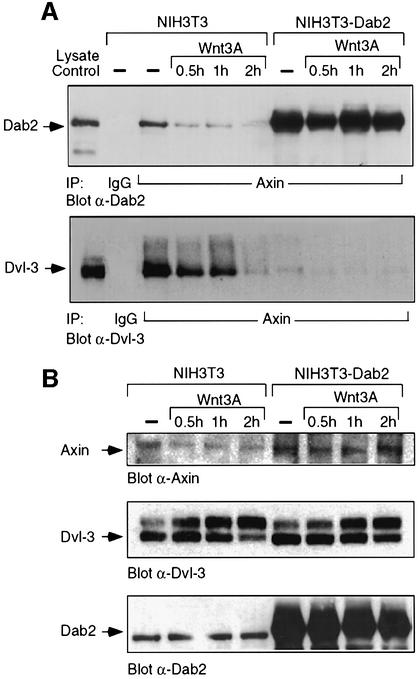
Dvl previously has been demonstrated to bind directly to axin through its DIX and PDZ domains, while axin has been shown to reside in a multimeric degradation complex containing APC, GSK3β and β-catenin (Ikeda et al., 1998). To investigate if Dab2 plays a role in modulating this complex, we first determined whether Dab2 can be found in association with axin. As shown in Figure 6A (top panel), axin is found in association with Dab2 in unstimulated NIH-3T3 cells, while Wnt-3A treatment results in a time-dependent dissociation of this interaction. In contrast, Dab2 overexpression results in greater interaction between axin and Dab2 and also prevents Wnt-3A-induced dissociation of the complex. Since interaction of Dvl with axin has been shown to be required for Wnt signaling (Li et al., 1999a), we next investigated whether Dab2 expression alters the association of Dvl-3 and axin. As shown in Figure 6A (lower panel), a pre-existing complex between Dvl-3 and axin is present in unstimulated NIH-3T3 cells, while Wnt-3A treatment promotes a time-dependent dissociation of axin and Dvl-3. In cells which overexpress Dab2, a dramatic reduction in the basal association of Dvl-3 and axin is observed, which decreases further following Wnt-3A stimulation. Examination of the cellular lysates for expression of these proteins reveals that while Dvl-3 protein levels are comparable, greater axin levels are present in NIH-3T3Dab2 cells (Figure 6B). These results thus prompted us to investigate if Dab2 binds directly to axin, thereby modulating its expression. As shown in Figure 6C, a GST construct containing the PID domain of Dab2, but not the mid-region of Dab2 (amino acids 335–610) or GST alone, could co-precipitate axin. To localize this interaction further to a specific domain, various axin constructs were in vitro translated and subjected to GSTpull-down assay utilizing the GST–PID construct of Dab2. As seen in Figure 6D, deletion of the N-terminal 194 amino acids of axin leads to a significant reduction in binding to the PID domain of Dab2, while binding to other constructs is maintained to various extents. Together, these results demonstrate a direct binding interaction between axin and Dab2 in vitro and in vivo, which may account for the observed displacement of Dvl-3 from the axin–β-catenin degradation complex in NIH-3T3Dab2 cells resulting in stabilization of axin levels.
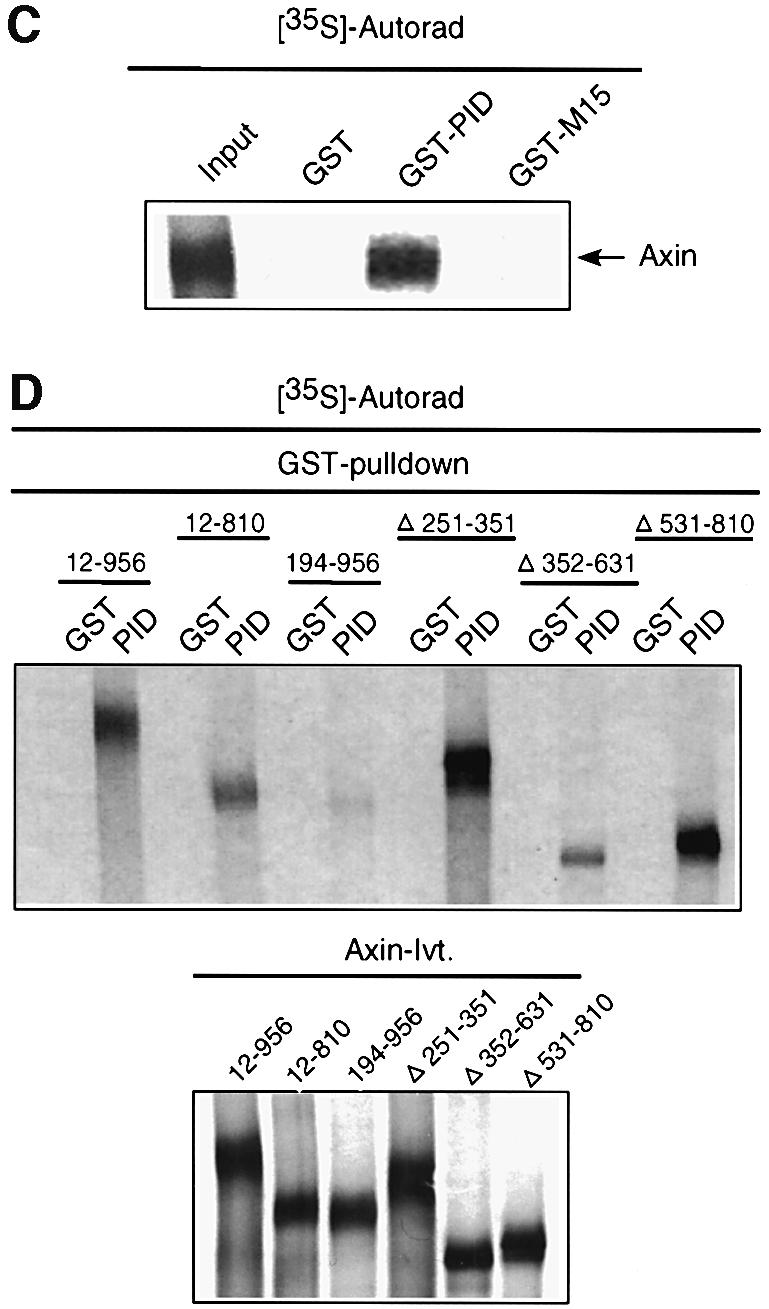
Fig. 6. Dab2 directly interacts with axin and alters Dvl-3–axin association. (A and B) Cell lysates derived from control (–) or Wnt-3A-stimulated NIH-3T3 and NIH-3T3Dab2 cells were subjected to immunoprecipitation with control IgG or α-axin antibody. Following resolution by SDS–PAGE, the presence of Dab2 (upper panel) or Dvl-3 (lower panel) in the immunocomplex was detected by western blot analysis (A). An aliquot of control NIH-3T3 cell lysate (lysate control) is utilized to denote the migration of Dab2 and Dvl-3. The expression of axin, Dvl-3 and Dab2 in control or Wnt-3A-stimulated NIH-3T3 and NIH-3T3Dab2 cells was analyzed by western blot for each of the proteins as indicated (B). (C) Full-length mouse axin was subjected to in vitro transcription/translation in the presence of [35S]methionine followed by incubation with control GST, GST–PID or GST–M15 beads. Following extensive washing, the reactions were resolved by SDS–PAGE and visualized by autoradiography. An aliquot of the input in vitro transcription/translation reaction is included as a control. (D) Full-length and various deletion constructs of axin were subjected to in vitro transcription/translation utilizing [35S]methionine and analyzed for interaction with GST–PID as described above (top panel). An aliquot of the in vitro translated product (Axin-Ivt.) was analyzed by SDS–PAGE to visualize the various constructs (bottom panel).
Fibroblasts conditionally null for Dab2 exhibit enhanced Wnt/β-catenin signaling
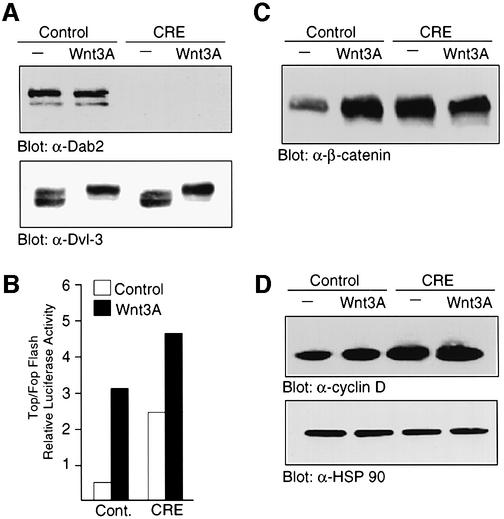
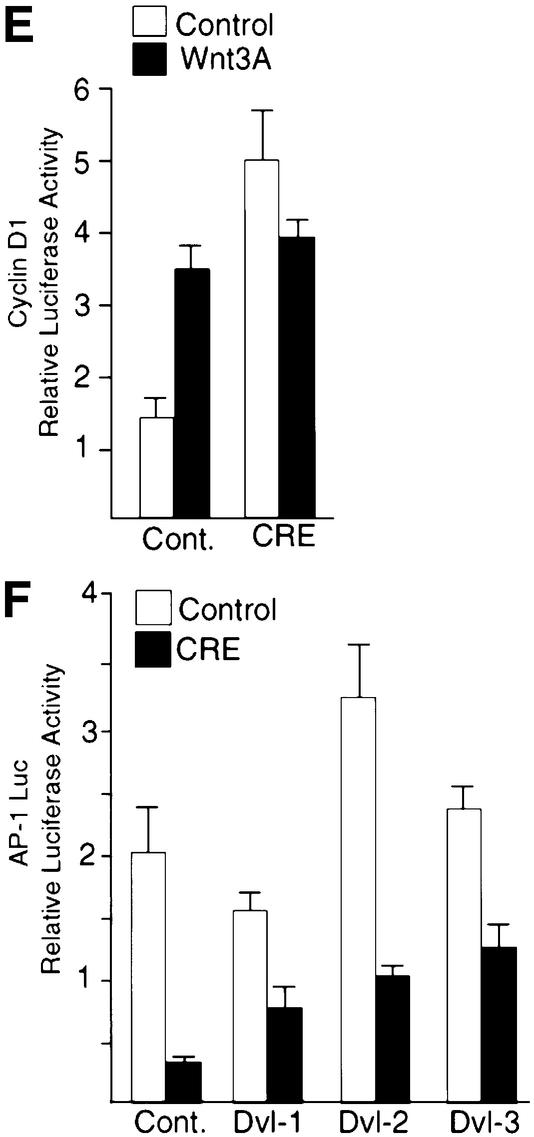
To assess the effect of Dab2 ablation on Wnt signaling, we examined Wnt-3A-induced signaling in MEFs derived from Dab2 conditional null animals. As shown in Figure 7A, infection of MEFs with virus expressing Cre recombinase results in the successful ablation of Dab2 protein expression, while infection with virus expressing the vector backbone alone had no effect on endogenous Dab2 levels (Figure 7A, upper panel). Both control and Dab2-deficient MEFs express comparable levels of Dvl-3, which becomes phosphorylated normally following Wnt-3A stimulation (Figure 7A, lower panel). Analysis of these cells for Wnt-3A-mediated signal transduction revealed that Dab2–/– cells exhibit higher basal and Wnt-induced levels of TOPFLASH activity as compared with control MEFs (Figure 7B). In control cells, Wnt-3A induces a significant accumulation of β-catenin in the nucleus; however, in Dab2–/– MEFs, basal nuclear β-catenin levels are elevated and Wnt-3A treatment of these cells fails to elicit any further accumulation (Figure 7C). While Wnt-3A treatment was able to stimulate induction of cyclin D1 promoter activity and protein expression ∼2-fold in control MEFs, basal expression of cyclin D1 protein and cyclin D1 promoter activity is elevated in Dab2–/– MEFs and again does not increase further following Wnt treatment (Figure 7D and E). To assess the effect of Dab2 ablation on the non-canonical PCP–CE pathway, JNK activation stimulated by the various Dvl isoforms was measured in control and Dab2–/– MEFs. Determination of AP-1 reporter activity revealed that cells which lack Dab2 exhibit both decreased basal and Dvl-stimulated luciferase induction (Figure 7F).
Fig. 7. Ablation of Dab2 leads to constitutive Wnt signaling. (A) MEFs derived from conditional null animals were infected with control virus or virus containing Cre recombinase. Successful ablation of Dab2 protein expression is demonstrated in control and Wnt-3A-stimulated cell lysates by western analysis for Dab2 (top panel). Cellular Dvl-3 levels were determined by western analysis following Wnt-3A stimulation in control and Dab2–/– MEFs (bottom panel). (B) Activation of the TOPFLASH and FOPFLASH luciferase reporter constructs was determined in control and Dab2–/– MEFs following stimulation with control or Wnt-3A-conditioned medium. Shown is the mean TOP/FOPFLASH ratio derived from duplicate assays of a representative experiment. (C) Control and Dab2–/– MEFs were treated with control or Wnt-3A-conditioned medium for 2 h followed by subcellular fractionation. Equal amounts of nuclear extracts were subjected to western analysis using a monoclonal antibody to β-catenin. (D) Control Dab2–/– MEFs were treated with control or Wnt-3A-conditioned medium for 2 h. Cell lysates were subjected to western immunoblotting with antibody to cyclin D1 (top panel) or to HSP90 (bottom panel) to demonstrate equal protein loading. (E) The activation of the cyclin D1 promoter luciferase reporter construct in control and Cre recombinase-infected MEFs was assayed following treatment of the cells with control or Wnt-3A-conditioned medium. Shown is the mean ± SD of duplicate assays from a representative experiment. (F) Control and Dab2–/– MEFs were co-transfected with AP-1 luciferase in the absence (cont.) or presence of Dvl-1, Dvl-2 or Dvl-3 as indicated. Luciferase activity was determined after 48 h of transfection. Shown is the mean ± SD of duplicates from a representative experiment.
To probe further the effect of Dab2 ablation on canonical Wnt/β-catenin signaling, we assessed axin– β-catenin and axin–Dvl-3 interactions following Wnt-3A stimulation in control and Dab2–/– MEFs. We find that Wnt-3A stimulation leads to a time-dependent dissociation of axin–β-catenin complexes in control MEFs, while this association remains unchanged in Dab2–/– MEFs (Figure 8A, top panel). In contrast, the association of Dvl-3 with axin following Wnt-3A treatment is transiently stimulated in control MEFs, while this association is greatly abrogated in Dab2–/– MEFs (Figure 8A, bottom panel). Analysis of cellular levels of these proteins in the two cell types reveals a marked decrease in expression of axin in Dab2–/– MEFs, while total cellular levels of Dvl-3 and β-catenin are comparable (Figure 8B). Together, these results thus suggest that ablation of Dab2 leads to decreased axin expression, liberating free β-catenin, and leading to constitutive canonical Wnt/β-catenin signaling.
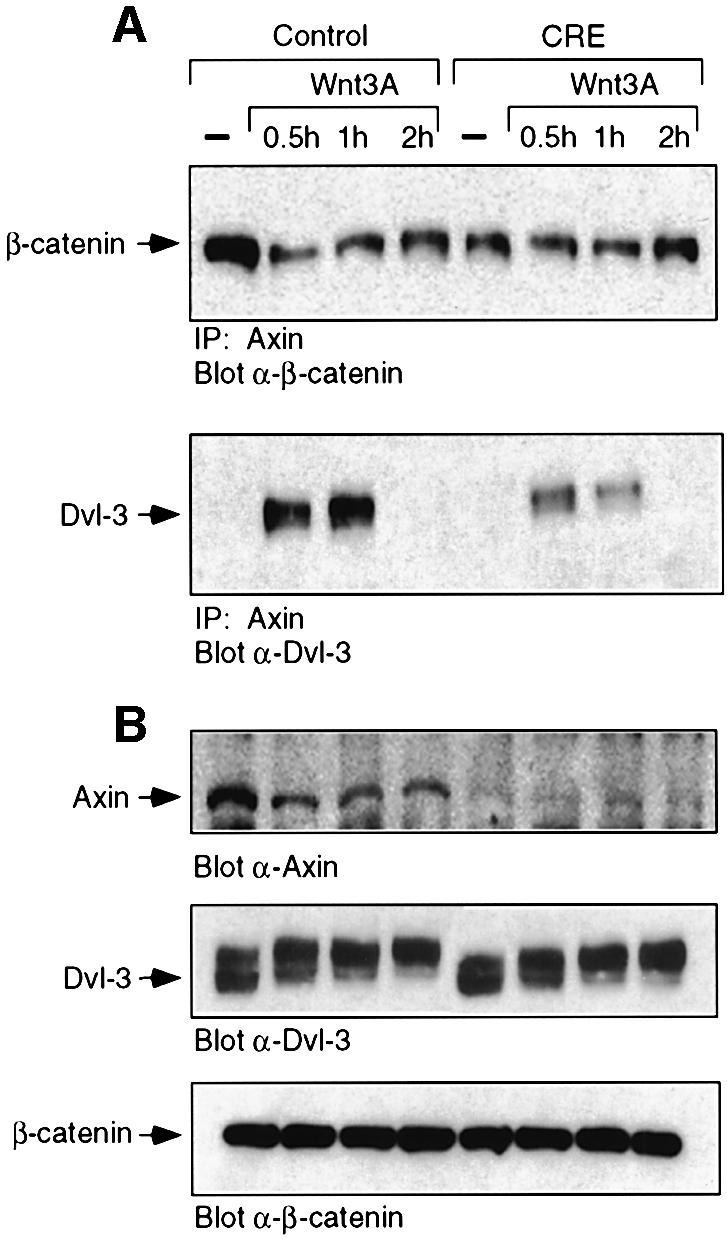
Fig. 8. Dab2 ablation leads to decreased axin and decreased axin– β-catenin–Dvl-3 interactions. (A) Control and Dab2–/– MEFs were treated with control (–) or Wnt-3A-conditioned medium for the times indicated. Cell lysates were prepared and subjected to immunoprecipitation with α-axin antibody. Following resolution by SDS–PAGE, the presence of β-catenin (top panel) or Dvl-3 (bottom panel) in the immunocomplex was detected by western blot analysis. The position of β-catenin and Dvl-3 is indicated by the arrow. (B) The expression of axin, Dvl-3 and β-catenin in the above lysates was determined by western analysis for each of the proteins as indicated.
Discussion
Dab2 is an adaptor molecule which has been shown to bind to clathrin-coated pits and participate in regulation of several signaling pathways including the Ras/MAPK and TGF-β signaling pathways (Xu et al., 1998; Hocevar et al., 2001). We report here that Dab2 directly interacts with two components of the β-catenin destruction complex, Dvl-3 and axin, and participates in regulation of the Wnt signaling pathway. Overexpression of Dab2 in NIH-3T3 cells was shown to inhibit Wnt-3A-stimulated accumulation of β-catenin, leading to decreased canonical Wnt/ β-catenin-mediated gene induction, while simultaneously potentiating non-canonical Wnt-5A-stimulated JNK activation. Ablation of the Dab2 protein was found to lead to increased nuclear β-catenin levels and elevated β-catenin/TCF/LEF-1-dependent gene induction. These results thus suggest that Dab2 plays an important, non-redundant, negative regulatory role in the canonical Wnt/β-catenin signaling pathway. This is similar to the inhibitory effect of Stbm and Nkd, several other recently identified Dvl-binding partners, on the canonical Wnt pathway (Yan et al., 2001; Park and Moon 2002). Control of Dvl-binding partners thus appears to be an important regulatory component of the Wnt signaling pathway.
The Dvl protein has been shown to interact directly with axin and Frat1, while GSK3β was shown to bind to axin and Frat1, but not Dvl (Li et al., 1999a; Fraser et al., 2002). A complex of axin, GSK3β, Dvl, Frat1 and β-catenin thus appears to exist in unstimulated cells (Li et al., 1999a). Here we identify that Dab2 can bind directly to Dvl-3 and axin, and that endogenous Dab2, Dvl-3 and axin can be found in association in unstimulated cells. Overexpression of Dab2 was found to lead to a decrease in the Dvl-3–axin association while simultaneously causing an increased level of Dab2 in association with axin. Our results also indicate that at least two populations of Dab2 must exist in the cell; Dab2 which is bound to axin and Dab2 which is bound to Dvl-3. Our studies suggest that these interactions may be mutually exclusive, in that Dvl-3 does not co-precipitate with axin in the presence of overexpressed Dab2. Indeed, Dab2 may displace bound Dvl-3 from axin, since it was shown previously that the PDZ domain of Dvl interacted with the N-terminal domain of axin (Li et al., 1999a), which we have identified as the site of interaction with Dab2. In the normal cellular setting, the endogenous Dab2–Dvl-3 interaction may limit the number of functional Dvl-3–axin interactions, thus reducing the ability of Wnt signals to reach axin and its associated regulatory kinases. This, in turn, limits the extent to which phosphorylation of β-catenin can be inhibited and the extent to which transcription can be induced. Overexpression of Dab2 would thus function to suppress Wnt signaling by further decreasing Dvl-3–axin interactions and maintaining axin–β-catenin–GSK3β interactions, while ablation of Dab2 would lead to constitutive Wnt signaling.
We observed that inhibition of GSK3β activity by LiCl was able to overcome the negative effect of Dab2 overexpression on TCF/LEF-1-mediated gene transcription. This may be explained by the decreased levels of Dab2 observed in cells treated with LiCl, and suggests that like axin, Dab2 phosphorylation by GSK3β is important for its stability (Yamamoto et al., 1999). In fact, Dab2 expression appears to regulate the protein level of axin, which again demonstrates a critical role for Dab2 in maintaining the axin–GSK3β–β-catenin interaction. This is supported by the data from Dab2–/– fibroblasts which display low levels of axin, high basal levels of nuclear β-catenin and elevated β-catenin/TCF/LEF-1-mediated transcriptional activity. An additional role for Dab2 may be to help localize axin to the plasma membrane, since an axin construct lacking the N-terminal region displayed diffuse cytosolic localization and weak plasma membrane association (Fagotto et al., 1999).
The Wnt-1 proto-oncogene was first identified as a protein which could initiate mammary tumors in mice (Nusse and Varmus, 1982), while the ability to cause cellular transformation was found to correlate with the ability to activate TCF/LEF-1-dependent transcription (Polakis, 2000). Since members of the TCF/LEF-1 transcription factor family require interaction with β-catenin to mediate gene transcription, controlling the level of nuclear β-catenin determines whether the Wnt signaling pathway is turned on or off. β-catenin is targeted for degradation by the ubiquitin–proteasome pathway following phosphorylation of four N-terminal S/T residues (Kitagawa et al., 1999). Recently, it has been elucidated that CKIα phosphorylates β-catenin at S45, which then allows the subsequent phosphorylation of S33, S37 and T41 by GSK3β (Amit et al., 2002; Liu et al., 2002). We report here that Dab2 prevents Wnt-stimulated accumulation of β-catenin when overexpressed in NIH-3T3 cells, and can decrease expression of full-length β-catenin and β-catenin/TCF/LEF-1-mediated transcriptional activity in HepG2 cells. Indeed, we observe increased levels of β-catenin phosphorylated at Ser45 and Ser33/37, indicating that increased degradation is taking place.
Mutations in the various components of the Wnt signaling pathway leading to increased nuclear β-catenin and increased β-catenin/TCF-mediated signaling have been demonstrated in many different types of human tumors (reviewed in Bienz and Clevers, 2000; Polakis, 2000). The results presented here suggest that mutations in Dab2 may also contribute to aberrant Wnt signaling. Dab2 was first identified as a protein whose expression was downregulated in ovarian carcinoma (Mok et al., 1994), and subsequently found to be downregulated in breast and prostate carcinoma as well (Schwahn and Medina, 1998; Tseng et al., 1998). Elevated β-catenin/TCF/LEF-1-mediated signaling has been observed in breast cancer cell lines (Lin et al., 2000), which in some cases has been attributed to mutations in known signaling components such as APC in the metastatic breast cancer cell line Du4475 (Schlosshauer et al., 2000). While MCF-7 cells have been reported to exhibit elevated β-catenin/TCF/LEF-1-mediated gene transcription, no mutations have been found in their APC, axin or β-catenin genes (Lin et al., 2000). Interestingly, MCF-7 cells express only low levels of Dab2; whether ectopic expression of Dab2 can inhibit β-catenin/TCF/LEF-1-mediated gene transcription in these cells currently is under investigation. Dab2 may thus provide an additional therapeutic target for intervention in colon or hepatic carcinoma where mutations in APC, β-catenin and axin are prevalent.
Materials and methods
Cell culture
Mv1Lu (CCL64) and NIH-3T3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% newborn calf serum. HEK293 and HepG2 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). To generate stable NIH-3T3 and HepG2 cell lines that overexpress Flag-tagged human Dab2, cells were co-transfected with WTDab2 (Hocevar et al., 2001) and pSV2Neo with Lipofectamine reagent (Invitrogen) according to the manufacturer’s protocols. Cells were selected in medium containing 500 µg/ml G418 and maintained as pools. Overexpression of Dab2 was verified by western analysis. To generate Dab2–/– MEFs, fibroblasts at passage 18–20 derived from a heterozygous disrupted and floxed Dab2 allelic embryo (Morris et al., 2002b) were infected with pBabepuro (Control) or pBabepuro-Cre recombinase virus. Cells were selected and maintained as pools in DMEM supplemented with 10% FBS and 1 µg/ml puromycin. Successful deletion of Dab2 was verified by PCR analysis, and loss of protein expression was verified by western analysis for Dab2.
DNA constructs and antibodies
The Wnt-1 and Wnt-5A expression plasmids and TOPFLASH and FOPFLASH luciferase reporter constructs were purchased from Upstate, and the AP-1-Luc plasmid was purchased from Stratagene. The –973 cyclin D1 promoter luciferase construct has been described previously (Herber et al., 1994). Expression constructs for Dvl-1, Dvl-2 and Dvl-3 in pcDNA3 (Semenov and Snyder, 1997), human Dab2 in pRK5 (Hocevar et al., 2001), mouse axin constructs (Fagotto et al., 1999), and GST–PID and GST–M15 constructs of Dab2 (Xu et al., 1998) have been described previously. The various deletion constructs of Dvl-3 were generated by standard PCR methods using human Dvl-3 as the template and inserted into the pCruz myc vector (Santa Cruz Biotechnology). The monoclonal antibody to Dab2/p96 was purchased from Transduction Laboratories. Monoclonal antibodies to Dvl-3 (sc-8027), cyclin D1 (sc-6281) and β-catenin (sc-7963), and polyclonal antibodies to axin (sc-8567), HSP90 (sc-7947) and TβRI (sc-398) were purchased from Santa Cruz Biotechnology. Phosphospecific antibodies to β-catenin (α-Ser33/37/Thr41 and α-Thr41/Ser45) were purchased from Cell Signaling Technology. A polyclonal antibody to Dvl-2 was generously provided by M.Semenov.
Production of Wnt-3A-conditioned medium
Mouse L cells transfected with Wnt-3A expression vector or blank vector were maintained, and conditioned medium was collected as described previously (Shibamoto et al., 1998). For Wnt-3A treatment of cells, the conditioned medium was diluted 1:1 with normal culture medium. The designation untreated or control treatment corresponds to the use of 1:1 diluted conditioned medium from L cells transfected with blank vector.
Small-pool cDNA screen and GST pull-down assays
Small-pool cDNA screening to identify binding partners for the PID domain of Dab2 was carried out essentially as previously described (Lustig et al., 1997). Briefly, a human liver cDNA library in pcDNA3 (Invitrogen) was amplified and separated into pools containing ∼100 different clones each. 35S-labeled proteins were generated using the TnT T7-Quick coupled transcription/translation system (Promega) utilizing a reaction volume of 25 µl. Each pool was then incubated with GST–PID and control GST beads in binding buffer for 2 h at 4°C, followed by extensive washing as described (Hocevar et al., 2001). Samples were resolved by SDS–PAGE and binding interactions visualized by autoradiography. Positive pools were subjected to rounds of dilution until a single positive clone was obtained, at which time the clone was amplified and sequenced.
Transient transfection and reporter gene measurements
For luciferase reporter measurements, NIH-3T3 and NIH-3T3Dab2 cells were transiently transfected with Fugene6 (Roche Diagnostics), while HepG2, HepG2Dab2 and MEFs were transfected with Lipofectamine (Invitrogen) according to the manufacturer’s protocols. Typically, 5 × 104 cells in a 24-well plate were transfected with 0.2 µg of DNA/well of the specific luciferase reporter along with 0.04 µg of DNA/well of SV40-RL as an internal control for transfection efficiency. After 18 h, the medium was changed to control, conditioned Wnt-3A medium, or medium containing 60 mM LiCl for an additional 24 h. Cells were lysed and luciferase activity determined using the Promega Dual Luciferase Assay System as per the manufacturer’s instructions in a Dynex model ML-2250 luminometer. Luciferase activity is expressed as the specific reporter activity divided by the SV40-RL activity. In addition, TOP/FOPFLASH ratios were derived and plotted to reflect activity attributable only to TCF/LEF-1/β-catenin-mediated transactivation.
Preparation of cell lysates, immunoprecipitation and western blot analysis
For immunoprecipitation and western blot analysis, cells were lysed in buffer D and immunoprecipitation was carried out as previously described (Hocevar et al., 1999). For generation of cytosolic, nuclear and membrane fractions, confluent cells from a 10 cm plate were resuspended in 1 ml of hypotonic lysis buffer [10 mM Tris–HCl pH 7.5, 10 mM NaCl, 0.1 mM EGTA, 1 mM dithiothreitol (DTT), 25 mM β-glycerophosphate and EDTA-free protease inhibitor cocktail Complete; Roche Diagnostics] and placed on ice for 15 min. Following Dounce homogenization to achieve cell lysis, samples were centrifuged at 179 g for 10 min to separate nuclei from cytosol. The supernatant was subjected further to centrifugation at 100 000 g for 1 h to separate cytosolic and membrane fractions. The nuclear pellet was resuspended in 100 µl of buffer C (25% glycerol, 20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA pH 8.0, 1 mM EGTA, 1 mM DTT, 25 mM β-glycerophosphate and EDTA-free protease inhibitor cocktail Complete; Roche Diagnostics), rocked for 20 min at 4°C, and centrifuged at 14 000 g for 20 min. The supernatant was collected and designated the nuclear fraction.
Acknowledgments
Acknowledgements
We thank Dr S.Takada for the generous provision of control and Wnt-3A-producing mouse L cells, Drs M.Semenov, M.Sabbah, E.Jho and F.Constantini for the generous provision of plasmids, and Dennis Wang for excellent technical assistance. This work was supported by grant CA55536 and CA80095 from the National Cancer Institute to P.H.H. and a Scientist Development Grant from the American Heart Association to B.A.H.
References
- Amit S., Hatzubai,A., Birman,Y., Andersen,J.S., Ben-Shushan,E., Mann,M., Ben-Neriah,Y. and Alkalay,I. (2002) Axin-mediated CKI phosphorylation of β-catenin at Ser45: a molecular switch for the Wnt pathway. Genes Dev., 16, 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. and Clevers,H. (2000) Linking colorectal cancer to Wnt signaling. Cell, 103, 311–320. [DOI] [PubMed] [Google Scholar]
- Boutros M., Paricio,N., Strutt,D.I. and Mlodzik,M. (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell, 94, 109–118. [DOI] [PubMed] [Google Scholar]
- Cadigan K.M. and Nusse,R. (1997) Wnt signaling: a common theme in animal development. Genes Dev., 11, 3286–3305. [DOI] [PubMed] [Google Scholar]
- Dale T. (1998) Signal transduction by the Wnt family of ligands. Biochem. J., 329, 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- delaCoste A. et al. (1998) Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc. Natl Acad. Sci. USA, 95, 8847–8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F., Jho,E., Zeng,L., Kurth,T., Joos,T., Kaufmann,C. and Constantini,F. (1999) Domains of axin involved in protein–protein interactions, Wnt pathway inhibition and intracellular localization. J. Cell Biol., 145, 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser E. et al. (2002) Identification of the axin and Frat binding region of glycogen synthase kinase-3. J. Biol. Chem., 277, 2176–2185. [DOI] [PubMed] [Google Scholar]
- He T.-C., Sparks,A.B., Rago,C., Hermeking,H., Zawel,L., da Costa,L.T., Morin,P.J., Vogelstein,B. and Kinzler,K.W. (1998) Identification of c-MYC as a target of the APC pathway. Science, 281, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Herber B., Truss,M., Beato,M. and Muller,R. (1994) Inducible regulatory elements in the human cyclin D1 promoter. Oncogene, 9, 1295–1304. [PubMed] [Google Scholar]
- Hocevar B.A., Brown,T.L. and Howe,P.H. (1999) TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J., 18, 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar B.A., Smine,A., Xu,X.-X. and Howe,P.H. (2001) The adaptor molecule Disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J., 20, 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Friess,H., Kleeff,J., Esposito,I., Zhu,Z., Liu,S., Mok,S.C., Zimmermann,A. and Buchler,M.W. (2001) DOC-2/hDab2 expression is up-regulated in primary pancreatic cancer but reduced in metastasis. Lab. Invest., 81, 863–873. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Kishida,S., Yamamoto,H., Murai,H., Koyama,S. and Kikuchi,A. (1998) Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J., 17, 1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S., Yamamoto,H., Hino,S.-I., Ikeda,S., Kishida,M. and Kikuchi,A. (1999) DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol. Cell. Biol., 19, 4414–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M., Hatakeyama,S., Shirane,M., Matsumoto,M., Ishida,N., Hattori,K., Nakamichi,I., Kikuchi,A. and Nakayama,K. (1999) An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J., 18, 2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J., Yang,Y., Axelrod,J.D., Beier,D.R., Perrimon,N. and Sussman,D.J. (1996) Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech. Dev., 58, 15–26. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker,N., Morin,P.J., van Wichen,D., de Weger,R., Kinzler,K.W., Vogelstein,B. and Clevers,H. (1997) Constitutive transcriptional activation by a β-catenin–Tcf complex in APC–/– colon carcinoma. Science, 275, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Li L., Yuan,H., Weaver,C.D., Mao,J., Farr,G.H.,III, Sussman,D.J., Jonkers,J., Kimelman,D. and Wu,D. (1999a) Axin and Frat1 interact with Dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J., 18, 4233–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yuan,H., Xie,W., Mao,J., Caruso,A.M., McMahon,A., Sussman,D.J. and Wu,D. (1999b) Dishevelled proteins lead to two signaling pathways. J. Biol. Chem., 274, 129–134. [DOI] [PubMed] [Google Scholar]
- Lin S.-Y., Xia,W., Wang,J.C., Kwong,K.Y., Spohn,B., Wen,Y., Pestell,R.G. and Hung,M.-C. (2000) β-Catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc. Natl Acad. Sci. USA, 97, 4262–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li,Y., Semenov,M., Han,C., Baeg,G.-H., Tan,Y., Zhang,Z., Lin,X. and He,X. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell, 108, 837–847. [DOI] [PubMed] [Google Scholar]
- Lustig K.D., Stukenberg,P.T., McGarry,T.J., King,R.W., Cryns,V.L., Mead,P.E., Zon,L.I., Yuan,J. and Kirschner,M.W. (1997) Small pool expression screening: identification of genes involved in cell cycle control, apoptosis and early development. Methods Enzymol., 283, 83–99. [DOI] [PubMed] [Google Scholar]
- Mishra S.K., Keyel,P.A., Hawryluk,J., Agostinelli,N.R., Watkins,S.C. and Traub,L.M. (2002) Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J., 21, 4915–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S.C., Wong,K.-K., Chan,R.K.W., Lau,C.C., Tsao,S.-W., Knapp,R.C. and Berkowitz,R.S. (1994) Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol. Oncol., 52, 247–252. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Kawachi,K., Kamakura,S., Masuyama,N., Yamanaka,H., Matsumoto,K., Kikuchi,A. and Nishida,E. (1999) Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem., 274, 30957–30962. [DOI] [PubMed] [Google Scholar]
- Morris S.M. and Cooper,J.A. (2001) Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic, 2, 111–123. [DOI] [PubMed] [Google Scholar]
- Morris S.M., Arden,S.D., Roberts,R.C., Kendrick-Jones,J., Cooper,J.A., Luzio,J.P. and Buss,F. (2002a) Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic, 3, 331–341. [DOI] [PubMed] [Google Scholar]
- Morris S.M., Tallquist,M.D., Rock,C.O. and Cooper,J.A. (2002b) Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J., 21, 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. and Varmus,H.E. (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell, 31, 99–109. [DOI] [PubMed] [Google Scholar]
- Park M. and Moon,R.T. (2002) The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nature Cell Biol., 4, 20–25. [DOI] [PubMed] [Google Scholar]
- Pawson T. and Scott,J.D. (1997) Signaling through scaffold, anchoring and adaptor proteins. Science, 278, 2075–2080. [DOI] [PubMed] [Google Scholar]
- Peters J.M., McKay,R.M., McKay,J.P. and Graff,J.M. (1999) Casein kinase I transduces Wnt signals. Nature, 401, 345–350. [DOI] [PubMed] [Google Scholar]
- Polakis P. (2000) Wnt signaling and cancer. Genes Dev., 14, 1837–1851. [PubMed] [Google Scholar]
- Sakanaka C., Leong,P., Xu,L., Harrison,S.D. and Williams,L.T. (1999) Casein kinase Iε in the Wnt pathway: regulation of β-catenin function. Proc. Natl Acad. Sci. USA, 96, 12548–12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosshauer P.W., Brown,S.A., Eisinger,K., Yan,Q., Guglielminetti,E.R., Parsons,R., Ellenson,L.H. and Kitajewski,J. (2000) APC truncation and increased β-catenin levels in a human breast cancer cell line. Carcinogenesis, 21, 1453–1456. [PubMed] [Google Scholar]
- Schwahn D.J. and Medina,D. (1998) p96, a MAPK-related protein, is consistently downregulated during mouse mammary carcinogenesis. Oncogene, 17, 1173–1178. [DOI] [PubMed] [Google Scholar]
- Semenov M.V. and Snyder,M. (1997) Human dishevelled genes constitute a DHR-containing multigene family. Genomics, 42, 302–310. [DOI] [PubMed] [Google Scholar]
- Shibamoto S., Higano,K., Takada,R., Ito,F., Takeichi,M. and Takada,S. (1998) Cytoskeletal reorganization by soluble Wnt-3a protein signaling. Genes Cells, 3, 659–670. [DOI] [PubMed] [Google Scholar]
- Shtutman M., Zhurinsky,J., Simcha,I., Albanese,C., D’Amico,M., Pestell,R. and Ben-Ze’ev,A. (1999) The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl Acad. Sci. USA, 96, 5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D.J., Klingensmith,J., Salinas,P., Adams,P.S., Nusse,R. and Perrimon,N. (1994) Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev. Biol., 166, 73–86. [DOI] [PubMed] [Google Scholar]
- Tree D.R.P., Shulman,J.M., Rousset,R., Scott,M.P., Gubb,D. and Axelrod,J.D. (2002) Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell, 109, 371–381. [DOI] [PubMed] [Google Scholar]
- Tsang M., Lijam,N., Yang,Y., Beier,D.R., Wynshaw-Boris,A. and Sussman,D.J. (1996) Isolation and characterization of mouse dishevelled-3. Dev. Dyn., 207, 253–262. [DOI] [PubMed] [Google Scholar]
- Tseng C.-P., Ely,B.D., Li,Y., Pong,R.-C. and Hsieh,H.-T. (1998) Regulation of rat DOC-2 gene during castration-induced rat ventral prostate degeneration and its growth inhibitory function in human prostatic carcinoma cells. Endocrinology, 139, 3542–3553. [DOI] [PubMed] [Google Scholar]
- Wharton K.A. Jr (2003) Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol., 253, 1–17. [DOI] [PubMed] [Google Scholar]
- Willert K., Brink,M., Wodarz,A., Varmus,H. and Nusse,R. (1997) Casein kinase 2 associates with and phosphorylates Dishevelled. EMBO J., 16, 3089–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-X., Yi,T., Tang,B. and Lambeth,J.D. (1998) Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene, 16, 1561–1569. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Moriguchi,T., Masuyama,N., Kusakabe,M., Hanafusa,H., Takada,R., Takada,S. and Nishida,E. (2002) JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep., 3, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Kishida,S., Kishida,M., Ikeda,S., Takada,S. and Kikuchi,A. (1999) Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J. Biol. Chem., 274, 10681–10684. [DOI] [PubMed] [Google Scholar]
- Yan D., Wallingford,J.B., Sun,T.-Q., Nelson,A.M., Sakanaka,C., Reinhard,C., Harland,R.M., Fantl,W.J. and Williams,L.T. (2001) Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc. Natl Acad. Sci. USA, 98, 3802–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S., van Leeuwen,F., Wodarz,A., Klingensmith,J. and Nusse,R. (1995) The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev., 9, 1087–1097. [DOI] [PubMed] [Google Scholar]
- Zhou J. and Hsieh,J.-T. (2001) The inhibitory role of DOC-2/DAB2 in growth factor receptor-mediated signal cascade. J. Biol. Chem., 276, 27793–27798. [DOI] [PubMed] [Google Scholar]